The hematopoietic microenvironment while crucial for maintaining healthy self-renewal and differentiation of hematopoietic stem and progenitor cells (HSPCs) may also be a source for deleterious signals that may cause damage to HSPCs leading to disease (1,2). Previous studies have shown that alterations to niche components can trigger or aid in disease progression. A study to generate an improved xenograft mouse model of myelodysplasia syndrome (MDS) found that co-transplantation of patient stem cells with stromal cells increased disease engraftment, suggesting that the stromal cells are important in disease initiation and maintenance (3). Acute myeloid leukemia (AML) was triggered in mice by activation of β-catenin in osteoblast which lead to overexpression of the Notch ligand Jagged1 and upregulation of the Notch receptor in HSPCs (4). In another study, myeloproliferative syndrome (MPS) was induced in retinoic acid receptor gamma mutants by defects in the stromal cells that transformed hematopoietic cells (5,6). In mice deletion of Dicer in osteoprogenitors caused MDS leading in rare cases to AML, this defect was linked to decreased expression of the Sbds gene, which is mutated in patients with Schwachman-Diamond syndrome (SDS) a leukemia predisposition disorder (7). Furthermore, deletion of Sbds in osterix+ mesenchymal stromal progenitors created a mouse SDS model that displayed apoptosis in HSPCs and myelodysplasia (7). Recently, Zambetti and colleagues identified the mechanism by which deletion of Sbds in this SDS mouse model causes disease. They found that increased inflammatory signals in the microenvironment caused genotoxic stress in both the mouse model and SDS patient samples (8).
By detailed characterization of the skeletal system in the SDS model Zambetti and colleagues were able to define a skeletal phenotype with reduced growth, trabecular area and bone volume recapitulating the human disease (8). Interestingly, they observed an increase in the mesenchymal progenitor pool and found by transcriptional profiling that these progenitors where impaired to differentiate into matrix depositing osteoblast, leading to the observed phenotype. Next, Zambetti and colleagues analyzed the HSPCs and found that while their numbers were unaffected the transcriptional profile was similar to profiles associated with leukemic evolution of human CD34+ cells, specifically pathways linked to mitochondrial abnormalities were triggered (8). Further analysis of mitochondria found increased membrane potential leading to increased levels of reactive oxygen species (ROS); which activated DNA damage response and repair pathways indicated by accumulation of Ser139-phosphorylated H2AX histone (γH2AX) (8). Depletion of S-phase cells indicated cell cycle arrest at G1-S. These findings suggest a highly stressed and genotoxic environment for HSPCs although competitive transplantation assays showed that short-term exposure to this microenvironment did not have long-term effects of HSPCs with proper DNA repair (8). This suggests that treatment of the microenvironment may be a possibility in cases where disease progression caused by microenvironment stressors is in its early phase.
Analysis of mesenchymal stromal cells of SDS model mice found that p53 was overexpressed. Moreover, creating double knockouts of Sbds and Trp53 partially rescued the skeletal phenotype and ameliorated the genotoxic stress on HSPCs (8). Next, Zambetti and colleagues looked for downstream targets of p53 by comparing the transcriptome of mesenchymal stromal cells from SDS mouse models to that of CD271+ mesenchymal cells from human SDS patients. An abundance of inflammatory genes were increased in both the SDS mouse model and human patient mesenchymal stromal cells. The researchers narrowed their search for p53 targets by including analysis of mesenchymal stromal cells of two related diseases MDS (pre-leukemic disorder) and Diamond-Blackfan anemia (DBA) a ribosomopathy similar to SDS but with lower incidence of AML. They found 11 genes that were differentially expressed in SDS and MDS but not in DBA suggesting that they are important in the evolution of AML (8). The DAMP genes S100A8 and S100A9 were included in this gene set and are downstream targets of p53. These genes are pro-inflammatory molecules that bind to the TLR4 receptor, which is present in HSPCs. TLR4 activates NF-κB and MAPK pathways, which were upregulated in SDS mouse model HSPCs. Exposure of mouse HSPCs (LKS) and human cord blood CD34+ cells to S100A8/9 caused increased DNA damage and apoptosis (8). Likewise, in vivo overexpression of S100A8/9 in mesenchymal cells caused increased DNA damage of transplanted HSPCs. Analysis of MDS patient mesenchymal stromal cells found that those with S100A8/9+ stromal cells had a higher incidence and shorter time to leukemia evolution than those with S100A8/9− stromal cells. This suggests that p53-S100A9/9−TLR4 activation causes genotoxic stress and leukemic evolution in MDS and SDS (8).
The study conducted by Zambetti and colleagues focused on the loss of Sbds in osterix+ mesenchymal stromal cell as a driving force for genotoxic stress and transformation of the HSPCs population. There are various other support cells that contribute to HSPCs microenvironment which play partially redundant roles in maintaining the HSPCs population. Even within the mesenchymal stromal population there are primitive, intermediate and mature progenitor cells that maintain the microenvironment and provide support for HSPCs and various definitions of the different populations (9-11). While there are differences in markers used and definition of osteoblast, this study and others show that the lack of a healthy osteoblast population is a recurring theme in multiple blood disorders and that maintenance of a healthy microenvironment is crucial for HSPC population integrity. In AML mouse models we observed increased intermediate and primitive mesenchymal stromal cells and reduced osteoblast (12). Others have also seen osteoblastic defects in AML mouse models as well as AML and MDS patients (13,14). Ablation of osteoblast in mice lead to increased engraftment of both AML and chronic myeloid leukemia (CML) (15). Moreover, treatment of AML mice with compounds that inhibit osteoblast loss increased survival (14). In T-cell acute lymphoblastic leukemia (T-ALL) Notch overexpression suppressed osteoblast differentiation (16). In contrast, in a myeloproliferative neoplasia (MPN) mouse model an increase in trabecular number and thickness was observed and functional osteoblast were replaced by altered osteoblast that overproduced pro-inflammatory cytokines and myeloid differentiation signals (17). Suggesting that both loss of osteoblast or overproduction of abnormal osteoblast can cause deleterious effects to HSPCs. The differences observed may be due to markers used to define osteoblast as several of the studies did see increases in osteoprogenitors.
Another recurring theme is increased pro-inflammatory signals in the diseased microenvironment. In the MPN mouse model while the skeletal abnormalities observed were opposite of those observed by Zambetti and others, the pro-inflammatory theme persisted with osteoblast resembling inflammatory myelofibrotic cells at the molecular level; expressing cellular adhesion, extracellular matrix remodeling and inflammatory genes reminiscent of this cell type (17). In MDS patients, stromal cells were found to overexpress pro-inflammatory signals and these properties were transferred to healthy stromal cells upon co-culture (3). Loss of Notch signaling in bone marrow endothelial cells lead to increased miR-155 and activation of pro-inflammatory NF-κB which resulted in myeloproliferative-like disease in mice (18). It is unclear if there is a causal relationship between pro-inflammatory signals and changes to osteoblast but evidence provided by Zambetti and others shows that both are key players in disease evolution and maintenance (Figure 1).
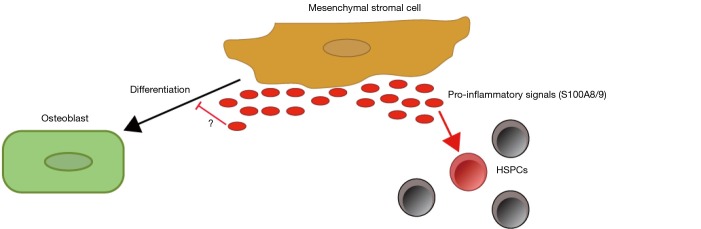
Figure 1.
Mesenchymal stromal cells of the Sbds mouse model secrete pro-inflammatory signals S100A8/9 causing genotoxic stress to the HSPCs. The mesenchymal stromal cells are also deficient in their ability to differentiate into osteoblast although it is unclear if the pro-inflammatory signals play a role in this defect.
These findings are pivotal in understanding the evolution of disease driven by microenvironment stressors. Although current treatments are still centered on elimination of the leukemia cells alone, this study adds to the growing research that suggests that treatment of AML and other cancers needs to focus on the microenvironment as a whole and not the specific cells that are being transformed. Disrupting the production of S1A008/9 with antibodies or other inhibitors may stop or delay evolution of MDS to AML. Post-irradiation or other forms of marrow ablation, transplanted mesenchymal stromal cells are capable of engrafting and providing substantial contributions in supporting HSPCs (9), allowing for the possibility of co-transplanting healthy mesenchymal stromal cells along with cord blood or marrow transplantations as a treatment option (19). If aberrant stromal components could be replaced by healthy ones survival may be increased in patients. This is an exciting study that provides a detailed mechanism of leukemic evolution and the possibility for future studies on microenvironment specific therapeutic targets.
Acknowledgements
Funding was provided by the American Cancer Society Grant 128766-RSG-15-162.
Provenance: This is an invited Commentary commissioned by Editor-in-Chief Zhizhuang Joe Zhao (Pathology Graduate Program, University of Oklahoma Health Sciences Center, Oklahoma City, USA).
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Schepers K, Campbell TB, Passegué E. Normal and leukemic stem cell niches: insights and therapeutic opportunities. Cell Stem Cell 2015;16:254-67. 10.1016/j.stem.2015.02.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Morrison SJ, Scadden DT. The bone marrow niche for haematopoietic stem cells. Nature 2014;505:327-34. 10.1038/nature12984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Medyouf H, Mossner M, Jann JC, et al. Myelodysplastic cells in patients reprogram mesenchymal stromal cells to establish a transplantable stem cell niche disease unit. Cell Stem Cell 2014;14:824-37. 10.1016/j.stem.2014.02.014 [DOI] [PubMed] [Google Scholar]
- 4.Kode A, Manavalan JS, Mosialou I, et al. Leukaemogenesis induced by an activating β-catenin mutation in osteoblasts. Nature 2014;506:240-4. 10.1038/nature12883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Walkley CR, Olsen GH, Dworkin S, et al. A microenvironment-induced myeloproliferative syndrome caused by retinoic acid receptor gamma deficiency. Cell 2007;129:1097-110. 10.1016/j.cell.2007.05.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Walkley CR, Shea JM, Sims NA, et al. Rb regulates interactions between hematopoietic stem cells and their bone marrow microenvironment. Cell 2007;129:1081-95. 10.1016/j.cell.2007.03.055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Raaijmakers MH, Mukherjee S, Guo S, et al. Bone progenitor dysfunction induces myelodysplasia and secondary leukaemia. Nature 2010;464:852-7. 10.1038/nature08851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zambetti NA, Ping Z, Chen S, et al. Mesenchymal Inflammation Drives Genotoxic Stress in Hematopoietic Stem Cells and Predicts Disease Evolution in Human Pre-leukemia. Cell Stem Cell 2016;19:613-27. 10.1016/j.stem.2016.08.021 [DOI] [PubMed] [Google Scholar]
- 9.Hu X, Garcia M, Weng L, et al. Identification of a common mesenchymal stromal progenitor for the adult haematopoietic niche. Nat Commun 2016;7:13095. 10.1038/ncomms13095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhou BO, Yue R, Murphy MM, et al. Leptin-receptor-expressing mesenchymal stromal cells represent the main source of bone formed by adult bone marrow. Cell Stem Cell 2014;15:154-68. 10.1016/j.stem.2014.06.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Méndez-Ferrer S, Michurina TV, Ferraro F, et al. Mesenchymal and haematopoietic stem cells form a unique bone marrow niche. Nature 2010;466:829-34. 10.1038/nature09262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kumar B, Garcia M, Weng L, et al. Acute Myeloid Leukemia-Derived Exosomes Transform Bone Marrow Niche into Leukemic Niche. Blood 2014;124:352. [Google Scholar]
- 13.Frisch BJ, Ashton JM, Xing L, et al. Functional inhibition of osteoblastic cells in an in vivo mouse model of myeloid leukemia. Blood 2012;119:540-50. 10.1182/blood-2011-04-348151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Krevvata M, Silva BC, Manavalan JS, et al. Inhibition of leukemia cell engraftment and disease progression in mice by osteoblasts. Blood 2014;124:2834-46. 10.1182/blood-2013-07-517219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bowers M, Zhang B, Ho Y, et al. Osteoblast ablation reduces normal long-term hematopoietic stem cell self-renewal but accelerates leukemia development. Blood 2015;125:2678-88. 10.1182/blood-2014-06-582924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang W, Zimmerman G, Huang X, et al. Aberrant Notch Signaling in the Bone Marrow Microenvironment of Acute Lymphoid Leukemia Suppresses Osteoblast-Mediated Support of Hematopoietic Niche Function. Cancer Res 2016;76:1641-52. 10.1158/0008-5472.CAN-15-2092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schepers K, Pietras EM, Reynaud D, et al. Myeloproliferative neoplasia remodels the endosteal bone marrow niche into a self-reinforcing leukemic niche. Cell Stem Cell 2013;13:285-99. 10.1016/j.stem.2013.06.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang L, Zhang H, Rodriguez S, et al. Notch-dependent repression of miR-155 in the bone marrow niche regulates hematopoiesis in an NF-κB-dependent manner. Cell Stem Cell 2014;15:51-65. 10.1016/j.stem.2014.04.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Noort WA, Kruisselbrink AB, in't Anker PS, et al. Mesenchymal stem cells promote engraftment of human umbilical cord blood-derived CD34(+) cells in NOD/SCID mice. Exp Hematol 2002;30:870-8. 10.1016/S0301-472X(02)00820-2 [DOI] [PubMed] [Google Scholar]