Introduction
Adalimumab is a recombinant human IgG1 monoclonal antibody specific for human tumor necrosis factor. We report a case of recurrent erythema multiforme (EM) recalcitrant to previous therapy that showed complete response to adalimumab.
Case report
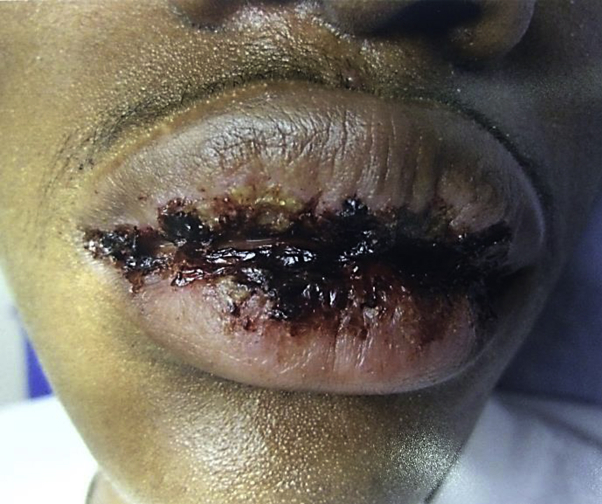
An African-American man in his late teens with glucose-6-phosphate dehydrogenase deficiency presented with a 2-year history of recurrent EM. Lesions waxed and waned in timing and intensity with recurrent palm, sole, and oral mucosal lesions most characteristic. The patient was experiencing separate episodes every 6 to 8 weeks of variable duration and would heal between flares. At his baseline dermatologic examination, the patient had multiple violaceous targetoid macules over his bilateral palms, soles, and volar forearms. Additionally, the patient had erosion and hemorrhagic crusting of the lips with additional presence of scattered buccal mucosal erosions (Fig 1). Biopsy findings of a targetoid macule were histologically consistent with EM. The patient had a documented history of a tender vesicular eruption of the upper labial mucosa consistent with herpes labialis treated with valacyclovir. He additionally tested positive for herpes simplex virus (HSV)-1 by plasma polymerase chain reaction (PCR) but did not have viral culture or lesional PCR completed. Further workup was negative for mycoplasma by both PCR and blood titers. Other potential etiologies of recurrent oral erosions and cutaneous lesions were considered in the workup of this patient, but he was otherwise healthy, not on any medications or supplements, and followed up with by a general pediatrician. Workup by a pediatric rheumatologist found no autoimmune or inflammatory etiologies on examination or through laboratory testing that could have contributed to this patient's findings.
Fig 1.
Recurrent EM in a teenage male at initial presentation.
Prior unsuccessful treatment regimens included suppressive-dose valacyclovir therapy over several months and multiple oral steroid regimens to control flares. Topical steroids were also trialed without success. The patient was on daily high-dose prednisone on presentation to our clinic. When the prednisone was tapered, the rash and mucositis were reproducible to the point that oral intake was severely limited.
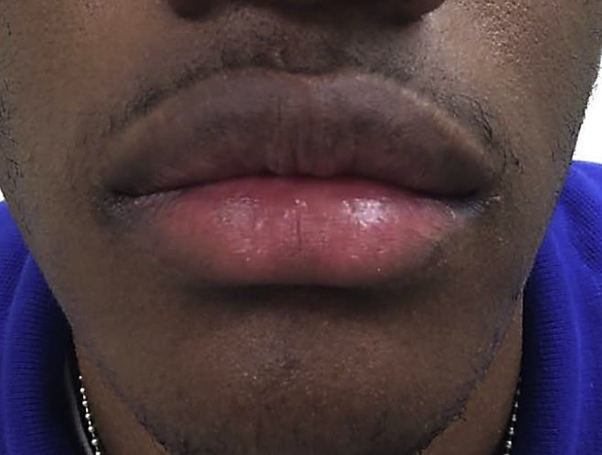
After lengthy discussions with the patient and family regarding further therapeutic options, the patient was started on a trial of adalimumab. Although other treatment options have more published data, adalimumab was ultimately chosen because of patient and parent desire to avoid oral medications, ease of injections, and the proposed mechanism of cytokine interaction. A dose of 40 mg subcutaneously every other week was initiated, and his current daily prednisone dose was stopped. After the initial injection, the patient showed rapid and complete clearance of all active cutaneous and mucosal lesions (Fig 2). Clearance was then maintained without cyclical flaring over a 6-month period with continued alternate week injections. At 6 months, the patient discontinued adalimumab because of lack of insurance coverage, and episodic flaring characterized by targetoid lesions and mucositis returned. Since then he was treated with mycophenolate mofetil and suppressive dose valacyclovir with continued intermittent flaring.
Fig 2.
Resolution of EM after initiation of adalimumab.
Discussion
EM is considered a hypersensitivity reaction with several inciting factors including medications and infections. HSV and mycoplasma pneumonia comprise the most common infectious etiologies and triggers of recurrent EM. It comprises a spectrum of clinical disease ranging from limited cutaneous involvement (EM minor) to more diffuse cutaneous and mucosal involvement (EM major). Although once thought to be on a spectrum with EM, the widespread body surface and mucosal involvement of Stevens-Johnson syndrome (SJS) and toxic epidermal necrolysis (TEN) are now considered by many to be distinct clinical entities.
EM can be broken down mechanistically into herpes-associated EM (HAEM) and drug-induced EM (DIEM).1, 2 Further elucidation and studies show that HAEM lesions are predominantly a T-helper 1–driven and interferon-γ–driven delayed type hypersensitivity process, whereas DIEM is a mechanistically distinct macrophage and tumor necrosis factor (TNF)-α–driven process.3 A similar process of a cell-poor infiltrate with macrophages and strong immunoreactivity for TNF-α is thought to contribute to the mechanistic processes of SJS and TEN.4 This understanding has led to the use of successful TNF-α–targeted biologics in the treatment of SJS and TEN.5, 6, 7
Initial treatment of EM minor has involved infection control including viral suppression in HAEM and treatment of bacterial infection in cases of mycoplasma pneumonia–associated EM. In recalcitrant to treatment or robust recurrences, immunosuppressive measures have also been used. Case reports and small case series in the literature highlight the use of different agents with variable degrees of success. The rapid, complete, and sustained clearance with a TNF-α inhibitor was somewhat surprising in our case given the well-described interferon-γ–driven pathway of viral-associated EM. Although the final cytokine contribution and interaction in EM is not fully elucidated, we postulate the interference with TNF-α could contribute to clearance in refractory cases of both HAEM and DIEM.
We present a case of recurrent EM considered secondary to HSV that was successfully treated with adalimumab. Several alternate therapeutic agents would have been applicable in our case, but the patient's and parent's desires to stop oral treatment out of frustration led to our decision to trial adalimumab. Caution must also be exercised when initiating treatment of EM with adalimumab, as no controlled trials have been completed, and hypersensitivity reactions including EM and SJS have been attributed to adalimumab in the literature.8, 9 In our case, treatment with adalimumab resulted in a rapid and complete clearance. Although prior studies suggested distinctive mechanisms, perhaps the final cell and cytokine interactions are not fully elucidated. Additional studies could prove beneficial for further clarification and understanding.
Footnotes
Funding sources: None.
Conflicts of interest: None declared.
References
- 1.Bastuji-Garin S., Rzany B., Stern R.S. Clinical classification of cases of toxic epidermal necrolysis, Stevens-Johnson syndrome, and erythema multiforme. Arch Dermatol. 1993;129(1):92–96. [PubMed] [Google Scholar]
- 2.Carrozzo M, Togliatto M, Gandolfo Erythema multiforme. A heterogeneous pathologic phenotype. Minerva Stomatol. 1999;48(5):217–226. [PubMed] [Google Scholar]
- 3.Kokuba H., Aurelian L., Burnett J. Herpes simplex virus associated erythema multiforme (HAEM) is mechanistically distinct from drug-induced erythema multiforme: interferon-gamma is expressed in HAEM lesions and tumor necrosis factor-alpha in drug-induced erythema multiforme lesions. J Invest Dermatol. 1999;113(5):808–815. doi: 10.1046/j.1523-1747.1999.00754.x. [DOI] [PubMed] [Google Scholar]
- 4.Paquet P., Nikkels A., Arrese J.E. Macrophages and tumor necrosis factor alpha in toxic epidermal necrolysis. Arch Dermatol. 1994;130(5):605–608. [PubMed] [Google Scholar]
- 5.Paradisi A., Abeni D., Bergamo F. Etanercept therapy for toxic epidermal necrolysis. J Am Acad Dermatol. 2014;71(2):278–283. doi: 10.1016/j.jaad.2014.04.044. [DOI] [PubMed] [Google Scholar]
- 6.Zarate-Correa L.K., Carrilo-Gomez D.C., Ramirez-Escobar A.F., Serrano-Reyes C. Toxic epidermal necrolysis successfully treated with infliximab. J Investig Allergol Clin Immunol. 2013;23(1):50–73. [PubMed] [Google Scholar]
- 7.Gubinelli E., Canzona F., Tonanzi T., Raskovic D., Didona B. Toxic epidermal necrolysis successfully treated with etanercept. J Dermatol. 2009;36(3):150–153. doi: 10.1111/j.1346-8138.2009.00616.x. [DOI] [PubMed] [Google Scholar]
- 8.Ahdout J., Haley J.C., Chiu M.W. Erythema multiforme during anti-tumor necrosis factor treatment for plaque psoriasis. J Am Acad Dermatol. 2010;62(5):874–879. doi: 10.1016/j.jaad.2009.04.048. [DOI] [PubMed] [Google Scholar]
- 9.Mounach A., Rezqi A., Nouijai A., Ghozlani I., Achemlal L., Maghraoui A.E., Bezza A. Stevens-Johnson syndrome complicating adalimumab therapy in rheumatoid arthritis disease. Rheumatol Int. 2013;33(5):1351–1353. doi: 10.1007/s00296-011-2212-4. [DOI] [PubMed] [Google Scholar]