Summary
Aging is characterized by genome instability, which contributes to cancer formation and cell lethality leading to organismal decline. The high levels of DNA double‐strand breaks (DSBs) observed in old cells and premature aging syndromes are likely a primary source of genome instability, but the underlying cause of their formation is still unclear. DSBs might result from higher levels of damage or repair defects emerging with advancing age, but repair pathways in old organisms are still poorly understood. Here, we show that premeiotic germline cells of young and old flies have distinct differences in their ability to repair DSBs by the error‐free pathway homologous recombination (HR). Repair of DSBs induced by either ionizing radiation (IR) or the endonuclease I‐SceI is markedly defective in older flies. This correlates with a remarkable reduction in HR repair measured with the DR ‐white DSB repair reporter assay. Strikingly, most of this repair defect is already present at 8 days of age. Finally, HR defects correlate with increased expression of early HR components and increased recruitment of Rad51 to damage in older organisms. Thus, we propose that the defect in the HR pathway for germ cells in older flies occurs following Rad51 recruitment. These data reveal that DSB repair defects arise early in the aging process and suggest that HR deficiencies are a leading cause of genome instability in germ cells of older animals.
Keywords: aging, double‐strand break repair, Drosophila melanogaster, homologous recombination, Rad51
Introduction
Genome instability and DNA damage are hallmarks of old cells (reviewed in Gorbunova & Seluanov, 2016). More specifically, aging is accompanied by increase in chromosome rearrangements (Ramsey et al., 1995; Dolle et al., 1997; Tucker et al., 1999), oxidative DNA damage (Hamilton et al., 2001), and DNA double‐strand breaks (DSBs) (Sedelnikova et al., 2004). Similarly, cells from patients affected by premature aging syndromes, such as Hutchinson–Gilford progeria syndrome and restrictive dermopathy, are characterized by high level of DSBs (Liu et al., 2005). At the same time, mutations in several DSB repair components lead to premature aging syndromes, such as ataxia telangiectasia and Werner syndrome (Savitsky et al., 1995; Yu et al., 1996; Gorbunova & Seluanov, 2016), and DSB induction in mouse tissues leads to aging phenotypes (White et al., 2015). These studies suggest that DSB repair defects might be a driving force for aging. Whether the high level of DSBs detected in old cells represents higher incidence of damage or diminished capacity for repair as the organism ages is still unclear.
Most DSBs result from endogenous cellular by‐products, such as free radicals and single‐strand breaks, the latter of which are converted to DSBs during replication. Accurate repair of DSBs is accomplished by homologous recombination (HR), in which a homologous DNA sequence is used as a template to restore the information lost at the break. HR repair is initiated by 5′ to 3′ resection of the broken double‐strand, which is facilitated by the Mre11‐Rad50‐Nbs1 complex and CtIP, resulting in 3′ protruding single‐stranded DNA (ssDNA) (Chiolo et al., 2011; Nimonkar et al., 2011). Rad51 is recruited to this ssDNA substrate, and the resulting nucleoprotein filament guides homology search and strand invasion (Sugawara et al., 1995; McIlwraith et al., 2000). Invasion into the donor sequence on the sister chromatid or the homologous chromosome results in the formation of a D‐loop, after which Rad51 is removed to ensure DNA synthesis and HR progression (Williams & Michael, 2010). Components that facilitate Rad51 disassembly include yeast and human Rad54 (Li & Heyer, 2009; Wright & Heyer, 2014), and the C. elegans Rad51 paralog RFS‐1 and DNA helicase HELQ‐1 (Ward et al., 2010). After repair synthesis, the double‐Holliday junction (dHJ) and synthesis‐dependent strand annealing (SDSA) pathways of HR diverge. During dHJ, the D‐loop is extended to form two Holliday junctions that can be resolved into either a crossover or a non‐crossover product (Szostak et al., 1983). In contrast, during SDSA the newly synthesized strand of the D‐loop dissociates from the donor sequence and re‐ligates to the second end of the original DSB, resulting exclusively in a non‐crossover product. Meiosis relies primarily on dHJ repair, while SDSA is the preferred pathway for mitotically dividing cells, and both pathways are largely error‐free. Furthermore, both homologous chromosomes and sister chromatids can be used as templates for repair, but the sister chromatid is the preferred template in S/G2 cell cycle phases of mitotically dividing cells (Rothkamm et al., 2003; Janssen et al., 2016). While several studies revealed that error‐prone DSB repair pathways become defective with age (Ren & Pena de Ortiz, 2002; Seluanov et al., 2004; Preston et al., 2006a; Vaidya et al., 2014), the impact of aging on HR in cultured cells and in vivo is still controversial (Hendricks et al., 2003; Preston et al., 2006b; Mao et al., 2012; White et al., 2013; Sukup‐Jackson et al., 2014).
Premeiotic cells of Drosophila melanogaster are an excellent system to study the effects of aging on HR. These dividing cells of adult flies are subjected to age‐related changes and mortality (Wallenfang et al., 2006), they largely rely on HR for DSB repair (Rong & Golic, 2003; Chan et al., 2011), and repair outcomes are easily detectable in the progeny (Rong & Golic, 2003; Preston et al., 2006b; Johnson‐Schlitz et al., 2007; Chan et al., 2011; Do et al., 2014). Further, those are arguably among the most important cells of adult organisms as their genome integrity is necessary for producing viable and healthy progeny. Lastly, the male germline develops early during embryogenesis and begins meiosis at pupariation (Lindsley, 1976). Thus, spermatogonia are fully developed by eclosure of adults, providing an ideal cell population to study the effects of aging on DSB repair starting from very ‘young’ adults – as early as 1 day old (d.o.) (Boyle et al., 2007; Toledano et al., 2012).
Previous studies in Drosophila premeiotic germ cells suggested that interhomolog HR repair is more efficient in older flies, potentially ruling out defective HR as a contributor to genome instability and repair defects in aging (Preston et al., 2006b). This study also demonstrated that ‘error‐prone’ repair pathways (i.e., nonhomologous end joining (NHEJ) and single‐strand annealing (SSA)) prevail in young flies, suggesting that, unexpectedly, DNA repair becomes more accurate as the organism ages (Preston et al., 2006b).
While we were able to recapitulate these results in a model of constitutive DSB induction, here we challenge this larger conclusion, showing that aging results in a remarkable defect in repairing ionizing radiation (IR)‐ or endonuclease‐induced DSBs in spermatogonia. Strikingly, induction of DSBs in the DR‐white reporter (Do et al., 2014) at various ages reveals a significant decrease in intrachromosomal or intersister chromatid HR in the spermatogonia of older organisms (8 d.o. and older), revealing a very early effect on the proficiency of HR repair. Interestingly, this defect correlates with higher expression of early HR components, and increased Rad51 recruitment to repair sites. This not only suggests that HR defects may be caused by deregulation of repair steps following Rad51 recruitment, but also that excessive Rad51 recruitment might contribute to this phenotype. Contrary to previous conclusions, this study uncovers a dramatic effect of aging in the ability of premeiotic cells to complete HR repair, suggesting HR deregulation as a major source of age‐dependent genomic instability and cell lethality in the male germline.
Results
Aging results in defective repair of IR‐induced DSBs in premeiotic germ cells
We investigated the efficiency of DSB repair in premeiotic germ cells (Fig. 1A) of male flies during aging, by determining the kinetics of DSB formation and resolution following IR (Chiolo et al., 2011). Testes were dissected and fixed at different time points after IR, and stained for γH2Av foci, a marker for DSBs (Fig. 1B; Chiolo et al., 2011). Quantification of γH2Av focus number indicates that DSBs form with similar kinetics in newly emerged (1 d.o) and older (8 d.o. and 29 d.o.) flies (Fig. 1C, time point 30 min). However, older flies display a higher number of γH2Av foci at both 2 and 8 h after IR, relative to 1‐d.o. flies, revealing defects in DSB resolution (Fig. 1B,C). Notably, there is no difference between young and old flies in the number of repair foci before IR (Fig. 1C, time point 0 h). This suggests that ‘spontaneous’ DSBs do not accumulate with age in these cells and do not significantly contribute to the higher number of ‘persistent’ DSBs observed in older flies. Similar repair defects were observed when Mu2/Mdc1 foci were used as a marker for DSBs (Dronamraju & Mason, 2009; Chiolo et al., 2011; Fig. S1, Supporting information). Further, repair defects are already present in 8‐d.o. flies (with intermediated levels detected in 5‐d.o. flies; Fig. S1, Supporting information), but no significant differences are observed between 8‐ and 29‐d.o. flies (Fig. 1C). We conclude that aging compromises DSB repair efficiency in premeiotic cells of adult flies and that this effect arises early on during the aging process.
Figure 1.
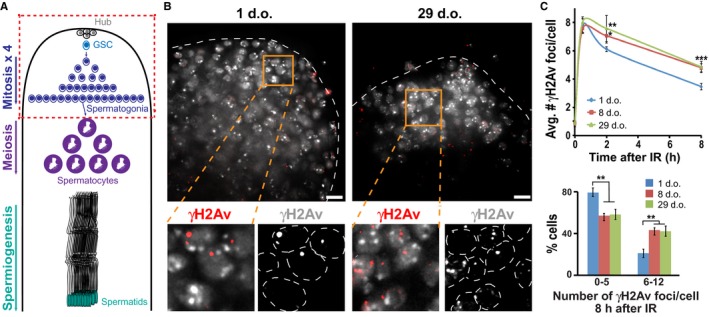
DSB repair is delayed in older animals. (A) Schematic view of Drosophila spermatogenesis, indicating the position of premeiotic cells in the testis (red rectangle) corresponding to the top images in B. GSC = germline stem cell. (B) Immunofluorescence (IF) analysis of premeiotic cells in testes dissected and fixed 8 h after 5 Gy IR shows more γH2Av foci in mitotically dividing spermatogonia of 29‐day old (d.o.) flies compared to those of 1‐d.o. flies. Scale bars = 5 μm. (C) Quantification of γH2Av foci in spermatogonia shows higher average number of γH2Av foci at 2 and 8 h after IR (top) and higher frequency of cells with more foci at 8 h after IR (bottom) in older flies relative to young flies. Error bars: SD. Top: *P = 0.04 (one‐tailed Mann–Whitney test); **P = 0.0056 and ***P < 0.001 (two‐tailed Mann–Whitney test). Bottom: **P < 0.005 (Welsh's test). All p values refer to comparisons with 1‐d.o. flies. Differences between 8‐ and 29‐d.o. flies were not significant. n = 85–168 nuclei from at least three independent testes/age/time point.
HR repair of premeiotic germ cells becomes defective as the organism ages
To investigate whether the repair defects observed in older flies are a consequence of defective HR, we analyzed repair outcomes with the DR‐white repair reporter assay (Do et al., 2014). In this system, two nonfunctional repeats of the white gene (Sce.white and iwhite) are inserted in a euchromatic locus on Chromosome 2 (Fig. 2A). Exposure to the I‐SceI meganuclease results in specific cleavage of both strands of the DNA in Sce.white, causing a DSB. Intrachromosomal or intersister chromatid HR repair using the iwhite sequence as a template restores the wild‐type SacI sequence of the white gene, resulting in red eyes in the progeny (Fig. 2A,B).
Figure 2.
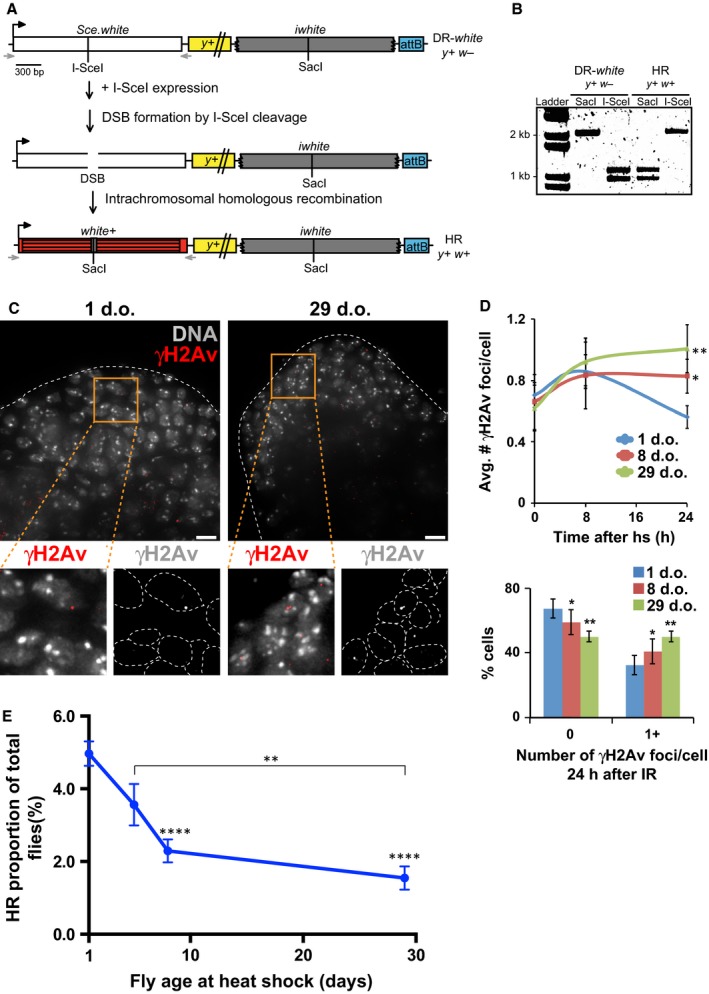
HR repair of I‐SceI induced DSBs decreases with age. DSB repair is measured by I‐SceI induced DSBs using the DR‐white reporter. (A) The DR‐white assay contains two nonfunctional direct repeats of the white gene. The first repeat, Sce.white, is nonfunctional due to the insertion of an I‐SceI recognition sequence into the wild‐type white cDNA. This results in a premature STOP codon. The second repeat, iwhite, is nonfunctional due to 5′ and 3′ truncations, but contains wild‐type white sequence, including a SacI cut site, at the location correspondent to the I‐SceI site in Sce.white. DR‐white flies are crossed with flies containing the I‐SceI transgene, which results in DSB formation at the I‐SceI recognition sequence. Repair by HR results in restoration of the wild‐type sequence and a red‐eyed fly (y + w +) in the progeny. (B) HR repair resulting in gene conversion of the wild‐type SacI sequence can be confirmed molecularly. Sce.white gene is amplified using primers indicated in (A) (gray arrows), followed by digestion of PCR product with SacI or I‐SceI. I‐SceI cleaves only intact Sce.white sequence. SacI cleaves only HR products. (C‐D) Flies containing the DR‐white chromosome and the hs‐I‐SceI transgene were heat‐shocked at the indicated ages. Testes were dissected at given time point and stained for γH2Av foci. (C) IF analysis shows more γH2Av foci in spermatogonia of 29‐d.o. flies compared to 1‐d.o. flies, at 24 h after heat shock. Scale bars = 5 μm. (D) Quantification of γH2Av focus number in spermatogonia fixed prior to and at different time points after heat shock shows higher number of γH2Av foci (top) and higher frequency of cells with one or more foci (bottom) in 8‐ and 29‐d.o. flies relative to 1‐d.o. flies, at 24 h after heat shock. Error bars: SEM; *P < 0.05 and **P = 0.005 by two‐tailed Mann–Whitney test. p values refer to comparisons with 1‐d.o. flies. Differences between 8‐ and 29‐d.o. flies were not significant. n = 38–167 nuclei from at least two independent testes/age/time point. (E) Flies containing the DR‐white reporter and hs‐I‐SceI transgene were aged to the given times and exposed to heat shock. After 11 days, flies were mated to females and F1 progeny scored for HR products. Data given are mean ± SEM of 73–123 germlines. **P < 0.01; ****P < 0.0001 for comparisons to 1 d.o., by one‐way ANOVA with multiple comparisons followed by Tukey–Kramer post hoc test. Differences between all other ages were not significant.
We first confirmed that within the DR‐white system, I‐SceI induces DSBs with similar efficiency in young and older flies by investigating repair focus kinetics. To induce DSBs at specific ages, adults that contain both the DR‐white reporter and a heat‐shock‐inducible I‐SceI transgene (hs‐I‐SceI) were aged and then heat‐shocked. Quantification of γH2Av foci in the premeiotic male germ cells revealed that after heat shock, focus numbers are initially similar in young and older flies (Fig. 2C,D, time point 8 h). This suggests that I‐SceI induction and DSB formation are equally efficient at different ages of the fly, validating the use of the DR‐white system for aging studies. We further observed that the γH2Av focus number decreases over time in young flies (1 d.o.), reflecting repair progression (Fig. 2D, time point 24 h). Conversely, γH2Av focus number remains high in older flies (Fig. 2C,D). We conclude that older animals (≥ 8 d.o.) have a reduced ability to repair I‐SceI‐induced DSBs in their germline, similar to their defect in repairing IR‐induced DSBs.
Next, we investigated whether repair defects in germ cells of older animals are a consequence of defective HR, by measuring repair outcomes in the progeny. Adult flies containing both the DR‐white chromosome and hs‐I‐SceI were aged to 1, 5, 8, and 29 days and then heat‐shocked. These males were crossed with control females 11 days later, allowing time for the spermatogonia affected by DSB induction and repair to develop into mature sperm (Lindsley, 1976). Notably, this timing corresponds to the peak of HR events detectable in the progeny (data not shown), consistent with the fact that HR is the main DSB repair pathway used in premeiotic cells but not in later differentiation stages (Rong & Golic, 2003; Preston et al., 2006a; Chan et al., 2011). A progressive decrease in the proportion of red‐eyed progeny was observed between 1 and 8 days, revealing that the frequency of repair by HR quickly declines with age (Fig. 2E). Similarly to γH2Av focus kinetics, there is not a significant difference between 8‐ and 29‐day‐old flies (Fig. 2E). Notably, spontaneous HR repair events do not significantly change across different ages (Table S1, Supporting information), suggesting that the decrease in proportion of red‐eyed progeny in older flies is due specifically to defects in HR repair of I‐SceI‐induced DSBs. In contrast to HR defects, we did not detect SSA defects in 8‐ to 29‐d.o. animals relative to 1‐d.o. animals (Fig. S2, Supporting information). We note, however, that SSA events detected with the DR‐white system are rare (about 10‐fold less relative to HR (Do et al., 2014)); thus, small changes in this repair pathway may be below detection level.
Importantly, these results are in striking contradiction with the previously reported increase in HR repair in old flies (Preston et al., 2006b). This discrepancy might depend on the different reporter assay (interhomolog HR vs. intrachromosomal or intersister‐chromatid HR) or the constitutive expression of I‐SceI used in previous experiments. We directly tested the second possibility by repeating the DR‐white repair assay in flies of different ages with constitutive expression of I‐SceI. In agreement with previous studies, constitutive I‐SceI expression leads to higher levels of HR products detected in the progeny as the organism ages (Fig. S3, Supporting information). This points to the constitutive vs timed activation of I‐SceI as the most likely explanation for the differences observed in the two studies. We suggest that constitutive expression of I‐SceI across development and differentiation favors the detection of HR products in the progeny, potentially skewing the data toward HR outcomes (see Discussion). Together, our data suggest that, contrary to previous conclusions, aging leads to a marked defect in HR repair of DSBs in premeiotic male germ cells.
HR repair defects in older animals correlate with increased expression of early HR components and Rad51 localization to repair foci
To gain insight on the mechanism causing HR defects in older animals, we investigated how aging affects the expression of HR genes, particularly at the ages where the proportion of HR progeny rapidly declines (1, 5, and 8 d.o). Flies containing both DR‐white and hs‐I‐SceI transgene were aged for 1, 5, and 8 days and then harvested. We analyzed the mRNA expression of genes required for different HR steps, particularly DSB detection and resection (rad50, ctip/CG5872, and blm), strand invasion (rad51/spnA), and D‐loop processing (rad54/okr).
Interestingly, we found statistically significant increases in relative mRNA expression levels of rad50, ctip, blm, and rad51, in 8‐d.o. flies relative to both 1‐d.o. and 5‐d.o. flies (Figs 3A and S4A, Supporting information). mRNA expression levels of rad51 remain elevated in 15‐ and 29‐d.o. flies (Fig. S4B, Supporting information). Accordingly, Rad50 protein levels dramatically increase in older flies (Fig. 3B). These responses were not observed for all HR genes, as Smc5 or Smc6 protein levels did not display major changes between 1‐ and 8‐d.o. flies (Fig. 3B). Changes in rad54 mRNA levels were also not significant (Fig. 3A), possibly reflecting high variability in rad54 expression in older flies.
Figure 3.
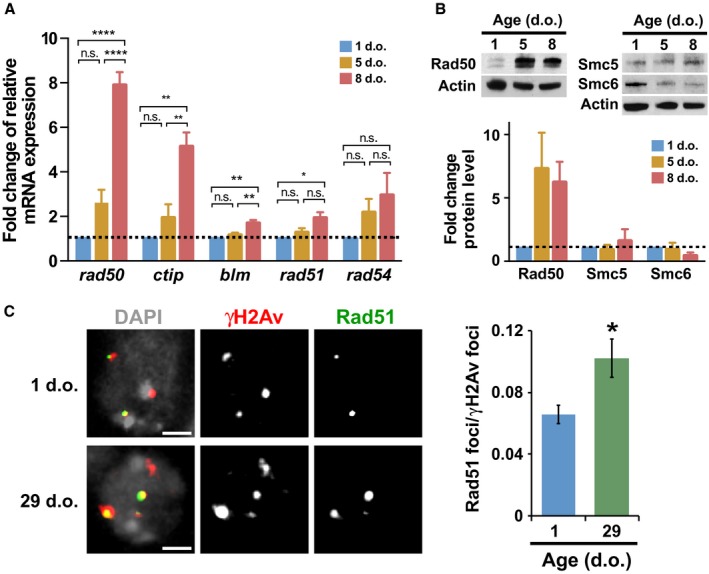
Expression of early HR components, and Rad51 localization to DSBs, increase in older animals. Flies containing the DR‐white reporter and the hs‐I‐SceI transgene were aged to the given times. (A) CT values for rad50, ctip, rad51, and rad54 were normalized to that of gapdh2 (CT) to determine relative expression. ΔΔCT values were calculated relative to 1‐d.o. flies for each gene to determine expression fold change (2‐ΔΔCT). Averages of the fold change are given; error bars are SEM of 3–10 biological replicates. *P < 0.05; ** P < 0.01; *** P < 0.001; **** P < 0.0001, by one‐way ANOVA with multiple comparisons, followed by Tukey‐Kramer post hoc test. (B) Western blot analysis of protein extracts from male flies of different ages shows Rad50, Smc5, and Smc6 protein levels relative to 1‐d.o. flies. Actin is used as loading control. Error bars: SD; n = 3 independent experiments; representative blots are shown. (C) Left: IF analysis of Drosophila testes dissected and fixed 4 h after 5 Gy IR shows examples of Rad51 foci colocalizing with γH2Av foci in mitotically dividing spermatogonia of 1‐ and 29‐day‐old (d.o.) flies. Scale bars = 1 μm. Right: Quantification of the ratio of Rad51 foci colocalizing with γH2Av foci over total γH2Av foci in spermatogonia shows an increase in older flies (29 d.o.) compared with young flies (1 d.o.). Error bars: SD; *P = 0.0166, Mann–Whitney test. n = 125 nuclei for 1‐d.o. flies and n = 116 nuclei for 29‐d.o. flies from at least three independent testes/age.
Notably, Rad51 overexpression has been previously linked to HR defects, aberrant recombination, and genome instability (Richardson et al., 2004; Paffett et al., 2005; Klein, 2008), as high levels of this protein might impede HR progression by interfering with Rad51 displacement after strand invasion. In agreement with this prediction, older flies display a higher number of Rad51 foci colocalizing with γH2Av foci at 4 h after IR (Fig. 3C). Given that Rad51 focus formation is not reduced in older flies, we conclude that early HR steps (resection and Rad51 recruitment) are not negatively affected by aging. Rather, we propose that the observed HR defects are caused by malfunctions of later steps. Specifically, overexpression of resection components and Rad51 might contribute to HR defects by overloading Rad51 and hindering the disassembly of the nucleoprotein filament after strand invasion.
Discussion
Many factors contribute to aging and this biological process encompasses all systems, ranging from the cellular level to the organismal level. One hallmark of aging is an increase in genome instability, as evidenced by higher levels of DNA damage and tumorigenesis in natural aging, and by the high level of genome instability characterizing premature aging syndromes. However, the mechanisms leading to genome instability in aging organisms remain unclear. Here, we have utilized Drosophila to measure the effects of physiological aging on HR repair at the cellular level within the context of aging organisms. We found that IR‐ and endonuclease‐induced DSBs persist in premeiotic germline cells of older animals and that repair by intrachromosomal or intersister HR becomes significantly defective in the mitotically dividing spermatogonia as the organism ages. Pronounced HR defects appear early in aging organisms, and excessive resection and/or the accumulation of Rad51 protein onto resected DSBs might contribute to these defects. We propose that high levels of genome instability that characterize older animals are, at least in part, a consequence of a decreased ability to repair DSBs through error‐free HR repair.
Data demonstrating increased γH2AX staining in older mammalian cells (Sedelnikova et al., 2004) concluded that DNA damage accumulates over time, which might reflect a combination of higher levels of spontaneous damage from oxidative reactions (Hamilton et al., 2001) and/or defective repair (Gorbunova et al., 2007; Gorbunova & Seluanov, 2016). However, in our experiments the baseline level of spontaneous DSBs in premeiotic cells is not significantly different between young and old flies, enabling a more direct evaluation of changes in repair efficiency. Further, shortly after DSB induction with IR or I‐SceI, the frequency of γH2Av foci was similar regardless of age, suggesting that early repair steps are not compromised in older flies. This also argues against major defects in DSB formation, or in DSB repair by NHEJ which typically occurs within ~30 min after IR (Mao et al., 2008). Rather, the persistence of γH2Av foci in older animals suggests a specific defect in DSB repair by slower pathways, such as HR.
The DR‐white system allows us to directly test if DSB repair by intrachromosomal or intersister chromatid recombination is affected with age. Using a constitutively active I‐SceI enzyme, the frequency of HR repair increases over time. In agreement, Preston, et al. showed that constitutive induction of I‐SceI in a Rr3 reporter leads to more interhomolog HR with age (Preston et al., 2006b). However, several caveats to these experiments warrant a guarded approach in interpreting these results. First, in both the Rr3 and the DR‐white systems, HR repair of DSBs results in the loss of the I‐SceI recognition sequence, while simple NHEJ without processing will restore the cleavable sequence. This intrinsically biases the observed output toward the terminal repair event that cannot be further cleaved by I‐SceI (such as HR). Thus, HR outcomes may be more frequently detected when I‐SceI is constitutively expressed, and the more prolonged expression of I‐SceI in older flies might explain the observed increase in HR products in the progeny. Second, constitutive DSB formation may result in a DNA damage response unique to this system where repeated break events occur at the same site. Last, constitutive DSB formation in the germline may eventually impact the pool of germline stem cells. Repair of a stem cell by HR would result in 100% of the progeny arising from that stem cell displaying the HR product phenotype for the remainder of the life of the male, resulting in an overall increase in observed HR frequency in the progeny population. In fact, this was observed on several occasions, where by 29 days of age, all of the progeny from a single germline were HR events (data not shown).
Due to these concerns, we used an inducible system to generate DSBs within a narrow timeframe, and the reproduction was timed so that observed progeny reflected the outcomes of repair within the premeiotic cell population. In contrast to what is observed in the constitutively active system, the heat‐shock‐induced DSBs were repaired by HR at decreased frequencies in older animals. Because of the ability to induce damage at a specific time point in the organism's lifespan, and because we observed similarly low baseline levels of damage across age groups, we suggest that this assay more accurately represents how DSBs are repaired in the spermatogonia of older animals. In agreement with our analysis of repair focus kinetics, these results suggest that defective HR repair significantly contributes to repair defects in older germ cells. Interestingly, HR defects have also been detected in presenescent human fibroblasts, an in vitro model of aging (Mao et al., 2012). Similarly, interchromosomal HR defects occur in somatic cells of older mice (White et al., 2013), suggesting that recombination defects might be a common problem in older cells. While more studies are required to directly address the effects of aging on interhomolog HR repair, our study is the first direct demonstration that intrachromosomal or intersister chromatid HR repair of DSBs induced in the germline of older animals declines, supporting the conclusion that HR defects may promote genome instability in older organisms (Fig. 4).
Figure 4.
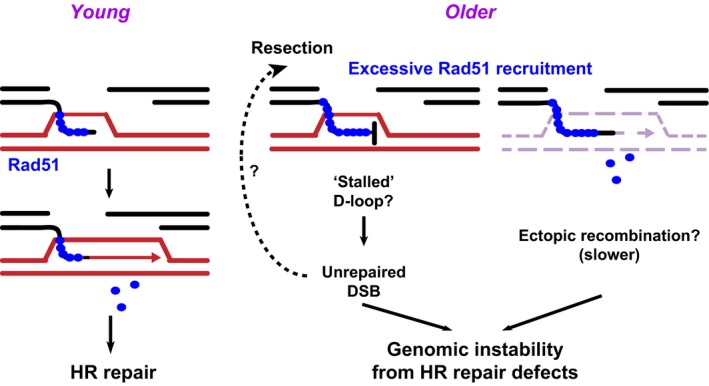
Model for HR defects in aging. In older flies, Rad51 overexpression and persistence at DSBs correlates with defective HR progression. The increased expression of resection components might aggravate this response by enhancing Rad51 nucleofilament formation. We propose that excessive Rad51 recruitment may counteract nucleofilament disassembly, resulting in persistent unrepaired DSBs and chromosome rearrangements. Excessive Rad51 may also trigger ectopic recombination (chromosome exchanges with nonhomologous chromosomes), which also typically occurs with delayed kinetics (Chung et al., 2010), and contributes to ‘persistent’ damage foci and genome instability.
What can be the cause of HR defects in older flies? Previous studies in presenescent human cells identified a reduction of Rad51 levels and focus formation, suggesting Rad51 recruitment as a limiting step for HR in old cells (Mao et al., 2012). However, neither Rad51 expression nor recruitment to repair foci is defective in the spermatogonia of older flies, suggesting that early HR steps are proficient in this context. This difference between presenescent human cells and aging flies suggests the interesting possibility that deregulation of more than one recombination step might contribute to HR defects during aging. One possibility is that the abnormally high levels of Rad51 in older flies interfere with Rad51 disassembly postsynapsis and repair completion (Fig. 4). Accordingly, we observed a greater frequency of Rad51 foci associated with γH2Av foci in older flies, possibly reflecting a defect in disassembling the nucleoprotein filament after strand invasion. Additionally, the increase in interhomolog HR observed by Preston et al. was associated with an increase in longer gene conversion tracts (Preston et al., 2006b), and defective Rad51 disassembly may contribute to this phenotype. What participates in the nucleofilament disassembly in Drosophila is still unknown, but homologues of Rad54 (okra) (Kooistra et al., 1999), HELQ‐1 (mus301) (McCaffrey et al., 2006), and several Rad51 paralogs exist in flies (Morris & Lehmann, 1999) and might play conserved roles in Rad51 displacement from the postsynaptic filament. Rad51 overexpression and persistent nucleofilaments could also induce ectopic recombination, further promoting genome rearrangements (Richardson et al., 2004; Klein, 2008), particularly if suppressors of Rad51‐mediated aberrant recombination (e.g., Smc5/6, Chiolo et al., 2011; Li et al., 2013) are not equally induced. Notably, the higher expression of genes required for early repair steps might aggravate these effects by channeling DSBs toward the HR pathway and/or by over‐resecting the DSBs.
While the cause of Rad51 overexpression is still unclear, high Rad51 levels have been proposed as a leading cause of chromosome aberrations in cancer cells (Maacke et al., 2000; Raderschall et al., 2002; Richardson et al., 2004). Our current data do not directly determine whether increased recruitment of Rad51 is a cause or consequence of defective HR. However, we propose that abnormally high expression of resection components and Rad51 might be a driving force for genome instability and cancer formation in old organisms and future studies will directly test this possibility.
In all our experiments, the most significant differences in HR repair were observed when comparing 1‐ and 8‐day‐old animals. These data suggest that the effects of aging on DSB repair are not linear with age. Rather, there may be a threshold that regulates HR repair efficiency in young animals, and once this is reached (for example, between 5 and 8 days), this efficiency is lost. Considering the median and mean lifespan of male Drosophila in standard culturing conditions in the laboratory is ~50 days (Linford et al., 2013), the age at which HR repair capacity decreases is relatively young. This may have implications for other organisms as well, suggesting that HR repair is defective even before ‘old age’. Together, our data revealed a striking defect in error‐free HR repair in premeiotic germ cells of older animals, suggesting HR defects as a cause of the characteristic cancer predisposition, infertility, and developmental defects in the progeny, observed in older organisms.
Experimental procedures
Drosophila stocks and maintenance
Drosophila were maintained on standard media at 25 °C, which was either Nutri‐fly Bloomington Formulation medium (Genesee Scientific; San Diego, CA, USA) or prepared as in (Ren et al., 2009). I‐SceI transgenic stocks included either a ubiquitin promoter for constitutive active expression (Preston et al., 2006a) or hsp70 promoter for heat‐shock induction (Rong & Golic, 2003). Standard genetic crosses were used to create DR‐white/I‐SceI males in a y w background. y ry or mGFP‐Mu2‐expressing flies were used in ionizing radiation experiments. For aging time points, flies were collected for 24 h before treatment or aging; thus, 1 d.o. age corresponds to flies 0–1 day old, as in previous studies (Boyle et al., 2007; Toledano et al., 2012). Similarly, 5 d.o. refers to 4‐ to 5‐d.o. flies, and so on. mGFP‐Mu2‐expressing flies are a kind gift from J. Mason (Dronamraju & Mason, 2009).
Immunofluorescence and imaging of male germline
For IR experiments, flies were exposed to 5 Gy using a 160‐kV X‐ray source (X‐RAD iR‐160, Precision X‐Ray) before testis dissection. For staining post‐I‐SceI induction, male testes were dissected and fixed at given time points after heat shock. Notably, I‐SceI induction requires more time to induce DSB formation relative to IR (compare Figs 2D to 1C). This is likely because of the time required to induce I‐SceI expression, which results in less synchronous DSB formation in the cell population (Janssen et al., 2016). Testes were dissected in PBS–Triton 0.1% (PBST) and fixed at room temperature in 4% paraformaldehyde in PBST for 8 min, rinsed three times in PBST, and blocked for 1 h at room temperature with milk 4% in PBST. Primary antibodies were incubated overnight at 4°C, and secondary antibodies were incubated at RT for 1 h, both in the blocking solution. Antibodies were as follows: anti‐γH2Av (Rockland, Cat. # 600‐401‐914, 1:1000), anti‐Rad51/SpnA (kind gift from J. Kadonaga, 1:1000), and anti‐GFP (Aves Labs, Cat #GFP‐1020, 1:500). For Rad51 and γH2Av colocalization experiments, primary antibodies were directly labeled with Alexa‐488 (Rad51) and Alexa‐555 (γH2Av) as described in Oegema et al. (2001). DNA staining, slide mounting, imaging with a DeltaVision microscope (Applied Precision/GE Healthcare), and image analysis with the softWoRx software were performed as previously described (Chiolo et al., 2011; Ryu et al., 2015).
Measuring HR and SSA repair with DR‐white assay
DR‐white was targeted in the genome using the attB sequence, and integration was confirmed using yellow (y+) transgene expression (Do et al., 2014). HR and SSA repair of I‐SceI‐induced DSBs in DR‐white was measured as previously described (Do et al., 2014). Briefly, I‐SceI expression results in cleavage of the I‐SceI recognition sequence in Sce.white. DSBs occur in the somatic and germline cells. To isolate single repair events and determine HR and SSA repair frequency, males containing repair events in their germline are crossed to y w virgin females. Repair by HR includes utilizing the downstream iwhite sequence restoring the wild‐type white sequence, which results in wild‐type white expression and red‐eyed progeny. Gene conversion of the wild‐type iwhite sequence is confirmed molecularly by amplification of the repair events as described previously (Do et al., 2014). Briefly, Sce.white was PCR‐amplified using Sce.white‐specific primers (Fig. 2A) (forward, 5′ GTTTTGGGTGGGTAAGCAGG; reverse, 5′ AGACCCACGTAGTCCAGC) using SapphireAmp Fast PCR Master Mix (Clontech, Mountain View, CA, USA). For I‐SceI and SacI digests, PCR products were directly digested (New England Biolabs, Ipswich, MA, USA). Repair by SSA results in loss of the yellow (y+) transgene. NHEJ with processing (e.g., loss of I‐SceI recognition sequence) products detected with the DR‐white system was negligible (data not shown).
DR‐white aging experiments
To obtain flies with inducible DSBs for aging, females containing the DR‐white reporter were crossed to males containing the heat‐inducible I‐SceI transgene (Wei & Rong, 2007) in large embryo collection cages in 24‐h increments to synchronize eclosion. Progeny of this cross, containing DR‐white and heat‐shock‐inducible I‐SceI transgene, were collected for 24‐h increments and then aged to given time points. To minimize losses while aging, 30 flies/vial were flipped onto fresh food twice a week.
For 5‐, 8‐ and 29‐d.o. experiments, male flies were outcrossed to y w virgins at a 1:1 ratio for 5 days (3 days for 5‐d.o. males) prior to heat shock to reset their germline. Females were removed 24 h before heat shock. Flies were then heat‐shocked at 37.5 °C for 36–38 min. To capture only repair events in the premeiotic germline, 1, 5, 8, and 29‐d.o. heat‐shocked males were aged for an additional 11 days at the conditions mentioned above and single males were crossed to 5–8 y w virgins. Progeny of this cross were scored. Only vials containing ≥ 20 progeny were included in analyses. Each vial represented one germline (n). Notably, flies scored for HR defects are expressed as a percentage of the total progeny; thus, potential fluctuations in the number of spermatogonia (and total progeny) with age are unlikely to contribute specifically to changes in HR frequencies.
To analyze accumulating repair events in aged animals with constitutively active I‐SceI transgene, aging experiments were performed similar to Preston, et al. (Preston et al., 2006b). Briefly, single male flies containing both DR‐white and the I‐SceI transgene constitutively activated by the ubiquitin promoter (Preston et al., 2006a) were crossed to new 4–5 y w virgins at 7‐day intervals. After 7 days, the parents were removed; females were discarded and males were crossed to 5 new y w females. Only males that survived and had productive progeny (≥ 20 progeny) at all four ages were included in analyses. Each vial represented one germline (n).
qPCR analysis of genes in HR pathway
Male flies containing both DR‐white and the hs‐I‐SceI transgene were synchronized and aged as above to 1, 5, and 8 days. Two flies were combined to represent one biological replicate, and five biological replicates per age were harvested. mRNA was purified by acid guanidinium thiocyanate–phenol–chloroform extraction with TRIzol® LS (Invitrogen; Carlsbad, CA, USA) and RNA Clean and Concentration‐5 (Zymo Research; Irvine, CA, USA). Contaminating DNA was removed by DNA‐free™ rDNase I treatment (Invitrogen). Reverse transcription was performed using SuperScript™ VILO Master Mix (Invitrogen), and quantitative real‐time polymerase chain reaction (qPCR) was primed with the RT2 SYBR Green Master Mix, using RT2 qPCR Primers for Drosophila melanogaster rad51, rpl32, rad50, CG5872 (ctip), gapdh2, and rad54 (cat. n.:(PPD10573A, PPD10569B, PPD05479A, PPD07756A, PPD01317A, and PPD02120A, respectively, from Qiagen; Hilden, Germany). gapdh2 and rpl32 were used for normalization due to low expression variation with age (Ling & Salvaterra, 2011). All qPCR measurements were obtained using the 7900HT Real‐Time Thermal Cycler and software (Applied BioSystems; Carlsbad, CA). CT values for each experimental gene were normalized to that of gapdh2 or rpl32 (CT) to determine relative expression. ΔΔCT values were calculated relative to 1‐d.o. flies within each experiment for each gene to determine expression fold change (). For rad51 mRNA analysis in Fig. S4B (Supporting information), an additional experiment was performed by aging flies 1, 5, 8, 15, and 29 days, and ΔΔCT values for 1‐, 5‐, and 8‐d.o. flies were combined.
Western blot analysis of protein levels
For each protein extract, three male flies were flash frozen and ground with a pestle in lysis buffer A (50 mm Tris, pH 7.8, 1% NP‐40, 150 mm NaCl) containing protease inhibitors (Complete, Roche), 2.5% 2‐mercaptoethanol, and 1 mM PMSF. A total of 250 U of Benzonase® nuclease was added to each sample, and the mix was incubated for 15–20 min on ice. The soluble lysate was recovered by centrifugation (10 min, 16873 × g 4 °C) and precipitated in acetone before resuspension in loading buffer (Laemmli). Samples were denatured by 5 min at 70 °C before running them on a TGX 4‐12% polyacrylamide gel (Bio‐Rad) and transferred onto nitrocellulose membrane. Anti‐Rad50 (kind gift from M. Gatti, 1:1000), anti‐Smc5 and anti‐Smc6 (SDI, 1:1000 (Chiolo et al., 2011)), and anti‐actin (Abcam, ab8224, 1:1000) antibodies were diluted in TBS–Tween 0.1% plus milk 4%. Secondary antibodies were from Thermo Scientific. Quantification of protein level in Fig. 3B was performed by measuring the band intensity of Western blot signals with Fiji, and levels were normalized to actin and then to 1‐d.o. samples.
Statistical analysis
All statistical analyses were performed using prism 6 (GraphPad Software; La Jolla, CA, USA), with the methods indicated in the legends.
Funding
This research was supported by the American Federation on Aging Research (J.R.L.), the National Institutes of Health Grant 1R15GM110454‐01 (J.R.L.), the National Institutes of Health Grant R01GM117376 (I.C.), The Rose Hills Foundation (I.C.), and the Edward Mallinckrodt Jr. Foundation (I.C.).
Author contributions
J.R.L., I.C., L.D., D.M., and H.E. designed experiments; J.R.L., D.M., H.E., F.S., L.D., and C.M.H. performed experiments; J.R.L., D.M., H.E., F.S., L.D., and I.C. analyzed data; E.J.B. provided statistical support; H.S. generated preliminary data that motivated experiments within this study; J.R.L., I.C., L.D., D.M., and H.E. wrote the manuscript.
Conflict of interest
None declared.
Supporting information
Fig. S1 Mu‐2 repair foci persist in older animals.
Fig. S2 SSA repair is not significantly affected by age.
Fig. S3 HR repair of constitutively‐induced DSBs increases with age.
Fig. S4 mRNA levels of early HR components increase in older flies.
Table S1 HR proportion of total flies from spontaneous events.
Acknowledgments
We are grateful to J. Kadonaga for anti‐Rad51 antibodies, to M. Gatti for anti‐Rad50 antibodies, to J. Mason for mGFP‐Mu2‐expressing flies, to Maria Purice and the Logan laboratory for feedback on qRT–PCR conditions, John Tower for sharing reagents, and to John Tower and Kathryn Kohl for insightful comments on the manuscript.
Contributor Information
Irene Chiolo, Email: chiolo@usc.edu.
Jeannine R. LaRocque, Email: jlk99@georgetown.edu
References
- Boyle M, Wong C, Rocha M, Jones DL (2007) Decline in self‐renewal factors contributes to aging of the stem cell niche in the Drosophila testis. Cell Stem Cell 1, 470–478. [DOI] [PubMed] [Google Scholar]
- Chan Y, Naujoks DA, Huen DS, Russell S (2011) Insect population control by homing endonuclease‐based gene drive: an evaluation in Drosophila melanogaster . Genetics 188, 33–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiolo I, Minoda A, Colmenares SU, Polyzos A, Costes SV, Karpen GH (2011) Double‐strand breaks in heterochromatin move outside of a dynamic HP1a domain to complete recombinational repair. Cell 144, 732–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung WH, Zhu Z, Papusha A, Malkova A, Ira G (2010) Defective resection at DNA double‐strand breaks leads to de novo telomere formation and enhances gene targeting. PLoS Genet. 6, e1000948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Do AT, Brooks JT, Le Neveu MK, LaRocque JR (2014) Double‐strand break repair assays determine pathway choice and structure of gene conversion events in Drosophila melanogaster . G3 4, 425–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolle ME, Giese H, Hopkins CL, Martus HJ, Hausdorff JM, Vijg J (1997) Rapid accumulation of genome rearrangements in liver but not in brain of old mice. Nat. Genet. 17, 431–434. [DOI] [PubMed] [Google Scholar]
- Dronamraju R, Mason JM (2009) Recognition of double strand breaks by a mutator protein (MU2) in Drosophila melanogaster . PLoS Genet. 5, e1000473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorbunova V, Seluanov A (2016) DNA double strand break repair, aging and the chromatin connection. Mutat. Res. 788, 2–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorbunova V, Seluanov A, Mao Z, Hine C (2007) Changes in DNA repair during aging. Nucleic Acids Res. 35, 7466–7474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton ML, Van Remmen H, Drake JA, Yang H, Guo ZM, Kewitt K, Walter CA, Richardson A (2001) Does oxidative damage to DNA increase with age? Proc. Natl Acad. Sci. USA 98, 10469–10474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendricks CA, Almeida KH, Stitt MS, Jonnalagadda VS, Rugo RE, Kerrison GF, Engelward BP (2003) Spontaneous mitotic homologous recombination at an enhanced yellow fluorescent protein (EYFP) cDNA direct repeat in transgenic mice. Proc. Natl Acad. Sci. USA 100, 6325–6330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssen A, Breuer GA, Brinkman EK, van der Meulen AI, Borden SV, van Steensel B, Bindra RS, LaRocque JR, Karpen GH (2016) A single double‐strand break system reveals repair dynamics and mechanisms in heterochromatin and euchromatin. Genes Dev. 30, 1645–1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson‐Schlitz DM, Flores C, Engels WR (2007) Multiple‐pathway analysis of double‐strand break repair mutations in Drosophila . PLoS Genet. 3, e50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein HL (2008) The consequences of Rad51 overexpression for normal and tumor cells. DNA Repair 7, 686–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kooistra R, Pastink A, Zonneveld JB, Lohman PH, Eeken JC (1999) The Drosophila melanogaster DmRAD54 gene plays a crucial role in double‐ strand break repair after P‐element excision and acts synergistically with Ku70 in the repair of X‐ray damage. Mol. Cell. Biol. 19, 6269–6275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Heyer WD (2009) RAD54 controls access to the invading 3′‐OH end after RAD51‐mediated DNA strand invasion in homologous recombination in Saccharomyces cerevisiae . Nucleic Acids Res. 37, 638–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Zhuo R, Tiong S, Di Cara F, King‐Jones K, Hughes SC, Campbell SD, Wevrick R (2013) The Smc5/Smc6/MAGE complex confers resistance to caffeine and genotoxic stress in Drosophila melanogaster . PLoS ONE 8, e59866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsley DTK (1976) Spermatogenesis In Genetics and Biology of Drosophila (Ashburner MWT. ed.). New York: Academic Press, pp. 225–294. [Google Scholar]
- Linford NJ, Bilgir C, Ro J, Pletcher SD (2013) Measurement of lifespan in Drosophila melanogaster . J. Vis. Exp. 71, 50068. doi: 10.3791/50068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling D, Salvaterra PM (2011) Robust RT‐qPCR data normalization: validation and selection of internal reference genes during post‐experimental data analysis. PLoS ONE 6, e17762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu B, Wang J, Chan KM, Tjia WM, Deng W, Guan X, Huang JD, Li KM, Chau PY, Chen DJ, Pei D, Pendas AM, Cadinanos J, Lopez‐Otin C, Tse HF, Hutchison C, Chen J, Cao Y, Cheah KS, Tryggvason K, Zhou Z (2005) Genomic instability in laminopathy‐based premature aging. Nat. Med. 11, 780–785. [DOI] [PubMed] [Google Scholar]
- Maacke H, Jost K, Opitz S, Miska S, Yuan Y, Hasselbach L, Luttges J, Kalthoff H, Sturzbecher HW (2000) DNA repair and recombination factor Rad51 is over‐expressed in human pancreatic adenocarcinoma. Oncogene 19, 2791–2795. [DOI] [PubMed] [Google Scholar]
- Mao Z, Bozzella M, Seluanov A, Gorbunova V (2008) Comparison of nonhomologous end joining and homologous recombination in human cells. DNA Repair 7, 1765–1771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao Z, Tian X, Van Meter M, Ke Z, Gorbunova V, Seluanov A (2012) Sirtuin 6 (SIRT6) rescues the decline of homologous recombination repair during replicative senescence. Proc. Natl Acad. Sci. USA 109, 11800–11805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCaffrey R, St Johnston D, Gonzalez‐Reyes A (2006) Drosophila mus301/spindle‐C encodes a helicase with an essential role in double‐strand DNA break repair and meiotic progression. Genetics 174, 1273–1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIlwraith MJ, Van Dyck E, Masson JY, Stasiak AZ, Stasiak A, West SC (2000) Reconstitution of the strand invasion step of double‐strand break repair using human Rad51 Rad52 and RPA proteins. J. Mol. Biol. 304, 151–164. [DOI] [PubMed] [Google Scholar]
- Morris J, Lehmann R (1999) Drosophila oogenesis: versatile spn doctors. Curr. Biol. 9, R55–R58. [DOI] [PubMed] [Google Scholar]
- Nimonkar AV, Genschel J, Kinoshita E, Polaczek P, Campbell JL, Wyman C, Modrich P, Kowalczykowski SC (2011) BLM‐DNA2‐RPA‐MRN and EXO1‐BLM‐RPA‐MRN constitute two DNA end resection machineries for human DNA break repair. Genes Dev. 25, 350–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oegema K, Desai A, Rybina S, Kirkham M, Hyman AA (2001) Functional analysis of kinetochore assembly in Caenorhabditis elegans . J. Cell Biol. 153, 1209–1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paffett KS, Clikeman JA, Palmer S, Nickoloff JA (2005) Overexpression of Rad51 inhibits double‐strand break‐induced homologous recombination but does not affect gene conversion tract lengths. DNA Repair 4, 687–698. [DOI] [PubMed] [Google Scholar]
- Preston CR, Flores CC, Engels WR (2006a) Differential usage of alternative pathways of double‐strand break repair in Drosophila . Genetics 172, 1055–1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preston CR, Flores C, Engels WR (2006b) Age‐dependent usage of double‐strand‐break repair pathways. Curr. Biol. 16, 2009–2015. [DOI] [PubMed] [Google Scholar]
- Raderschall E, Stout K, Freier S, Suckow V, Schweiger S, Haaf T (2002) Elevated levels of Rad51 recombination protein in tumor cells. Cancer Res. 62, 219–225. [PubMed] [Google Scholar]
- Ramsey MJ, Moore DH II, Briner JF, Lee DA, Olsen L, Senft JR, Tucker JD (1995) The effects of age and lifestyle factors on the accumulation of cytogenetic damage as measured by chromosome painting. Mutat. Res. 338, 95–106. [DOI] [PubMed] [Google Scholar]
- Ren K, Pena de Ortiz S (2002) Non‐homologous DNA end joining in the mature rat brain. J. Neurochem. 80, 949–959. [DOI] [PubMed] [Google Scholar]
- Ren C, Finkel SE, Tower J (2009) Conditional inhibition of autophagy genes in adult Drosophila impairs immunity without compromising longevity. Exp. Gerontol. 44, 228–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson C, Stark JM, Ommundsen M, Jasin M (2004) Rad51 overexpression promotes alternative double‐strand break repair pathways and genome instability. Oncogene 23, 546–553. [DOI] [PubMed] [Google Scholar]
- Rong YS, Golic KG (2003) The homologous chromosome is an effective template for the repair of mitotic DNA double‐strand breaks in Drosophila . Genetics 165, 1831–1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothkamm K, Kruger I, Thompson LH, Lobrich M (2003) Pathways of DNA double‐strand break repair during the mammalian cell cycle. Mol. Cell. Biol. 23, 5706–5715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryu T, Spatola B, Delabaere L, Bowlin K, Hopp H, Kunitake R, Karpen GH, Chiolo I (2015) Heterochromatic breaks move to the nuclear periphery to continue recombinational repair. Nat. Cell Biol. 17, 1401–1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savitsky K, Bar‐Shira A, Gilad S, Rotman G, Ziv Y, Vanagaite L, Tagle DA, Smith S, Uziel T, Sfez S, Ashkenazi M, Pecker I, Frydman M, Harnik R, Patanjali SR, Simmons A, Clines GA, Sartiel A, Gatti RA, Chessa L, Sanal O, Lavin MF, Jaspers NGJ, Taylor AMR, Arlett CF, Miki T, Weissman SM, Lovett M, Collins FS, Shiloh Y (1995) A single ataxia telangiectasia gene with a product similar to PI‐3 kinase. Science 268, 1749–1753. [DOI] [PubMed] [Google Scholar]
- Sedelnikova OA, Horikawa I, Zimonjic DB, Popescu NC, Bonner WM, Barrett JC (2004) Senescing human cells and ageing mice accumulate DNA lesions with unrepairable double‐strand breaks. Nat. Cell Biol. 6, 168–170. [DOI] [PubMed] [Google Scholar]
- Seluanov A, Mittelman D, Pereira‐Smith OM, Wilson JH, Gorbunova V (2004) DNA end joining becomes less efficient and more error‐prone during cellular senescence. Proc. Natl Acad. Sci. USA 101, 7624–7629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugawara N, Ivanov EL, Fishman‐Lobell J, Ray BL, Wu X, Haber JE (1995) DNA structure‐dependent requirements for yeast RAD genes in gene conversion. Nature 372, 84–86. [DOI] [PubMed] [Google Scholar]
- Sukup‐Jackson MR, Kiraly O, Kay JE, Na L, Rowland EA, Winther KE, Chow DN, Kimoto T, Matsuguchi T, Jonnalagadda VS, Maklakova VI, Singh VR, Wadduwage DN, Rajapakse J, So PT, Collier LS, Engelward BP (2014) Rosa26‐GFP direct repeat (RaDR‐GFP) mice reveal tissue‐ and age‐dependence of homologous recombination in mammals in vivo . PLoS Genet. 10, e1004299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szostak JW, Orr‐Weaver TL, Rothstein RJ, Stahl FW (1983) The double‐strand‐break repair model for recombination. Cell 33, 25–35. [DOI] [PubMed] [Google Scholar]
- Toledano H, D'Alterio C, Czech B, Levine E, Jones DL (2012) The let‐7‐Imp axis regulates ageing of the Drosophila testis stem‐cell niche. Nature 485, 605–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker JD, Spruill MD, Ramsey MJ, Director AD, Nath J (1999) Frequency of spontaneous chromosome aberrations in mice: effects of age. Mutat. Res. 425, 135–141. [DOI] [PubMed] [Google Scholar]
- Vaidya A, Mao Z, Tian X, Spencer B, Seluanov A, Gorbunova V (2014) Knock‐in reporter mice demonstrate that DNA repair by non‐homologous end joining declines with age. PLoS Genet. 10, e1004511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallenfang MR, Nayak R, DiNardo S (2006) Dynamics of the male germline stem cell population during aging of Drosophila melanogaster . Aging Cell 5, 297–304. [DOI] [PubMed] [Google Scholar]
- Ward JD, Muzzini DM, Petalcorin MI, Martinez‐Perez E, Martin JS, Plevani P, Cassata G, Marini F, Boulton SJ (2010) Overlapping mechanisms promote postsynaptic RAD‐51 filament disassembly during meiotic double‐strand break repair. Mol. Cell 37, 259–272. [DOI] [PubMed] [Google Scholar]
- Wei DS, Rong YS (2007) A genetic screen for DNA double‐strand break repair mutations in Drosophila . Genetics 177, 63–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White RR, Sung P, Vestal CG, Benedetto G, Cornelio N, Richardson C (2013) Double‐strand break repair by interchromosomal recombination: an in vivo repair mechanism utilized by multiple somatic tissues in mammals. PLoS ONE 8, e84379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White RR, Milholland B, de Bruin A, Curran S, Laberge RM, van Steeg H, Campisi J, Maslov AY, Vijg J (2015) Controlled induction of DNA double‐strand breaks in the mouse liver induces features of tissue ageing. Nat. Commun. 6, 6790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams AB, Michael WM (2010) Eviction notice: new insights into Rad51 removal from DNA during homologous recombination. Mol. Cell 37, 157–158. [DOI] [PubMed] [Google Scholar]
- Wright WD, Heyer WD (2014) Rad54 functions as a heteroduplex DNA pump modulated by its DNA substrates and Rad51 during D loop formation. Mol. Cell 53, 420–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu C‐, Oshima J, Fu Y‐, Wijsman EM, Hisama F, Alisch R, Metthews S, Nakura J, Miki T, Ouais S, Martin GM, Mulligan J, Schellenberg GD (1996) Positional cloning of the Werner's syndrome gene. Science 272, 258–262. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1 Mu‐2 repair foci persist in older animals.
Fig. S2 SSA repair is not significantly affected by age.
Fig. S3 HR repair of constitutively‐induced DSBs increases with age.
Fig. S4 mRNA levels of early HR components increase in older flies.
Table S1 HR proportion of total flies from spontaneous events.


