Abstract
Objective
As Ureaplasmas may be pathogens in preterm infants, this study was conducted to determine the incidence of invasive disease with Ureaplasma parvum and Ureaplasma urealyticum and the relationship with adverse outcomes in a prospective cohort of very low birth weight (VLBW) infants.
Study Design
DNA was extracted from the cord or venous blood and cerebrospinal fluid (CSF) samples obtained from 313 VLBW infants. PCR was performed using primers for the mba gene to detect all 14 serovars and then repeated for all positive samples using species-specific primers.
Result
Ureaplasma species were detected in serum and/or CSF samples from 74 of 313 (23.6%) infants. U. parvum was the predominant species (70%). Presence of Ureaplasma was significantly associated with elevated interleukin-1β in cord blood (odds ratio (OR) 2.6, 1.05 to 6.45, P = 0.039). Ureaplasma serum-positive infants had a 2.3-fold increased risk of intraventicular hemorrhage ≥ grade 3 (OR 2.50; 1.06 to 5.89, P = 0.036).
Conclusion
Invasive Ureaplasma occurs commonly in VLBW infants and may increase the risk for severe intraventricular hemorrhage.
Keywords: prematurity, chorioamnionitis, PCR, cranial ultrasound
Introduction
The genital mycoplasma Ureaplasma urealyticum was previously subtyped into two biovars and 14 serovars. On the basis of the significant genotypic differences between the two biovars, it has been proposed that the species previously known as U. urealyticum be separated into two new species, Ureaplasma parvum (biovar 1, containing serovars 1, 3, 6 and 14) and U. urealyticum (biovar 2, containing serovars 2, 4, 5 and 7 to 13).1 The common characteristics of all serovars include lack of a cell wall, small genome size, limited biosynthetic abilities, hydrolysis of urea and mucosa association in the human host.2
As Ureaplasma is a commensal in the adult female genital tract, it has been considered of low virulence. However, it has been associated with multiple obstetrical complications including infertility, stillbirth, preterm delivery, histologic chorioamnionitis, neonatal morbidity and perinatal death.2 U. parvum and U. urealyticum are the most common organisms isolated from amniotic fluid and infected placentas.3 Ureaplasma respiratory tract colonization has been associated with respiratory distress syndrome,4 persistent pulmonary hypertension5 and bronchopulmonary dysplasia (BPD).6,7 Some studies suggest serovars 4 and 8 are more frequently associated with disease.8 Although the relationship of Ureaplasma respiratory tract colonization with BPD has been extensively studied, less is known concerning the incidence of invasive disease (detection in blood and/or cerebrospinal fluid (CSF)) and the relationship with neonatal outcomes. In the prospective Alabama Preterm Birth Study cohort, Ureaplasma was detected in 17% cord blood cultures,3 but genotyping and CSF cultures were not performed. Detection of the organism by culture in CSF has been described in small studies9,10 and case reports.11–14
Previous studies in human infants15–17 and experimental animal18,19 and in vitro20 models demonstrated that Ureaplasma stimulates the host inflammatory response. We conducted a prospective study to determine whether Ureaplasma infection in blood or CSF is associated with inflammation in the placenta, blood or CSF, and increases the risk for BPD or cranial ultrasound (CUS) abnormalities in infants ≤32 weeks gestation. In the initial analysis of this cohort, we observed that elevated inflammatory cytokines in serum and CSF were associated with an abnormal CUS.21 Serum and CSF samples from this cohort were analyzed by PCR to determine the incidence of invasive Ureaplasma in very low birth weight (VLBW) infants and to determine the contribution of the two species to invasive disease. We hypothesized that invasive Ureaplasma would be associated with systemic and/or CNS inflammation and neonatal morbidities such as intraventricular hemorrhage (IVH).
Study design
Sample
Eligible infants were inborn infants with gestational age <33 weeks and birth weight <1501 g admitted to the neonatal intensive care units at the University of Maryland Medical Center and Mercy Medical Center (Baltimore, MD, USA) between May 1999 and December 2002. Parent consent was obtained, and the University of Maryland School of Medicine and Mercy Medical Center Institutional Review Boards approved the study protocol. Sample size calculation and cohort description are provided in the prior report.21
Specimen extraction and PCR
After informed consent was obtained, serum was obtained from available cord blood (2 ml) or, if cord blood was not available, venous blood (1 ml) was drawn aseptically within 12 h of delivery, the serum separated and aliquots stored at −80 °C for later analysis of cytokines by ELISA or processed for DNA extraction and amplification. CSF was obtained within 72 h of birth from 189 of 313 (60%) of infants as part of an evaluation for sepsis. A stringent protocol for the processing of blood and CSF samples, DNA extraction and PCR was followed to prevent contamination and hence false-positives. This included prepping the cord and skin sites with betadine; physical separation of areas for sample preparation, amplification and product detection; use of filter-plugged tips; and inclusions of water, extracted with the specimens, as a negative control in each PCR run. DNA was extracted from serum and CSF (200 µl each) samples using the QiAmp DNA Blood Mini kits (Qiagen, Valencia, CA, USA) according to the manufacturer’s protocol. PCR was first performed with primers directed against the 5′ region of the multiple-banded antigen (mba) gene to identify all positive samples.22 The 50 µl reaction mixture consisted of 10 µl 10× Opti-prime buffer #11 (Stratagene, La Jolla, CA, USA), 30 pmol each of the primers UMS-125 (GTATTTGCAATCTTTATATGTTTTCG) and UMA 226 (CAGCTGATGTAAGTGCAGCATTAAATT), 200 µm each dNTP, 2.5 U Platinum Taq DNA polymerase (Invitrogen, Carlsbad, CA, USA) and 10 µl sample template. The thermal cycling parameters for the first reaction were 94 °C for 2 min, then 40 cycles of 94 °C for 30 s, 58 °C for 45 s, 72 °C for 45 s and 72 °C for 7 min for final extension. All positive samples were subjected to a second PCR reaction using species-specific primers according to the algorithm proposed by Kong et al.1 Thermal cycling reaction parameters were the same as above except that the annealing temperature was 56 °C for each set of species-specific primers. All PCRs included a positive control, equivalent to 100 color changing units of Ureaplasma. After PCR, 10 µl of each sample and controls was run on a 2% ethidium bromide-stained agarose gel to determine whether the expected product had been amplified.
Cytokines
The cytokines interleukin (IL)-1β, tumor necrosis factor-α and IL-6 were measured in serum and CSF samples as previously described.21
Placental pathology
Placental studies were performed on 262 (84%) infants. The placental sections were reviewed in a blinded manner according to a standard protocol.23 Histologic chorioamnionitis was separated into maternal and fetal response and assigned a stage according to the scheme proposed by Redline et al.23
Neonatal outcomes
An infant was assigned the diagnosis of BPD if the infant was receiving supplemental oxygen or positive pressure support at 36-week post-menstrual age. Serial CUS examinations were performed as previously described.21
Statistical analysis
The t-test and analysis of variance was used to compare continuous variables and the χ2 or Fisher exact test was used to compare categorical variables. Univariate odds ratios (ORs) and 95% confidence intervals were calculated for neonatal outcomes including BPD and specific CUS diagnoses. As the range of cytokine concentrations was large, for analyses, cytokine data expressed as pg ml−1 were dichotomized using the median of serum and CSF IL-6 concentrations and serum IL-1β concentrations for the entire sample and the lower cutoff of the ELISA sensitivity for tumor necrosis factor-α concentrations and CSF IL-1β concentrations. For determining whether there were interactions of Ureaplasma PCR status with the inflammatory variables for each outcome, interaction terms were generated and included in regression models. All statistical analyses were performed using STATA (Stata Corp., College Station, TX, USA). A P-value <0.05 was considered significant. The P-values were not corrected for multiple comparisons.
Results
Cohort characteristics
Of 442 eligible infants admitted to the University of Maryland Medical Center and Mercy Medical Center during the study period, 313 were enrolled after parental consent was obtained. Of the remaining 129 infants, consent was declined for 24 infants, and in 105, there was no cord blood available, age was greater than 12 h when screened, and a lumbar puncture was deferred or not indicated. Infants not enrolled had similar race, sex, gestational age and obstetrical factors as enrolled infants.
Ureaplasma species were detected in serum and/or CSF samples from 74 of 313 (23.6%) infants. This included 46 of 246 serum samples (29/152, 19.1% cord blood; 17/94, 18.1% venous blood) and 36 of 189 (19.1%) CSF samples. Eight of 122 infants (6.6%) with paired blood and CSF were PCR-positive in both compartments. For 95 infants who were serum Ureaplasma PCR-negative, the paired CSF sample was PCR-positive in 19 cases (20%).
Serum or CSF Ureaplasma PCR-positive infants were similar to PCR-negative infants for birth weight, gestational age and race (Table 1). It is of interest to note that membrane rupture duration was <1 h in 31% of serum and 22% of CSF Ureaplasma PCR-positive infants, suggesting a possible preexisting rather than ascending infection.24,25 Forty-six percent serum PCR-positive and 44% CSF PCR-positive infants were delivered by cesarean section, indicating that operative delivery did not prevent invasive Ureaplasma.
Table 1.
Characteristics of the study cohorta
| Variable | Serum Ureaplasma PCR positive N = 46 |
Serum Ureaplasma PCR negative N = 200 |
CSF Ureaplasma PCR positive N = 36 |
CSF Ureaplasma PCR negative N = 153 |
|---|---|---|---|---|
| Birth weight (g) | 951 ± 242 | 996 ± 289 | 1075 ± 276 | 993 ± 271 |
| Gestational age (week) | 27.3 ± 2.3 | 27.6 ± 2.5 | 27.4 ± 2.2 | 28.4 ± 2.6 |
| Black race | 36 (78) | 142 (71) | 26 (72) | 114 (75) |
| Preterm labor | 41 (89) | 159 (80) | 33 (91) | 131 (86) |
| PPROM | 20 (44) | 79 (40) | 19 (53) | 80 (53) |
| Duration ROM < 1 h | 14 (31) | 79 (40) | 8 (22) | 40 (26) |
| Cesarean section | 21 (46) | 117 (59) | 16 (44) | 70 (46) |
Abbreviations: CSF, cerebrospinal fluid; PPROM, preterm premature rupture of membranes; ROM, rupture of membranes.
Data are presented as mean ± s.d. or number positive (%).
All samples PCR-positive with primers for the common mba gene were reamplified using species-specific primers. As shown in Table 2, U. parvum was the most common species detected in serum and CSF. Both species were detected in the same sample in three serum and two CSF samples. Eight serum and five CSF samples could not be further genotyped possibly due to the degradation of the bacterial DNA or presence of inhibitors in the samples.
Table 2.
Ureaplasma species distribution in serum and CSF positive samplesa
| Ureaplasma parvum | Ureaplasma urealyticum | Both species | Unknown | |
|---|---|---|---|---|
| Serum, N = 46 |
31 (67) | 4 (9) | 3 (7) | 8 (17) |
| CSF, N = 36 |
26 (72) | 3 (8) | 2 (6) | 5 (14) |
Abbreviation: CSF, cerebrospinal fluid.
Serum and CSF samples PCR-positive for the common mba gene were genotyped using species-specific primers. Eight serum and five CSF samples could not be further genotyped.
Relationship of invasive Ureaplasma and respiratory tract colonization
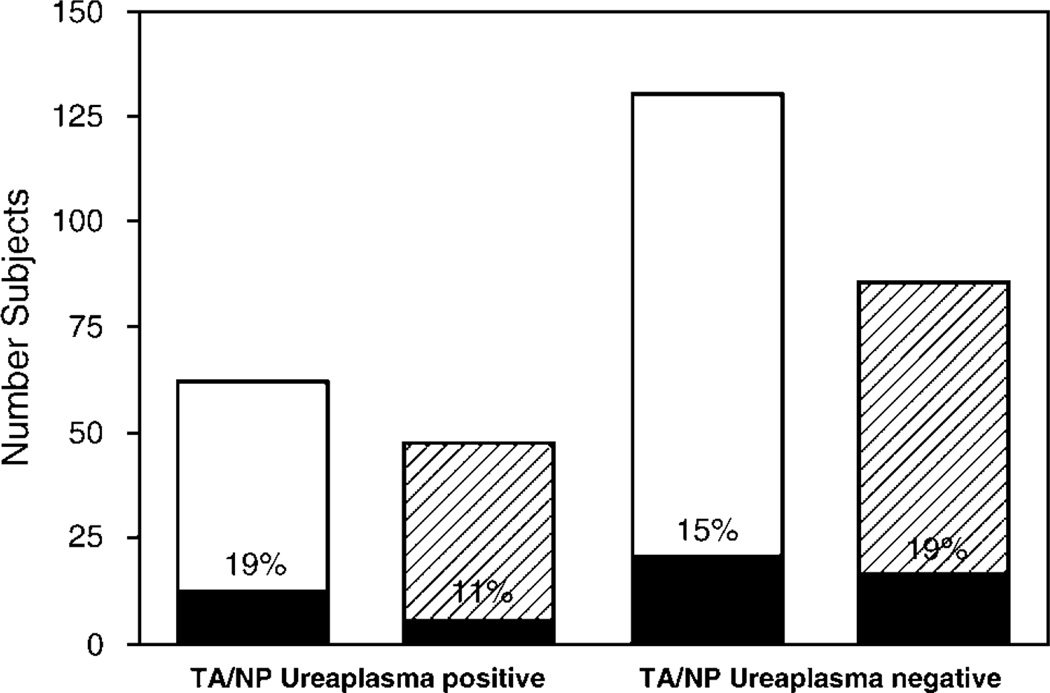
As Ureaplasma respiratory colonization occurs commonly in VLBW infants,2,15 we analyzed the incidence of invasive Ureaplasma in a subset of the study cohort with known Ureaplasma respiratory colonization status. Tracheal aspirate or nasopharyngeal Ureaplasma cultures were performed during the first week of life in 232 infants with a 35% culture-positive rate. As shown in Figure 1, 19% serum and 11% CSF samples from infants with culture-confirmed Ureaplasma respiratory colonization and 15% serum and 19% CSF samples from infants without Ureaplasma respiratory colonization were PCR-positive. In all cases, the Ureaplasma detected in respiratory samples was the same species as the one detected in serum or CSF from the same infant.
Figure 1.
Relationship of Ureaplasma respiratory tract colonization in the first week of life with detection of Ureaplasma in serum and cerebrospinal fluid (CSF) by PCR. PCR to detect Ureaplasma species in serum (white bars) and CSF (hatched bars) was performed in a subset of infantss with known respiratory tract colonization status (tracheal aspirate (TA) or nasopharyngeal (NP)) in the first week of life. The number of PCR-positive infants in each group is shown in black.
Relationship of invasive Ureaplasma and inflammation
Histologic chorioamnionitis was present in 65% Ureaplasma serum PCR-positive and 68% CSF PCR-positive cases (Table 3). Fetal vasculitis was detected in 51% serum PCR-positive and 57% CSF PCR-positive cases. Thirty-three percent of infants exposed to long-standing or necrotizing amnionitis (maternal stage 5) were serum PCR-positive (Figure 2a). Fetal vasculitis was present in all of the serum PCR-positive infants with maternal stage 5 histologic chorioamnionitis. In contrast, the maternal stage in CSF PCR-positive infants was less advanced than in CSF PCR-negative infants (Figure 2b), possibly suggesting a shorter duration of exposure to intrauterine infection/inflammation.
Table 3.
Relationship of inflammatory factors and invasive Ureaplasma
| Serum PCR(+) N = 46 |
Serum PCR(−) N = 200 |
OR (95% CI) | P-value | CSF PCR(+) N = 36 |
CSF PCR(−) N = 153 |
OR (95% CI) | P-value | |
|---|---|---|---|---|---|---|---|---|
| Histologic chorioamnionitisa | 65b | 64 | 0.92 (0.46–1.85) | 0.819 | 68 | 70 | 0.92 (0.38–2.22) | 0.851 |
| WBC > 9.2 × 103 | 47 | 53 | 0.8 (0.425–1.52) | 0.50 | 56 | 54 | 1.09 (0.52–2.27) | 0.814 |
| CSF WBC > 9.5 | 27 | 29 | 0.89 (0.365–2.19) | 0.807 | 26 | 22 | 1.22 (0.52–2.87) | 0.645 |
| Cord serum IL-6 ≥ 18.2 pg ml−1 | 52 | 49 | 1.11 (0.49–2.5) | 0.808 | 60 | 48 | 1.16 (0.41–6.34) | 0.495 |
| Venous serum IL-6 ≥ 18.2 pg ml−1 | 36 | 53 | 0.48 (0.144–1.62) | .239 | 60 | 52 | 1.41 (0.34–5.94) | 0.638 |
| Combined serum IL-6 ≥ 18.2 pg ml−1 | 46 | 51 | 0.85 (0.435–1.66) | 0.63 | 62 | 50 | 1.63 (0.61–4.3) | 0.328 |
| Cord serum IL-1β ≥ 0.322 pg ml−1 | 70 | 48 | 2.6 (1.05–6.45) | 0.039 | 78 | 59 | 2.41 (0.45–12.6) | 0.301 |
| Venous serum IL-1β ≥ 0.322 pg ml−1 | 46 | 43 | 1.128 (0.325–3.91) | 0.850 | 56 | 50 | 1.25 (0.27–5.73) | 0.774 |
| Combined serum IL-1β ≥ 0.322 pg ml−1 | 63 | 46 | 1.92 (0.94–3.93) | 0.07 | 68 | 56 | 1.73 (0.6–5.01) | 0.31 |
| Cord serum TNF-α ≥ 3 pg ml−1 | 38 | 46 | 0.76 (0.314–1.83) | 0.54 | 40 | 33 | 1.37 (0.34–5.52) | 0.66 |
| Venous serum TNF-α ≥ 3 pg ml−1 | 36 | 38 | 1.127 (0.325–3.91) | 0.85 | 33 | 10 | 0.94 (0.24–3.70) | 0.931 |
| Combined serum TNF-α ≥ 3 pg ml−1 | 38 | 43 | 0.80 (0.386–1.69) | 0.57 | 40 | 26 | 1.93 (0.69–5.44) | 0.213 |
| CSF IL-6 ≥ 6.3 pg ml−1 | 36 | 56 | 0.44 (0.179–1.11) | 0.082 | 53 | 46 | 1.32 (0.60–2.92) | 0.49 |
| CSF IL-1β ≥ 0.78 pg ml−1 | 25 | 37 | 0.56 (0.200–1.56) | 0.266 | 36 | 36 | 1.0 (0.41–2.45) | 0.99 |
| CSF TNF-α ≥ 3 pg ml−1 | 16 | 19 | 0.80 (0.241–2.62) | 0.707 | 24 | 21 | 1.18 (0.46–3.05) | 0.73 |
Abbreviations: CI, confidence interval; CSF, cerebrospinal fluid; IL, interleukin; OR, odds ratio; TNF, tumor necrosis factor.
Based on review of 262 placentas.
Percentage positive.
Figure 2.
Relationship of maternal stage of chorioamnionitis and detection of Ureaplasma species in serum (a) and cerebrospinal fluid (CSF) (b). Number of study infants exposed to different maternal stages of chorioamnionitis23 with (black) and without Ureaplasma (white). Percentages of Ureaplasma PCR-positive for each stage are noted in each bar. P = 0.008, serum PCR positive vs serum PCR-negative; P = 0.01, CSF PCR-positive vs CSF PCR-negative.
As experimental animal18,19 and in vitro20 models have demonstrated that Ureaplasma stimulates a proinflammatory cytokine response, we analyzed the relationship of invasive Ureaplasma with inflammatory cytokines in serum and CSF (Table 3). When data from cord and venous samples were combined, there was a trend toward increased serum IL-1β in serum PCR-positive infants (OR 1.92, 0.94 to 3.93; P = 0.07). However, when the analysis was limited to IL-1β concentrations in cord samples alone, an elevated IL-1β was detected in 70% of Ureaplasma serum PCR-positive compared with 48% cord serum PCR-negative infants (OR 2.6, 1.05 to 6.45, P = 0.039). No other cytokine or white blood count in serum or CSF was associated with invasive Ureaplasma.
Relationship with outcomes
There was no difference in survival between Ureaplasma PCR-positive and PCR-negative infants (Table 4). Although the incidence of BPD was higher in respiratory colonized than noncolonized infants (41 vs 23%, P = 0.003), there was no difference in incidence of BPD between infants with and without Ureaplasma detected in serum or CSF. There was a trend toward an increase in all IVH grades in Ureasplasma serum PCR-positive infants (OR 1.7, 0.91 to 3.3; P = 0.092). The risk of severe IVH (≥ grade 3) was 2.32-fold higher in serum PCR-positive than PCR-negative infants. This difference remained significant after adjustment for gestational age (OR 2.50; 1.06 to 5.89, P = 0.036). U. parvum was the species detected in all PCR-positive infants with severe IVH. Interactions of inflammatory variables and invasive Ureaplasma for adverse outcomes were examined in regression analyses. Only an interaction of Ureaplasma in serum detected by PCR and elevated serum IL-1β was significant with a 5.36-fold increased risk for severe IVH (1.43 to 20.13, P = 0.013).
Table 4.
Relationship of invasive Ureaplasma with neonatal outcomes
| Serum PCR(+) N = 46 |
Serum PCR(−) N = 200 |
OR (95% CI) | P-value | CSF PCR(+) N = 36 |
CSF PCR(−) N = 153 |
OR (95% CI) | P-value | |
|---|---|---|---|---|---|---|---|---|
| Survival | 97a | 92 | 4.06 (0.53–31.5) | 0.179 | 94 | 95 | 0.84 (0.17–4.25) | 0.837 |
| BPD | 20 | 23 | 0.81 (0.36–1.81) | 0.605 | 14 | 23 | 0.58 (0.21–1.61) | 0.292 |
| IVH | 54 | 42 | 1.7 (0.91–3.30) | 0.092 | 44 | 49 | 0.87 (0.42–1.8) | 0.704 |
| IVH ≥ grade 3 | 24 | 12 | 2.32 (1.04–5.19) | 0.039 | 6 | 16 | 0.29 (0.66–1.29) | 0.105 |
| PVL | 9 | 7 | 1.2 (0.38–3.87) | 0.741 | 3 | 7 | 0.36 (0.04–2.85) | 0.330 |
Abbreviations: BPD, bronchopulmonary dysplasia; CI, confidence interval; CSF, cerebrospinal fluid; IVH, intraventricular hemorrhage; PVL, periventricular leukomalacia; OR, odds ratio.
Percentage positive.
Discussion
Recently, Goldenberg et al.3 demonstrated that the incidence of Ureaplasma in cord blood from VLBW infants by culture techniques is 17% and is associated with markers of inflammation. We confirm and extend these observations in this study. We demonstrated in a large prospective cohort that Ureaplasma species not only colonize the respiratory tract, but also invade the bloodstream and cross the immature blood–brain barrier in 23% VLBW infants and that U. parvum is the predominant species. Overall, almost half of the cohort was Ureaplasma positive in one or more compartments (respiratory, blood, CNS), confirming that this organism is the most common pathogen affecting this population. The duration of rupture of membranes was < 1 h in 30% of Ureaplasma-positive infants, suggesting the presence of pre-existing infection rather than ascending infection in some of these infants.24,25
In this study, U. parvum was the predominant species detected in serum and CSF. This is in agreement with the predominance of the U. parvum serovars in clinical samples obtained from vaginal cultures in women and tracheal aspirates8 and CSF from preterm infants.10 In contrast to Abele-Horn et al.,8 who observed a higher incidence of BPD in infants with respiratory colonization with U. urealyticum, there were no differences in this study in the outcomes of infants infected by the different species.
Although Ureaplasma is a commensal organism in the adult female genital tract, there is previous strong clinical evidence that it can cause invasive disease in the preterm neonate. Ureaplasma has been isolated by culture techniques from blood, CSF, tracheal aspirates and lung and brain tissue of newborn infants.13,14,26 The prevalence of Ureaplasma detected in CSF from preterm infants for whom a lumbar puncture was performed for suspected meningitis was 8%,9 suggesting that Ureaplasma is one of the most common organism isolated from CSF in this high-risk population. It is likely that culture techniques underestimate the true rate of Ureaplasma infection.
In this study, detection of Ureaplasma by PCR in serum, but not CSF, increased the risk for severe IVH by twofold. This is in contrast to the study by Waites et al.9 that isolated Ureaplasma from the CSF of six infants with severe IVH and from three with hydrocephalus. Ureaplasma was also cultured from brain tissue of preterm twins who died of IVH.14 Our result differs from the Alabama Preterm Birth study that found no association of combined Ureaplasma and/or Mycoplasma hominis culture status with IVH severity.3 This difference may be explained by the differences in study populations and rates of severe IVH, culture vs PCR-based detection methods and analysis of combined mycoplasma species vs Ureaplasma alone.
Inflammation initiated outside the CNS may contribute to IVH pathogenesis. Ureaplasma PCR-positive serum was associated with amnionitis and elevated IL-1β, suggesting that the observed brain injury in affected infants may result, in part, from intrauterine infection that elicits maternal and fetal inflammatory responses and activates the systemic and CNS inflammatory cascades. In juvenile rhesus monkeys, intravenous administration of IL-1β induces brain-derived IL-6 in CSF,27 confirming that the brain responds to peripheral activation. Furthermore, systemic inflammatory cytokines may exacerbate injury mediated by excitatory neurotransmitters such as glutamate released in response to hypoxia-ischemia.28 In a model of ibotenate-induced excitotoxic brain injury in newborn mice, systemic pretreatment with IL-1β, IL-6 or tumor necrosis factor-α exacerbates ibotenate-induced brain lesions.28 We have previously shown that Ureaplasma directly stimulates cord blood monocyte IL-1β release in vitro20 and that Ureaplasma respiratory colonization in preterm infants is associated with elevated lung lavage IL-1β in vivo.15 These observations support the contention that Ureaplasma can elicit an immune response that may be detrimental to the host.
There are a number of study limitations. First, lumbar punctures were not performed on all infants, so some infants with Ureaplasma-positive CSF may have been missed. Second, only a single sample of either cord blood or venous blood drawn within 12 h (for those infants without an available cord sample) was analyzed for each infant. Therefore, the rate of clearance of Ureaplasma from the blood and CSF cannot be determined as there was only a single sampling time point. Finally, the timing of the first CUS scan at 3 to 7 days did not allow us to determine the timing of IVH. Despite these limitations, results of this study suggest that Ureaplasma-invasive disease is more prevalent than previously suspected and that it may be a contributing factor in the pathogenesis of IVH. To develop preventative strategies, future studies will need to determine the timing and route of transmission of invasive Ureaplasma and host factors that increase the susceptibility of some preterm infants to bloodstream and CNS invasion by this pathogen.
Acknowledgments
The University of Maryland Special Research Initiative Support Program and National Institute of Health Grant R01 HL71113 (RMV) supported this work.
References
- 1.Kong F, Ma Z, James G, Gordon S, Gilbert GL. Species identification and subtyping of Ureaplasma parvum and Ureaplasma urealyticum using PCR-based assays. J Clin Microbiol. 2000;38:1175–1179. doi: 10.1128/jcm.38.3.1175-1179.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Waites KB, Katz B, Schelonka RL. Mycoplasmas and Ureaplasmas as neonatal pathogens. Clin Microbiol Rev. 2005;18:757–789. doi: 10.1128/CMR.18.4.757-789.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goldenberg RL, Andrews WW, Goepfert AR, Faye-Petersen O, Cliver SP, Carlo WA, et al. The Alabama Preterm Birth Study: umbilical cord blood Ureaplasma urealyticum and Mycoplasma hominis cultures in very preterm newborn infants. Am J Obstet Gynecol. 2008;198:43.e1–43.e5. doi: 10.1016/j.ajog.2007.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Abele-Horn M, Peters J, Genzel-Boroviczeny O, Wolff C, Zimmermann A, Gottschling W. Vaginal Ureaplasma urealyticum colonization: influence on pregnancy outcome and neonatal morbidity. Infection. 1997;25:286–291. doi: 10.1007/BF01720398. [DOI] [PubMed] [Google Scholar]
- 5.Waites KB, Crouse DT, Philips JB, Canupp KC, Cassell GH. Ureaplasmal pneumonia and sepsis associated with persistent pulmonary hypertension of the newborn. Pediatrics. 1989;83:79–85. [PubMed] [Google Scholar]
- 6.Wang EL, Ohlsson A, Kellner JD. Association of Ureaplasma urealyticum colonization with chronic lung disease of prematurity: results of a metaanalysis. J Pediatr. 1995;127:640–644. doi: 10.1016/s0022-3476(95)70130-3. [DOI] [PubMed] [Google Scholar]
- 7.Schelonka RL, Katz B, Waites KB, Benjamin DK., Jr Critical appraisal of the role of Ureaplasma in the development of bronchopulmonary dysplasia with metaanalytic techniques. Pediatr Infect Dis J. 2005;24(12):1033–1039. doi: 10.1097/01.inf.0000190632.31565.83. [DOI] [PubMed] [Google Scholar]
- 8.Abele-Horn M, Wolff C, Dressel P, Pfaff F, Zimmermann A. Association of Ureaplasma urealyticum biovars with clinical outcome for neonates, obstetric patients, and gynecological patients with pelvic inflammatory disease. J Clin Microbiol. 1997;35:1199–1202. doi: 10.1128/jcm.35.5.1199-1202.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Waites KB, Crouse DT, Nelson KG, Rudd PT, Canupp KC, Ramsey C, et al. Chronic Ureaplasma urealyticum and Mycoplasma hominis infections of central nervous system in preterm infants. Lancet. 1988;2:17–21. doi: 10.1016/s0140-6736(88)91002-1. [DOI] [PubMed] [Google Scholar]
- 10.Zheng X, Watson HL, Waites KB, Cassell GH. Serotype diversity and antigen variation among invasive isolates of Ureaplasma urealyticum from neonates. Infect Immun. 1992;60:3472–3474. doi: 10.1128/iai.60.8.3472-3474.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Garland SM, Murton LJ. Neonatal meningitis caused by Ureaplasma urealyticum. Pediatr Infect Dis J. 1987;6(9):868–870. doi: 10.1097/00006454-198709000-00019. [DOI] [PubMed] [Google Scholar]
- 12.Heggie AD, Jacobs MR, Butler VT, Baley JE, Boxerbaum B. Frequency and significance of isolation of Ureaplasma urealyticum and Mycoplasma hominis from cerebrospinal fluid and tracheal aspirate specimens from low birth weight infants. J Pediatr. 1994;124:956–961. doi: 10.1016/s0022-3476(05)83192-0. [DOI] [PubMed] [Google Scholar]
- 13.Waites KB, Crouse DT, Cassell GH. Systemic neonatal infection due to Ureaplasma urealyticum. Clin Infect Dis. 1993;17(Suppl 1):S131–S135. doi: 10.1093/clinids/17.supplement_1.s131. [DOI] [PubMed] [Google Scholar]
- 14.Ollikainen J, Hiekkaniemi H, Korppi M, Katila ML, Heinonen K. Ureaplasma urealyticum cultured from brain tissue of preterm twins who died of intraventricular hemorrhage. Scand J Infect Dis. 1993;25:529–531. doi: 10.3109/00365549309008538. [DOI] [PubMed] [Google Scholar]
- 15.Patterson AM, Taciak V, Lovchik J, Fox RE, Campbell AB, Viscardi RM. Ureaplasma urealyticum respiratory tract colonization is associated with an increase in IL-1β and TNF-α relative to IL-6 in tracheal aspirates of preterm infants. Pediatr Infect Dis J. 1998;17:321–328. doi: 10.1097/00006454-199804000-00011. [DOI] [PubMed] [Google Scholar]
- 16.Viscardi RM, Manimtim WM, Sun CCJ, Duffy L, Cassell GH. Lung pathology in premature infants with Ureaplasma urealyticum infection. Pediatr Devel Pathol. 2002;5:141–150. doi: 10.1007/s10024001-0134-y. [DOI] [PubMed] [Google Scholar]
- 17.Viscardi R, Manimtim W, He JR, Hasday JD, Sun CC, Joyce B, et al. Disordered pulmonary myofibroblast distribution and elastin expression in preterm infants with Ureaplasma urealyticum pneumonitis. Pediatr Dev Pathol. 2006;9(2):143–151. doi: 10.2350/10-05-0112.1. [DOI] [PubMed] [Google Scholar]
- 18.Viscardi RM, Kaplan J, Lovchik JC, He JR, Hester L, Rao S, et al. Characterization of a murine model of Ureaplasma urealyticum pneumonia. Infect Immun. 2002;70:5721–5729. doi: 10.1128/IAI.70.10.5721-5729.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yoder BA, Coalson JJ, Winter VT, Siler-Khodr T, Duffy LB, Cassell GH. Effects of antenatal colonization with Ureaplasma urealyticum on pulmonary disease in the immature baboon. Pediatr Res. 2003;54:797–807. doi: 10.1203/01.PDR.0000091284.84322.16. [DOI] [PubMed] [Google Scholar]
- 20.Manimtim WM, Hasday JD, Hester L, Fairchild KD, Lovchik JC, Viscardi RM. Ureaplasma urealyticum modulates endotoxin-induced cytokine release by human monocytes derived from preterm and term newborns and adults. Infect Immun. 2001;69(6):3906–3915. doi: 10.1128/IAI.69.6.3906-3915.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Viscardi RM, Muhumuza CK, Rodriguez A, Fairchild KD, Sun CC, Gross GW, et al. Inflammatory markers in intrauterine and fetal blood and cerebrospinal fluid compartments are associated with adverse pulmonary and neurologic outcomes in preterm infants. Pediatr Res. 2004;55:1009–1017. doi: 10.1203/01.pdr.0000127015.60185.8a. [DOI] [PubMed] [Google Scholar]
- 22.Nelson S, Matlow A, Johnson G, Th’ng C, Dunn M, Quinn P. Detection of Ureaplasma urealyticum in endotracheal tube aspirates from neonates by PCR. J Clin Microbiol. 1998;36:1236–1239. doi: 10.1128/jcm.36.5.1236-1239.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Redline RW, Wilson-Costello D, Borawski E, Fanaroff AA, Hack M. Placental lesions associated with neurologic impairment and cerebral palsy in very low-birth-weight infants. Arch Pathol Lab Med. 1998;122:1091–1098. [PubMed] [Google Scholar]
- 24.Dammann O, Allred EN, Genest DR, Kundsin RB, Leviton A. Antenatal mycoplasma infection, the fetal inflammatory response and cerebral white matter damage in very-low-birthweight infants. Paediatr Perinat Epidemiol. 2003;17(1):49–57. doi: 10.1046/j.1365-3016.2003.00470.x. [DOI] [PubMed] [Google Scholar]
- 25.McElrath TF, Allred EN, Leviton A. Prolonged latency after preterm premature rupture of membranes: an evaluation of histologic condition and intracranial ultrasonic abnormality in the neonate born at < 28 weeks of gestation. Am J Obstet Gynecol. 2003;189(3):794–798. doi: 10.1067/s0002-9378(03)00814-7. [DOI] [PubMed] [Google Scholar]
- 26.Ollikainen J, Hiekkaniemi H, Korppi M, Sarkkinen H, Heinonen K. Ureaplasma urealyticum infection associated with acute respiratory insufficiency and death in premature infants. J Pediatr. 1993;122:756–760. doi: 10.1016/s0022-3476(06)80022-3. [DOI] [PubMed] [Google Scholar]
- 27.Reyes TM, Coe CL. The proinflammatory cytokine network: interactions in the CNS and blood of rhesus monkeys. Am J Physiol. 1998;274:R139–R144. doi: 10.1152/ajpregu.1998.274.1.R139. (Regulatory Integrative Comp Physiol 43) [DOI] [PubMed] [Google Scholar]
- 28.Dommergues MA, Patkai J, Renauld JC, Evrard P, Gressens P. Proinflammatory cytokines and interleukin-9 exacerbate excitotoxic lesions of the newborn murine neopallium. Ann Neurol. 2000;47:54–63. [PubMed] [Google Scholar]