Post-colonoscopy colorectal cancer or so-called “interval cancer” has emerged as one of new research topics, attracting much attention of endoscopists, gastroenterologists, and oncologists alike (1-4). In addition to lesions missed during the index colonoscopy due to technical issues (e.g., skills of endoscopists, quality of bowel preparation) or tumor morphology (e.g., sessile serrated adenoma/polyps) (5), some tumors arising after colonoscopy may have rapidly-growing biological behavior with distinct molecular and pathological features (1-4). Colonoscopy has remained the cornerstone of colorectal cancer screening which can provide primary prevention, early detection, and pathological diagnosis of neoplastic lesions, potentially leading to the reduction in colorectal cancer incidence and mortality (4,6). In the U.S., screening colonoscopy has been increasingly utilized since Medicare, a national social insurance program, initiated coverage of this procedure in 2001 (6). The U.S. Preventive Services Task Force (USPSTF) statement in 2016 recommends screening of the colon and rectum for average-risk asymptomatic individuals aged 50–75 years, and refers to positive family history, male sex, and black race as risk factors for colorectal cancer (7). Follow-up colonoscopy after negative findings or polypectomy is scheduled according to the number, size, and/or pathological findings of polyps on the index colonoscopy (8). However, a fraction of people develop colorectal cancer between the index and subsequent surveillance procedures. In a meta-analysis of 12 observational studies, the pooled prevalence of interval cancer was 3.7% [95% confidence interval (CI), 2.8–4.9%] among all colorectal cancer cases (1). This analysis also suggests that interval cancer is more likely to be located in the proximal colon, and old age and positive family history of colorectal cancer are potential risk factors (1). Accumulating evidence indicates specific molecular and pathological features associated with post-colonoscopy colorectal cancer. Our previous study based on two U.S. prospective cohort studies has shown not only the effectiveness of colonoscopy on risk reduction of colorectal cancer incidence and mortality but also distinct molecular alterations in post-colonoscopy tumors (4). Among 1,815 incident colorectal cancer patients during the study period of more than 22 years for 88,902 participants, we examined tumor molecular features of 62 patients diagnosed within 5 years after the index colonoscopy. Compared with tumors diagnosed more than 5 years after colonoscopy or without any prior colonoscopy, post-colonoscopy tumors were more likely to show high-degree microsatellite instability [MSI-high; odds ratio (OR), 2.10; 95% CI, 1.10–4.02], high-degree CpG island methylator phenotype (CIMP-high; OR, 2.19; 95% CI, 1.14–4.21), and high-level long interspersed nucleotide element-1 (LINE-1) methylation (OR for 30% increase, 3.21; 95% CI, 1.29–8.00) (4). Post-colonoscopy colorectal cancer was not significantly associated with KRAS, BRAF or PIK3CA mutation. Other retrospective studies also affirmed that high-level MSI and CIMP were more frequently observed in post-colonoscopy colorectal cancer (1-3). These molecular features of post-colonoscopy colorectal cancer implicate the role of the serrated pathway in early carcinogenesis after colonoscopy (5). A better understanding of the underlying etiologies of those distinct neoplasms would help improve surveillance strategies focusing on high-risk populations and thereby optimize the benefits from colonoscopy examinations for cancer screening and prevention.
In an article published recently in Gastroenterology (9), Stoffel and colleagues reported a nationwide population-based study in Denmark and gave new insights into this particular group of colorectal neoplasms. Utilizing unique resources of nationwide patient and cancer registry databases which could capture a vast majority of colonoscopy procedures throughout the country, Stoffel et al. successfully evaluated characteristics of colorectal neoplasms diagnosed at varying time-points after the index colonoscopy. Among 10,365 patients diagnosed as incident colorectal cancer during the 5-year study period [2007–2011], post-colonoscopy cancers (N=725, 7.0%) were limited to those with a history of colonoscopy >180 days before cancer diagnosis. In line with prior studies (1-4), post-colonoscopy cancer was more likely to be located in the proximal colon (OR, 2.34; 95% CI, 1.90–2.89) and to exhibit MSI-high (OR, 1.26; 95% CI, 1.00–1.59), compared with cancer with no prior colonoscopy. This study also reported other clinical features of post-colonoscopy tumors including older age at diagnosis, positive family history of colorectal cancer, and earlier disease stage, but no significant association with sex. Furthermore, the large sample size enabled a time-course evaluation of cancer phenotypes after the index colonoscopy. A higher prevalence of the proximal tumor location or MSI-high phenotype in post-colonoscopy colorectal cancer was observed in tumors identified up to 10 years after colonoscopy, but appeared more pronounced during the interval of 3–6 years after colonoscopy. Interestingly, when stratified by the tumor location, the trend of the association of post-colonoscopy cancer with MSI-high over time appeared to be more evident in distal colorectal cancer than in proximal cancer. As Stoffel et al. advocated, the heterogeneity in clinical and molecular features of tumors identified at different time-points after colonoscopy suggests that post-colonoscopy colorectal cancer may represent a heterogeneous group of neoplasms which potentially differ in the process of carcinogenesis and progression. These findings underpin the importance of consideration of the tumor heterogeneity in post-colonoscopy cancer research. Their detailed molecular analysis of 85 post-colonoscopy cases at a single institution found BRAF, KRAS/NRAS, and PIK3CA mutations in 16, 23, and 16 cases (19%, 27%, and 19%), respectively, and features of Lynch syndrome in six cases (7%), although a comparative group was lacking in the analysis. As a procedural factor, the index colonoscopy tended to be incomplete in cases diagnosed within 1 year after colonoscopy than in those diagnosed thereafter. Along with a higher proportion of proximal or MSI-high tumors, these findings highlight the importance of meticulous endoscopic observation of the proximal colon. We have attested to “the colorectal continuum theory”: i.e., the prevalence of certain molecular alterations of colorectal cancer (e.g., CIMP, MSI, BRAF mutation) may increase (or decrease) gradually along the colorectum axis rather than having an abrupt transition at the splenic flexure (10). Taken together, efforts to observe the colorectum endoscopically as further as possible may help maximize the survival benefits from colonoscopy surveillance.
How can we utilize these findings for personalized screening and prevention strategies after the baseline colonoscopy? We assert that molecular pathological epidemiology (MPE) can establish a basis of risk stratification for colonoscopy screening and tailored management after negative colonoscopy or polypectomy in the context of precision medicine. MPE is an integrative field of research which has been derived from efforts to incorporate the methodology of molecular pathology into population-based epidemiologic research (11,12). Conventional epidemiology typically examines the association of an epidemiologic exposure with an overall single disease entity (e.g., “colorectal cancer”) on the assumption that people diagnosed with the disease would represent homogeneous patterns of disease course based on common etiologies and pathogenesis. Beyond this conventional approach, MPE research attempts to decipher differential associations of an exposure with several distinct subtypes classified by molecular or pathological features of the disease (e.g., “MSI-high tumors” or “microsatellite stable/MSI-low tumors”) (11,12). Namely, the underlying paradigm of MPE is “the unique disease principle”: i.e., among people assigned with a particular disease name, each individual may bear a unique pathologic process based on different genetic and epigenetic alterations in cells which arise from a complex network with the surrounding microenvironment (13). There has been an increasing awareness that colorectal cancer represents a heterogeneous mixture of neoplasms which arise through stepwise accumulation of varying combinations of molecular alterations in the colorectal epithelium (11). Furthermore, accessibility to precursor lesions via endoscopy and availability of molecular diagnostic assays has allowed colorectal premalignant lesions to serve as a unique practical model for MPE research (14). Importantly, MPE research can provide biological evidence on etiologies and pathogenesis of diseases, thereby uncovering causal relationships in human diseases (11,12). Recently, the trend of precision medicine driven by the U.S. National Institutes of Health has cast light on MPE research for the disease heterogeneity. Post-colonoscopy colorectal cancer has distinct molecular features compared with all incident cancer, and hence, we need to fully consider this heterogeneity to generate effective surveillance strategies. Another important notion derived from the MPE paradigm is the “etiologic field effect” concept: i.e., etiologic factors may generate a field of tissue microenvironmental alterations, potentially promoting tumor development within the field through shared carcinogenic pathways (15). This discipline underscores the importance of prudent colonoscopy surveillance even after complete resection of precursor lesions.
MPE research can unveil differential associations of a particular exposure (or prevention strategy) with respective tumor subtypes. If lifestyle and genetic factors increase the risk of tumor subtypes that are less preventable by colonoscopy and more common in post-colonoscopy cancers, we may need to modify surveillance strategies after the initial colonoscopy (Figure 1). As an example, our previous MPE study has shown that cigarette smoking may predispose people to an increased risk of CIMP-high colorectal cancer, but not to that of the CIMP-negative/low cancer (16). These findings have served as supportive evidence for smoking-related colorectal carcinogenesis through DNA methylation alterations. Post-colonoscopy colorectal cancer may be associated with high-level CIMP and MSI, suggesting that colonoscopy surveillance might be less effective in reducing the risk of CIMP-high and MSI-high subtypes. In addition, serrated lesions, sessile serrated adenomas in particular, have been recognized as precursors of CIMP-high colorectal carcinoma (14,17). Owing to sessile morphological appearance, these lesions are difficult to be detected and removed endoscopically (5). Taken together, smokers are susceptible to the development of CIMP-high tumors, and may need an additional or alternative strategy of colorectal cancer screening.
Figure 1.
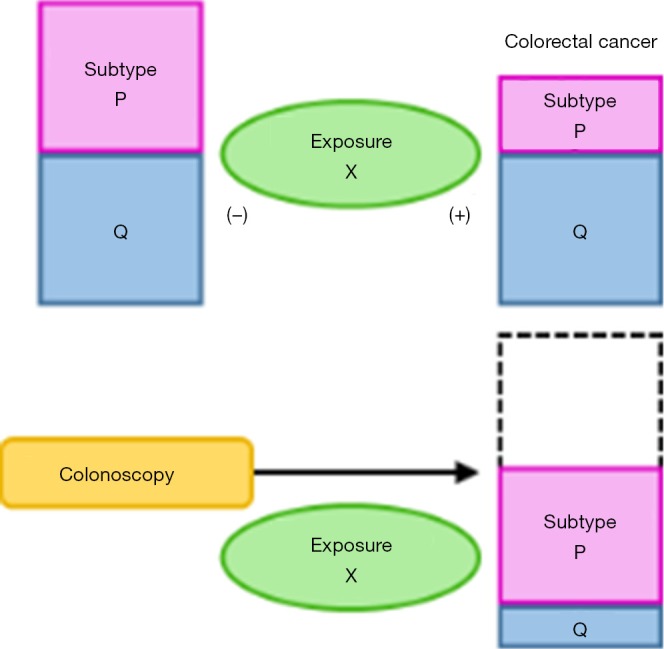
The paradigm of molecular pathological epidemiology (MPE) and application of MPE research for post-colonoscopy colorectal cancer. (A) MPE research on colorectal cancer. By classifying colorectal cancer into subtypes based on molecular or pathological features, MPE can assess differential associations of an exposure with the risk of each subtype. Here, exposure X is associated with a lower risk of subtype P, but not with the risk of subtype Q. Note that, although we simplified our illustration using an example of two subtypes, more than two categorical or ordinal subtypes can be examined in MPE; (B) assuming that post-colonoscopy colorectal cancer is more likely to be subtype P, modification of exposure X might become an effective prevention strategy for post-colonoscopy cancer. Short-interval follow-up colonoscopy may be recommended for individuals who have low-level of exposure X. The arrow indicates disease process with time. MPE, molecular pathological epidemiology.
Microbial MPE is an emerging subfield of MPE, which addresses differential associations of an exposure with individual disease subtypes classified by characteristics of the inherent microbiota and investigates the etiologic heterogeneity (18). The colon and rectum harbor by far the largest number of microorganisms in the human body, and the dysregulation of the intestinal ecosystem including host cells and microorganisms may contribute to chronic inflammation and carcinogenesis in the colorectum. Among a wide spectrum of bacteria, Fusobacterium nucleatum (F. nucleatum) has emerged as a microbial pathogen that contributes to the initiation and evolution of colorectal cancer (19). Accumulating evidence suggests that F. nucleatum may be associated with CIMP-high and MSI-high colorectal cancer (17,19). Our recent study attests a gradual (rather than abrupt) increase of the proportion of tumors containing high-level F. nucleatum from the rectum to cecum, supporting “the colorectal continuum model (20)”. In addition, F. nucleatum may be associated with colorectal carcinogenesis through serrated pathways (17) and suppression of T cells in the tumor microenvironment (19). On the other hand, lifestyle factors (e.g., diet, smoking), medications (e.g., antibiotics), and probiotics can influence the network of the intestinal microbiota. Modulation of those modifiable factors can have a potential to prevent F. nucleatum-positive colorectal cancer after colonoscopy or accelerate personalization of surveillance strategies taking into account factors associated with the enrichment of F. nucleatum in the colon and rectum. Investigations of viruses and other bacteria, such as Bifidobacterium, enterotoxigenic Escherichia coli, Lactobacillus, and Bacteroides are also warranted (21).
Pharmaco-MPE can also provide evidence for more sophisticated cancer prevention strategies after colonoscopy (12). Pharmacoepidemiology is a field of public health where effects of medications on disease incidence and health outcomes are investigated. Pharmaco-MPE investigates the association of a medication of interest with specific disease subtypes (12), potentially providing biological evidence of drug effects. Aspirin is not only a widely-used nonsteroidal anti-inflammatory drug (NSAID) but also a promising chemoprevention agent against colorectal cancer, at least in part, through inhibition of PTGS2 (cyclooxygenase-2). The USPSTF recommendation statement in 2016 suggests the use of low-dose aspirin for primary prevention of colorectal cancer among adults with a substantial cardiovascular risk (22). A strength of colonoscopy screening is sample acquisition from the normal background epithelium as well as tumor tissue. A pharmaco-MPE study has shown that a reduced risk of colorectal cancer associated with aspirin use may be augmented in individuals with high mRNA expression for HPGD [hydroxyprostaglandin dehydrogenase 15-(NAD), or 15-PGDH], the primary enzyme to catabolize prostaglandins produced by PTGS2, in the normal colorectal mucosa. These findings suggest a potential of HPGD as a biomarker to predict benefits from aspirin chemoprevention (23). Although it is warranted to investigate whether these differential associations of aspirin with colorectal cancer incidence can be extrapolated to the setting of post-colonoscopy cancer, it may be possible to further refine strategies of aspirin use through the investigation of molecular markers in the normal mucosa obtained during the baseline colonoscopy.
Immuno-MPE is a new discipline derived from the MPE concept, which categorizes a disease into subtypes by parameters of host immune response (12). Findings from immune-MPE research can inform cancer immunoprevention research through identification of potential immunomodulators. High-level MSI status in colorectal cancer, which is commonly observed in post-colonoscopy colorectal cancer, is characterized by intense immune response to the tumor. Emerging evidence points to DNA mismatch repair deficiency and resultant high-level neoantigen load, which estimates immunogenic peptides in the tumor microenvironment, as the underlying mechanism linking MSI with high-level immune response (24). A immuno-MPE study has shown that the inverse association of high-level plasma 25-hydroxyvitamin D [25(OH)D], a standard indicator of systemic vitamin D status, with colorectal incidence appears to be more pronounced for tumors with intense histopathological immune response than for tumors with poor immune response (25). This study support that antitumor effects of vitamin D may be in part mediated by local immune cells that can enzymatically convert 25(OH)D to a bioactive form, 1,25-dihydroxyvitamin D (also known as calcitriol). These findings implicate a possibility of plasma 25(OH)D modulation by means of sun exposure and vitamin D supplementation for effective prevention of post-colonoscopy tumors with MSI-driven immune enhancement.
In summary, the current large population-based study by Stoffel et al. supports the existing evidence on specific molecular features of post-colonoscopy colorectal cancer. In order to optimize cancer prevention strategies after colonoscopy, we should take into account the specific features of interval cancers which distinguish themselves from others. MPE research, which essentially addresses the disease heterogeneity in human population, can provide rationales for future research on post-colonoscopy colorectal cancer. Given the relative rarity of post-colonoscopy colorectal cancer, however, worldwide collaborative efforts that can link databases covering detailed endoscopic and pathological findings would be useful to fully investigate this group of neoplasms. We propose post-colonoscopy MPE research, which can play a key role in discovering insights into post-colonoscopy colorectal cancer in the era of precision medicine.
Acknowledgements
Funding: This work was supported by U.S. National Institutes of Health (NIH) grants (R35 CA197735 to Shuji Ogino and K07 CA190673 to Reiko Nishihara); by Nodal Award from the Dana-Farber Harvard Cancer Center (to Shuji Ogino); and by The Friends of the Dana-Farber Cancer Institute. Tsuyoshi Hamada was supported by a fellowship grant from the Mitsukoshi Health and Welfare Foundation.
Disclaimer: The content is solely the responsibility of the authors and does not necessarily represent the official views of NIH. The funders had no role in decision to submit the manuscript to publication, or preparation of the manuscript.
Provenance: This is a Guest Editorial commissioned by Section Editor Qiang Shi, MD, PhD (Zhongshan Hospital, Fudan University, Shanghai, China).
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Singh S, Singh PP, Murad MH, et al. Prevalence, risk factors, and outcomes of interval colorectal cancers: a systematic review and meta-analysis. Am J Gastroenterol 2014;109:1375-89. 10.1038/ajg.2014.171 [DOI] [PubMed] [Google Scholar]
- 2.Sawhney MS, Farrar WD, Gudiseva S, et al. Microsatellite instability in interval colon cancers. Gastroenterology 2006;131:1700-5. 10.1053/j.gastro.2006.10.022 [DOI] [PubMed] [Google Scholar]
- 3.Arain MA, Sawhney M, Sheikh S, et al. CIMP status of interval colon cancers: another piece to the puzzle. Am J Gastroenterol 2010;105:1189-95. 10.1038/ajg.2009.699 [DOI] [PubMed] [Google Scholar]
- 4.Nishihara R, Wu K, Lochhead P, et al. Long-term colorectal-cancer incidence and mortality after lower endoscopy. N Engl J Med 2013;369:1095-105. 10.1056/NEJMoa1301969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rex DK, Ahnen DJ, Baron JA, et al. Serrated lesions of the colorectum: review and recommendations from an expert panel. Am J Gastroenterol 2012;107:1315-29; quiz 1314, 1330. [DOI] [PMC free article] [PubMed]
- 6.Wu BU, Longstreth GF, Ngor EW. Screening colonoscopy versus sigmoidoscopy: implications of a negative examination for cancer prevention and racial disparities in average-risk patients. Gastrointest Endosc 2014;80:852-61. e1-2. [DOI] [PubMed]
- 7.US Preventive Services Task Force , Bibbins-Domingo K, Grossman DC, et al. Screening for Colorectal Cancer: US Preventive Services Task Force Recommendation Statement. JAMA 2016;315:2564-75. 10.1001/jama.2016.5989 [DOI] [PubMed] [Google Scholar]
- 8.Lieberman DA, Rex DK, Winawer SJ, et al. Guidelines for colonoscopy surveillance after screening and polypectomy: a consensus update by the US Multi-Society Task Force on Colorectal Cancer. Gastroenterology 2012;143:844-57. 10.1053/j.gastro.2012.06.001 [DOI] [PubMed] [Google Scholar]
- 9.Stoffel EM, Erichsen R, Frøslev T, et al. Clinical and Molecular Characteristics of Post-Colonoscopy Colorectal Cancer: A Population-based Study. Gastroenterology 2016;151:870-878.e3. 10.1053/j.gastro.2016.07.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yamauchi M, Morikawa T, Kuchiba A, et al. Assessment of colorectal cancer molecular features along bowel subsites challenges the conception of distinct dichotomy of proximal versus distal colorectum. Gut 2012;61:847-54. 10.1136/gutjnl-2011-300865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ogino S, Chan AT, Fuchs CS, et al. Molecular pathological epidemiology of colorectal neoplasia: an emerging transdisciplinary and interdisciplinary field. Gut 2011;60:397-411. 10.1136/gut.2010.217182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ogino S, Nishihara R, VanderWeele TJ, et al. Review Article: The Role of Molecular Pathological Epidemiology in the Study of Neoplastic and Non-neoplastic Diseases in the Era of Precision Medicine. Epidemiology 2016;27:602-11. 10.1097/EDE.0000000000000471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ogino S, Lochhead P, Chan AT, et al. Molecular pathological epidemiology of epigenetics: emerging integrative science to analyze environment, host, and disease. Mod Pathol 2013;26:465-84. 10.1038/modpathol.2012.214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lochhead P, Chan AT, Giovannucci E, et al. Progress and opportunities in molecular pathological epidemiology of colorectal premalignant lesions. Am J Gastroenterol 2014;109:1205-14. 10.1038/ajg.2014.153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lochhead P, Chan AT, Nishihara R, et al. Etiologic field effect: reappraisal of the field effect concept in cancer predisposition and progression. Mod Pathol 2015;28:14-29. 10.1038/modpathol.2014.81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nishihara R, Morikawa T, Kuchiba A, et al. A prospective study of duration of smoking cessation and colorectal cancer risk by epigenetics-related tumor classification. Am J Epidemiol 2013;178:84-100. 10.1093/aje/kws431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ito M, Kanno S, Nosho K, et al. Association of Fusobacterium nucleatum with clinical and molecular features in colorectal serrated pathway. Int J Cancer 2015;137:1258-68. 10.1002/ijc.29488 [DOI] [PubMed] [Google Scholar]
- 18.Hamada T, Keum N, Nishihara R, et al. Molecular pathological epidemiology: new developing frontiers of big data science to study etiologies and pathogenesis. J Gastroenterol 2016. [Epub ahead of print]. 10.1007/s00535-016-1272-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mima K, Sukawa Y, Nishihara R, et al. Fusobacterium nucleatum and T Cells in Colorectal Carcinoma. JAMA Oncol 2015;1:653-61. 10.1001/jamaoncol.2015.1377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mima K, Cao Y, Chan AT, et al. Fusobacterium nucleatum in Colorectal Carcinoma Tissue According to Tumor Location. Clin Transl Gastroenterol 2016;7:e200. 10.1038/ctg.2016.53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.O'Keefe SJ. Diet, microorganisms and their metabolites, and colon cancer. Nat Rev Gastroenterol Hepatol 2016;13:691-706. 10.1038/nrgastro.2016.165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bibbins-Domingo K. Aspirin Use for the Primary Prevention of Cardiovascular Disease and Colorectal Cancer: U.S. Preventive Services Task Force Recommendation Statement. Ann Intern Med 2016;164:836-45. 10.7326/M16-0577 [DOI] [PubMed] [Google Scholar]
- 23.Fink SP, Yamauchi M, Nishihara R, et al. Aspirin and the risk of colorectal cancer in relation to the expression of 15-hydroxyprostaglandin dehydrogenase (HPGD). Sci Transl Med 2014;6:233re2. 10.1126/scitranslmed.3008481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Giannakis M, Mu XJ, Shukla SA, et al. Genomic Correlates of Immune-Cell Infiltrates in Colorectal Carcinoma. Cell Rep 2016;17:1206. 10.1016/j.celrep.2016.10.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Song M, Nishihara R, Wang M, et al. Plasma 25-hydroxyvitamin D and colorectal cancer risk according to tumour immunity status. Gut 2016;65:296-304. 10.1136/gutjnl-2014-308852 [DOI] [PMC free article] [PubMed] [Google Scholar]


