Abstract
Antifreeze proteins (AFPs) are biological antifreezes with unique properties, including thermal hysteresis (TH), ice recrystallization inhibition (IRI), and interaction with membranes and/or membrane proteins. These properties have been utilized in the preservation of biological samples at low temperatures. Here, we review the structure and function of marine-derived AFPs, including moderately active fish AFPs and hyperactive polar AFPs. We also survey previous and current reports of cryopreservation using AFPs. Cryopreserved biological samples are relatively diverse ranging from diatoms and reproductive cells to embryos and organs. Cryopreserved biological samples mainly originate from mammals. Most cryopreservation trials using marine-derived AFPs have demonstrated that addition of AFPs can improve post-thaw viability regardless of freezing method (slow-freezing or vitrification), storage temperature, and types of biological sample type.
Keywords: antifreeze proteins, ice-binding proteins, ice recrystallization inhibition, cryoprotectant, slow-freezing, vitrification
1. Introduction
Antifreeze proteins (AFPs) are biological antifreeze materials originally found in polar fish; AFPs can bind to ice and subsequently inhibit the growth of the ice crystals. Fish can inhabit ice-laden or cold seawater below the freezing point (−0.7 °C) of their blood serum by virtue of AFPs [1,2,3,4]. However, in a literal sense, the term AFP is a misnomer since AFP does not stop freezing of the blood serum or solution containing AFP. Hence, the term ice-binding protein (IBP) has been proposed to include any protein that binds to ice including AFPs [5]. The term IBP has a bit more nuance than the term AFP. The term ice structuring protein (ISP), which is not used frequently, is synonymous with AFP. However, AFPs are a subset of the larger class of IBPs that includes ice nucleating proteins. In short, all AFPs are IBPs, but not all IBPs are AFPs. In this review, the terms AFP and IBP are used interchangeably.
Marine organisms known to possess or express AFPs, as shown in Figure 1, include bacteria [6,7,8,9], fungi [10,11,12], crustacean [13], microalgae [14,15,16,17,18,19], and fish [20]. Propelled by next-generation sequencing (NGS) technologies, identification of antifreeze genes from marine organisms has advanced rapidly within the last few years. However, until now, other than fish AFPs, only a few AFPs have been thoroughly characterized from Colwellia sp. [21], Flavobacterium frigoris [7], Glaciozyma antarctica [12,22], Navicula glaciei [16], Fragilariopsis cylindrus [23,24], and Chaetocero neoglacile [15]. The unique function of AFPs, i.e., enabling fish to survive in subfreezing environments, has inspired the researchers in academia and industries to examine the potential applications of AFPs as a potential cryoprotective agents or cryoprotectants (CPAs) in the cryopreservation of biological samples [25,26,27,28,29,30,31]. In this review, we discuss the biophysical and biochemical aspects of marine-derived AFPs as well as investigate past and current research of the practical applications of AFPs in cryopreservation. We also describe the possible role of AFPs in cryopreservation.
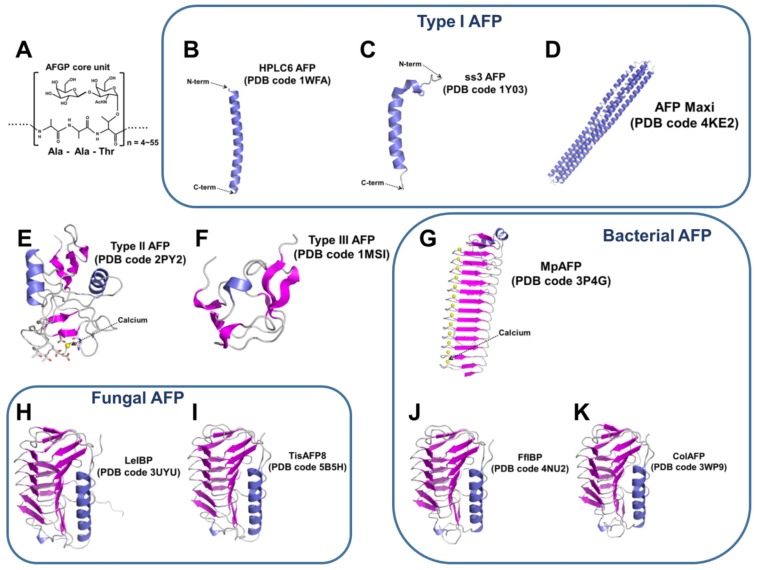
Figure 1.
Structural diversity of AFPs: (A) core unit structure of antifreeze glycoproteins (AFGPs); (B) Type I HPLC6 AFP structure; (C) Type I ss3 AFP structure; (D) the structure of AFP Maxi from winter flounder, Pseudopleuronectes americanus; (E) calcium-dependent type II AFP structure; (F) Type III HPLC12 AFP structure; (G) the structure of MpAFP from Marinomonas primoryensis; (H) the structure of LeIBP from Glaciozyma sp. AY30; (I) the structure of TisAFP8 from Typhula ishikariensis; (J) the structure of FfIBP from Flavobacterium frigoris PS I; and (K) the structure of ColAFP from Colwellia sp. strain SLW05.
2. AFP Properties: Thermal Hysteresis (TH), Ice Recrystallization Inhibition (IRI), and Interaction with Biological Membranes
Generally, AFPs can be characterized based on two properties: TH and IRI. However, interaction of AFPs with membranes should not be ruled out. In this section, the unique features of AFPs and their contribution to cryopreservation are discussed briefly (for more an in-depth biophysical discussion on these properties, refer to recent reviews [32,33]).
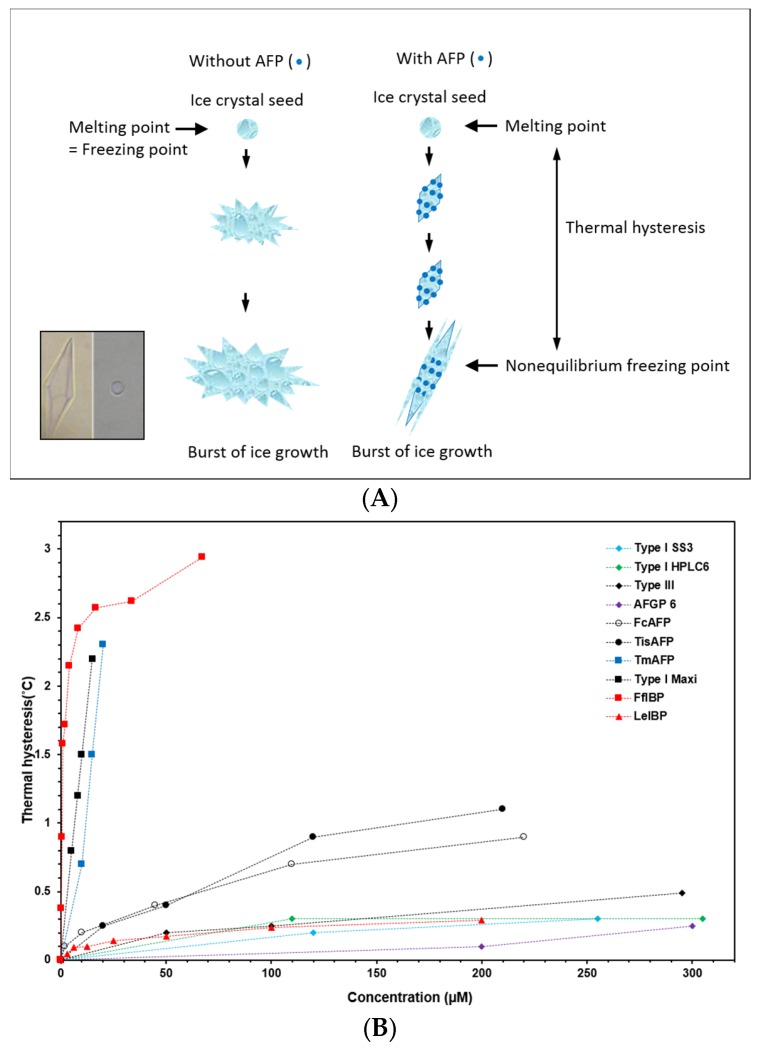
TH refers to the difference between melting and freezing points of a solution. In AFP-containing solution, the temperature gap can be created by irreversible binding of AFPs to ice crystals and subsequent inhibition of their growth until the temperature decreases to the non-equilibrium freezing point [32,34,35,36,37,38,39]. Below the non-equilibrium freezing point, the burst of the ice crystal can be observed (Figure 2A). During the TH gap, AFPs bind to the specific planes of ice crystals, shaping a unique ice morphology. For example, type I AFPs bind to the prism plane of ice and creates a hexagonal bipyramidal shape [35,40], whereas hyperactive FfIBP binds to prism and basal planes and generates a lemon shape [41]. Moderately active AFPs bind to prism and/or pyramidal planes [40,42,43], whereas hyperactive AFPs are able to bind to the basal plane of ice crystals [42,44]. The ice morphology shaped by AFPs is a hallmark of binding of AFPs to ice (inset of Figure 2A) [20]. TH has been used to describe the activity of AFPs quantitatively. For most fish AFPs, the observed TH activity is approximately 1 °C [20,45]. This temperature gap provides enough cushion against seawater during the winter season (−1.9 °C) for polar fish to survive in cold environments. In addition to fish AFPs, many marine AFPs are associated with sea ice [7,13,14,16,21,23,46]. Unlike the blood plasma of polar fish, seawater in brine channels in sea ice undergoes freezing to ice. Hence, AFPs from sea-ice associated bacteria, microalgae, and eukaryotic protists are secreted into the surrounding environment to protect themselves from freezing, and some of them are hyperactive (Figure 2B) [7,21,41].
Figure 2.
(A) Cartoon illustration of TH phenomenon. In the left panel, the ice starts to grow rapidly as temperature drops. However, as shown in the right panel, AFPs adsorb irreversibly on to the specific planes of ice surface, inhibiting the further growth of ice until the temperature reaches nonequilibrium freezing point. This adsorption-inhibition mechanism by AFPs separates melting and freezing points of solution. The inset shows the bipyramidal and lemon ice morphologies created by moderately active type I AFP (left) and hyperactive FfIBP (right), respectively; (B) Comparison of TH activities of AFPs from various organisms. TH activity of marine-derived FfIBP (from Flavobacterium frigoris), and type I-Hyp (from Pseudopleuronectes americanus) are comparable to hyperactive insect and fungal AFPs, TmAFP and TisAFP, respectively, of non-marine origin. Other marine AFPs are moderately. Abbreviations are as follows: TmAFP, Tenebrio molitor AFP; TisAFP, Thyphula ishikariensis AFP; FcAFP, Fragilariopsis cylindrus AFP; and LeIBP, Glaciozyma (formerly known as Leucosporidium) sp. IBP.
The second function of AFPs, which may be more useful for cryopreservation, is IRI. Ice recrystallization (IR), as depicted in Figure 3, explains a thermodynamically favorable process in which the formation of larger ice grains takes place at the expense of smaller ones with a high internal energy [47,48]. Eventually, the larger ice crystals formed because of this phenomenon can be fatal to the cryopreserved cells as well as the organisms inhabiting polar or cold regions [49,50]. Fortunately for these organisms, AFPs can inhibit IR at very low concentrations. The AFP-dependent IRI mechanism remains to be elucidated; however, similar to TH activity, IRI is attributed to the binding of AFPs to ice [5,45,51]. AFPs at the interface between the grain boundaries bind to the surface of ice grains and inhibit the growth process [50]. The IRI is more likely to be a key property for cold-tolerant organisms to survive in extremely cold environments [47,52,53,54,55]. To this end, IRI is eventually thought to defend membranes against freezing injury [27,28,29,30,31,56,57]. IRI activity was first analyzed using a splat cooling assay developed by Knight [58]. In splat assays, a small droplet of a solution is expelled from a height of 2 m onto a very cold (−70 °C) metal plate and freezes instantaneously as a polycrystalline wafer. The ice is then annealed at −6 °C over a certain period of time, allowing ice recrystallization to occur. Modified methods have been proposed wherein the ice grains are generated from a few-microliter sample placed between coverslips by flash freezing [54,55] or where the sample inside 10 μL glass capillary undergoes freezing and annealing [59]. However, the IRI result was only semi-quantitatively reported by presenting the IRI endpoint, expressed as mg/mL or μM, where IRI is no longer observed [54,55,58,59]. To assess IRI activity quantitatively, Jackman et al. employed domain recognition software to measure and report the mean grain size (MGS) of the 10 largest ice crystals after the annealing period [60]. This method displayed percent MGS as a function of AFP relative to the control. Very recently, Voets’ group adopted an automated image analysis using the circle Hough transform (CHT) algorithm with a modified splat assay [55] to quantitatively evaluate IRI [61,62]. The CHT is a basic technique for detecting circular objects in a digital image. They attempted to include all ice crystal images instead of only the 10 largest ice crystals in the calculation, which obviously makes the quantitative evaluation of IR kinetics more statistically significant. In this method, the inflection point of the kinetic curve was presented as an IRI endpoint.
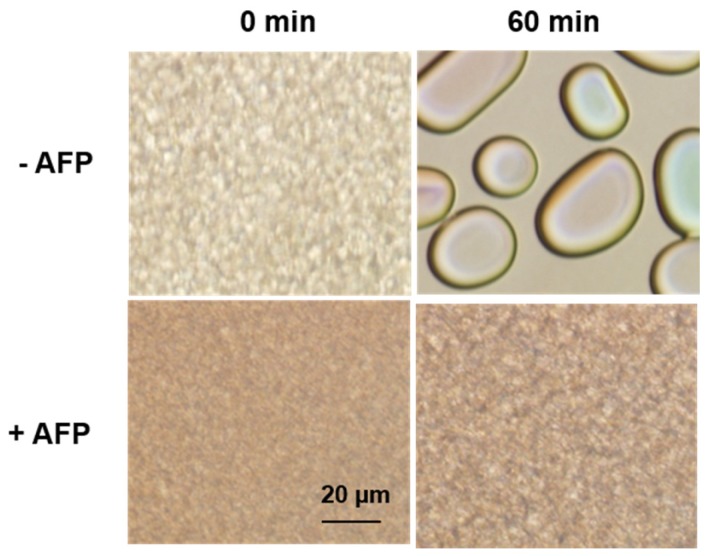
Figure 3.
Results of ice recrystallization inhibition (IRI) assay using modified splat assay. In this assay, AFP containing solution was mixed with 30% sucrose in a 1:1 ratio. The mixed solution was spotted between two coverslips and flash frozen. Then, the sample was placed at −6 °C stage and the changes were observed over a specific period of time-in this case 30 min. As in upper panel, larger ice grains grow as expense of smaller ice crystals, while the growth was halted in lower panel in the presence of AFPs. All subfigures are drawn in the same scale.
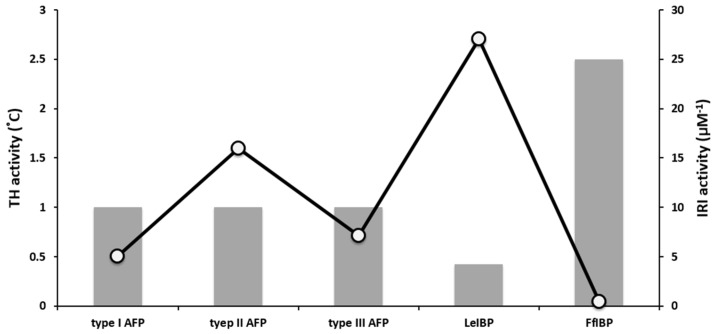
Both TH and IRI properties are based on the affinity of AFPs for ice. Intriguingly, however, TH activity is not always proportional to IRI activity (Figure 4), which remains to be elucidated [63]. In the comparison of TH and IRI activities of hyperactive insect, bacterial, and fish AFPs with moderately active fish AFPs, Yu et al. reported that the TH hyperactivity of AFPs was not reflected in their IRI activity [63]. This was corroborated by other marine-derived AFPs, i.e., LeIBP and FfIBP [7,10,41,44,64]. Olijve et al. also demonstrated that type III AFP and its mutant showed different TH values but almost the same IRI activity [61]. The hyperactive FfIBP showed less activity in IRI, compared to the moderately active LeIBP [7,44]. These results implied that TH activity was not necessarily translated into improvements in the cryopreservation efficiency of biological samples [65,66,67,68,69]. Therefore, the utilization of AFPs in cryopreservation cannot be considered from their TH activity only.
Figure 4.
A graph of TH and IRI activities of marine-derived AFPs. TH values, represented as a bar, are from Figure 2B. The IRI activities (O) are expressed as the reciprocal of endpoint of each AFP. The endpoint indicates the lowest concentration at which the AFP shows IRI activity. Higher IRI value means more effective in IRI. The LeIBP is weaker in TH but higher in IRI activity, but vice versa in FfIBP. This plot demonstrates that the TH values are not proportional to IRI activities.
Along with the IRI feature, the interaction of AFPs with membranes (or proteins in membranes) also may also ameliorate the cryoinjury of cells. In the early study of Rubinsky and his colleagues, fish AFPs were found to protect cell membranes during hypothermic storage [70]. As membranes are cooled to low temperatures, one mechanism of injury is often thermotropic phase transition partly due to weakened hydrophobic interactions [71,72,73,74,75]. During the transition from liquid crystalline to gel phase, membranes become leaky, resulting in the loss of intracellular contents [76]. It is not entirely clear what causes leakage during the phase transition; however, this process may be related to defects in packing of the hydrocarbon chains during the coexistence of gel and liquid crystalline domains [71]. Since the phase transition temperature of each lipid depends on the degree of unsaturation of lipid tails and the number of carbons in the lipid alkane chains, model membranes with diverse compositions, such as dielaidoylphosphatidylcholine (DEPC), dielaidoylphos-phatidylethanolamine (DEPE), and dielaidoylphosphatidylglycerol (DEPG), have been used in order to better understand the nature of the interactions between AFPs and cell membranes [72,73,75,77,78,79,80,81,82]. The results showed that these interactions were lipid specific, i.e., the lipid composition of the bilayer dictates whether or not a certain AFP or antifreeze glycoprotein (AFGP) will protect/interact with the membranes [61,62,64,65,66,67,68,69]. Other reports have indicated that the cryoprotective effects of AFPs arose from their interaction with membrane proteins, such as potassium and calcium ion channels [83,84,85,86]. However, in some cases, the addition of AFPs in cryopreservation medium induces leakage from cryopreserved cells [87,88,89,90,91,92,93,94,95]. These results implied that protection against freezing damage by AFPs depends on the type of membrane and the type of AFP [80,96].
3. Marine-Derived AFPs
3.1. Fish AFPs
Two scientists, Scholander and DeVries, first observed that some fish inhabiting the polar oceans could survive in cold water that occasionally reached sub-zero temperatures [1,3,97]. Following this observation, they attempted to elucidate how these fish could survive in icy water, reaching temperatures below the freezing point of fish blood. When cooling was increased even further, they observed that the growth of ice crystals was sluggish and delayed due to the presence of glycoproteins that depress the noncolligative freezing point of solutions [98]. These proteins were designated AFGPs [3]. Thereafter, nonglycosylated AFPs (type I AFPs) were found in the winter flounder, Pseudopleuronectes americanus [99]. In addition, several types of AFPs have been discovered and classified within distinct groups (classified into types I, II, III, and IV) in the Arctic and Antarctic regions. Even though the AFP types are fundamentally different in terms of their primary sequences and three-dimensional structures, they all have equivalent properties allowing them to bind to ice and depress the freezing point of the solutions. Moreover, these different types of AFPs do not seem to share any common ancestor genes.
AFGPs contain a three amino-acid (Ala-Ala-Thr) repeating sequence motif with a disaccharide connected to the hydroxyl group of the threonine residue [100]. However, there are sequence variations at the first residue position; sometimes, the first Ala residue is replaced by a Pro, Thr, or Arg. There are eight AFGPs (AFGP1-8), named according to the number of repeating units. AFGP1 has about 50 repeating units and therefore the highest molecular weight (33.7 kDa), whereas AFGP8 has the lowest molecular weight (2.6 kDa), with only four repeating units. Typically, the antifreeze activities of AFGPs are proportional to the number of repeating units. It is thought that high-molecular-weight AFGPs cover a wider ice surface and inhibit ice growth more efficiently than smaller AFGPs [47,101,102,103,104]. Recent studies have also shown that carbohydrate moieties are important for AFGP activity. Structural studies using nuclear magnetic resonance (NMR) have revealed that carbohydrate moieties and Ala residues are located on opposite sides. This feature confers AFGPs with a helical shape and amphipathic characteristics. Consequently, AFGPs show strong recrystallization properties. However, there are several limitations regarding their commercial utilization toward cryopreservation. Natural polar fish sources are not sufficient to prepare large quantities of AFGPs, and chemical synthesis is difficult to establish in large-scale mass production systems. In contrast, AFPs can be prepared in large quantities by recombinant protein expression techniques. For that reason, AFPs have been more broadly used for application studies than AFGPs. This review focuses on marine AFPs used for cryopreservation applications.
3.1.1. Type I AFPs
Type I AFPs are found in many flounders and sculpins. Type I AFP HPLC6 from winter flounder has been the most extensively studied. This protein possesses 37 amino acids and its sequences are composed of 11 amino acid repeating units [20,105]. Moreover, this protein also has a high Ala residue content, making up 23 of 37 residues. The molecular structure of HPLC6 (PDB code 1WFA) was determined using the X-ray crystallography, which showed that HPLC6 AFP is an α-helical protein with amphipathic characteristics. Another type I AFP has been isolated from the shorthorn sculpin (Myoxocephalus Scorpius; ss3 AFP), also displaying a high Ala content (21 Ala residues among a total of 33 residues). The structure of the ss3 AFP (PDB code 1Y03) was determined by NMR spectroscopy. The overall structure of ss3 AFP is similar to that of HPLC6 AFP; however, ss3 AFP contains a Pro residue at position 4, inducing a helix kink. Recently, the four-repeat containing isoform AFP9 and a much larger type I AFP (a 195-residue protein, AFP Maxi) were discovered in winter flounder (Pseudopleuronectes americanus). These two proteins exhibit significantly higher TH activities than HPLC6 AFP. Moreover, the increased size of the AFP may induce higher antifreeze activity by facilitating binding to multiple ice crystals and increasing coverage of the ice surface. Furthermore, analysis of the AFP Maxi structure (PDB code 4KE2) revealed that this protein folds into a dimeric four-helix bundle and that its ordered water may be involved in ice binding, thereby enhancing its antifreeze activity.
The ice-binding mechanism of type I AFP was previously investigated through an ice-etching experiment, which is used to identify AFP binding sites. A simple crystal growth and etching technique allows the identification of the crystallographic planes where the binding occurs [40]. Furthermore, ice etching has also been used to identify the ice-binding planes of AFPs and enhanced green fluorescent protein (EGFP) fusion constructs allow their clear visualization. In 1991, Knight et al. reported that type I AFPs from winter flounder (Pseudopleuronectes americanus) and Alaskan plaice (Pleuronectes quadritaberulatus) adsorb onto the {2 0 −2 1} pyramidal planes of ice, whereas the sculpin (Myoxocephalus scorpius) AFP adsorbs onto {2 −1 −1 0}, the secondary prism planes [40]. This finding suggests that each type I AFP has a unique ice-binding mechanism depending on its sequence length and composition. Moreover, ice-binding sites of type I AFPs were analyzed by site-directed mutagenesis, truncated variants, and molecular docking studies [106,107,108,109,110,111]. Currently, it is generally accepted that ice-binding sites of type I AFPs are located on their Ala-rich hydrophobic faces.
3.1.2. Type II AFPs
Type II AFPs are found in sea raven, smelt, herring, and long snout poacher. Type II AFPs are globular cysteine-rich fish AFPs with molecular weights ranging from 11 to 24 kDa. The overall structure of type II AFPs shows numerous similarities with C-type lectin-like domains (CTLDs). Type II AFPs have two α-helices and nine β-strands with specific cysteines forming disulfide bonds. Those disulfide bonds are known for their capacity to increase the structural stability of type II AFPs [112,113,114,115,116]. Structural comparison studies between various groups of type II AFPs showed that even if their amino acid sequence similarity is low, overall, their structures are similar, and they display the same functions. These results indicate that type II AFPs evolved from the backbones of CTLDs [117].
Type II AFPs are distinguished by their dependence on calcium ions to enable their antifreeze activities. Herring and two types of smelts produce Ca2+-dependent type II AFPs. Herring type II AFP (hAFP) has close structural similarities with lithostathine (PDB code: 1qdd; root mean square deviation [RMSD] = 1.7 Å for 122 Cα atoms) and mannose-binding protein (PDB code: 1sl6; RMSD = 2.2 Å for 124 Cα atoms). However, these two proteins have no ice-binding activities. Likewise, hAFP has no carbohydrate-binding activity. Thus, this high similarity in carbon backbone structure along with different activities indicates a divergent evolutionary pattern. Another difference between hAFP and C-type lectin protein is the number of cysteine bonds. hAFP has five disulfide bonds, whereas C-type lectin only possesses three or four. Thr96, Leu97, Thr98, and Thr115 residues are important for ice-binding. Interestingly, all of these residues are located near the Ca2+ binding site. Therefore, the results obtained from these investigations suggest that Ca2+ binding in hAFP is critical for forming an ice-binding state structure and increasing ice-binding activity [115]. Sea raven and long snout poacher produce Ca2+-independent type II AFPs. Structural comparisons between Ca2+-dependent and -independent type II AFPs showed that several residues near the Ca2+ binding site are different. Gln92, Asp94, Glu99, and Asn113 residues of hAFP are substituted with Lys95, Asn97, Asp102, and Asp116 residues, respectively, in long snout poacher AFP (lpAFP). Through these studies, the critical amino acids for Ca2+ binding were identified. These amino acids could be important indicators allowing the distinction between Ca2+-dependent and-independent type II AFPs [116]. Additionally, a type II AFP was found in Japanese smelt (Hypomesus nipponensis; HniAFP), which does not inhabit polar regions, but instead is found in fresh waters in regions near the middle latitudes. Interestingly, HniAFP can bind to Ca2+, but its ice binding activity does not depend on this feature; indeed, despite adding ethylenediaminetetraacetic acid (EDTA) to remove Ca2+, its antifreeze activity was not affected [118].
3.1.3. Type III AFPs
Type III AFPs are small globular proteins with an average molecular weight of 6.5 kDa, found in Antarctic eelepout (Macrozoarces americanus) and wolf fish [119,120]. Type III AFPs can be divided into two groups, quaternary-amino-ethyl (QAE) and sulfopropyl (SP) sephadex-binding isoforms, based on both their sequence similarities and affinities for SP and QAEs [121]. QAEs can be further categorized into QAE1 and QAE2 subgroups [122]. According to some studies, QAE1 isoforms have higher TH activities than the QAE2 and SP isoforms. SP and QAE2 isoforms are incapable of stopping ice growth [123,124]. The structures of type III AFPs have been extensively studied, and about 40 models have been solved and deposited in the protein data bank (http://www.rcsb.org/pdb/) to date. Among these, the three-dimensional structure of HPLC12 AFP, belonging to the QAE1 subgroup, was the first to be determined, showing a globular β-sandwich consisting of two antiparallel triple-stranded β-sheets [125,126,127]. Although type III AFPs are mainly composed of several loops, they form stable structures through hydrophobic interactions and a number of hydrogen bonds at the center of the structure. Type III AFPs were found to be active over a broad pH range (2–11), indicating that the protein fold is stable even at extreme pH, which would normally cause protein denaturation [125]. Recent studies have shown that temperature treatment at 80 °C and pressure treatment at 400 MPa (duration of 1 min for both treatments) did not influence the IRI activity of type III AFPs [128]. Interestingly, sialic acid synthase (SAS) has a C-terminal antifreeze-like domain similar to that of type III AFPs. However, these two homologous proteins have very different temperature-dependent stabilities, activities, and backbone dynamics. While type III AFPs are mostly rigid, with a few residues showing slow motions, SAS is remarkably flexible at low temperature [129,130]. These two proteins, displaying different functions, may have evolved from a common structural ancestor.
The most widely accepted hypothesis to describe the mechanism through which type III AFPs interact with ice crystals involves the Thr18 residue located on the flat surface; this residue is thought to be responsible for the recognition and interaction with the primary prism planes of ice. AFPs cover water-accessible ice surfaces, thereby inhibiting ice growth. Several reports have shown that putative ice-binding residues (Gln9, Asn14, Thr15, Ala16, Thr18, and Gln44) are capable of significantly altering TH activity and ice crystal morphology [125,126,131,132]. Notably, the replacement of Thr18 by Asn causes a significant loss of TH activity (90% loss). Computer simulation studies have emphasized that hydrophobic interactions within ice-binding sites are also important for the antifreeze activity of the protein [132,133]. When hydrophobic residues, such as Leu19, Val20, and Val41, were replaced with Ala, a 20% loss in activity was observed. Double mutants (L19A/V41A and L10A/I13A) showed more than 50% loss of activity compared with the activity of the wild-type protein [124]. Ice-etching studies revealed a more complex ice-binding mechanism within type III AFPs, showing that they could interact with both the primary prism and a pyramidal plane of ice [1]. While the QAE1 isoform is able to bind both the primary prism and a pyramidal plane of ice, the SP and QAE2 isoforms can only bind pyramidal ice planes [134]. Interestingly, a triple mutant of the inactive QAE2 isoform (V9Q/V19L/G20V) is able to bind to the primary prism ice plane and shows full TH activity, similar to the QAE1 isoform [135]. More recently, NMR experiments with inactive QAE2-like mutants containing the V20G mutation were reported. These experiments showed that the mutants exhibited increased conformational flexibility and were incapable of binding to the primary prism plane of ice crystals. These results suggested that inactive type III AFPs may be unable to anchor water molecules via H-bond interactions in the first 310 helix (residues 18–22) and therefore have no antifreeze activity [136].
Interestingly, two almost identical type III AFP domains tied by linker residues, designated RD3, were found in nature in the Antarctic eelpout, Rhigophila dearborni [137,138,139]. RD3 possesses 5.9-fold higher activity than a single domain in the range of 0 to 0.5 mM. This high activity at low concentrations may be related to the need for much smaller concentrations of AFP for cryopreservation, as mentioned below.
3.2. Fungal AFPs
To date, various mushrooms and Basidiomycetous psychrophilic yeast species have been screened and reported to have antifreeze activities. Only two mushrooms (enoki and shiitake), one snow mold fungus (Typhula ishikariensis), and two yeast organisms (Glaciozyma antarctica and Glaciozyma sp. AY30) have been characterized both genomically and for their antifreeze properties [9,10,140,141].
Lee et al. were the first to report the antifreeze activity of a protein isolated from the psychrophilic yeast Glaciozyma sp. AY30, itself isolated from an ice core sample of a freshwater pond near the Dasan station, Ny-Ålesund, Svalbard archipelago, Norway, and named LeIBP [10]. LeIBP contains a right-handed β-helical structure, which provides the advantage of a broad-range interaction surface for ice binding [44,64]. The ice-binding site of LeIBP was determined to be a B-face using site-directed mutagenesis experiments [64]. Moreover, the codon-optimized LeIBP (pLeIBP) was constructed and subjected to high-level expression in the Pichia pastoris system [142]. In pilot-scale fermentation (700 L), pLeIBP was secreted into culture medium, and the yield was 300 mg/L. The TH activity of pLeIBP was about 0.42 °C, which was similar to that of LeIBP expressed in E. coli. The availability of large quantities of pLeIBP allowed us to use this protein in further application studies [65,66,67,143,144,145].
Snow mold fungus (Typhula ishikariensis) secretes seven antifreeze protein isoforms composing the TisAFPs [141]. Among them, the structures of TisAFP6 (PDB code 3VN3) and TisAFP8 (PDB code 5B5H) were determined and their antifreeze mechanisms were characterized [146]. The results suggested that TisAFP8 has a more adapted shape and higher hydrophobicity to allow ice binding than TisAFP6, which may possess a higher TH activity. Notably, the overall structures of LeIBP (PDB code 3UYU), TisAFP6, and TisAFP8 are very similar, with RMSD values within 0.73 Å when superimposed.
Glaciozyma antarctica AFP (Afp1), described by Hashim et al., possesses both TH and RI activities and shows 30% sequence similarity with TisAFPs [12]. Amino-acid sequence analysis showed that Afp1 contains four α-helices. Shah et al. confirmed the antifreeze activity of each helical peptide [22]. In addition, the NMR structures of the peptides were determined and the ice-binding model was generated using a molecular dynamics method. The results indicated that the Afp1 peptides work like type I AFPs. In 2014, another Glaciozyma antarctica AFP (Afp4) was identified and characterized [147]. The Afp4 sequence shows the highest amino acid similarity (93%) to LeIBP. A recombinant Afp4 protein changed ice crystals into hexagonal shaped crystals and showed a TH value of 0.8 °C at a protein concentration of 5 mg/mL.
3.3. Diatom AFPs
Studies aiming to identify new AFP genes from polar sea diatoms (Chaetoceros neogracile, Berkeleya sp., Navicula sp., Fragilariopsis sp., and Nitzschia frustulum) have been performed, and further gene expression studies have shown that the expression of AFP genes is regulated in response to stress conditions, such as cold temperature and high salinity [16,17,23,24,148]. Thus, AFP genes may play an important role in the environmental adaptation of diatoms. In 2009, Gwak et al. first produced recombinant antifreeze protein (Cn-AFP) from a marine diatom, C. neogracile, and characterized its antifreeze activity [15]. The TH value of the mature form of Cn-AFP is 0.8 °C, whereas pre-mature Cn-AFP has a 16-fold lower TH activity, indicating that the signal peptide induces improper folding of Cn-AFP or masks the ice-binding site.
3.4. Bacterial AFPs
In 2004, Gilbert et al. published an interesting finding showing bacterial AFP screening results obtained from Antarctic lake bacteria [149]. The authors managed to culture 866 bacterial isolates from an Antarctic lake and found RI activity in 19 of these isolates. The first bacterial IBP gene (~25 kDa) was identified, and the protein purified through ice affinity purification, in the sea ice gram-negative bacterium Colwellia strain SLW05 [8]. In 2008, other bacterial IBPs (54 kDa) were isolated from a deep Antarctic ice core of the subglacial Lake Vostok, at a depth of 3519 m (GenBank EU694412) [140]. The sequence of the protein is similar to those of IBPs previously found in sea ice habitats, even though the protein is longer. In addition, uncharacterized proteins similar to IBPs were found in sea ice bacteria Polaribacter irgensii (ZP_01118128; sequence identity: 61%, sequence similarity: 75%), Psychromonas ingrahamii (ZP_01349469; sequence identity: 59%, sequence similarity: 71%), and marine bacterium Shewanella frididimarina (YP_749708; sequence identity: 52%, sequence similarity: 69%).
The first bacterial AFP structure was solved using a protein isolated from an Antarctic lake bacterium (Marinomonas primoryensis; MpAFP) [150]. MpAFP is a 1.5-MDa protein with calcium-dependent antifreeze activity [6]. The solved MpAFP structure (PDB code 3P4G) shows a calcium-bound β-helical fold and bound water molecules, which fit well onto the ice crystal lattice. Therefore, this structure may explain the anchored clathrate mechanism of AFPs when binding to ice.
Recently, another IBP (FfIBP) from the Antarctic bacterium F. frigoris PS1 was identified from sea ice on the shore of McMurdo Sound (GenBank accession no. AHKF00000000.1) and characterized [41,151]. FfIBP shares 56% sequence similarity with LeIBP, but displays an antifreeze activity that is up to 10-fold higher than that of LeIBP. Structural and functional characterization of FfIBP revealed that this protein displays regular motifs (T-A/G-X-T/N motif) and more regularly aligned ice-binding residues on its IBS than LeIBP [7]. These structural differences may confer FfIBP with higher TH activity.
In 2014, structural and biochemical data on an AFP from Colwellia sp. strain SLW05 (ColAFP) were published [21]. Interestingly, the ColAFP structure is similar to those of LeIBP, TisAFP, and FfIBP, displaying a β-helical structure. In addition, the alignment of sequences and phylogenetic trees of the bacterial AFPs with those of other AFPs and IBPs suggests that eukaryotic IBPs could have been acquired from bacteria by horizontal gene transfer (HGT) [151]. One theory in favor of HGT is “restricted occurrence”, which suggests that the same small set of organisms can be found in different locations [152]. IBPs seem to satisfy this criterion because hundreds of organisms have IBPs or IBP-like genes. Another potential explanation involves virus-mediated transformation of IBP genes. For example, Arctic cryoconite holes are built on snow, glaciers, or ice caps where viruses are abundant; these viruses are able to infect a broad range of bacterial species and other organisms, suggesting that viruses in the environment may play a role in the exchange of genetic material [153].
Furthermore, a new bacterial AFP [154] with high IRI activity [155] was reported very recently. Metagenomic sequencing of the Antarctic psychrophilic marine ciliate Euplotes focardii revealed two sequences encoding IBPs, designated as EFsymbAFP and EFsymbIBP, obtained from its putative bacterial symbiont [154,155]. These IBPs seem to be structurally similar to TisIBP, LeIBP, and FfIBP [154]. Of these, N-terminal 23 residue-deleted EFsymbAFP was recombinantly expressed in E. coli and characterized [155]. Its TH activity was 0.53 °C at 50 μM, but its IRI activity was in the nanomolar range, as determined by Voets method. This value is the lowest observed to date. The recombinant protein also effectively protected bacterial cells from freezing damage. Further investigations of this IBP will provide more insight into the relationships among IRI and TH and the evolution of IBP.
4. Cryopreservation Using AFPs as a Potential Cryoprotectants (CPAs)
4.1. Cryopreservation and Ice Recrystallization
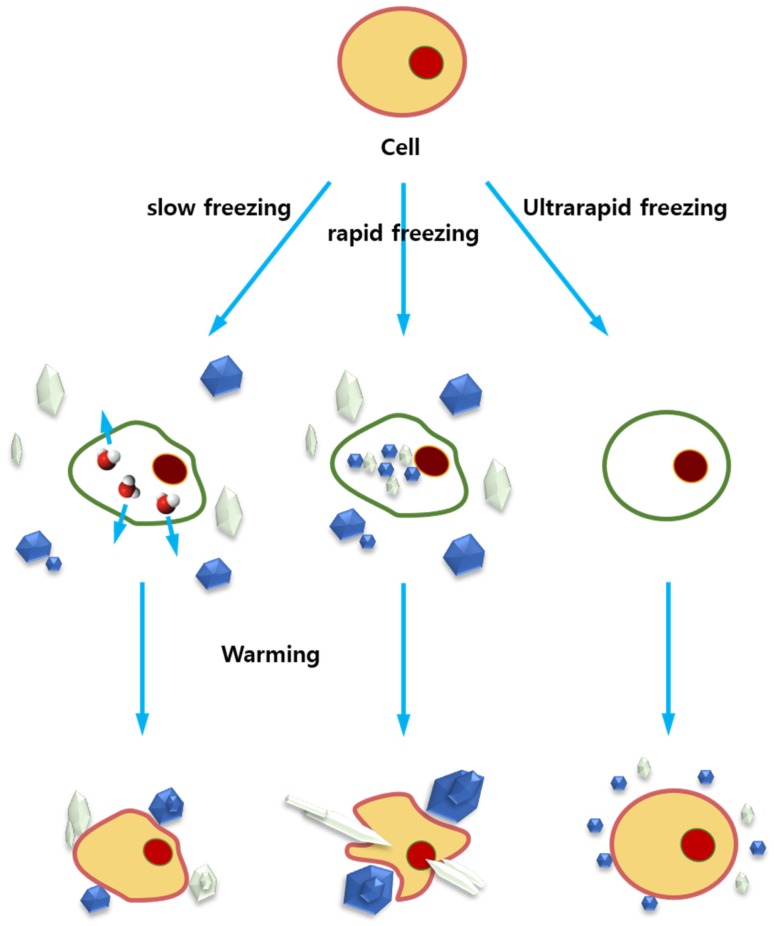
Cryopreservation is an important technique used to store various types of cells, tissues, and organs at very low temperature, usually in liquid nitrogen (−196 °C) [156], and has become crucial in cell biology and regenerative medicine [157,158]. However, cells are not always viable after thawing [145,159]. The freezing and thawing process during cryopreservation causes cryo-injury to cells (Figure 5). Currently, two methods, i.e., slow-freezing [156] and vitrification [160], are commonly adopted in cryopreservation. Prior to addressing the role of AFPs in cryopreservation, we will discuss the association of cryo-injury with freezing with regard to methods other than decreased temperature.
Figure 5.
Schematic illustration of freezing rate and ice recrystallization during warming. In slow freezing process, the extracellular ice starts to form below the equilibrium freezing point. Subsequently, water is expelled from inside the cell by osmotic pressure, eventually eliminating the intracellular ice formation. Fast freezing process, however, causes the intracellular ice formation since water cannot leave the cell quickly. In ultrarapid cooling, such as vitrification process, theoretically no bulk ice will form in the presence of higher concentration of CPAs. The ice formed during freezing will become problematic, when the cryopreserved cells are thawed (or warmed). They start to grow bigger: a process known as ice recrystallization. This process is fatal to the cells. Even in vitrification, ice can form during the warming. Therefore, freezing rate should be optimized depending of cell type, CPAs used, etc. The addition of AFPs in freezing media seems to alleviate the ice formation and recrystallization.
During the slow-freezing process, since the solute concentration inside a cell is higher than that in the medium, the cell is supercooled and ice forms extracellularly [156]. The growth of extracellular ice leaves the unfrozen fraction highly concentrated with salt, leading to dehydration of the cell and destabilization of cellular membranes simultaneously due to osmotic pressure. Incomplete dehydration inside the cell allows intracellular ice formation, which is believed to be detrimental to cells. Eventually the further growth of extracellular ice may cause rupture of the cell membrane. In addition, recrystallization of intracellular and extracellular ice during the thawing process may further damage the cryopreserved cells. Since cell-penetrating CPAs, such as dimethylsulfoxide (DMSO) and glycerol, reduce ice formation by replacing water outside and within the cell as well as stabilize the membranes, the addition of CPAs can increase the post-thaw viability of cryopreserved cells.
Vitrification is a process in which a liquid turns into an amorphous glass solid in the absence of crystallization [160]. Vitrification of cells requires very high concentrations of CPAs and ultrafast cooling rates to completely avoid fatal intracellular and extracellular ice formation [160,161]. In addition to the osmotic stress and chemical toxicity caused by high CPA concentrations, however, vitrification is also associated with ice recrystallization during thawing. In both cases, ice recrystallization during thawing seems to be one of major cold damages. In this context, AFPs are believed to play a crucial role in inhibiting ice recrystallization, improving the cryopreservation efficiency.
4.2. AFPs in Cryopreservation
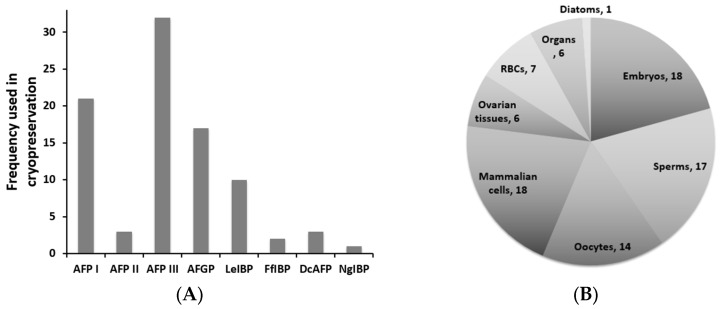
The first application of marine AFPs to the protection of membranes at hypothermic temperatures was made in 1990 using AFGP from Antarctic and Arctic fishes [83]. Since then, marine-derived AFPs have been tested for cryopreservation on numerous occasions. Almost all reports of cryopreservation using AFPs are summarized in Table 1. Of eight AFPs, including nonmarine insect DcAFP, as shown in Figure 6A, type III AFP has been tested most in cryopreservation, followed by type I AFP, AFGP, and LeIBP. This is because type III AFP is easy to produce recombinantly compared with other fish AFPs and because it has been studied longer than other marine-derived AFPs, such as LeIBP and FfIBP. The results listed in Table 1 also showed that hyperactive AFPs do not always ensure better cryopreservation efficiency [65,66,145]. For example, moderately active LeIBP protects mouse ovarian tissue more effectively than 10-fold hyperactive FfIBP [65]. The same result was obtained in human cell line cryopreservation (Hak Jun Kim, unpublished result), consistent with the observation that hyperactive AFPs do not ensure increased IRI activity (Figure 4) [63]. The AFP concentration used in cryopreservations was also determined empirically (Table 1). The IRI endpoint, sometimes expressed as mg/mL, does not indicate the effective amount of AFP in cryopreservation, and the solubility of AFPs and the molar concentration in the freezing medium should also be considered [145]. Quite frequently, higher concentrations of AFPs lead to a decrease in the post-thaw survival of cryopreserved cells, which may be due to the formation of destructive needle-like ice at high AFP concentrations [65,66,68,69,92,162,163].
Table 1.
Lists of AFPs used in cryopreservation of biological samples.
| AFPs | Origin Species of AFPs | Cryopreserved Biological Samples | AFP Quantities Used | Freezing Methods | References | |
|---|---|---|---|---|---|---|
| Organisms | Sample Types | |||||
| III | Fish | Turbot (Scophthalmus maximus) | Embryos | 20 nL (final conc. 0.77 mg/mL) of 10 mg/mL type III AFP injected in yolk sac 0.23914 mm3) | Vitrification | [166] |
| I/III | Fish | Gilthead seabream (Sparus aurata) | Sperm | 1 μg/mL | Vitrification | [167] |
| I/III/AFGP | Fish | Bull | Sperm | 0.1, 1, 10, and 100 μg/mL | Cryopreservation | [168] |
| LeIBP | Yeast | Boar | Sperm | 0.01, 0.1, and 1 mg/mL | Cryopreservation | [169] |
| III | Fish | Mouse | Ovarian tissue | 0.1, 1, and 10 mg/mL | Vitrification | [65] |
| FfIBP | Bacteria | |||||
| LeIBP | Yeast | |||||
| AFGP | Fish | Buffalo | Sperm | 0.1, 1, and 10 μg/mL | Vitrification | [170] |
| III | Fish | Buffalo | Sperm | 0.01, 0.1, 1, and 10 mg/mL | Cryopreservation | [171] |
| III | Fish | Bovine | Embryos | 10 mg/mL | Hypothermic | [172] |
| AFGP | Fish | Pig | Oocyte | 40 mg/mL | Hypothermic | [83] |
| I | Winter flounder | Bovine | Oocyte | 20 mg/mL | Hypothermic | [70] |
| II | Sea raven | |||||
| III | Eel pout | |||||
| III | Notched-fin eelpout | Human | HepG2 | 2–10 mg/mL | Hypothermic | [173] |
| I/III/AFGP | Fish | Rat | RIN-5F cells (insulinoma) | 10 mg/mL | Hypothermic | [174] |
| I | Winter flounder | Sheep | Embryo | 1 or 10 mg/mL | Hypothermic | [175] |
| III | Ocean pout | |||||
| III | Fish | Rabbit | Sperm | 0.1, 1, 10, and 100 μg/mL | Vitrification | [176] |
| Embryo | 100, 500, and 1000 μg/mL | |||||
| III | Fish | Mouse | Oocyte | 500 ng/mL | Vitrification | [177] |
| LeIBP | Yeast | Diatom | Diatom | 0.1 mg/mL | Cryopreservation | [143] |
| AFGP | Fish | Carp | Spermatozoa | 2–10 mg/mL | Hypothermic | [178] |
| I | Fish | Mouse | Pronuclear embryos, 4-cell embryos | 0.1 and 1.0 mg/mL | Vitrification | [92] |
| III | 0.1 mg/mL | |||||
| I/III | Fish | Sea bream | Spermatozoa | 0.1, 1, and 10 μg/mL | Cryopreservation | [165] |
| AFGP | Fish | Equine | Embryos | 20 mg/mL | Hypothermic/Cryopreservation | [91] |
| DcAFP | Insect | Mouse | A10 smooth muscle cell | 1 μg/mL | Cryopreservation | [179] |
| AFGP | Gadus morhua | Mouse | Embryos | 20 mg/mL | Vitrification | [180] |
| III | Fish | Mouse | Ovarian tissue | 0, 5, and 20 mg/mL | Vitrification | [67] |
| III | Fish | Mouse | Mature oocyte | 2.5 mg/mL | Vitrification | [181] |
| I | Fish | Rat | Hippocampal slice cultures | 10 mg/mL | Hypothermic | [182] |
| AFGP | Fish | Pig | Oocyte | 40 mg/mL | Vitrification | [183] |
| AFGP | Fish | Mouse | Embryos | 20 mg/mL | Vitrification | [183] |
| LeIBP | Yeast | Human | Red blood cells | 0.4–0.8 mg/mL | Cryopreservation | [144] |
| III | Fish | Rat | Heart | 3, 5, and 15 mg/mL | Hypothermic | [184] |
| AFGP | Fish | Rat | Cardiomyoctes | 0.5–10 mg/mL | Hypothermic (−4 °C) | [162] |
| III | Fish | Mouse | Oocytes | 500 ng/mL | Cryopreservation | [185] |
| AFGP | Fish | Rat | Cardiac | 10 μg/mL, 10 and 15 mg/mL | Hypothermic | [164] |
| I/III | Fish | Zebra fish | Embryo | 40 μg/mL | Hypothermic | [186] |
| NgIBP | Diatom | Human | Red blood cells | 25, 50, and 77 μg/mL | Cryopreservation | [18] |
| I/III | Fish | Zebra fish | Embryo | 40 μg/mL | Vitrification/Cryopreservation | [187] |
| DcAFP | Insect | Centipede | Gut cells | 0.02 mg/mL | Cryopreservation | [188] |
| III | Fish | Rat | Hearts | 15 mg/mL | Hypothermic | [189] |
| AFGP | Fish | Mouse | Oocytes | 1 mg/mL | Cryopreservation | [190] |
| I/III | Fish | Mouse | Blastocysts | 0.1, 1.0 mg/mL | Cryopreservation | [191] |
| I/III/AFGP | Fish | Mouse | Spermatozoa | 1–100 μg/mL | Cryopreservation | [192] |
| AFGP | Synthetic | Rat | Islet cell | 500 μg/mL | Cryopreservation | [193] |
| I/II/III/AFGP | Fish | Mouse | Oocytes | 20 mg/mL | Vitrification | [194] |
| I | Fish | Human | Myelogenous leukemia cells | 0~1000 μg/mL | Cryopreservation | [163] |
| III | Ocean pout | Chimpanzee (Pan troglodytes) | Spermatozoa | 1, 10, and 100 μg/mL | Cryopreservation | [195] |
| I | Fish | Seabream | Embryos | 20 nL of 10 mg/mL type I AFP injected | Vitrification | [196] |
| III | Fish | Turbot | Embryos | 10 mg/mL | Hypothermic | [166] |
| I | Fish | Rat | Liver | 1 mg/mL | Hypothermic | [94] |
| I/III | Fish | Rat | Hearts | 10, 15, and 20 mg/mL | Hypothermic | [69] |
| I/II/III | Fish | Human | Red blood cells | 0–1.54 mg/mL | Cryopreservation | [197] |
| I | Fish | Human | Red blood cells | 5–160 μg/mL | Cryopreservation | [68] |
| III | Eel pout | Mouse | Oocyte | 0.1 mg/mL | Vitrification | [66] |
| FfIBP | Bacteria | 0.05 mg/mL | ||||
| LeIBP | Yeast | 0.1 mg/mL | ||||
| III | Eel pout | Bovine | Oocyte | 0.5–1 μg/mL | Vitrification | [198] |
| AFGP8 | Fish | Bovine | Oocyte | 1 mM (2.6 mg/mL) | Vitrification | [199] |
| DcAFP | Beetle Dendroides canadensis | Buffalo | Semen | 0.1, 1.0, and 10 μg/mL | Cryopreservation | [200] |
| LeIBP | Yeast | Human | Cell lines | 0.1 mg/mL | Cryopreservation | [145] |
Figure 6.
Cryopreservation research using AFPs: (A) frequency of AFPs used in cryopreservation; and (B) types and frequency of biological samples in cryopreservation using AFPs.
Cryopreserved biological samples are relatively diverse ranging from diatoms and reproductive cells to embryos and organs (Figure 6B). Most cryopreserved biological samples originated from mammals. Most cryopreservation trials using AFPs have demonstrated that the addition of AFPs could improve post-thaw viability, regardless of the freezing method (slow-freezing or vitrification), storage temperature, and biological sample, but several reports showed no beneficial effects [68,87,88,89,90,91,92,93,94,164,165].
In the cryopreservation of cell lines, AFPs have been used as additives to conventional freezing medium to reduce the high amount of cytotoxic CPAs and reduce freezing damage [31]. Some of the cell types tested for cryopreservation with the addition of AFPs include sperms [167,168,169,170,171,176,178,200], oocytes [66,70,83,177,181,183,185,190,198,199], human liver cells [173], RIN-5F insulin tumor cells [174], diatoms [143], red blood cells [18,144,197], muscle cells [162,179], gut cell [188], islet cells [193], E. coli [83], and human cell lines [145] including HeLa cells, NIH/3T3 cells, preosteoblasts (MC3T3-E1 cells), and human ketatinocytes (HaCaT cells). Thus, the addition of AFPs seems to mainly enhance the cryopreservation efficiency regardless of cell type and freezing method, with a handful of exceptions [68,89,90,162,164]. Notably, these exceptions appear to be related to the concentration of AFPs used; indeed, at higher concentrations, AFPs form needle-like ice, which penetrates and destroys cells during freezing [68,143,144,145,162,193,201]. The amount used in cryopreservation also differs between AFPs. LeIBP, which shows lower TH activity, but higher IRI activity than fish AFPs has been used in the range of 0.1–0.8 mg/mL in red blood cells [144], diatoms [143], oocytes [66], and mammalian cell lines [145], whereas fish AFPs have been used at concentrations lower than 0.1 mg/mL, depending on the cell type (Table 1). Interestingly, in the vitrification of mouse oocytes, 0.05 mg/mL FfIBP is more effective at maintaining in murine oocyte quality and embryo development than 0.1 mg/mL LeIBP and 0.1 mg/mL type III AFP [66]. Since the results obtained vary between studies, the utilization of AFPs in cryopreservation needs fine-tuning depending on the type of AFPs, cells, freezing media, and storage temperature.
Embryos from fish [166,186,187,196,202], cows [172,203], sheep [175], rabbits [176], mice [67,92,183,191], and horses [91] were preserved in the presence of AFPs. Early attempts with equine and mouse embryos demonstrated that fish AFPs had negligible effects [91,92]; however, fish embryos subjected to microinjection or incubation in type I AFP solution showed significantly increased survival after chilling at 4 °C or −10 °C. Vitrified 5-somite embryos in type I AFP solution showed similar survival to that of cells recovered from unfrozen embryos [187]. Similarly, AFPs can help improve the survival of embryos preserved at hypothermic temperatures [150,153,154]. These promising results may fuel research in not only hypothermic storage but also vitrification of other embryos such as mammalian embryos.
Lee and colleagues evaluated the beneficial effects of AFPs in vitrification of mouse ovarian tissues [65,67]. Ovarian tissues treated with type III AFPs showed significantly higher intact follicle ratios and lower apoptotic follicle rates than control tissues. The transplanted vitrified-warmed ovaries showed higher intact follicle ratios [65]. In another attempt, all AFP-treated groups had significantly improved follicle preservation with decreasing efficiency in the order of LeIBP > FfIBP > type III AFP [65].
Few studies have evaluated the potential use of AFPs in the hypothermic storage of organs [94,164,184,204]. The TH activity of AFPs has been exploited for subzero preservation of organs. As anticipated, the presence of AFPs decreases cold-induced injury during the hypothermic storage of rat livers [204] and mammalian hearts [69,205] by decreasing the ice formation [189,204,206]. In contrast, Wang et al. reported that higher concentrations of AFGPs have adverse effects on heart preservation [164].
5. Conclusions and Perspective
Thanks to their unique properties as biological antifreezes, AFPs have attracted interest from researchers in academia and biomedical fields. In this review, we surveyed the past and current trends in cryopreservation applications of AFPs. The first property of freezing point depression, termed TH, has typically been utilized primarily in the hypothermic storage of tissues and organs. Due to the complexity and size of tissues and organs, more advancement is needed to achieve effective hypothermic storage of these biological materials. The ability to inhibit ice recrystallization is known to neutralize the catastrophic large icy environment for the cryopreserved cells during freezing and/or warming. The third and less characterized function of AFPs is the interaction with cellular membranes and/or integral membrane proteins. It is not likely that these interactions themselves can confer the cryopreserved cells with post-thaw viability. However, AFPs are thought to augment the viability or cryopreservation efficiency of the cells together with the other two features, particularly IRI.
Our physicochemical understanding of unique binding of AFPs to ice crystal has been the main focus of scientists within the last five decades [51,207]. Relatively few studies have evaluated the application of AFPs in cryopreservation. This is mainly because AFPs are expensive to obtain. Therefore, prior and current cryopreservation research has been limited only to moderately active fish AFPs. Additionally, the applications of AFPs has still only partly characterized based on empirical features, similar to other CPAs [31]. In other words, researchers still need to determine the optimal working concentrations of AFPs in cryopreservation; neither TH nor IRI can provide this information.
For the application of AFPs to be practical, a few questions should be addressed. First, mass production of AFPs should be established. Currently, only type III AFP has been produced on the industrial scale owing to its use as an ingredient in ice cream. However, advancements in molecular biology and genomics have improved our ability to produce genes and proteins easily, expanding AFP-related research. Indeed, a mass production system for LeIBP, Glaciozyma IBP, has been reported [142]. Additionally, the LeIBP has been shown to yield better post-thaw viability in several studies compared with that of other marine-derived AFPs [65,66,143,144,145]. Second, the behaviors of AFPs in freezing medium should be characterized thoroughly. Typically, freezing medium contains high concentrations of chemicals, such as DMSO, ethylene glycol, polyvinylpyrrolidone, and polyethylene glycol, which may destabilize AFPs, leading to loss of function [208]. Third, functionalized AFPs should be engineered and developed to overcome the limitations of natural counterparts. Mother nature has suggested the use of RD3 and an IBP from Vostok glacial bacterium [137,209]. In both cases, connecting two almost homologous domains increases the TH value cooperatively compared with their monomeric AFPs [139,209]. Studies from the laboratories of Tsuda, Davies, and Holland have demonstrated that the multimerization of native type III AFP can increase TH activity [210,211,212]. Recently, Steven et al. claimed the dendrimer-like AFPs showed higher TH values [213], and Phippen et al. demonstrated 12 AFP-fused protein cage nanoparticles that increased the TH value to more than 50-fold that of monomeric AFP [214]. A few groups have attempted to synthesize AFP or AFGP derivatives to elucidate the underlying mechanism of action and to develop practical applications [215,216,217,218,219,220,221]. Another interesting approach is the development of cell-internalizable or -penetrating AFPs. AFPs are usually nonpenetrating, such that the internal ice formation should be inhibited by high amounts of cytotoxic CPAs. Cell-internalizable AFPs may also reduce the amount of CPAs in freezing medium, eventually increasing the efficiency of cryopreservation.
Finally, it is encouraging that many research groups studying AFP worldwide have started expanding their research into cryopreservation using AFPs. We hope these concerted efforts will accelerate the development of biomedical application of AFPs.
Acknowledgments
We thank the anonymous reviewers for their careful reading of our manuscript and their insightful comments and suggestions. This work is supported by the Polar Genomics 101 Project: Genome analysis of polar organisms and establishment of application platform (PE17080) funded by the Korea Polar Research Institute (KOPRI), and National Fisheries Research and Development Institute (R2017013 to Y.B.H).
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.DeVries A.L., Wohlschlag D.E. Freezing resistance in some Antarctic fishes. Science. 1969;163:1073–1075. doi: 10.1126/science.163.3871.1073. [DOI] [PubMed] [Google Scholar]
- 2.DeVries A.L., Komatsu S.K., Feeney R.E. Chemical and physical properties of freezing point-depressing glycoproteins from Antarctic fishes. J. Biol. Chem. 1970;245:2901–2908. [PubMed] [Google Scholar]
- 3.DeVries A.L. Glycoproteins as biological antifreeze agents in antarctic fishes. Science. 1971;172:1152–1155. doi: 10.1126/science.172.3988.1152. [DOI] [PubMed] [Google Scholar]
- 4.DeVries A.L. Freezing resistance in fishes of the Antarctic penninsula. Antarct. J. US. 1969;4:104–105. [Google Scholar]
- 5.Davies P.L. Ice-binding proteins: A remarkable diversity of structures for stopping and starting ice growth. Trends Biochem. Sci. 2014;39:548–555. doi: 10.1016/j.tibs.2014.09.005. [DOI] [PubMed] [Google Scholar]
- 6.Gilbert J.A., Davies P.L., Laybourn-Parry J. A hyperactive, Ca2+-dependent antifreeze protein in an Antarctic bacterium. FEMS Microbiol. Lett. 2005;245:67–72. doi: 10.1016/j.femsle.2005.02.022. [DOI] [PubMed] [Google Scholar]
- 7.Do H., Kim S.J., Kim H.J., Lee J.H. Structure-based characterization and antifreeze properties of a hyperactive ice-binding protein from the Antarctic bacterium Flavobacterium frigoris PS1. Acta Crystallogr. D Biol. Crystallogr. 2014;70:1061–1073. doi: 10.1107/S1399004714000996. [DOI] [PubMed] [Google Scholar]
- 8.Raymond J.A., Fritsen C., Shen K. An ice-binding protein from an Antarctic sea ice bacterium. FEMS Microbiol. Ecol. 2007;61:214–221. doi: 10.1111/j.1574-6941.2007.00345.x. [DOI] [PubMed] [Google Scholar]
- 9.Singh P., Hanada Y., Singh S.M., Tsuda S. Antifreeze protein activity in Arctic cryoconite bacteria. FEMS Microbiol. Lett. 2014;351:14–22. doi: 10.1111/1574-6968.12345. [DOI] [PubMed] [Google Scholar]
- 10.Lee J.K., Park K.S., Park S., Park H., Song Y.H., Kang S.H., Kim H.J. An extracellular ice-binding glycoprotein from an Arctic psychrophilic yeast. Cryobiology. 2010;60:222–228. doi: 10.1016/j.cryobiol.2010.01.002. [DOI] [PubMed] [Google Scholar]
- 11.Boo S.Y., Wong C.M.V.L., Rodrigues K.F., Najimudin N., Murad A.M.A., Mahadi N.M. Thermal stress responses in Antarctic yeast, Glaciozyma antarctica PI12, characterized by real-time quantitative PCR. Polar Biol. 2013;36:381–389. doi: 10.1007/s00300-012-1268-2. [DOI] [Google Scholar]
- 12.Hashim N.H., Bharudin I., Nguong D.L., Higa S., Bakar F.D., Nathan S., Rabu A., Kawahara H., Illias R.M., Najimudin N., et al. Characterization of Afp1, an antifreeze protein from the psychrophilic yeast Glaciozyma antarctica PI12. Extremophiles. 2013;17:63–73. doi: 10.1007/s00792-012-0494-4. [DOI] [PubMed] [Google Scholar]
- 13.Kiko R. Acquisition of freeze protection in a sea-ice crustacean through horizontal gene transfer? Polar Biol. 2010;33:543–556. doi: 10.1007/s00300-009-0732-0. [DOI] [Google Scholar]
- 14.Jung W., Gwak Y., Davies P.L., Kim H.J., Jin E. Isolation and characterization of antifreeze proteins from the antarctic marine microalga Pyramimonas gelidicola. Mar. Biotechnol. 2014;16:502–512. doi: 10.1007/s10126-014-9567-y. [DOI] [PubMed] [Google Scholar]
- 15.Gwak I.G., Jung W., Kim H.J., Kang S.H., Jin E. Antifreeze protein in Antarctic marine diatom, Chaetoceros neogracile. Mar. Biotechnol. 2009;12:630–639. doi: 10.1007/s10126-009-9250-x. [DOI] [PubMed] [Google Scholar]
- 16.Janech M., Krell A., Mock T., Kang J.-S., Raymond J. Ice-binding proteins from sea ice diatoms (bacillariophyceae) J. Phycol. 2006;42:410–416. doi: 10.1111/j.1529-8817.2006.00208.x. [DOI] [Google Scholar]
- 17.Krell A., Beszteri B., Dieckmann G., Glöckner G., Valentin K., Mock T. A new class of ice-binding proteins discovered in a salt-stress-induced cDNA library of the psychrophilic diatom Fragilariopsis cylindrus (Bacillariophyceae) Eur. J. Phycol. 2008;43:423–433. doi: 10.1080/09670260802348615. [DOI] [Google Scholar]
- 18.Kang J.S., Raymond J.A. Reduction of freeze-thaw-induced hemolysis of red blood cells by an algal ice-binding protein. Cryo Lett. 2004;25:307–310. [PubMed] [Google Scholar]
- 19.Raymond J.A., Janech M., Fritsen C. Novel ice-binding proteins from a psychrophilic antarctic alga (Chlamydomonadaceae, chlorophyceae) J. Phycol. 2009;45:130–136. doi: 10.1111/j.1529-8817.2008.00623.x. [DOI] [PubMed] [Google Scholar]
- 20.Davies P.L., Hew C.L. Biochemistry of fish antifreeze proteins. FASEB J. 1990;4:2460–2468. doi: 10.1096/fasebj.4.8.2185972. [DOI] [PubMed] [Google Scholar]
- 21.Hanada Y., Nishimiya Y., Miura A., Tsuda S., Kondo H. Hyperactive antifreeze protein from an Antarctic sea ice bacterium Colwellia sp. has a compound ice-binding site without repetitive sequences. FEBS J. 2014;281:3576–3590. doi: 10.1111/febs.12878. [DOI] [PubMed] [Google Scholar]
- 22.Shah S.H.H., Kar R.K., Asmawi A.A., Rahman M.B.A., Murad A.M.A., Mahadi N.M., Basri M., Rahman R.N.Z.A., Salleh A.B., Chatterjee S., et al. Solution structures, dynamics, and ice growth inhibitory activity of peptide fragments derived from an antarctic yeast protein. PLoS ONE. 2012;7:e49788. doi: 10.1371/journal.pone.0049788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bayer-Giraldi M., Weikusat I., Besir H., Dieckmann G. Characterization of an antifreeze protein from the polar diatom Fragilariopsis cylindrus and its relevance in sea ice. Cryobiology. 2011;63:210–219. doi: 10.1016/j.cryobiol.2011.08.006. [DOI] [PubMed] [Google Scholar]
- 24.Uhlig C., Kabisch J., Palm G.J., Valentin K., Schweder T., Krell A. Heterologous expression, refolding and functional characterization of two antifreeze proteins from Fragilariopsis cylindrus (Bacillariophyceae) Cryobiology. 2011;63:220–228. doi: 10.1016/j.cryobiol.2011.08.005. [DOI] [PubMed] [Google Scholar]
- 25.Hew C.L., Davies P.L., Fletcher G. Antifreeze protein gene transfer in Atlantic salmon. Mol. Mar. Biol. Biotechnol. 1992;1:309–317. [PubMed] [Google Scholar]
- 26.Wohrmann A.P. Antifreeze glycopeptides of the high-Antarctic silverfish Pleuragramma antarcticum (Notothenioidei) Comp. Biochem. Physiol. C Pharmacol. Toxicol. Endocrinol. 1995;111:121–129. doi: 10.1016/0742-8413(95)00007-T. [DOI] [PubMed] [Google Scholar]
- 27.Barrett J. Thermal hysteresis proteins. Int. J. Biochem. Cell Biol. 2001;33:105–117. doi: 10.1016/S1357-2725(00)00083-2. [DOI] [PubMed] [Google Scholar]
- 28.Ben R.N. Antifreeze glycoproteins-preventing the growth of ice. Chembiochem. 2001;2:161–166. doi: 10.1002/1439-7633(20010302)2:3<161::AID-CBIC161>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 29.Bouvet V., Ben R.N. Antifreeze glycoproteins. Cell Biochem. Biophys. 2003;39:133–144. doi: 10.1385/CBB:39:2:133. [DOI] [PubMed] [Google Scholar]
- 30.Harding M.M., Anderberg P.I., Haymet A.D. “Antifreeze” glycoproteins from polar fish. Eur. J. Biochem. 2003;270:1381–1392. doi: 10.1046/j.1432-1033.2003.03488.x. [DOI] [PubMed] [Google Scholar]
- 31.Fuller B.J. Cryoprotectants: The essential antifreezes to protect life in the frozen state. Cryo Lett. 2004;25:375–388. [PubMed] [Google Scholar]
- 32.Kristiansen E., Zachariassen K.E. The mechanism by which fish antifreeze proteins cause thermal hysteresis. Cryobiology. 2005;51:262–280. doi: 10.1016/j.cryobiol.2005.07.007. [DOI] [PubMed] [Google Scholar]
- 33.Bar Dolev M., Braslavsky I., Davies P.L. Ice-binding proteins and their function. Annu. Rev. Biochem. 2016;85:515–542. doi: 10.1146/annurev-biochem-060815-014546. [DOI] [PubMed] [Google Scholar]
- 34.Raymond J.A., DeVries A.L. Freezing behavior of fish blood glycoproteins with antifreeze properties. Cryobiology. 1972;9:541–547. doi: 10.1016/0011-2240(72)90176-9. [DOI] [PubMed] [Google Scholar]
- 35.Raymond J.A., DeVries A.L. Adsorption inhibition as a mechanism of freezing resistance in polar fishes. Proc. Natl. Acad. Sci. USA. 1977;74:2589–2593. doi: 10.1073/pnas.74.6.2589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wilson P.W., Beaglehole D., Devries A.L. Antifreeze glycopeptide adsorption on single crystal ice surfaces using ellipsometry. Biophys. J. 1993;64:1878–1884. doi: 10.1016/S0006-3495(93)81559-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wilson P.W. Explaining thermal hysteresis by the Kelvin effect. Cryo Lett. 1993;14:31–36. [Google Scholar]
- 38.Wilson P.W., Leader J.P. Stabilization of supercooled fluids by thermal hysteresis proteins. Biophys. J. 1995;68:2098–2107. doi: 10.1016/S0006-3495(95)80389-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Celik Y., Drori R., Pertaya-Braun N., Altan A., Barton T., Bar-Dolev M., Groisman A., Davies P.L., Braslavsky I. Microfluidic experiments reveal that antifreeze proteins bound to ice crystals suffice to prevent their growth. Proc. Natl. Acad. Sci. USA. 2013;110:1309–1314. doi: 10.1073/pnas.1213603110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Knight C.A., Cheng C.C., DeVries A.L. Adsorption of alpha-helical antifreeze peptides on specific ice crystal surface planes. Biophys. J. 1991;59:409–418. doi: 10.1016/S0006-3495(91)82234-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Do H., Lee J.H., Lee S.G., Kim H.J. Crystallization and preliminary X-ray crystallographic analysis of an ice-binding protein (FfIBP) from Flavobacterium frigoris PS1. Acta Crystallogr. Sect. F Struct. Biol. Cryst. Commun. 2012;68:806–809. doi: 10.1107/S1744309112020465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Drori R., Celik Y., Davies P.L., Braslavsky I. Ice-binding proteins that accumulate on different ice crystal planes produce distinct thermal hysteresis dynamics. J. R. Soc. Interface. 2014;11:2014526. doi: 10.1098/rsif.2014.0526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pertaya N., Marshall C.B., Celik Y., Davies P.L., Braslavsky I. Direct visualization of spruce budworm antifreeze protein interacting with ice crystals: Basal plane affinity confers hyperactivity. Biophys. J. 2008;95:333–341. doi: 10.1529/biophysj.107.125328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Park K.S., Do H., Lee J.H., Park S.I., Kim E.J., Kim S.J., Kang S.H., Kim H.J. Characterization of the ice-binding protein from Arctic yeast Leucosporidium sp. AY30. Cryobiology. 2012;64:286–296. doi: 10.1016/j.cryobiol.2012.02.014. [DOI] [PubMed] [Google Scholar]
- 45.Fletcher G.L., Hew C.L., Davies P.L. Antifreeze proteins of teleost fishes. Annu. Rev. Physiol. 2001;63:359–390. doi: 10.1146/annurev.physiol.63.1.359. [DOI] [PubMed] [Google Scholar]
- 46.Jung W., Campbell R.L., Gwak Y., Kim J.I., Davies P.L., Jin E. New cysteine-rich ice-binding protein secreted from Antarctic microalga, Chloromonas sp. PLoS ONE. 2016;11:e0154056. doi: 10.1371/journal.pone.0154056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Knight C.A., DeVries A.L., Oolman L.D. Fish antifreeze protein and the freezing and recrystallization of ice. Nature. 1984;308:295–296. doi: 10.1038/308295a0. [DOI] [PubMed] [Google Scholar]
- 48.Knight C.A., Wen D., Laursen R.A. Nonequilibrium antifreeze peptides and the recrystallization of ice. Cryobiology. 1995;32:23–34. doi: 10.1006/cryo.1995.1002. [DOI] [PubMed] [Google Scholar]
- 49.Raymond J.A., Fritsen C.H. Semipurification and ice recrystallization inhibition activity of ice-active substances associated with Antarctic photosynthetic organisms. Cryobiology. 2001;43:63–70. doi: 10.1006/cryo.2001.2341. [DOI] [PubMed] [Google Scholar]
- 50.Raymond J.A., Knight C.A. Ice binding, recrystallization inhibition, and cryoprotective properties of ice-active substances associated with Antarctic sea ice diatoms. Cryobiology. 2003;46:174–181. doi: 10.1016/S0011-2240(03)00023-3. [DOI] [PubMed] [Google Scholar]
- 51.Jia Z., Davies P.L. Antifreeze proteins: An unusual receptor-ligand interaction. Trends Biochem. Sci. 2002;27:101–106. doi: 10.1016/S0968-0004(01)02028-X. [DOI] [PubMed] [Google Scholar]
- 52.Knight C.A., Duman J.G. Inhibition of recrystallization of ice by insect thermal hysteresis proteins: A possible cryoprotective role. Cryobiology. 1986;23:256–262. doi: 10.1016/0011-2240(86)90051-9. [DOI] [Google Scholar]
- 53.Worrall D., Elias L., Ashford D., Smallwood M., Sidebottom C., Lillford P., Telford J., Holt C., Bowles D. A carrot leucine-rich-repeat protein that inhibits ice recrystallization. Science. 1998;282:115–117. doi: 10.1126/science.282.5386.115. [DOI] [PubMed] [Google Scholar]
- 54.Smallwood M., Worrall D., Byass L., Elias L., Ashford D., Doucet C.J., Holt C., Telford J., Lillford P., Bowles D.J. Isolation and characterization of a novel antifreeze protein from carrot (Daucus carota) Biochem. J. 1999;340:385–391. doi: 10.1042/bj3400385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sidebottom C., Buckley S., Pudney P., Twigg S., Jarman C., Holt C., Telford J., McArthur A., Worrall D., Hubbard R., et al. Heat-stable antifreeze protein from grass. Nature. 2000;406:256. doi: 10.1038/35018639. [DOI] [PubMed] [Google Scholar]
- 56.Hew C.L., Yang D.S. Protein interaction with ice. Eur. J. Biochem. 1992;203:33–42. doi: 10.1111/j.1432-1033.1992.tb19824.x. [DOI] [PubMed] [Google Scholar]
- 57.Wohrmann A. Antifreeze glycoproteins in fishes: Structure, mode of action and possible applications. Tierarztl. Prax. 1996;24:1–9. [PubMed] [Google Scholar]
- 58.Knight C.A., Hallett J., DeVries A.L. Solute effects on ice recrystallization: An assessment technique. Cryobiology. 1988;25:55–60. doi: 10.1016/0011-2240(88)90020-X. [DOI] [PubMed] [Google Scholar]
- 59.Tomczak M.M., Marshall C.B., Gilbert J.A., Davies P.L. A facile method for determining ice recrystallization inhibition by antifreeze proteins. Biochem. Biophys. Res. Commun. 2003;311:1041–1046. doi: 10.1016/j.bbrc.2003.10.106. [DOI] [PubMed] [Google Scholar]
- 60.Jackman J., Noestheden M., Moffat D., Pezacki J.P., Findlay S., Ben R.N. Assessing antifreeze activity of AFGP 8 using domain recognition software. Biochem. Biophys. Res. Commun. 2007;354:340–344. doi: 10.1016/j.bbrc.2006.12.225. [DOI] [PubMed] [Google Scholar]
- 61.Olijve L.L.C., Oude Vrielink A.S., Voets I.K. A simple and quantitative method to evaluate ice recrystallization kinetics using the circle Hough Transform algorithm. Cryst. Growth Des. 2016;16:4190–4195. doi: 10.1021/acs.cgd.5b01637. [DOI] [Google Scholar]
- 62.Olijve L.L.C., Meister K., DeVries A.L., Duman J.G., Guo S., Bakker H.J., Voets I.K. Blocking rapid ice crystal growth through nonbasal plane adsorption of antifreeze proteins. Proc. Natl. Acad. Sci. USA. 2016;113:3740–3745. doi: 10.1073/pnas.1524109113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yu S.O., Brown A., Middleton A.J., Tomczak M.M., Walker V.K., Davies P.L. Ice restructuring inhibition activities in antifreeze proteins with distinct differences in thermal hysteresis. Cryobiology. 2010;61:327–334. doi: 10.1016/j.cryobiol.2010.10.158. [DOI] [PubMed] [Google Scholar]
- 64.Lee J.H., Park A.K., Do H., Park K.S., Moh S.H., Chi Y.M., Kim H.J. Structural basis for the antifreeze activity of an ice-binding protein from an Arctic yeast. J. Biol. Chem. 2012;287:11460–11468. doi: 10.1074/jbc.M111.331835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lee J., Kim S.K., Youm H.W., Kim H.J., Lee J.R., Suh C.S., Kim S.H. Effects of three different types of antifreeze proteins on mouse ovarian tissue cryopreservation and transplantation. PLoS ONE. 2015;10:e0126252. doi: 10.1371/journal.pone.0126252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lee H.H., Lee H.J., Kim H.J., Lee J.H., Ko Y., Kim S.M., Lee J.R., Suh C.S., Kim S.H. Effects of antifreeze proteins on the vitrification of mouse oocytes: Comparison of three different antifreeze proteins. Hum. Reprod. 2015;30:2110–2119. doi: 10.1093/humrep/dev170. [DOI] [PubMed] [Google Scholar]
- 67.Lee J.R., Youm H.W., Lee H.J., Jee B.C., Suh C.S., Kim S.H. Effect of antifreeze protein on mouse ovarian tissue cryopreservation and transplantation. Yonsei Med. J. 2015;56:778–784. doi: 10.3349/ymj.2015.56.3.778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Carpenter J.F., Hansen T.N. Antifreeze protein modulates cell survival during cryopreservation: Mediation through influence on ice crystal growth. Proc. Natl. Acad. Sci. USA. 1992;89:8953–8957. doi: 10.1073/pnas.89.19.8953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Amir G., Rubinsky B., Kassif Y., Horowitz L., Smolinsky A.K., Lavee J. Preservation of myocyte structure and mitochondrial integrity in subzero cryopreservation of mammalian hearts for transplantation using antifreeze proteins-an electron microscopy study. Eur. J. Cardiothorac. Surg. 2003;24:292–297. doi: 10.1016/S1010-7940(03)00306-3. [DOI] [PubMed] [Google Scholar]
- 70.Rubinsky B., Arav A., Fletcher G.L. Hypothermic protection-a fundamental property of “antifreeze” proteins. Biochem. Biophys. Res. Commun. 1991;180:566–571. doi: 10.1016/S0006-291X(05)81102-7. [DOI] [PubMed] [Google Scholar]
- 71.Quinn P.J. A lipid-phase separation model of low-temperature damage to biological membranes. Cryobiology. 1985;22:128–146. doi: 10.1016/0011-2240(85)90167-1. [DOI] [PubMed] [Google Scholar]
- 72.Hays L.M., Feeney R.E., Crowe L.M., Crowe J.H., Oliver A.E. Antifreeze glycoproteins inhibit leakage from liposomes during thermotropic phase transitions. Proc. Natl. Acad. Sci. USA. 1996;93:6835–6840. doi: 10.1073/pnas.93.13.6835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tomczak M.M., Hincha D.K., Estrada S.D., Wolkers W.F., Crowe L.M., Feeney R.E., Tablin F., Crowe J.H. A mechanism for stabilization of membranes at low temperatures by an antifreeze protein. Biophys. J. 2002;82:874–881. doi: 10.1016/S0006-3495(02)75449-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tomczak M.M., Vigh L., Meyer J.D., Manning M.C., Hincha D.K., Crowe J.H. Lipid unsaturation determines the interaction of AFP type I with model membranes during thermotropic phase transitions. Cryobiology. 2002;45:135–142. doi: 10.1016/S0011-2240(02)00122-0. [DOI] [PubMed] [Google Scholar]
- 75.Tablin F., Oliver A.E., Walker N.J., Crowe L.M., Crowe J.H. Membrane phase transition of intact human platelets: Correlation with cold-induced activation. J. Cell. Physiol. 1996;168:305–313. doi: 10.1002/(SICI)1097-4652(199608)168:2<305::AID-JCP9>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 76.Crowe J.H., Crowe L.M. Water and carbohydrate interactions with membranes: Studies with infrared spectroscopy and differential scanning calorimetry methods. Methods Enzymol. 1986;127:696–703. doi: 10.1016/0076-6879(86)27054-8. [DOI] [PubMed] [Google Scholar]
- 77.Hays L.M., Crowe J.H., Wolkers W., Rudenko S. Factors affecting leakage of trapped solutes from phospholipid vesicles during thermotropic phase transitions. Cryobiology. 2001;42:88–102. doi: 10.1006/cryo.2001.2307. [DOI] [PubMed] [Google Scholar]
- 78.Tomczak M.M., Hincha D.K., Estrada S.D., Feeney R.E., Crowe J.H. Antifreeze proteins differentially affect model membranes during freezing. Biochim. Biophys. Acta. 2001;1511:255–263. doi: 10.1016/S0005-2736(01)00281-4. [DOI] [PubMed] [Google Scholar]
- 79.Kun H., Byk G., Mastai Y. Effects of antifreeze protein fragments on the properties of model membranes. Adv. Exp. Med. Biol. 2009;611:85–86. doi: 10.1007/978-0-387-73657-0_38. [DOI] [PubMed] [Google Scholar]
- 80.Wu Y., Fletcher G.L. Efficacy of antifreeze protein types in protecting liposome membrane integrity depends on phospholipid class. Biochim. Biophys. Acta. 2001;1524:11–16. doi: 10.1016/S0304-4165(00)00134-3. [DOI] [PubMed] [Google Scholar]
- 81.Kun H., Mastai Y. Isothermal calorimetry study of the interactions of type I antifreeze proteins with a lipid model membrane. Protein Pept. Lett. 2009;17:739–743. doi: 10.2174/092986610791190354. [DOI] [PubMed] [Google Scholar]
- 82.Tomczak M.M., Hincha D.K., Crowe J.H., Harding M.M., Haymet A.D. The effect of hydrophobic analogues of the type I winter flounder antifreeze protein on lipid bilayers. FEBS Lett. 2003;551:13–19. doi: 10.1016/S0014-5793(03)00843-3. [DOI] [PubMed] [Google Scholar]
- 83.Rubinsky B., Arav A., Mattioli M., Devries A.L. The effect of antifreeze glycopeptides on membrane potential changes at hypothermic temperatures. Biochem. Biophys. Res. Commun. 1990;173:1369–1374. doi: 10.1016/S0006-291X(05)80939-8. [DOI] [PubMed] [Google Scholar]
- 84.Negulescu P.A., Rubinsky B., Fletcher G.L., Machen T.E. Fish antifreeze proteins block Ca entry into rabbit parietal cells. Am. J. Physiol. 1992;263:C1310–C1313. doi: 10.1152/ajpcell.1992.263.6.C1310. [DOI] [PubMed] [Google Scholar]
- 85.Arav A., Rubinsky B., Seren E., Roche J.F., Boland M.P. The role of thermal hysteresis proteins during cryopreservation of oocytes and embryos. Theriogenology. 1994;41:107–112. doi: 10.1016/S0093-691X(05)80055-X. [DOI] [Google Scholar]
- 86.Rubinsky B., Mattioli M., Arav A., Barboni B., Fletcher G.L. Inhibition of Ca2+ and K+ currents by “antifreeze” proteins. Am. J. Physiol. 1992;262:R542–R545. doi: 10.1152/ajpregu.1992.262.3.R542. [DOI] [PubMed] [Google Scholar]
- 87.Wang J.H., Bian H.W., Huang C.N., Ge J.G. Studies on the application of antifreeze proteins in cryopreservation of rice suspension cells. Shi Yan Sheng Wu Xue Bao. 1999;32:271–276. [PubMed] [Google Scholar]
- 88.Wang L.H., Wusteman M.C., Smallwood M., Pegg D.E. The stability during low-temperature storage of an antifreeze protein isolated from the roots of cold-acclimated carrots. Cryobiology. 2002;44:307–310. doi: 10.1016/S0011-2240(02)00036-6. [DOI] [PubMed] [Google Scholar]
- 89.Ishiguro H., Rubinsky B. Influence of fish antifreeze proteins on the freezing of cell suspensions with cryoprotectant penetrating cells. Int. J. Heat Mass Transf. 1998;41:1907–1915. doi: 10.1016/S0017-9310(97)00368-2. [DOI] [Google Scholar]
- 90.Payne S.R., Oliver J.E., Upreti G.C. Effect of antifreeze proteins on the motility of ram spermatozoa. Cryobiology. 1994;31:180–184. doi: 10.1006/cryo.1994.1021. [DOI] [PubMed] [Google Scholar]
- 91.Lagneaux D., Huhtinen M., Koskinen E., Palmer E. Effect of anti-freeze protein (AFP) on the cooling and freezing of equine embryos as measured by DAPI-staining. Equine Vet. J. Suppl. 1997;25:85–87. doi: 10.1111/j.2042-3306.1997.tb05108.x. [DOI] [PubMed] [Google Scholar]
- 92.Shaw J.M., Ward C., Trounson A.O. Evaluation of propanediol, ethylene glycol, sucrose and antifreeze proteins on the survival of slow-cooled mouse pronuclear and 4-cell embryos. Hum. Reprod. 1995;10:396–402. doi: 10.1093/oxfordjournals.humrep.a135951. [DOI] [PubMed] [Google Scholar]
- 93.Mezhevikina L.M., Karanova M.V. The use of antifreeze glycoproteins in the freezing in liquid nitrogen of early mouse embryos. Izv. Akad. Nauk. Seriia Biol. Akad. Nauk. 1994;2:172–177. [PubMed] [Google Scholar]
- 94.Soltys K.A., Batta A.K., Koneru B. Successful nonfreezing, subzero preservation of rat liver with 2,3-butanediol and type I antifreeze protein. J. Surg. Res. 2001;96:30–34. doi: 10.1006/jsre.2000.6053. [DOI] [PubMed] [Google Scholar]
- 95.Zhang E., Zhang L., Wang B., Yan B., Wang Q. Cryopreservation of marine diatom algae by encapsulation-vitrification. Cryo Lett. 2009;30:224–231. [PubMed] [Google Scholar]
- 96.Larese A., Acker J., Muldrew K., Yang H.Y., McGann L. Antifreeze proteins induce intracellular nucleation. Cryoletters. 1996;17:175–182. [Google Scholar]
- 97.Scholander P.F., Van Dam L., Kanwisher J.W., Hammel H.T., Gordon M.S. Supercooling and osmoregulation in Arctic fish. J. Cell. Physiol. 1957;49:5–24. doi: 10.1002/jcp.1030490103. [DOI] [Google Scholar]
- 98.Komatsu S.K., DeVries A.L., Feeney R.E. Studies of the structure of freezing point-depressing glycoproteins from an Antarctic fish. J. Biol. Chem. 1970;245:2909–2913. [PubMed] [Google Scholar]
- 99.Duman J.G., Devries A.L. Freezing resistance in winter flounder Pseudopleuronectes americanus. Nature. 1974;247:237–238. doi: 10.1038/247237a0. [DOI] [Google Scholar]
- 100.Slaughter D., Fletcher G.L., Ananthanarayanan V.S., Hew C.L. Antifreeze proteins from the sea raven, Hemitripterus americanus. Further evidence for diversity among fish polypeptide antifreezes. J. Biol. Chem. 1981;256:2022–2026. [PubMed] [Google Scholar]
- 101.Feeney R.E., Yeh Y. Antifreeze proteins from fish bloods. Adv. Protein Chem. 1978;32:191–282. doi: 10.1016/s0065-3233(08)60576-8. [DOI] [PubMed] [Google Scholar]
- 102.Burcham T.S., Osuga D.T., Chino H., Feeney R.E. Analysis of antifreeze glycoproteins in fish serum. Anal. Biochem. 1984;139:197–204. doi: 10.1016/0003-2697(84)90405-6. [DOI] [PubMed] [Google Scholar]
- 103.Kao M.H., Fletcher G.L., Wang N.C., Hew C.L. The relationship between molecular weight and antifreeze polypeptide activity in marine fish. Can. J. Zool. 1986;64:578–582. doi: 10.1139/z86-085. [DOI] [Google Scholar]
- 104.Ahlgren J.A., Cheng C.C., Schrag J.D., DeVries A.L. Freezing avoidance and the distribution of antifreeze glycopeptides in body fluids and tissues of Antarctic fish. J. Exp. Biol. 1988;137:549–563. doi: 10.1242/jeb.137.1.549. [DOI] [PubMed] [Google Scholar]
- 105.Hew C.L., Wang N.C., Yan S., Cai H., Sclater A., Fletcher G.L. Biosynthesis of antifreeze polypeptides in the winter flounder. Eur. J. Biochem. 1986;160:267–272. doi: 10.1111/j.1432-1033.1986.tb09966.x. [DOI] [PubMed] [Google Scholar]
- 106.Chao H., Houston M.E., Jr., Hodges R.S., Kay C.M., Sykes B.D., Loewen M.C., Davies P.L., Sönnichsen F.D. A diminished role for hydrogen bonds in antifreeze protein binding to ice. Biochemistry. 1997;36:14652–14660. doi: 10.1021/bi970817d. [DOI] [PubMed] [Google Scholar]
- 107.Haymet A.D., Ward L.G., Harding M.M., Knight C.A. Valine substituted winter flounder “antifreeze”: Preservation of ice growth hysteresis. FEBS Lett. 1998;430:301–306. doi: 10.1016/S0014-5793(98)00652-8. [DOI] [PubMed] [Google Scholar]
- 108.Loewen M.C., Chao H., Houston M.E., Jr., Baardsnes J., Hodges R.S., Kay C.M., Sykes B.D., Sönnichsen F.D., Davies P.L. Alternative roles for putative ice-binding residues in type I antifreeze protein. Biochemistry. 1999;38:4743–4749. doi: 10.1021/bi982602p. [DOI] [PubMed] [Google Scholar]
- 109.Zhang W., Laursen R.A. Structure-function relationships in a type I antifreeze polypeptide. The role of threonine methyl and hydroxyl groups in antifreeze activity. J. Biol. Chem. 1998;273:34806–34812. doi: 10.1074/jbc.273.52.34806. [DOI] [PubMed] [Google Scholar]
- 110.Vasina E.N., Paszek E., Nicolau D., Jr., Nicolau D.V. The BAD project: Data mining, database and prediction of protein adsorption on surfaces. Lab Chip. 2009;9:891–900. doi: 10.1039/B813475H. [DOI] [PubMed] [Google Scholar]
- 111.Baardsnes J., Kondejewski L.H., Hodges R.S., Chao H., Kay C., Davies P.L. New ice-binding face for type I antifreeze protein. FEBS Lett. 1999;463:87–91. doi: 10.1016/S0014-5793(99)01588-4. [DOI] [PubMed] [Google Scholar]
- 112.Ewart K.V., Yang D.S., Ananthanarayanan V.S., Fletcher G.L., Hew C.L. Ca2+-dependent antifreeze proteins. Modulation of conformation and activity by divalent metal ions. J. Biol. Chem. 1996;271:16627–16632. doi: 10.1074/jbc.271.28.16627. [DOI] [PubMed] [Google Scholar]
- 113.Gronwald W., Loewen M.C., Lix B., Daugulis A.J., Sönnichsen F.D., Davies P.L., Sykes B.D. The solution structure of type II antifreeze protein reveals a new member of the lectin family. Biochemistry. 1998;37:4712–4721. doi: 10.1021/bi972788c. [DOI] [PubMed] [Google Scholar]
- 114.Drickamer K. C-type lectin-like domains. Curr. Opin. Struct. Biol. 1999;9:585–590. doi: 10.1016/S0959-440X(99)00009-3. [DOI] [PubMed] [Google Scholar]
- 115.Liu Y., Li Z., Lin Q., Kosinski J., Seetharaman J., Bujnicki J.M., Sivaraman J., Hew C.L. Structure and evolutionary origin of Ca2+-dependent herring type II antifreeze protein. PLoS ONE. 2007;2:e548. doi: 10.1371/journal.pone.0000548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Nishimiya Y., Kondo H., Takamichi M., Sugimoto H., Suzuki M., Miura A., Tsuda S. Crystal structure and mutational analysis of Ca2+-independent type II antifreeze protein from longsnout poacher, Brachyopsis rostratus. J. Mol. Biol. 2008;382:734–746. doi: 10.1016/j.jmb.2008.07.042. [DOI] [PubMed] [Google Scholar]
- 117.Ewart K.V., Lin Q., Hew C.L. Structure, function and evolution of antifreeze proteins. Cell. Mol. Life Sci. 1999;55:271–283. doi: 10.1007/s000180050289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Yamashita Y., Miura R., Takemoto Y., Tsuda S., Kawahara H., Obata H. Type II antifreeze protein from a mid-latitude freshwater fish, Japanese smelt (Hypomesus nipponensis) Biosci. Biotechnol. Biochem. 2003;67:461–466. doi: 10.1271/bbb.67.461. [DOI] [PubMed] [Google Scholar]
- 119.Yeh Y., Feeney R.E. Antifreeze proteins: Structures and mechanisms of function. Chem. Rev. 1996;96:601–618. doi: 10.1021/cr950260c. [DOI] [PubMed] [Google Scholar]
- 120.Antson A.A., Smith D.J., Roper D.I., Lewis S., Caves L.S., Verma C.S., Buckley S.L., Lillford P.J., Hubbard R.E. Understanding the mechanism of ice binding by type III antifreeze proteins. J. Mol. Biol. 2001;305:875–889. doi: 10.1006/jmbi.2000.4336. [DOI] [PubMed] [Google Scholar]
- 121.Hew C.L., Wang N.C., Joshi S., Fletcher G.L., Scott G.K., Hayes P.H., Buettner B., Davies P.L. Multiple genes provide the basis for antifreeze protein diversity and dosage in the ocean pout, Macrozoarces americanus. J. Biol. Chem. 1988;263:12049–12055. [PubMed] [Google Scholar]
- 122.Nishimiya Y., Sato R., Takamichi M., Miura A., Tsuda S. Co-operative effect of the isoforms of type III antifreeze protein expressed in Notched-fin eelpout, Zoarces elongatus Kner. FEBS J. 2005;272:482–492. doi: 10.1111/j.1742-4658.2004.04490.x. [DOI] [PubMed] [Google Scholar]
- 123.DeLuca C.I., Chao H., Sönnichsen F.D., Sykes B.D., Davies P.L. Effect of type III antifreeze protein dilution and mutation on the growth inhibition of ice. Biophys. J. 1996;71:2346–2355. doi: 10.1016/S0006-3495(96)79476-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Baardsnes J., Davies P.L. Contribution of hydrophobic residues to ice binding by fish type III antifreeze protein. Biochim. Biophys. Acta. 2002;1601:49–54. doi: 10.1016/S1570-9639(02)00431-4. [DOI] [PubMed] [Google Scholar]
- 125.Chao H., Sönnichsen F.D., DeLuca C.I., Sykes B.D., Davies P.L. Structure-function relationship in the globular type III antifreeze protein: Identification of a cluster of surface residues required for binding to ice. Protein Sci. 1994;3:1760–1769. doi: 10.1002/pro.5560031016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Jia Z., DeLuca C.I., Chao H., Davies P.L. Structural basis for the binding of a globular antifreeze protein to ice. Nature. 1996;384:285–288. doi: 10.1038/384285a0. [DOI] [PubMed] [Google Scholar]
- 127.Sönnichsen F.D., DeLuca C.I., Davies P.L., Sykes B.D., Sönnichsen F.D., DeLuca C.I., Davies P.L., Sykes B.D. Refined solution structure of type III antifreeze protein: Hydrophobic groups may be involved in the energetics of the protein–ice interaction. Structure. 1996;4:1325–1337. doi: 10.1016/S0969-2126(96)00140-2. [DOI] [PubMed] [Google Scholar]
- 128.Leiter A., Rau S., Winger S., Muhle-Goll C., Luy B., Gaukel V. Influence of heating temperature, pressure and pH on recrystallization inhibition activity of antifreeze protein type III. J. Food Eng. 2016;187:53–61. doi: 10.1016/j.jfoodeng.2016.04.019. [DOI] [Google Scholar]
- 129.Hamada T., Ito Y., Abe T., Hayashi F., Guntert P., Inoue M., Kigawa T., Terada T., Shirouzu M., Yoshida M., et al. Solution structure of the antifreeze-like domain of human sialic acid synthase. Protein Sci. 2006;15:1010–1016. doi: 10.1110/ps.051700406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Choi Y.-G., Park C.-J., Kim H.-E., Seo Y.-J., Lee A.-R., Choi S.-R., Lee S.S., Lee J.-H. Comparison of backbone dynamics of the type III antifreeze protein and antifreeze-like domain of human sialic acid synthase. J. Biomol. NMR. 2015;61:137–150. doi: 10.1007/s10858-014-9895-2. [DOI] [PubMed] [Google Scholar]
- 131.DeLuca C.I., Davies P.L., Ye Q., Jia Z. The effects of steric mutations on the structure of type III antifreeze protein and its interaction with ice. J. Mol. Biol. 1998;275:515–525. doi: 10.1006/jmbi.1997.1482. [DOI] [PubMed] [Google Scholar]
- 132.Graether S.P., DeLuca C.I., Baardsnes J., Hill G.A., Davies P.L., Jia Z. Quantitative and qualitative analysis of type III antifreeze protein structure and function. J. Biol. Chem. 1999;274:11842–11847. doi: 10.1074/jbc.274.17.11842. [DOI] [PubMed] [Google Scholar]
- 133.Chen G., Jia Z. Ice-binding surface of fish type III antifreeze. Biophys. J. 1999;77:1602–1608. doi: 10.1016/S0006-3495(99)77008-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Garnham C.P., Natarajan A., Middleton A.J., Kuiper M.J., Braslavsky I., Davies P.L. Compound ice-binding site of an antifreeze protein revealed by mutagenesis and fluorescent tagging. Biochemistry. 2010;49:9063–9071. doi: 10.1021/bi100516e. [DOI] [PubMed] [Google Scholar]
- 135.Garnham C.P., Nishimiya Y., Tsuda S., Davies P.L. Engineering a naturally inactive isoform of type III antifreeze protein into one that can stop the growth of ice. FEBS Lett. 2012;586:3876–3881. doi: 10.1016/j.febslet.2012.09.017. [DOI] [PubMed] [Google Scholar]
- 136.Choi S., Seo Y., Kim M., Eo Y., Ahn H., Lee A., Park C., Ryu K., Cheong H., Lee S.S. NMR study of the antifreeze activities of active and inactive isoforms of a type III antifreeze protein. FEBS Lett. 2016;590:4202–4212. doi: 10.1002/1873-3468.12451. [DOI] [PubMed] [Google Scholar]
- 137.Wang X., DeVries A.L., Cheng C.H. Antifreeze peptide heterogeneity in an antarctic eel pout includes an unusually large major variant comprised of two 7 kDa type III AFPs linked in tandem. Biochim. Biophys. Acta. 1995;1247:163–172. doi: 10.1016/0167-4838(94)00205-U. [DOI] [PubMed] [Google Scholar]
- 138.Miura K., Ohgiya S., Hoshino T., Nemoto N., Suetake T., Miura A., Spyracopoulos L., Kondo H., Tsuda S. NMR analysis of type III antifreeze protein intramolecular dimer. Structural basis for enhanced activity. J. Biol. Chem. 2001;276:1304–1310. doi: 10.1074/jbc.M007902200. [DOI] [PubMed] [Google Scholar]
- 139.Miura K., Ohgiya S., Hoshino T., Nemoto N., Odaira M., Nitta K., Tsuda S. Determination of the solution structure of the N-domain plus linker of Antarctic eel pout antifreeze protein RD3. J. Biochem. 1999;126:387–394. doi: 10.1093/oxfordjournals.jbchem.a022462. [DOI] [PubMed] [Google Scholar]
- 140.Raymond J.A., Christner B.C., Schuster S.C. A bacterial ice-binding protein from the Vostok ice core. Extremophiles. 2008;12:713–717. doi: 10.1007/s00792-008-0178-2. [DOI] [PubMed] [Google Scholar]
- 141.Xiao N., Suzuki K., Nishimiya Y., Kondo H., Miura A., Tsuda S., Hoshino T. Comparison of functional properties of two fungal antifreeze proteins from Antarctomyces psychrotrophicus and Typhula ishikariensis. FEBS J. 2010;277:394–403. doi: 10.1111/j.1742-4658.2009.07490.x. [DOI] [PubMed] [Google Scholar]
- 142.Lee J.H., Lee S.G., Do H., Park J.C., Kim E., Choe Y.H., Han S.J., Kim H.J. Optimization of the pilot-scale production of an ice-binding protein by fed-batch culture of Pichia pastoris. Appl. Microbiol. Biotechnol. 2013;97:3383–3393. doi: 10.1007/s00253-012-4594-y. [DOI] [PubMed] [Google Scholar]
- 143.Koh H.Y., Lee J.H., Han S.J., Park H., Lee S.G. Effect of the antifreeze protein from the Arctic yeast Leucosporidium sp. AY30 on cryopreservation of the marine diatom Phaeodactylum tricornutum. Appl. Biochem. Biotechnol. 2015;175:677–686. doi: 10.1007/s12010-014-1337-9. [DOI] [PubMed] [Google Scholar]
- 144.Lee S.G., Koh H.Y., Lee J.H., Kang S.H., Kim H.J. Cryopreservative effects of the recombinant ice-binding protein from the arctic yeast Leucosporidium sp. on red blood cells. Appl. Biochem. Biotechnol. 2012;167:824–834. doi: 10.1007/s12010-012-9739-z. [DOI] [PubMed] [Google Scholar]
- 145.Kim H.J., Shim H.E., Lee J.H., Kang Y.-C., Hur Y.B. Ice-binding protein derived from Glaciozyma can improve the viability of cryopreserved mammalian cells. J. Microbiol. Biotechnol. 2015;25:1989–1996. doi: 10.4014/jmb.1507.07041. [DOI] [PubMed] [Google Scholar]
- 146.Kondo H., Hanada Y., Sugimoto H., Hoshino T., Garnham C.P., Davies P.L., Tsuda S. Ice-binding site of snow mold fungus antifreeze protein deviates from structural regularity and high conservation. Proc. Natl. Acad. Sci. USA. 2012;109:9360–9365. doi: 10.1073/pnas.1121607109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Hashim N.H.F., Sulaiman S., Bakar F.D.A., Illias R.M., Kawahara H., Najimudin N., Mahadi N.M., Murad A.M.A. Molecular cloning, expression and characterisation of Afp4, an antifreeze protein from Glaciozyma antarctica. Polar Biol. 2014;37:1495–1505. doi: 10.1007/s00300-014-1539-1. [DOI] [Google Scholar]
- 148.Bayer-Giraldi M., Uhlig C., John U., Mock T., Valentin K., Bayer-Giraldi M., Uhlig C., John U., Mock T., Valentin K. Antifreeze proteins in polar sea ice diatoms: Diversity and gene expression in the genus Fragilariopsis. Environ. Microbiol. 2010;12:1041–1052. doi: 10.1111/j.1462-2920.2009.02149.x. [DOI] [PubMed] [Google Scholar]
- 149.Gilbert J.A., Hill P.J., Dodd C.E., Laybourn-Parry J. Demonstration of antifreeze protein activity in Antarctic lake bacteria. Microbiology. 2004;150:171–180. doi: 10.1099/mic.0.26610-0. [DOI] [PubMed] [Google Scholar]
- 150.Garnham C.P., Gilbert J.A., Hartman C.P., Campbell R.L., Laybourn-Parry J., Davies P.L. A Ca2+-dependent bacterial antifreeze protein domain has a novel beta-helical ice-binding fold. Biochem. J. 2008;411:171–180. doi: 10.1042/BJ20071372. [DOI] [PubMed] [Google Scholar]
- 151.Raymond J.A., Kim H.J. Possible role of horizontal gene transfer in the colonization of sea ice by algae. PLoS ONE. 2012;7:e35968. doi: 10.1371/journal.pone.0035968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Gogarten J.P., Doolittle W.F., Lawrence J.G. Prokaryotic evolution in light of gene transfer. Mol. Biol. Evol. 2002;19:2226–2238. doi: 10.1093/oxfordjournals.molbev.a004046. [DOI] [PubMed] [Google Scholar]
- 153.Anesio A.M., Mindl B., Laybourn-Parry J., Hodson A.J., Sattler B. Viral dynamics in cryoconite holes on a high Arctic glacier (Svalbard) J. Geophys. Res. Biogeosci. 2007;112:G04S31. doi: 10.1029/2006JG000350. [DOI] [Google Scholar]
- 154.Pucciarelli S., Chiappori F., Devaraj R.R., Yang G., Yu T., Ballarini P., Miceli C. Identification and analysis of two sequences encoding ice-binding proteins obtained from a putative bacterial symbiont of the psychrophilic Antarctic ciliate Euplotes focardii. Antarct. Sci. 2014;26:491–501. doi: 10.1017/S0954102014000017. [DOI] [Google Scholar]
- 155.Mangiagalli M., Bar-Dolev M., Tedesco P., Natalello A., Kaleda A., Brocca S., Pascale D., Pucciarelli S., Miceli C., Bravslavsky I. Cryo-protective effect of an ice-binding protein derived from Antarctic bacteria. FEBS J. 2016 doi: 10.1111/febs.13965. [DOI] [PubMed] [Google Scholar]
- 156.Mazur P. Freezing of living cells: Mechanisms and implications. Am. J. Physiol. 1984;247:C125–C142. doi: 10.1152/ajpcell.1984.247.3.C125. [DOI] [PubMed] [Google Scholar]
- 157.Naaldijk Y., Staude M., Fedorova V., Stolzing A. Effect of different freezing rates during cryopreservation of rat mesenchymal stem cells using combinations of hydroxyethyl starch and dimethylsulfoxide. BMC Biotechnol. 2012;12:49. doi: 10.1186/1472-6750-12-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Fowler A., Toner M. Cryo-injury and biopreservation. Ann. N. Y. Acad. Sci. 2006;1066:119–135. doi: 10.1196/annals.1363.010. [DOI] [PubMed] [Google Scholar]
- 159.Chaytor J.L., Tokarew J.M., Wu L.K., Leclre M., Tam R.Y., Capicciotti C.J., Guolla L., Von Moos E., Findlay C.S., Allan D.S., et al. Inhibiting ice recrystallization and optimization of cell viability after cryopreservation. Glycobiology. 2012;22:123–133. doi: 10.1093/glycob/cwr115. [DOI] [PubMed] [Google Scholar]
- 160.Fahy G.M., MacFarlane D.R., Angell C.A., Meryman H.T. Vitrification as an approach to cryopreservation. Cryobiology. 1984;21:407–426. doi: 10.1016/0011-2240(84)90079-8. [DOI] [PubMed] [Google Scholar]
- 161.Wowk B., Leitl E., Rasch C.M., Mesbah-Karimi N., Harris S.B., Fahy G.M. Vitrification enhancement by synthetic ice blocking agents. Cryobiology. 2000;40:228–236. doi: 10.1006/cryo.2000.2243. [DOI] [PubMed] [Google Scholar]
- 162.Mugnano J.A., Wang T., Layne J.R., Jr., DeVries A.L., Lee R.E., Jr. Antifreeze glycoproteins promote intracellular freezing of rat cardiomyocytes at high subzero temperatures. Am. J. Physiol. 1995;269:R474–R479. doi: 10.1152/ajpregu.1995.269.2.R474. [DOI] [PubMed] [Google Scholar]
- 163.Hansen T.N., Smith K.M., Brockbank K.G. Type I antifreeze protein attenuates cell recoveries following cryopreservation. Transpl. Proc. 1993;25:3182–3184. [PubMed] [Google Scholar]
- 164.Wang T., Zhu Q., Yang X., Layne J.R., Jr., Devries A.L. Antifreeze glycoproteins from antarctic notothenioid fishes fail to protect the rat cardiac explant during hypothermic and freezing preservation. Cryobiology. 1994;31:185–192. doi: 10.1006/cryo.1994.1022. [DOI] [PubMed] [Google Scholar]
- 165.Zilli L., Beirão J., Schiavone R., Herraez M.P., Gnoni A., Vilella S. Comparative proteome analysis of cryopreserved flagella and head plasma membrane proteins from sea bream spermatozoa: Effect of antifreeze proteins. PLoS ONE. 2014;9:e99992. doi: 10.1371/journal.pone.0099992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Robles V., Cabrita E., Anel L., Herraez M.P. Microinjection of the antifreeze protein type III (AFPIII) in turbot (Scophthalmus maximus) embryos: Toxicity and protein distribution. Aquaculture. 2006;261:1299–1306. doi: 10.1016/j.aquaculture.2006.07.047. [DOI] [Google Scholar]
- 167.Beirão J., Zilli L., Vilella S., Cabrita E., Schiavone R., Herraez M.P. Improving sperm cryopreservation with antifreeze proteins: Effect on gilthead seabream (Sparus aurata) plasma membrane lipids. Biol. Reprod. 2012;86:59. doi: 10.1095/biolreprod.111.093401. [DOI] [PubMed] [Google Scholar]
- 168.Prathalingam N.S., Holt W.V., Revell S.G., Mirczuk S., Fleck R.A., Watson P.F. Impact of antifreeze proteins and antifreeze glycoproteins on bovine sperm during freeze-thaw. Theriogenology. 2006;66:1894–1900. doi: 10.1016/j.theriogenology.2006.04.041. [DOI] [PubMed] [Google Scholar]
- 169.Kim J.S., Yoon J.H., Park G.H., Bae S.H., Kim H.J., Kim M.S., Hwang Y.J., Kim D.Y. Influence of antifreeze proteins on boar sperm DNA damaging during cryopreservation. Dev. Biol. 2011;356:195. doi: 10.1016/j.ydbio.2011.05.260. [DOI] [Google Scholar]
- 170.Qadeer S., Khan M.A., Ansari M.S., Rakha B.A., Ejaz R., Iqbal R., Younis M., Ullah N., DeVries A.L., Akhter S. Efficiency of antifreeze glycoproteins for cryopreservation of Nili-Ravi (Bubalus bubalis) buffalo bull sperm. Anim. Reprod. Sci. 2015;157:56–62. doi: 10.1016/j.anireprosci.2015.03.015. [DOI] [PubMed] [Google Scholar]
- 171.Qadeer S., Khan M.A., Ansari M.S., Rakha B.A., Ejaz R., Husna A.U., Ashiq M., Iqbal R., Ullah N., Akhter S. Evaluation of antifreeze protein III for cryopreservation of Nili-Ravi (Bubalus bubalis) buffalo bull sperm. Anim. Reprod. Sci. 2014;148:26–31. doi: 10.1016/j.anireprosci.2014.04.013. [DOI] [PubMed] [Google Scholar]
- 172.Ideta A., Aoyagi Y., Tsuchiya K., Nakamura Y., Hayama K., Shirasawa A., Sakaguchi K., Tominaga N., Nishimiya Y., Tsuda S. Prolonging hypothermic storage (4 °C) of bovine embryos with fish antifreeze protein. J. Reprod. Dev. 2015;61:1–6. doi: 10.1262/jrd.2014-073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Hirano Y., Nishimiya Y., Matsumoto S., Matsushita M., Todo S., Miura A., Komatsu Y., Tsuda S. Hypothermic preservation effect on mammalian cells of type III antifreeze proteins from notched-fin eelpout. Cryobiology. 2008;57:46–51. doi: 10.1016/j.cryobiol.2008.05.006. [DOI] [PubMed] [Google Scholar]
- 174.Kamijima T., Sakashita M., Miura A., Nishimiya Y., Tsuda S. Antifreeze protein prolongs the life-time of insulinoma cells during hypothermic preservation. PLoS ONE. 2013;8:e73643. doi: 10.1371/journal.pone.0073643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Baguisi A., Arav A., Crosby T.F., Roche J.F., Boland M.P. Hypothermic storage of sheep embryos with antifreeze proteins: Development in vitro and in vivo. Theriogenology. 1997;48:1017–1024. doi: 10.1016/S0093-691X(97)00328-2. [DOI] [PubMed] [Google Scholar]
- 176.Nishijima K., Tanaka M., Sakai Y., Koshimoto C., Morimoto M., Watanabe T., Fan J., Kitajima S. Effects of type III antifreeze protein on sperm and embryo cryopreservation in rabbit. Cryobiology. 2014;69:22–25. doi: 10.1016/j.cryobiol.2014.04.014. [DOI] [PubMed] [Google Scholar]
- 177.Jo J.W., Jee B.C., Lee J.R., Suh C.S. Effect of antifreeze protein supplementation in vitrification medium on mouse oocyte developmental competence. Fertil. Steril. 2011;96:1239–1245. doi: 10.1016/j.fertnstert.2011.08.023. [DOI] [PubMed] [Google Scholar]
- 178.Karanova M.V., Pronina N.D., Tsvetkova L.I. The effect of antifreeze glycoproteins on survival and quality of fish spermatozoa under the conditions of long-term storage at +4 degree C. Izv. Akad. Nauk. Ser. Biol. 2002;1:88–92. [PubMed] [Google Scholar]
- 179.Halwani D.O., Brockbank K.G., Duman J.G., Campbell L.H. Recombinant Dendroides canadensis antifreeze proteins as potential ingredients in cryopreservation solutions. Cryobiology. 2014;68:411–418. doi: 10.1016/j.cryobiol.2014.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Karanova M.V., Mezhevikina L.M., Petropavlov N.N. Study of cryoprotective properties of antifreeze glycoproteins from the white sea cod Gadus morhua on low temperature freezing of mouse embryos. Biofizika. 1994;40:1341–1347. [PubMed] [Google Scholar]
- 181.Wen Y., Zhao S., Chao L., Yu H., Song C., Shen Y., Chen H., Deng X. The protective role of antifreeze protein 3 on the structure and function of mature mouse oocytes in vitrification. Cryobiology. 2014;69:394–401. doi: 10.1016/j.cryobiol.2014.09.006. [DOI] [PubMed] [Google Scholar]
- 182.Rubinsky L., Raichman N., Lavee J., Frenk H., Ben-Jacob E., Bickler P.E. Antifreeze protein suppresses spontaneous neural activity and protects neurons from hypothermia/re-warming injury. Neurosci. Res. 2010;67:256–259. doi: 10.1016/j.neures.2010.04.004. [DOI] [PubMed] [Google Scholar]
- 183.Rubinsky B., Arav A., Devries A.L. The cryoprotective effect of antifreeze glycopeptides from antarctic fishes. Cryobiology. 1992;29:69–79. doi: 10.1016/0011-2240(92)90006-N. [DOI] [PubMed] [Google Scholar]
- 184.Amir G., Rubinsky B., Smolinsky A.K., Lavee J. Successful use of ocean pout thermal hysteresis protein (antifreeze protein III) in cryopreservation of transplanted mammalian heart at subzero temperature. J. Hear Lung Transplant. 2002;21:137. doi: 10.1016/S1053-2498(01)00662-3. [DOI] [Google Scholar]
- 185.Jo J.W., Jee B.C., Suh C.S., Kim S.H. The Beneficial Effects of antifreeze proteins in the vitrification of immature mouse oocytes. PLoS ONE. 2012;7:e37043. doi: 10.1371/journal.pone.0037043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Martínez-Páramo S., Pérez-Cerezales S., Robles V., Anel L., Herraez M.P. Incorporation of antifreeze proteins into zebrafish embryos by a non-invasive method. Cryobiology. 2008;56:216–222. doi: 10.1016/j.cryobiol.2008.03.003. [DOI] [PubMed] [Google Scholar]
- 187.Martínez-Páramo S., Barbosa V., Pérez-Cerezales S., Robles V., Herraez M.P. Cryoprotective effects of antifreeze proteins delivered into zebrafish embryos. Cryobiology. 2009;58:128–133. doi: 10.1016/j.cryobiol.2008.11.013. [DOI] [PubMed] [Google Scholar]
- 188.Tursman D., Duman J.G. Cryoprotective effects of thermal hysteresis protein on survivorship of frozen gut cells from the freeze-tolerant centipede Lithobius forficatus. J. Exp. Zool. 1995;272:249–257. doi: 10.1002/jez.1402720402. [DOI] [Google Scholar]
- 189.Amir G., Rubinsky B., Horowitz L., Miller L., Leor J., Kassif Y., Mishaly D., Smolinsky A.K., Lavee J. Prolonged 24-hour subzero preservation of heterotopically transplanted rat hearts using antifreeze proteins derived from arctic fish. Ann. Thorac. Surg. 2004;77:1648–1655. doi: 10.1016/j.athoracsur.2003.04.004. [DOI] [PubMed] [Google Scholar]
- 190.O’Neil L., Paynter S.J., Fuller B.J., Shaw R.W., DeVries A.L. Vitrification of mature mouse oocytes in a 6 M Me2SO solution supplemented with antifreeze glycoproteins: The effect of temperature. Cryobiology. 1998;37:59–66. doi: 10.1006/cryo.1998.2098. [DOI] [PubMed] [Google Scholar]
- 191.Shaw J.M., Ward C., Trounson A.O. Survival of mouse blastocysts slow cooled in propanediol or ethylene glycol is influenced by the thawing procedure, sucrose and antifreeze proteins. Theriogenology. 1995;43:1289–1300. doi: 10.1016/0093-691X(95)00114-N. [DOI] [Google Scholar]
- 192.Koshimoto C., Mazur P. Effects of warming rate, temperature, and antifreeze proteins on the survival of mouse spermatozoa frozen at an optimal rate. Cryobiology. 2002;45:49–59. doi: 10.1016/S0011-2240(02)00105-0. [DOI] [PubMed] [Google Scholar]
- 193.Matsumoto S., Matsusita M., Morita T., Kamachi H., Tsukiyama S., Furukawa Y., Koshida S., Tachibana Y., Nishimura S., Todo S. Effects of synthetic antifreeze glycoprotein analogue on islet cell survival and function during cryopreservation. Cryobiology. 2006;52:90–98. doi: 10.1016/j.cryobiol.2005.10.010. [DOI] [PubMed] [Google Scholar]
- 194.Arav A., Rubinsky B., Fletcher G., Seren E. Cryogenic protection of oocytes with antifreeze proteins. Mol. Reprod. Dev. 1993;36:488–493. doi: 10.1002/mrd.1080360413. [DOI] [PubMed] [Google Scholar]
- 195.Younis A.I., Rooks B., Khan S., Gould K.G. The effects of antifreeze peptide III (AFP) and insulin transferrin selenium (ITS) on cryopreservation of chimpanzee (Pan troglodytes) spermatozoa. J. Androl. 1998;19:207–214. [PubMed] [Google Scholar]
- 196.Robles V., Barbosa V., Herraez M.P., Martinez-Paramo S., Cancela M.L. The antifreeze protein type I (AFP I) increases seabream (Sparus aurata) embryos tolerance to low temperatures. Theriogenology. 2007;68:284–289. doi: 10.1016/j.theriogenology.2007.05.003. [DOI] [PubMed] [Google Scholar]
- 197.Chao H., Davies P.L., Carpenter J.F. Effects of antifreeze proteins on red blood cell survival during cryopreservation. J. Exp. Biol. 1996;199:2071–2076. doi: 10.1242/jeb.199.9.2071. [DOI] [PubMed] [Google Scholar]
- 198.Chaves D.F., Campelo I.S., Silva M.M.A.S., Bhat M.H., Teixeira D.I.A., Melo L.M., Souza-Fabjan J.M.G., Mermillod P., Freitas V.J.F. The use of antifreeze protein type III for vitrification of in vitro matured bovine oocytes. Cryobiology. 2016;73:324–328. doi: 10.1016/j.cryobiol.2016.10.003. [DOI] [PubMed] [Google Scholar]
- 199.Bouvet V.R., Lorello G.R., Ben R.N. Aggregation of antifreeze glycoprotein fraction 8 and its effect on antifreeze activity. Biomacromolecules. 2006;7:565–571. doi: 10.1021/bm050605t. [DOI] [PubMed] [Google Scholar]
- 200.Qadeer S., Khan M.A., Shahzad Q., Azam A., Ansari M.S., Rakha B.A., Ejaz R., Husna A.U., Duman J.G., Akhter S. Efficiency of beetle (Dendroides canadensis) recombinant antifreeze protein for buffalo semen freezability and fertility. Theriogenology. 2016;86:1662–1669. doi: 10.1016/j.theriogenology.2016.05.028. [DOI] [PubMed] [Google Scholar]
- 201.Liu S., Wang W., Moos E., Jackman J., Mealing G., Monette R., Ben R.N. In vitro studies of antifreeze glycoprotein (AFGP) and a C-linked AFGP analogue. Biomacromolecules. 2007;8:1456–1462. doi: 10.1021/bm061044o. [DOI] [PubMed] [Google Scholar]
- 202.Martinez-Paramo S., Perez-Cerezales S., Barbosa V., Robles V., Herraez M.P. Advances on fish embryo cryopreservation using antifreeze proteins. Biol. Reprod. 2008;78:152. [Google Scholar]
- 203.Ideta A., Aoyagi Y., Tsuchiya K., Kamijima T., Nishimiya Y., Tsuda S. A simple medium enables bovine embryos to be held for seven days at 4 °C. Sci. Rep. 2013;3:1173. doi: 10.1038/srep01173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Lee C.Y., Rubinsky B., Fletcher G.L. Hypothermic preservation of whole mammalian organs with antifreeze proteins. Cryo-Letters. 1992;13:59–66. [Google Scholar]
- 205.Amir G., Horowitz L., Rubinsky B., Yousif B.S., Lavee J., Smolinsky A.K. Subzero nonfreezing cryopresevation of rat hearts using antifreeze protein I and antifreeze protein III. Cryobiology. 2004;48:273–282. doi: 10.1016/j.cryobiol.2004.02.009. [DOI] [PubMed] [Google Scholar]
- 206.Rubinsky B., Arav A., Hong J.S., Lee C.Y. Freezing of mammalian livers with glycerol and antifreeze proteins. Biochem. Biophys. Res. Commun. 1994;200:732–741. doi: 10.1006/bbrc.1994.1512. [DOI] [PubMed] [Google Scholar]
- 207.Davies P.L., Sykes B.D. Antifreeze proteins. Curr. Opin. Struct. Biol. 1997;7:828–834. doi: 10.1016/S0959-440X(97)80154-6. [DOI] [PubMed] [Google Scholar]
- 208.Arakawa T., Kita Y., Timasheff S.N. Protein precipitation and denaturation by dimethyl sulfoxide. Biophys. Chem. 2007;131:62–70. doi: 10.1016/j.bpc.2007.09.004. [DOI] [PubMed] [Google Scholar]
- 209.Wang C., Oliver E.E., Christner B.C., Luo B.-H. Functional Analysis of a bacterial antifreeze protein indicates a cooperative effect between its two ice-binding domains. Biochemistry. 2016;55:3975–3983. doi: 10.1021/acs.biochem.6b00323. [DOI] [PubMed] [Google Scholar]
- 210.Nishimiya Y., Ohgiya S., Tsuda S. Artificial multimers of the type III antifreeze protein. Effects on thermal hysteresis and ice crystal morphology. J. Biol. Chem. 2003;278:32307–32312. doi: 10.1074/jbc.M304390200. [DOI] [PubMed] [Google Scholar]
- 211.Baardsnes J., Kuiper M.J., Davies P.L. Antifreeze protein dimer: When two ice-binding faces are better than one. J. Biol. Chem. 2003;278:38942–38947. doi: 10.1074/jbc.M306776200. [DOI] [PubMed] [Google Scholar]
- 212.Can O., Holland N.B. Utilizing avidity to improve antifreeze protein activity: A type III antifreeze protein trimer exhibits increased thermal hysteresis activity. Biochemistry. 2013;52:8745–8752. doi: 10.1021/bi401345b. [DOI] [PubMed] [Google Scholar]
- 213.Stevens C.A., Drori R., Zalis S., Braslavsky I., Davies P.L. Dendrimer-linked antifreeze proteins have superior activity and thermal recovery. Bioconjug. Chem. 2015;26:1908–1915. doi: 10.1021/acs.bioconjchem.5b00290. [DOI] [PubMed] [Google Scholar]
- 214.Phippen S.W., Stevens C.A., Vance T.D.R., King N.P., Baker D., Davies P.L. Multivalent display of antifreeze proteins by fusion to self-assembling protein cages enhances ice-binding activities. Biochemistry. 2016;55:6811–6820. doi: 10.1021/acs.biochem.6b00864. [DOI] [PubMed] [Google Scholar]
- 215.Bang J.K., Lee J.H., Murugan R.N., Lee S.G., Do H., Koh H.Y., Shim H.E., Kim H.C., Kim H.J. Antifreeze peptides and glycopeptides, and their derivatives: Potential uses in biotechnology. Mar. Drugs. 2013;11:2013–2041. doi: 10.3390/md11062013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Balcerzak A.K., Capicciotti C.J., Briard J.G., Ben R.N. Designing ice recrystallization inhibitors: From antifreeze (glyco) proteins to small molecules. RSC Adv. 2014;4:42682–42696. doi: 10.1039/C4RA06893A. [DOI] [Google Scholar]
- 217.Liu S., Ben R.N. C-linked galactosyl serine AFGP analogues as potent recrystallization inhibitors. Org. Lett. 2005;7:2385–2388. doi: 10.1021/ol050677x. [DOI] [PubMed] [Google Scholar]
- 218.Garner J., Harding M.M. Design and synthesis of antifreeze glycoproteins and mimics. Chembiochem. 2010;11:2489–2498. doi: 10.1002/cbic.201000509. [DOI] [PubMed] [Google Scholar]
- 219.Hachisu M., Hinou H., Takamichi M., Tsuda S., Koshida S., Nishimura S. One-pot synthesis of cyclic antifreeze glycopeptides. Chem. Commun. 2009 doi: 10.1039/b815917c. [DOI] [PubMed] [Google Scholar]
- 220.Garner J., Harding M.M. Design and synthesis of alpha-helical peptides and mimetics. Org. Biomol. Chem. 2007;5:3577–3585. doi: 10.1039/b710425a. [DOI] [PubMed] [Google Scholar]
- 221.Can O., Holland N.B. Conjugation of type I antifreeze protein to polyallylamine increases thermal hysteresis activity. Bioconjug. Chem. 2011;22:2166–2171. doi: 10.1021/bc2004318. [DOI] [PubMed] [Google Scholar]