Abstract
Strategies targeting intracellular negative regulators such as immune checkpoint inhibitors (ICPIs) have demonstrated significant antitumor activity across a wide range of solid tumors. In the clinical practice, the radiological effect of immunotherapeutic agents has raised several more relevant and complex challenges for the determination of their imaging-based response at single patient level. Accordingly, it has been suggested that the conventional Response Evaluation Criteria in Solid Tumors assessment alone, based on dimensional evaluation provided by computed tomography (CT), tends to underestimate the benefit of ICPIs at least in a subset of patients, supporting the need of immune-related response criteria. Different from CT, very few data are available for the evaluation of immunotherapy by means of 18F-fluoro-2-deoxy-D-glucose positron emission tomography (FDG-PET). Moreover, since the antineoplastic activity of ICPIs is highly related to the activation of T cells against cancer cells, FDG accumulation might cause false-positive findings. Yet, discrimination between benign and malignant processes represents a huge challenge for FDG-PET in this clinical setting. Consequently, it might be of high interest to test the complex and variegated response to ICPIs by means of PET and thus it is worthwhile to ask if a similar introduction of immune-related PET-based criteria could be proposed in the future. Finally, PET might offer a new insight into the biology and pathophysiology of ICPIs thanks to a growing number of non-invasive immune-diagnostic approaches based on non-FDG tracers.
Keywords: Immune checkpoint inhibitors, Positron emission tomography, Computed tomography, 18F-fluoro-2-deoxy-D-glucose, Non-18F-fluoro-2-deoxy-D-glucose tracers
Core tip: In the clinical practice, the radiological interpretation of immunotherapy effects represents a huge challenge at single patient level. However, although the computed tomography-based response evaluation for immune checkpoint inhibitors (ICPIs) is feasible thanks to the introduction of immune-related response criteria, very few data are available for the potential role of 18F-fluoro-2-deoxy-D-glucose positron emission tomography (FDG-PET). Due to the intrinsic nature of FDG accumulation pathophysiology, it might be central to test the complex and variegated response to ICPIs by means of PET. Finally, PET might offer a new insight into the biology of ICPIs thanks to a growing number of non-invasive immune-diagnostic approaches based on non-FDG tracers.
TEXT
The function of the immune system is characterized by multiple checkpoints aiming to avoid its over-activation against healthy cells (self-tolerance)[1]. Cancer cells may take advantage of these checkpoints to escape detection by the immune system. Some of these checkpoints such as cytotoxic T-lymphocyte-associated antigen 4 (CTLA-4) and programmed cell death protein 1 (PD-1) have been extensively studied as targets in the frame of the so-called cancer immunotherapy[1]. CTLA-4 counteracts the activity of the T cell co-stimulatory receptor CD28 and actively delivers inhibitory signals to the T cell[2]. PD-1 has two ligands, PD1 ligand 1 (PDL1) and PDL2, and its inhibitory effect is accomplished through a dual mechanism of promoting apoptosis in antigen specific T-cells in lymph nodes while simultaneously reducing apoptosis in regulatory T cells (suppressor T cells)[3]. In the last few years, the blockade of immune checkpoints has disclosed the potential of the antitumor immune response in a fashion that is transforming human cancer therapeutics. CTLA4 antibodies such as ipilimumab and tremelimumab have been tested in the last ten years in different types of cancer, starting with patients with advanced melanoma[4]. Ipilimumab was the first therapy to demonstrate a survival benefit for patients with metastatic melanoma. In a study by Hodi et al[5], ipilimumab significantly improved overall survival in patients with previously treated metastatic melanoma and the drug was approved by the United States Food and Drug Administration (FDA) for the treatment of advanced melanoma in 2011[5]. Similarly, nivolumab, a humanized anti-PD-1 monoclonal antibody, has demonstrated durable responses in several phase III trials and has received FDA approval in specific clinical settings in patients with melanoma, renal cell cancer, Hodgkin’s lymphoma, bladder cancer, and non-small cell lung cancer (NSCLC)[6-9]. Figure 1 summarizes the mechanisms of action of the two FDA approved immune checkpoint inhibitors (ICPIs).
Figure 1.
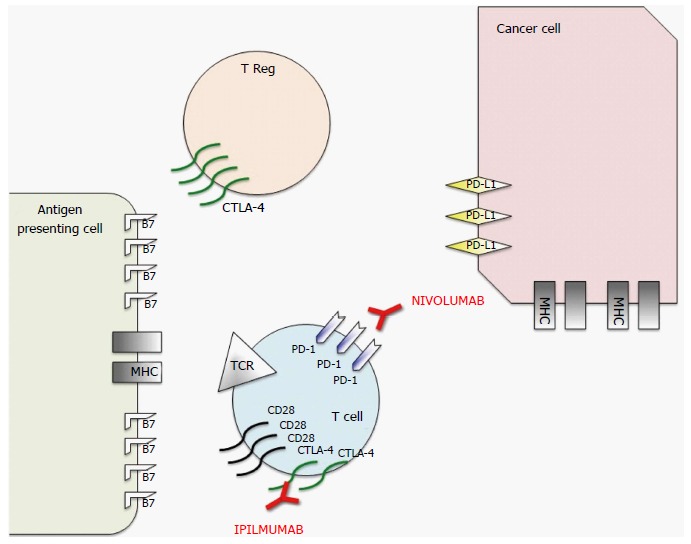
Schematic representation of mechanism of action of nivolumab and ipilimumab, two Food and Drug Administration approved immune checkpoint inhibitors. To prevent autoimmunity, numerous checkpoint pathways regulate the activation of T cells at multiple steps (process known as peripheral tolerance). Central in this process are the cytotoxic T-lymphocyte-associated antigen 4 (CTLA-4) and programmed death 1 (PD-1) immune checkpoints pathways. CTLA-4 is potentially able to stop autoreactive T cells at the initial stage of naive T-cell activation, typically in lymph nodes, while PD-1 regulates previously activated T cells at the later stages of an immune response in peripheral tissues. The binding between T-cell receptor (TCR), which is expressed on T cell surface, with major histocompatibility complex (MHC) expressed on antigen presenting cells (APCs) provides specificity to T-cell activation. However, T cell activation requires more than one stimulatory signal. Among them a central role is played by the binding between B7 molecules (APC) with CD28 (T-Cell). CTLA-4 is a CD28 homolog which does not produce a stimulatory signal but inhibits TCR-MHC binding and thus the T-Cell activation. Different from T-cells in which the amount of CTLA-4 is low, T-Regs highly express CTLA-4. In these cells CTLA-4 might play a role in their suppressive functions. PD-1 is a member of the B7/CD38 family of protein, which is able to bind with two different ligands: Programmed death ligand 1 (PD-L1) and programmed death ligand 2 (PD-L2). PD-1 activation in a T-cell prevents the phosphorylation of key TCR signaling intermediates and thus T-cell activation, resulting in suboptimal control of infections and cancers. Therefore, even though they act at different phases of T-cell activation, the negative effect of PD-1 and CTLA-4 on T-cell activity is similar. Moreover, different from CTLA-4, PD-1 expression is not specific in T-cells, but can be observed also in B-cells and myeloid cells. The rationale for immune checkpoint inhibition (represented in red) for cancer treatment is that CTLA-4 and PD1 pathways are strictly related to cancer survival and thus targeting these molecules or their ligands with monoclonal antibodies permits to impact on cancer growth. Therefore, even if the exact mechanism of action of these monoclonal antibodies in the antitumor response remains unclear, research data suggest that it is at least partially related to an activation and proliferation of T-cells regardless of TCR specificity (due to the inhibition of the inhibitory activity of these checkpoints), which enhances the anti-cancer immune reaction.
Evaluation of response to ICPIs
Historically, the Response Evaluation Criteria in Solid Tumors (RECIST) has been validated and used to evaluate antitumor responses to chemotherapeutic agents[10] (Table 1 for a more detailed description). These criteria are based on dimensional evaluation and rely on the fact that the cytotoxic effect of chemotherapeutic agents tends to translate into measurable effects in terms of tumor shrinkage from baseline. Furthermore, published studies indicated that achieving a response according to RECIST criteria is predictive of remission and improved survival in specific settings[11]. Conversely, both RECIST and their revised 1.1 version assumed that an early increase in tumor growth and/or the appearance of new lesions correspond to progressive disease (PD), testifying drug failure and indicating the need of ongoing treatment cessation[10].
Table 1.
Key features of positron emission tomography Response Criteria in Solid Tumors, European Organization for Research and Treatment of Cancer 1999, Response Evaluation Criteria in Solid Tumors 1.1 and immune related Response Criteria
| Category | PERCIST | EORTC 1999 | RECIST 1.1 | irRC |
| Target lesions | The hottest single tumor lesion (SUL peak) at baseline 18F-FDG PET | The most 18F-FDG-avid lesions (SUV BSA). Number of lesions not specified | Maximum, 5 | Maximum, 15 lesions |
| New lesion | Results in progressive disease at first appearance | Results in progressive disease at first appearance | Results in progressive disease at first appearance | Up to 10 new visceral and 5 cutaneous lesions may be added to the sum of the products of the two largest perpendicular diameters of all index lesions at any time point |
| Complete response | CMR: Complete resolution of 18F-FDG uptake within the target lesion (< mean liver activity and indistinguishable from background/blood pool and no new 18F-FDG-avid lesions) | CMR: Complete absence of 18F-FDG uptake | Disappearance of all target and nontarget lesions Nodes must regress to < 10 mm short axis No new lesions Confirmation required | |
| Partial response | PMR: A reduction of a minimum of 30% in the target tumor 18F- FDG SUL peak | PMR: A decrease in SUV > 25% | ≥ 30% decrease in tumor burden compared to baseline Confirmation required | ≥ 50% decrease in tumor burden compared with baseline1 Confirmation required |
| Progressive disease | PMD: A 30% increase in 18F-FDG SUL peak or advent of new 18F-FDG-avid lesions | PMD: An increase in SUV > 25% or appearance of new lesions | ≥ 20% + 5 mm absolute increase in tumor burden compared with nadir Appearance of new lesions or progression of nontarget lesions | ≥ 25% increase in tumor burden compared with baseline, nadir or reset baseline1 New lesions added to tumor burden Confirmation required |
| Stable disease | SMD: Disease other than CMR, PMR or PMD | SMD: Increase in SUV by < 25% or decrease in SUV by < 15% | Neither partial response nor progressive disease | |
If an increase in tumor burden is observed at the first scheduled assessment, the baseline is reset to the value observed at the first assessment. PERCIST: PET Response Criteria in Solid Tumors; EORTC: European Organization for Research and Treatment of Cancer; RECIST: Response Evaluation Criteria in Solid Tumors; irRC: Immune related Response Criteria; CMR: Complete metabolic response; PMR: Partial metabolic response; PMD: Progressive metabolic disease; SMD: Stable metabolic disease; SUL: SUV normalized to lean body mass; SUV BSA: SUV normalized for body surface area; SUV: Standardized uptake value.
Some exceptions for the use of these criteria have been already suggested in patients treated with target therapies such as tyrosine kinase inhibitors as in this group of patients the lack of tumor shrinkage in the presence of a stable disease has been identified as a potential surrogate end point for improved clinical outcome[12]. However, in the clinical practice, the radiological effect of immunotherapeutic agents has raised several more relevant and complex challenges for the determination of their imaging-based response at single patient level[13]. In published studies, while some patients have responded to ICPIs with the expected tumor shrinkage (chemo-like response) or with a stable disease (target therapy-like response), other distinct immune-related patterns of response have been identified. In particular, an initial increase in tumor size, development of new lesions and then a delayed objective response were also observed in patients treated with immunotherapeutic agents[13]. Specifically, in some patients, the initial increase in total tumor burden was proven to be due to inflammatory cell infiltrates by means of biopsy. In these patients the initial pseudo-progression was followed by a decrease in tumor burden or even disease regression.
As RECIST criteria were not suitable to catch these atypical responses, the so-called immune-related response criteria (irRC) have been proposed to provide more rigorous characterization of all patterns of response observed in the phase II development program for ipilimumab in melanoma[13-15] (Table 1). The main differences between RECIST 1.1 and irRC rely on the fact that, according to irRC, appearance of new lesions (PD according to the RECIST criteria) will only result in progressive disease in case of a significant (≥ 25%) increase in total tumor burden with respect to baseline. Moreover, different from conventional criteria, if irRC-based PD is evident, it requires further confirmation after one month with the aim of capturing delayed response.
Recently, Hodi et al[16] evaluated atypical response patterns and reported the overall survival data in correlation with irRC and RECIST criteria in the context of a retrospective analysis of 327 melanoma patients treated with the PD-1 inhibitor pembrolizumab. This study indicated that the conventional RECIST assessment alone tends to underestimate the benefit of PD-1 inhibitor therapy in a subset of patients, supporting a need of immune-related response evaluation[16]. IrRC are thus increasingly proposed, but they have not been validated yet in the context of clinical trials and most trials involving ICPIs continue to use RECIST 1.1 to obtain standardized endpoints for regulatory approvals[15].
However, although the irRC seem better than RECIST, the former has some limitations. The irRC use the bidimensional measurements in line with WHO criteria that are now rarely used in clinical trials and replaced by the unidimensional measurement of the larger axis of target lesions (RECIST 1.0 and 1.1). The bidimensional measurements introduce a greater variability than unidimensional measurements and make it difficult to compare the responses with studies using the RECIST criteria.
Is there a role for 18F-fluoro-2-deoxy-D-glucose positron emission tomography in the evaluation of ICPIs?
While optimal CT-based response criteria for ICPIs are in the path of their identification, very few data are available for the evaluation of immunotherapy by means of 18F-fluoro-2-deoxy-D-glucose positron emission tomography (18F-FDG-PET), one of the most used imaging techniques in oncology. 18F-FDG-PET is currently the most widely used molecular imaging modality in the clinical practice for staging and restaging of several cancers. 18F-FDG-PET is clinically indicated before and after treatment in patients with Hodgkin’s lymphoma and NSCLC and it is used in patients with melanoma for specific clinical indications[17-19]. The use of 18F-FDG-PET in post-treatment settings is based on the assumption that tumor size changes are only the final step in a sequence of complex metabolic and functional processes during and after treatment[20]. Two different types of criteria have been proposed for the identification of 18F-FDG-PET-based response in solid tumors: The European Organization for Research and Treatment of Cancer (EORTC) and the PET Response Criteria in Solid Tumors (PERCIST) criteria[21,22] (Table 1). Both criteria target the most metabolically active part of patient’s tumor burden, which is regarded as the most viable and aggressive disease site. In both cases, the so-called standardized uptake value (SUV) is measured at baseline and after treatment. However, they differ for some relevant aspects. The EORTC criteria were published in 1999 and are based on the evaluation of a lesion-specific region of interest (ROI) chosen as the most 18F-FDG-avid at baseline and followed in the after-treatment scans[22]. The PERCIST criteria were proposed in 2009 by Wahl et al[21] and rely on the use of a 1 cm3 ROI on the most 18F-FDG-avid part of the single most metabolically active lesion at each PET/CT scan (which is not necessarily located in the same lesion in all scans).
Relatively few papers have compared the two methods in solid tumors and good agreement, similar responses and survival outcomes have been highlighted in the available studies[23]. However, for the EORTC criteria, no recommendations on the number of target lesions or on whether computing SUV max or average SUV for response calculation are given while the PERCIST criteria recommend the use of lean body mass for SUV normalization (SUL). In this framework, some studies have demonstrated a higher accuracy with respect to RECIST for both metabolic response based criteria in patients treated with target therapies such as erlotinib. This finding is due to the relative lower tumor shrinkage characterizing this type of treatment[24]. Similarly, an 18F-FDG-PET-based five-point scale (5-PS), the so-called Deauville criteria, has been demonstrated to be superior to CT-based response by scoring images in the assessment of response at the middle and end of treatment in HD patients[18]. Again these findings testify that functional changes always precede morphological changes in the course of pathological processes. In this regard it might be of interest to test the complex and variegated response to ICPIs by means of PET-based criteria. In fact, on one hand, functional imaging may capture different features of treatment with ICPIs in terms of entity and time course of response. On the other hand, it has been reported that the initial increase in tumor size, later followed by tumor volume reduction in part of the patients treated with ICPIs, is due to inflammatory cell infiltrates. Accordingly, given the well-known high metabolic activity characterizing inflammatory cells, this feature may also hamper the evaluation of 18F-FDG-PET-based response to ICPIs. Sachpekidis et al[20] evaluated the role of 18F-FDG-PET/CT after two cycles of ipilimumab in predicting the final response to therapy in 22 patients with metastatic melanoma. They evaluated response to treatment by means of the EORTC criteria and found that 18F-FDG-PET/CT after two cycles of ipilimumab is predictive of the final treatment outcome in patients with progressive metabolic disease (PMD) and stable metabolic disease (SMD)[20]. However, two patients were initially falsely classified as early SMD, but they later demonstrated new metastatic lesions, “upgrading” them to late PMD. Similarly, early evaluation by means of 18F-FDG-PET did not identify responders to treatment as the two patients eventually characterized with PMR were initially classified with early PMD due to new lesions[20]. In fact, both RECIST 1.1 and PET-based criteria consider the identification of new (metabolically active) lesions as progressive disease. Therefore, presently proposed PET-based metabolic criteria suffer from at least one of the same limitations that have resulted in the underestimation of response to treatment with ICPIs by means of RECIST 1.1. Similarly, in the phase 2 study by Younes et al[9], nivolumab resulted in frequent responses in patients with classical Hodgkin’s lymphoma after failure of ASCT and brentuximab vedotin. Most of these responses were maintained through the reported follow-up period with an acceptable safety profile. In this study 18F-FDG-PET was performed at baseline and at weeks 17 and 25. A negative 18F-FDG-PET scan, visually assessed by an independent radiological review committee (IRRC), was required for confirmation of complete remission. The study demonstrated a general reduction of tumor burden. Yet, discordance in complete remission between IRRC and investigator assessments was largely based on the interpretation of 18F-FDG-PET scans and standardized uptake values were not collected as part of this study. The vast majority of other available data on the potential utility of 18F-FDG-PET after ICPIs are case reports more often describing underlying challenges of monitoring radiologic response in these patients and showing 18F-FDG-PET features of inflammatory reactions. PET-highlighted autoimmune pancolitis, splenic sarcoidosis-like lesion and exacerbation of sarcoidosis as a potential confounder in the assessment of tumor response in a melanoma patient treated with ipilimumab have all been described[25-27]. Similarly, Koo et al[26] illustrated a series of inflammatory reactions with avid FDG uptake in patients treated with ipilimumab, including those with thyroiditis, hypophysitis, granulomatous inflammation in the lymph nodes and skin, and enterocolitis.
Accordingly, the potential and challenges of 18F-FDG-PET imaging in the evaluation of patients treated with ICPIs still need to be clarified and deeply addressed. Given the relatively greater experience of CT-based evaluation in this setting and the fact that irRC CT-based criteria seem to better in capturing response to ICPIs, it is worthwhile to ask if a similar modification of PET-based criteria could be proposed in the future.
Potential new PET-based approaches to evaluate the effect of ICPIs
As mentioned above, due to its intrinsic nature, 18F-FDG-PET displays not only cancer cell’s metabolic activity but also inflammation. Since the antineoplastic activity of ICPIs is highly related to the activation of T cells against cancer cells, 18F-FDG accumulation might cause false-positive findings. Yet, discrimination between benign and malignant processes represents a huge challenge for 18F-FDG-PET in this clinical setting. Together with the need of the clinicians to discriminate between responders and non-responders, allowing individual therapy optimization and avoiding adverse effects brought about by ineffective therapy, several studies have been recently conducted to explore the possible role of non-FDG radiotracers in the field of ICPIs. These studies, mainly performed with labeled monoclonal antibodies, open the new era of the so-called “Immuno-PET”. Accordingly, in 2014, Higashikawa et al[28] developed a molecular imaging probe that is able to evaluate CTLA-4 expression prior to CTLA-4 targeting in cancer. This 64Cu labeled radiotracer is basically composed of DOTA protein together with a CTLA-4 specific antibody and is able to display CTLA-4 expression in vivo. Similarly, specific experimental radiotracers were proposed for the visualization of PD-1 and PD-L1 cellular expression[29-32]. Maute et al[29] measured PD-L1 expression by radiolabeling a PD-L1 high affinity protein (HAC) with 64Cu and tested its feasibility in a living mouse, while Hettich et al[30] developed two 64Cu labeled immunoPET tracers for imaging of both PD-1 and PD-L1. Also one SPECT study with radiolabeled anti-murine PD-L1 in mice has been conducted[32]. More recently, a 89Zr labeled CD3 PET imaging agent was proposed by Larimer et al[33]. CD3 is a part of the TCR complex that serves as a global T lymphocyte marker. By serving as a marker of total T-cell infiltration, CD3 may represent a more direct approach than pre-treatment biopsy or genetic screening to monitoring tumor immune response, by directly examining active recruitment of T cells responsible for cancer cell death. In this study the authors showed that CD3 PET imaging revealed two distinct groups of mice, stratified by PET signal intensity. While high-CD3 PET uptake was correlated with subsequent reduced tumor volume, low uptake was predictive of suboptimal response. Altogether these non-invasive approaches allow simultaneous imaging of the entire cancer mass and associated metastases, which may differ from the primary tumor in CTLA-4, PD-1 or PD-L1 expression status. Immune imaging can be used for repeated assessment of the same tumor at different time points (e.g., before and after treatment), thereby yielding a richer set of diagnostic information that would be difficult or impossible to achieve with traditional approaches. Furthermore, although further investigations are needed before their potential introduction in the clinical setting, these non-invasive immune-diagnostic approaches might yield novel insights into the biology and pathophysiological importance of ICPIs as cancer therapeutics.
Footnotes
Conflict-of-interest statement: The authors have no conflicts of interest related to this publication to disclose.
Manuscript source: Invited manuscript
Specialty type: Radiology, nuclear medicine and medical imaging
Country of origin: Italy
Peer-review report classification
Grade A (Excellent): A, A
Grade B (Very good): 0
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
Peer-review started: August 23, 2016
First decision: October 21, 2016
Article in press: November 29, 2016
P- Reviewer: Morris DLL, Palumbo B S- Editor: Ji FF L- Editor: Wang TQ E- Editor: Wu HL
References
- 1.Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12:252–264. doi: 10.1038/nrc3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Walunas TL, Lenschow DJ, Bakker CY, Linsley PS, Freeman GJ, Green JM, Thompson CB, Bluestone JA. CTLA-4 can function as a negative regulator of T cell activation. Immunity. 1994;1:405–413. doi: 10.1016/1074-7613(94)90071-x. [DOI] [PubMed] [Google Scholar]
- 3.Francisco LM, Sage PT, Sharpe AH. The PD-1 pathway in tolerance and autoimmunity. Immunol Rev. 2010;236:219–242. doi: 10.1111/j.1600-065X.2010.00923.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.O’Day SJ, Hamid O, Urba WJ. Targeting cytotoxic T-lymphocyte antigen-4 (CTLA-4): a novel strategy for the treatment of melanoma and other malignancies. Cancer. 2007;110:2614–2627. doi: 10.1002/cncr.23086. [DOI] [PubMed] [Google Scholar]
- 5.Hodi FS, O’Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, Gonzalez R, Robert C, Schadendorf D, Hassel JC, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Giri A, Walia SS, Gajra A. Clinical Trials Investigating Immune Checkpoint Inhibitors in Non-Small-Cell Lung Cancer. Rev Recent Clin Trials. 2016;11:297–305. doi: 10.2174/1574887111666160724181330. [DOI] [PubMed] [Google Scholar]
- 7.Carlo MI, Voss MH, Motzer RJ. Checkpoint inhibitors and other novel immunotherapies for advanced renal cell carcinoma. Nat Rev Urol. 2016;13:420–431. doi: 10.1038/nrurol.2016.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ball MW, Allaf ME, Drake CG. Recent advances in immunotherapy for kidney cancer. Discov Med. 2016;21:305–313. [PubMed] [Google Scholar]
- 9.Younes A, Santoro A, Shipp M, Zinzani PL, Timmerman JM, Ansell S, Armand P, Fanale M, Ratanatharathorn V, Kuruvilla J, et al. Nivolumab for classical Hodgkin’s lymphoma after failure of both autologous stem-cell transplantation and brentuximab vedotin: a multicentre, multicohort, single-arm phase 2 trial. Lancet Oncol. 2016;17:1283–1294. doi: 10.1016/S1470-2045(16)30167-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 11.von Minckwitz G, Sinn HP, Raab G, Loibl S, Blohmer JU, Eidtmann H, Hilfrich J, Merkle E, Jackisch C, Costa SD, et al. Clinical response after two cycles compared to HER2, Ki-67, p53, and bcl-2 in independently predicting a pathological complete response after preoperative chemotherapy in patients with operable carcinoma of the breast. Breast Cancer Res. 2008;10:R30. doi: 10.1186/bcr1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tsujino K, Shiraishi J, Tsuji T, Kurata T, Kawaguchi T, Kubo A, Takada M. Is response rate increment obtained by molecular targeted agents related to survival benefit in the phase III trials of advanced cancer? Ann Oncol. 2010;21:1668–1674. doi: 10.1093/annonc/mdp588. [DOI] [PubMed] [Google Scholar]
- 13.Wolchok JD, Hoos A, O’Day S, Weber JS, Hamid O, Lebbé C, Maio M, Binder M, Bohnsack O, Nichol G, et al. Guidelines for the evaluation of immune therapy activity in solid tumors: immune-related response criteria. Clin Cancer Res. 2009;15:7412–7420. doi: 10.1158/1078-0432.CCR-09-1624. [DOI] [PubMed] [Google Scholar]
- 14.Hodi FS, Sznol M, Kluger HM, McDermott DF, Carvajal RD, Lawrence DP, Topalian SL, Atkins MB, Powderly JD, Sharfman WH, et al. Long term survival of ipilimumab-naive patients (pts) with advanced melanoma (MEL) treated with nivolumab (anti-PD-1, BMS-936558, ONO-4538) in a phase I trial. ASCO Annual Meeting 2014 May 30- Jun 3; Chicago, Illinois, USA. J Clin Oncol. 2014;32:5s (suppl; abstr 9002). [Google Scholar]
- 15.Chiou VL, Burotto M. Pseudoprogression and Immune-Related Response in Solid Tumors. J Clin Oncol. 2015;33:3541–3543. doi: 10.1200/JCO.2015.61.6870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hodi FS, Hwu WJ, Kefford R, Weber JS, Daud A, Hamid O, Patnaik A, Ribas A, Robert C, Gangadhar TC, et al. Evaluation of Immune-Related Response Criteria and RECIST v1.1 in Patients With Advanced Melanoma Treated With Pembrolizumab. J Clin Oncol. 2016;34:1510–1517. doi: 10.1200/JCO.2015.64.0391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gould MK, Donington J, Lynch WR, Mazzone PJ, Midthun DE, Naidich DP, Wiener RS. Evaluation of individuals with pulmonary nodules: when is it lung cancer? Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2013;143:e93S–120S. doi: 10.1378/chest.12-2351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cheson BD, Fisher RI, Barrington SF, Cavalli F, Schwartz LH, Zucca E, Lister TA. Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: the Lugano classification. J Clin Oncol. 2014;32:3059–3068. doi: 10.1200/JCO.2013.54.8800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Morbelli S, Capitanio S, De Carli F, Bongioanni F, De Astis E, Miglino M, Verardi MT, Buschiazzo A, Fiz F, Marini C, et al. Baseline and ongoing PET-derived factors predict detrimental effect or potential utility of 18F-FDG PET/CT (FDG-PET/CT) performed for surveillance in asymptomatic lymphoma patients in first remission. Eur J Nucl Med Mol Imaging. 2016;43:232–239. doi: 10.1007/s00259-015-3164-9. [DOI] [PubMed] [Google Scholar]
- 20.Sachpekidis C, Larribere L, Pan L, Haberkorn U, Dimitrakopoulou-Strauss A, Hassel JC. Predictive value of early 18F-FDG PET/CT studies for treatment response evaluation to ipilimumab in metastatic melanoma: preliminary results of an ongoing study. Eur J Nucl Med Mol Imaging. 2015;42:386–396. doi: 10.1007/s00259-014-2944-y. [DOI] [PubMed] [Google Scholar]
- 21.Wahl RL, Jacene H, Kasamon Y, Lodge MA. From RECIST to PERCIST: Evolving Considerations for PET response criteria in solid tumors. J Nucl Med. 2009;50 Suppl 1:122S–150S. doi: 10.2967/jnumed.108.057307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Young H, Baum R, Cremerius U, Herholz K, Hoekstra O, Lammertsma AA, Pruim J, Price P. Measurement of clinical and subclinical tumour response using [18F]-fluorodeoxyglucose and positron emission tomography: review and 1999 EORTC recommendations. European Organization for Research and Treatment of Cancer (EORTC) PET Study Group. Eur J Cancer. 1999;35:1773–1782. doi: 10.1016/s0959-8049(99)00229-4. [DOI] [PubMed] [Google Scholar]
- 23.Skougaard K, Nielsen D, Jensen BV, Hendel HW. Comparison of EORTC criteria and PERCIST for PET/CT response evaluation of patients with metastatic colorectal cancer treated with irinotecan and cetuximab. J Nucl Med. 2013;54:1026–1031. doi: 10.2967/jnumed.112.111757. [DOI] [PubMed] [Google Scholar]
- 24.Stefano A, Russo G, Ippolito M, Cosentino S, Murè G, Baldari S, Sabini MG, Sardina D, Valastro LM, Bordonaro R, et al. Evaluation of erlotinib treatment response in non-small cell lung cancer using metabolic and anatomic criteria. Q J Nucl Med Mol Imaging. 2014 May 9; Epub ahead of print. [PubMed] [Google Scholar]
- 25.Goethals L, Wilgenhof S, De Geeter F, Everaert H, Neyns B. 18F-FDG PET/CT imaging of an anti-CTLA-4 antibody-associated autoimmune pancolitis. Eur J Nucl Med Mol Imaging. 2011;38:1390–1391. doi: 10.1007/s00259-011-1749-5. [DOI] [PubMed] [Google Scholar]
- 26.Koo PJ, Klingensmith WC, Lewis KD, Bagrosky BM, Gonzalez R. Anti-CTLA4 antibody therapy related complications on FDG PET/CT. Clin Nucl Med. 2014;39:e93–e96. doi: 10.1097/RLU.0b013e318292a775. [DOI] [PubMed] [Google Scholar]
- 27.Perng P, Marcus C, Subramaniam RM. (18)F-FDG PET/CT and Melanoma: Staging, Immune Modulation and Mutation-Targeted Therapy Assessment, and Prognosis. AJR Am J Roentgenol. 2015;205:259–270. doi: 10.2214/AJR.14.13575. [DOI] [PubMed] [Google Scholar]
- 28.Higashikawa K, Yagi K, Watanabe K, Kamino S, Ueda M, Hiromura M, Enomoto S. 64Cu-DOTA-anti-CTLA-4 mAb enabled PET visualization of CTLA-4 on the T-cell infiltrating tumor tissues. PLoS One. 2014;9:e109866. doi: 10.1371/journal.pone.0109866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Maute RL, Gordon SR, Mayer AT, McCracken MN, Natarajan A, Ring NG, Kimura R, Tsai JM, Manglik A, Kruse AC, et al. Engineering high-affinity PD-1 variants for optimized immunotherapy and immuno-PET imaging. Proc Natl Acad Sci USA. 2015;112:E6506–E6514. doi: 10.1073/pnas.1519623112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hettich M, Braun F, Bartholomä MD, Schirmbeck R, Niedermann G. High-Resolution PET Imaging with Therapeutic Antibody-based PD-1/PD-L1 Checkpoint Tracers. Theranostics. 2016;6:1629–1640. doi: 10.7150/thno.15253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Heskamp S, Hobo W, Molkenboer-Kuenen JD, Olive D, Oyen WJ, Dolstra H, Boerman OC. Noninvasive Imaging of Tumor PD-L1 Expression Using Radiolabeled Anti-PD-L1 Antibodies. Cancer Res. 2015;75:2928–2936. doi: 10.1158/0008-5472.CAN-14-3477. [DOI] [PubMed] [Google Scholar]
- 32.Josefsson A, Nedrow JR, Park S, Banerjee SR, Rittenbach A, Jammes F, Tsui B, Sgouros G. Imaging, Biodistribution, and Dosimetry of Radionuclide-Labeled PD-L1 Antibody in an Immunocompetent Mouse Model of Breast Cancer. Cancer Res. 2016;76:472–479. doi: 10.1158/0008-5472.CAN-15-2141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Larimer BM, Wehrenberg-Klee E, Caraballo A, Mahmood U. Quantitative CD3 PET Imaging Predicts Tumor Growth Response to Anti-CTLA-4 Therapy. J Nucl Med. 2016;57:1607–1611. doi: 10.2967/jnumed.116.173930. [DOI] [PMC free article] [PubMed] [Google Scholar]


