Abstract
Demosponges of the order Verongida such as Ianthella basta exhibit skeletons containing spongin, a collagenous protein, and chitin. Moreover, Verongida sponges are well known to produce bioactive brominated tyrosine derivatives. We recently demonstrated that brominated compounds do not only occur in the cellular matrix but also in the skeletons of the marine sponges Aplysina cavernicola and I. basta. Further investigations revealed the amino acid composition of the skeletons of A. cavernicola including the presence of several halogenated amino acids. In the present work, we investigated the skeletal amino acid composition of the demosponge I. basta, which belongs to the Ianthellidae family, and compared it with that of A. cavernicola from the Aplysinidae family. Seventeen proteinogenic and five non-proteinogenic amino acids were detected in I. basta. Abundantly occurring amino acids like glycine and hydroxyproline show the similarity of I. basta and A. cavernicola and confirm the collagenous nature of their sponging fibers. We also detected nine halogenated tyrosines as an integral part of I. basta skeletons. Since both sponges contain a broad variety of halogenated amino acids, this seems to be characteristic for Verongida sponges. The observed differences of the amino acid composition confirm that spongin exhibits a certain degree of variability even among the members of the order Verongida.
Keywords: demosponges, Ianthella basta, skeletons, spongin, amino acid composition, halogenated amino acids, GC-MS
1. Introduction
The oceans represent the biggest habitat on earth, which is however, not yet fully explored. Consequently, the marine habitat is one of increasing interest for the research and further exploration of this understudied environment. For example, a huge diversity of bioactive natural products is found in marine organisms. To date, approximately 28,000 of these marine natural products have been discovered, and sponges are the main producers of such compounds [1,2].
Sponges (Porifera), representing one of the oldest metazoans, are often a prominent component of benthic communities [3]. They can be found in polar, temperate and tropical regions [4,5]. Sponges are sessile filterfeeders which cannot escape or actively fight predators [6,7]. Therefore, they developed morphological and chemical defense mechanisms, providing them with protection against various predators [8,9,10,11,12,13].
Based on their skeletal composition, sponges can be divided into three classes: calcareous sponges (Calcarea), glass sponges (Hexactinellida), and demosponges (Demospongia) [14]. Recently, a fourth class, the Homoscleromorpha, was phylogenetically defined [15,16].
Demospongiae represent the largest class of sponges [17]. Their skeletons are made from proteins [18], polysaccharides [19,20], and/or inorganic components such as siliceous spicules [14,21]. Demosponges are subdivided into three orders: Dendroceratida, Dictyoceratida, and Verongida. The skeletons of all of these orders exhibit spongin, a collagenous protein [22].
Till now, spongin is not really well defined. Nevertheless, it has remarkable properties. Spongin is considered to be a halogenated scleroprotein [22] of collagenous character. Spongin fibers are more resistant than collagen fibers against enzymatic digestion [23]. Exposito et al. showed in genomic and cDNA studies that spongin possesses the typical collagen sequence motif Gly-Xaa-Yaa [24]. Glycine is thus the most abundant amino acid found in spongin. Apart from glycine, the amino acids serine, arginine, lysine, valine and cysteine are reported to occur in spongin [25]. Additionally, Ehrlich et al. showed that the polysaccharide chitin appears as an integral skeleton component in Verongida sponge skeletons [19,20,26], forming a chitin scaffold of the same overall morphology as the integer skeleton.
It is not yet clear how spongin is connected with these chitin scaffolds in sponge skeletons. However, there are a number of examples of so-called chitin-protein complexes in nature showing that proteins are often strongly—mostly covalently—bound to chitin [27,28,29]. Hackman suggested that proteins are covalently bound to chitin in different insects and crustaceans [27]. Furthermore, Blackwell and Weih developed a model for the three-dimensional structure of an insect chitin-protein complex with chitin fibrils surrounded by layers of proteins [28]. These examples lead to the assumption that spongin in the skeletons of the Verongida sponges is also strongly—maybe covalently—bound to the chitin scaffolds.
Furthermore, sponges of the order Verongida are well-known for the biosynthesis of characteristic bioactive natural products, especially brominated tyrosine derivatives like bastadins from Ianthella basta (I. basta) [30,31,32,33], aerothionin from Aplysina cavernicola (A. cavernicola) [34], or psammaplins from Aplysinella sp. [11]. These compounds are biosynthesized from brominated tyrosines [35]. Initial investigations show that brominated substances are present even in the sponge skeletons [36,37]. Since brominated tyrosines can inhibit chitinase activity [38], these compounds could have a protective effect preventing undesired skeletal degradation.
Until now the full amino acid composition of sponges was only determined for three species. This includes the commercial unbleached sponge Hippospongia equina (H. equina) and the bath sponge Spongia officinalis obliqua (S. officinalis obliqua). Both of the sponges belong to the order Dictyoceratida [39,40]. Furthermore, we have recently determined the skeletal amino acid composition of the Verongida sponge A. cavernicola [41]. These studies revealed the presence of halogenated tyrosines in addition to non-halogenated amino acids. 3,5-Diiodotyrosine was found in H. equina [39], while 3-Monoiodo-tyrosine, 3,5-Diiodotyrosine, and 3,5-Dibromotyrosine could be detected in S. officinalis obliqua [40]. Compared to these two Dictyoceratida sponges, the Verongida sponge A. cavernicola exhibits a surprising variety of halogenated, i.e., brominated, iodated, and chlorinated amino acids [41].
The goal of the present study is to examine the amino acid composition of the sponge skeleton of another Verongida sponge and to compare it with the other three known amino acid compositions of sponges. The results should reveal whether or not there is a significant difference between the Verongida sponges from two different families. This may encourage future investigations of further sponge species.
The order Verongida comprises four families distinguished mainly by the structure and composition of their spongin fibers [42,43]. Aplysinidae is the largest verongid family (63 species from three genera: Aplysina, Verongula, and Aiolochroia). This family is defined by an anastomosing fiber skeleton with both pith and bark elements [18]. Ianthellidae is the second largest verongid family (12 species from three genera: Ianthella, Anomoianthella, and Hexadella). This family is distinguished from other Verongida families by the presence of eurypylous choanocyte chambers. Aplysinellidae consists of nine species from three genera (Aplysinella, Porphyria, and Suberea). It is defined by a dendritic fiber skeleton with both pith and bark elements. Pseudoceratinidae consists of four species from a single genus (Pseudoceratina) and is defined by a dendritic fiber skeleton with only pith elements. We have chosen I. basta as a characteristic representative of the Ianthellidae family, which represents the second largest among the four Verongida sponge families.
Based on previous work on A. cavernicola [41], we optimized the methods for the effective isolation of the sponge skeletons of I. basta and the complete extraction of amino acids from the skeletons with Ba(OH)2. The determined amino acid composition of I. basta was finally compared with the amino acid composition of A. cavernicola skeletons as well as with the amino acid composition of the Dictyoceratida sponges H. equina and S. officinalis obliqua.
2. Results and Discussion
2.1. General Chemical Characterization of the I. basta Skeleton and Comparison with A. cavernicola
Morphological differences between the skeletons of the two Verongida sponges I. basta and A. cavernicola are shown in underwater and light microscopic images (Figure 1a–d). I. basta grows in large fan or funnel shapes (Figure 1a) which is reflected in its planar, netlike skeleton structure (Figure 1b). A. cavernicola grows thick and incrusting with numerous short tubes (Figure 1c), which is supported by its three-dimensional skeleton (Figure 1d).
Figure 1.
Underwater and light microscopic images of the sponges and their isolated skeletons after extraction; (a,b) I. basta; (c,d) A. cavernicola (photos: (a) Peter Schupp, (c) Carsten Thoms).
The SEM images of the isolated I. basta skeletons (Figure 2) reveal a fiber system. These fibers consist of several concentric layers similar to the structural organization previously described for A. cavernicola skeletons (see [44]). The central channel of the fiber is filled with the pith, a porous material.
Figure 2.
Scanning electron microscopy (SEM) images of the skeletons of I. basta.
Table 1 summarizes various chemical parameters from the two sponges. Interestingly, the amount of skeletal material related to the entire sponge is more than one order of magnitude higher in I. basta than in A. cavernicola. An explanation for the higher amount of skeletal material in I. basta might be a need for mechanical stability because of its two-dimensional skeleton structure and the large size of the sponges (more than 2 m maximum diameter) [45].
Table 1.
General chemical characterization of the skeletons of I. basta in comparison to the skeletons of A. cavernicola.
| Parameter | I. basta | A. cavernicola |
|---|---|---|
| percentage of skeleton in the sponge | 50.1 ± 20.0 wt. % | 3.1 ± 1.3 wt. % |
| percentage of chitin in the skeleton | 17.1 ± 1.4 wt. % | 8.0 ± 1.4 wt. % |
| percentage of other saccharides in the skeleton | 3–4 wt. % | 1–2 wt. % |
| content of calcium in the skeleton | 15.5 mg/g | 3.5 mg/g |
| estimated content of calcium carbonate in the skeleton | 3.9 wt. % | 0.9 wt. % |
| content of silicon in the skeleton | <1.7 mg/g | <1.7 mg/g |
| estimated content of protein in the skeleton | ≤77 wt. % | ≤90 wt. % |
| content of sulfur in the skeleton | 14.4 mg/g | 11.8 mg/g |
| halogens present in the skeleton | Br, Cl, I | Br, Cl, I |
| bromine content in the skeleton [44] | 51 ± 4 mg/g | 40 ± 3 mg/g |
The chitin content in I. basta skeletons is also higher than in A. cavernicola. Campbell [46] showed that chitin is responsible for the flexibility and not for the strength of chitin-protein complexes of insects. If this conclusion also applies here, it would lead to the assumption that the sponge skeleton of I. basta has to be more flexible than the skeletons of A. cavernicola. Given that the sponges can be found at depths as shallow as 5 m, where wave exposure certainly affects large sponges, a flexible skeleton might be a prerequisite to successfully colonize and persist on shallow reef slopes.
Furthermore, the percentage of saccharides in the skeletons other than chitin (estimated by the so-called resorcin method [47], see Materials and Methods) is also higher in I. basta, but in general much lower than the amount of chitin.
Ehrlich et al. [48] reported the presence of amorphous silicate and crystalline calcium carbonate in the form of aragonite as skeletal components of the Verongida sponge Verongula giganteum. In order to test this possibility for I. basta, the calcium and silicon contents of the skeletons were determined by ICP-OES. The calcium concentrations are low. Assuming that the calcium in the skeletons occurs as calcium carbonate, an amount of ca. 4% for I. basta and ca. 1% for A. cavernicola can be estimated. The silicon content is below the detection limit. These results show that both sponges, I. basta and A. cavernicola, do not exhibit the silica-aragonite-chitin-biocomposites found in Verongula giganteum.
The content of sulfur is of the same order for the skeletons of both samples. EDX measurements confirm that chlorine and iodine are present in both skeletons in addition to bromine. The bromine content (determined as described previously in [44]) is significantly higher in I. basta skeletons than in A. cavernicola.
Finally, we estimated the protein content of the skeletons as a remaining fraction after the consideration of the other analyses. It is significantly higher in A. cavernicola than in I. basta—mainly due to the higher amount of chitin in I. basta. Since I. basta also exhibits a higher content of bromine, we have to conclude that I. basta skeletons contain a higher overall number of brominated compounds than A. cavernicola. The question arises whether or not these brominated compounds are the same for both Verongida sponges. In order to answer this question, GC-MS analyses were performed.
2.2. Skeletal Amino Acid Composition
2.2.1. GC-MS Analysis of the Skeletal Amino Acid Composition
The isolated skeletons of I. basta were treated with a saturated Ba(OH)2 solution to extract the skeleton-bound amino acids and allow their analysis by GC-MS. The protocol for this extraction was initially developed for the sponge A. cavernicola [41]. It had to be adapted and optimized for the more resistant skeletons of I. basta. In contrast to A. cavernicola, the complete extraction of I. basta skeletons required a significantly higher temperature (100 °C versus 37 °C) because of the stronger hydrolysis resistance of the I. basta spongin fibers. This emphasizes the different characters of the two different sponge skeletons.
The dissolved amino acids were derivatized using MTBSTFA and analyzed as described for A. cavernicola [41]. The derivatization method was optimized such that a maximum number of amino acids with their broad range of structures is completely converted. The same method as for A. cavernicola was then applied for I. basta. Since the analysis methods for both sponges were exactly the same, the results are well comparable.
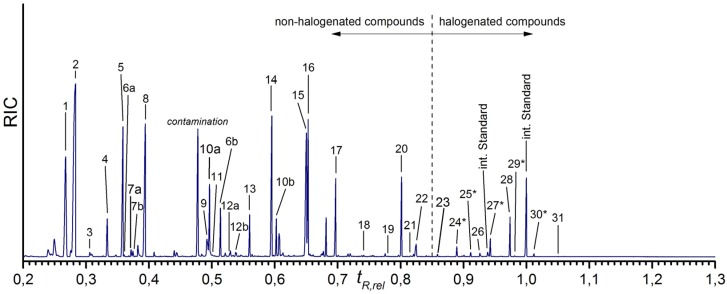
The chromatogram of the GC-MS measurements is shown in Figure 3. The assignment of the peaks is given in Table 2. It was carried out as described in detail in [41], using the information in EI mass spectra and confirmed for most of the peaks by retention times of standard compounds. The structure of a few halogenated amino acids could not be verified with pure standards due to the lack of availability of these reference compounds. For these compounds, the identification was based on their mass spectra and is also described in detail in [41].
Figure 3.
Gas chromatogram of the MTBSTFA-derivatized Ba(OH)2 extract of the isolated I. basta sponge skeletons. The relative retention time is related to the second peak of the internal standard 5-bromotryptophan. Components with * could not be verified with pure standards due to the lack of availability of these reference compounds.
Table 2.
Amino acids detected as TBDMS-derivatives in the Ba(OH)2 extract of isolated I. basta sponge skeletons. Components with * could not be verified with pure standards due to the lack of availability of these reference compounds.
| Peak | Amino Acid | Proteinogenic | Halogenated |
|---|---|---|---|
| 1 | Alanine | X | |
| 2 | Glycine | X | |
| 3 | α-Aminobutyric Acid (AABA) | ||
| 4 | Valine | X | |
| 5 | Leucine | X | |
| 6a | Serine (2 TBDMS) | X | |
| 7a | Isoleucine | X | |
| 7b | Isoleucine | X | |
| 8 | Proline | X | |
| 9 | Oxoproline | ||
| 10a | Hydroxyproline (2 TBDMS) | ||
| 11 | Methionine | X | |
| 6b | Serine (3 TBDMS) | X | |
| 12a | Threonine (3 TBDMS) | X | |
| 12b | Threonine (3 TBDMS) | X | |
| 13 | Phenylalanine | X | |
| 14 | Aspartic Acid | X | |
| 10b | Hydroxyproline (3 TBDMS) | ||
| 15 | Glutamic Acid | X | |
| 16 | Ornithine | ||
| 17 | Lysine | X | |
| 18 | Arginine | X | |
| 19 | Histidine | X | |
| 20 | Tyrosine | X | |
| 21 | Tryptophan | X | |
| 22 | Hydroxylysine | ||
| 23 | 3-Monochlorotyrosine | X | |
| 24 * | Monobromotyrosine | X | |
| 25 * | Dichlorotyrosine | X | |
| 26 | 3-Monoiodotyrosine | X | |
| 27 * | Monobromo-monochlorotyrosine | X | |
| 28 | 3,5-Dibromotyrosine | X | |
| 29 * | Monochloro-monoiodotyrosine | X | |
| 30 * | Monobromo-monoiodotyrosine | X | |
| 31 | 3,5-Diiodotyrosine | X |
Obviously, the isolated I. basta sponge skeletons contain a variety of amino acids. The gas chromatogram can be divided into two parts: below a relative retention time of tR,rel = 0.85, twenty-two different amino acids could be observed. Seventeen of these amino acids could be assigned to proteinogenic amino acids. Some of the amino acids give rise to multiple peaks. This is caused by different reasons. Serine (peaks 6a, 6b) and hydroxyproline (peaks 10a, 10b) exhibit two peaks due to different degrees of derivatization. In each case, the first peak is caused by the incomplete derivatization of the molecule while the second peak is caused by the completely derivatized amino acid. Isoleucine (peaks 7a, 7b) and threonine (peaks 12a, 12b), however, yield two peaks of the same TBDMS derivative. As described previously (see [41]), the existence of two stereocenters in isoleucine as well as in threonine leads to two different diastereomers [49,50], which can be separated by gas chromatography.
The largest peak of the non-halogenated amino acids is due to glycine (peak 2). Furthermore, the proteinogenic amino acids alanine (peak 1), leucine (peak 5), proline (peak 8), aspartic acid (peak 14), glutamic acid (peak 15), lysine (peak 17), and tyrosine (peak 20) occur in larger quantities. In contrast, valine (peak 4), serine (peaks 6a, 6b), and phenylalanine (peak 13) have lower intensities. Isoleucine (peaks 7a, 7b), methionine (peak 11), threonine (peaks 12a, 12b), arginine (peak 18), histidine (peak 19), and tryptophan (peak 21) occur in the smallest amounts, where methionine, histidine and tryptophan are only found as traces.
Additionally, the non-proteinogenic amino acids AABA (peak 3), oxoproline (peak 9), hydroxyproline (peaks 10a, 10b), hydroxylysine (peak 22), as well as ornithine (peak 16), could be detected in the Ba(OH)2 extract of the I. basta skeletons. Ornithine (peak 16) occurs in a large amount, while hydroxyproline (peaks 10a, 10b) is present in smaller amounts. The other non-proteinogenic amino acids AABA (peak 3), oxoproline (peak 9) and hydroxylysine (peak 22), exhibit the smallest amounts.
Various halogenated amino acids occur at relative retention times larger than 0.85. Nine different halogenated amino acids can be identified, all of them are tyrosine derivatives. The monohalogenated amino acids 3-monochlorotyrosine (peak 23), monobromotyrosine (peak 24*) and 3-monoiodotyro-sine (peak 26), the purely dihalogenated amino acids dichlorotyrosine (peak 25*), 3,5-dibromo-tyrosine (peak 28), and 3,5-diiodotyrosine (peak 31) as well as the mixed halogenated amino acids monobromo-monochlorotyrosine (peak 27*), monochloro-monoiodotyrosine (peak 29*), and monobromo-monoiodotyrosine (peak 30*) can be detected. 3,5-dibromotyrosine (peak 28) represents the main component of the halogenated amino acids. Furthermore, monobromo-monochlorotyrosine (peak 27*) also exhibits a relatively high amount. All other halogenated tyrosines are present in smaller amounts; the lowest concentrations are found for monochloro-monoiodotyrosine (peak 29*) and 3,5-diiodotyrosine (peak 31).
2.2.2. Comparison of the Found Amino Acid Composition with the Literature
The found amino acid composition of the I. basta skeletons was compared with the previously determined amino acid composition of A. cavernicola skeletons [41] from the order Verongida, and with that of the sponges H. equina [39] as well as S. officinalis obliqua [40], both belonging to the order Dictyoceratida. All these sponges are from the Demospongia class. The results are given in Table 3.
Table 3.
Comparison of detected amino acids with the known amino acid composition of spongin reported in the literature. Amino acids which are only found in I. basta are marked in orange; those only found in A. cavernicola are marked in green.
| Halogenation State | Amino Acid | Ianthella basta | Aplysina cavernicola [41] | Hippospongia equina [39] | Spongia officinalis obliqua [40] |
|---|---|---|---|---|---|
| Alanine | X | X | X | X | |
| Non-halogenated | α-Aminobutyric Acid (AABA) | X | X | X | |
| γ-Aminobutyric Acid (GABA) | X | ||||
| Arginine | X | X | X | ||
| Aspartic Acid | X | X | X | X | |
| Cystine | X | ||||
| Glutamic Acid | X | X | X | X | |
| Glycine | X | X | X | X | |
| Histidine | X | X | X | ||
| Hydroxylysine | X | ||||
| Hydroxyproline | X | X | X | X | |
| Isoleucine | X | ||||
| Leucine | X | X | X | X | |
| Lysine | X | X | X | X | |
| Methionine | X | X | X | ||
| Ornithine | X | X | X | ||
| Oxoproline | X | X | |||
| Phenylalanine | X | X | X | ||
| Proline | X | X | X | X | |
| Serine | X | X | X | ||
| Threonine | X | X | X | ||
| Tryptophan | X | X | X | X | |
| Tyrosine | X | X | X | X | |
| Valine | X | X | X | X | |
| Halogenated | Monobromohistidine | X | |||
| Monobromotyrosine | X | X | |||
| 3-Monochlorotyrosine | X | X | |||
| 3-Monoiodotyrosine | X | X | X | ||
| Monochloro-monoiodotyrosine | X | X | |||
| Monobromo-monochlorotyrosine | X | X | |||
| Monobromo-monoiodotyrosine | X | X | |||
| Dichlorotyrosine | X | X | |||
| 3,5-Dibromotyrosine | X | X | X | ||
| 3,5-Diiodotyrosine | X | X | X | X |
Since the extraction of the amino acids was carried out with an alkaline Ba(OH)2 solution in all cases, the amino acid compositions are well comparable. First of all, it can be stated that the skeletal amino acid composition of the Verongida sponges I. basta and A. cavernicola is relatively similar. There are, however, also some differences: I. basta skeletons exhibit the non-halogenated amino acids hydroxylysine and isoleucine, which cannot be found in A. cavernicola skeletons. Monobromohistidine, in turn, cannot be detected in I. basta skeletons but are in the skeletons of A. cavernicola. Striking differences occur between the amino acid composition of the two Verongida sponges and the Dictyoceratida sponges H. equina and S. officinalis obliqua, especially with respect to halogenated amino acids. Most of the characteristic halogenated amino acids are only observed in the skeletons of the Verongida sponges I. basta and A. cavernicola. The halogenated amino acids monobromotyrosine, 3-monochlorotyrosine, monochloro-monoiodotyrosine, monobromo-monochlorotyrosine and dichlorotyrosine could not be identified in H. equina and S. officinalis obliqua. Interestingly, the amino acid monobromo-monochlorotyrosine is occurring in the gastropod mollusk Buccinum undatum [51].
In addition, the results of the analysis of I. basta skeletons support the hypothesis [41] that the presence of AABA or GABA is species-specific. H. equina is the only sponge in which GABA occurs. In the other sponges AABA was found instead of GABA. Moreover, cystine only occurs in H. equina but not in the other examined species.
The proteinogenic amino acids alanine, aspartic acid, glutamic acid, glycine, leucine, lysine, proline, tryptophan, tyrosine, valine and the halogenated amino acid 3,5-diiodotyrosine have been identified in the skeletons of I. basta as well as in A. cavernicola, in the sponge H. equina and in the sponge S. officinalis obliqua. Moreover, the non-proteinogenic amino acids hydroxyproline, oxoproline and ornithine were found in all four sponge samples. The presence of hydroxyproline is well known for collagen [52]. This observation, together with the very high amount of glycine in both sponge skeletons, emphasizes the collagenous character of spongin. Oxoproline and ornithine were also found in Aplysina aerophoba [53], which is the closest relative to A. cavernicola.
Since both H. equina and S. officinalis obliqua are members of the order Dictyoceratida while I. basta and A. cavernicola belong to the order Verongida it is quite possible that the observed amino acid composition is genus specific.
For further comparison, the relative amounts of the detected amino acids were estimated for I. basta and A. cavernicola which were analyzed exactly by the same method by referring the individual peak intensities to that of the most intensive peak (cf. Table 4). Glycine was the most abundant amino acid in both sponge skeletons. Alanine, lysine, ornithine, proline, and tyrosine were present in large amounts in both samples. In contrast, phenylalanine and valine occurred in smaller amounts in both cases. Methionine, arginine and tryptophan were only detected in minute amounts.
Table 4.
Comparison of the relative amino acid amounts in I. basta and A. cavernicola skeletons. Relative amounts were estimated referring the peak heights in the gas chromatogram to the most intense peak (++++: very large amount (100%–80%); +++: large amount (80%–30%); ++: small amount (30%–10%); +: smallest amount (<10%); -: not present). Amino acids only found in I. basta are marked in orange, those only found in A. cavernicola are marked in green.
| Halogenation State | Amino Acids | Amounts in | |
|---|---|---|---|
| Ianthella basta | Aplysina cavernicola [41] | ||
| Non-halogenated | Glycine | ++++ | ++++ |
| Alanine | +++ | +++ | |
| Aspartic Acid | +++ | ++ | |
| Glutamic Acid | +++ | ++ | |
| Hydroxyproline | +++ | +++ | |
| Leucine | +++ | + | |
| Lysine | +++ | +++ | |
| Ornithine | +++ | +++ | |
| Proline | +++ | +++ | |
| Tyrosine | +++ | +++ | |
| Oxoproline | ++ | + | |
| Phenylalanine | ++ | + | |
| Serine | ++ | ++++ | |
| Valine | ++ | ++ | |
| Arginine | + | + | |
| α-Aminobutyric Acid (AABA) | + | + | |
| Histidine | + | ++ | |
| Hydroxylysine | + | - | |
| Isoleucine | + | - | |
| Methionine | + | + | |
| Threonine | + | +++ | |
| Tryptophan | + | + | |
| Monobromo-monochlorotyrosine | ++ | +++ | |
| 3,5-Dibromotyrosine | ++ | +++ | |
| Monobromotyrosine | + | ++ | |
| Halogenated | 3-Monochlorotyrosine | + | + |
| 3-Monoiodotyrosine | + | + | |
| Monochloro-monoiodotyrosine | + | + | |
| Monobromo-monoiodotyrosine | + | + | |
| Dichlorotyrosine | + | ++ | |
| 3,5-Diiodotyrosine | + | + | |
| Monobromohistidine | - | + | |
However, the compositions of the sponge skeletons from I. basta and A. cavernicola also exhibit interesting differences with respect to the non-halogenated amino acids. Histidine, serine, and threonine were present in much larger amounts in A. cavernicola skeletons than in I. basta. For aspartic acid, glutamic acid and phenylalanine the situation is opposite. Much larger amounts of these amino acids were detected in the skeletons of I. basta than in A. cavernicola. Moreover, the amino acids hydroxylysine and small amounts of isoleucine were found in the I. basta skeletons, with hydroxylysine supporting the collagenous nature of I. basta spongin. These amino acids were not identified in the skeletons of A. cavernicola. Furthermore, the amount of leucine is much higher in the skeletons of I. basta than in A. cavernicola.
Within the group of halogenated amino acids, 3,5-dibromotyrosine and monobromo-monochlorotyrosine were most abundant. Smaller amounts were detected for all iodated tyrosines. The major difference was recognized in the dichlorotyrosine amounts, which were larger in the skeletons of A. cavernicola.
3. Materials and Methods
3.1. Sponge Samples
Sponges were collected in July 2013 while snorkeling at depths ranging from 8 to 12 m at Western Shoals, Apra Harbor, Guam (13.27.018 N; 144.39.120 E). Specimens were inspected in regards to their health and only healthy and intact specimens were collected. Sponges were kept in large Ziploc bags during collection and transported in a cooler to the Guam marine laboratories. Specimens were frozen at −20 °C and freeze-dried prior to their transport to the laboratories in Dresden, Germany (see also [20]).
3.2. Extraction of the Skeletons
I. basta skeletons were isolated following the protocol described previously [41]. Small pieces of I. basta (5–10 cm2) were soaked in 40 mL of distilled water for two weeks. Subsequently, the samples were transferred into freshly distilled water for 24 h. This procedure was repeated two times under continuous shaking.
3.3. Light Microscopy
Small pieces of the purified chitin-scaffolds were put on a sample holder. Microscopic studies were carried out on a Keyence BZ-8000K microscope (Keyence, Osaka, Japan). The exposure time was 1/230 s.
3.4. Scanning Electron Microscopy (SEM) and Energy Dispersive X-ray Spectroscopy (EDX)
Dried pieces of the skeletons were fixed on a sample holder and coated with carbon. The SEM images were recorded on a ZEISS DSM 982 GEMINI field emission scanning electron microscope (ZEISS, Oberkochen, Germany) using an acceleration voltage of 2 kV. Furthermore, EDX spectra were recorded with an acceleration voltage of 15 keV.
3.5. Extraction of the Chitin-Based Scaffold
The pure chitin skeletons were uncovered by an alkaline extraction according to [26]. Five to Thirty-five mg of isolated and freeze dried samples were treated with 2.5 M NaOH at 37 °C for 7 days. The remaining fibrous skeletal material was neutralized. In a second step, the samples were treated with 20% acetic acid at 37 °C for 24 h. Subsequently, the remaining fibrous skeleton material was neutralized and freeze dried again. The percentage of chitin in the skeletons was determined gravimetrically.
3.6. Estimation of the Content of Other Saccharides
The content of saccharides beside chitin in the skeletons was estimated using the resorcin method [47]. Therefore, 4 mg of A. cavernicola or 1.5 mg of I. basta were soaked with 1 mL pure water, 1 mL of a solution of resorcin (6 mg/mL) and 5 mL of sulfuric acid (75%) for 40 min at 95 °C and then quenched for 30 min in a darkened water bath. For calibration, soaked standard solutions of a 1:1:1 mixture of glucose, glucuronic acid and mannose containing defined amounts of chitin and protein (lysozyme) to simulate the matrix of skeletons were used. For quantification, the UV/Vis spectrometer Cary 50 (Varian, Palo Alto, CA, USA) was used with the following conditions: wavelength 300–800 nm, 0.0125 s per scan, data interval 1 nm, scan rate 4800 nm.
3.7. Determination of Calcium, Silicon and Sulfur Contents by ICP-OES
Digestion for calcium and sulfur determination: 2–18 mg of the skeleton were weighted into a micro vessel and digested with 450 μL of a mixture of HNO3 (65%), HF (47%–51%) and HCl for 15 min using microwaves at 1600 W and stepwise heating to 130 °C.
Complexation: 1.5 mL of a saturated solution of H3BO3 was added to the digested sample for complexing fluorides. Complexation was accomplished as follows: power 800 W, temperature 110 °C, time 10 min.
Digestion for silicon determination: 3 mL of HNO3 (65%) and 2 mL H2O2 were added to 30 mg of sponge skeletons. Microwave digestion was accomplished using the following parameters: at the beginning 400 W at 50 °C, followed by stepwise heating to 180 °C using 800 W in 60 min.
Finally, all digested samples were filled up to 10 mL with ultrapure water.
Measurement: The samples were measured with an ICP optical emission spectrometer Perkin–Elmer Optima 7000 DV using the analytical lines at 317.933 nm for calcium, at 180.669 nm for sulfur and at 212.412 nm, 251.611 nm and 288.158 nm for silicon. The following operating parameters were used: plasma argon 15 L/min, auxiliary argon 0.2 L/min, nebulizer argon 0.65 L/min, RF-power 1300 W and pump rate 1.3 L/min.
3.8. Ba(OH)2 Extraction of the Amino Acids
30 mg of the dried isolated skeletons were treated with 7.5 mL of saturated Ba(OH)2 solution containing 2 mg 5-bromotryptophan as internal standard at 100 °C for 5 days. Subsequently, the Ba(OH)2 solution was neutralized with H2SO4 and centrifuged. The supernatant was removed and freeze dried.
3.9. GC-MS Measurements of the Skeleton Extracts
One mg from each of the dried Ba(OH)2 extracts was soaked in 20 μL 2.5 M HCl and dried under a gentle stream of nitrogen. Subsequently, the residues were soaked twice in 40 μL EtOH and dried under nitrogen. Fifty μL acetonitrile and 50 μL MTBSTFA were added to the dry residues. The mixtures were sonicated for 30 s and then heated to 70 °C for 30 min. One μL of the resulting solution was injected into the GC-MS.
Analyses were carried out on an Agilent Technologies 6890N gas chromatograph directly coupled to an Agilent Technologies 5973N mass spectrometer (Agilent Technologies, Santa Clara, CA, USA). GC separations were performed on a SPB®-5 capillary GC column (Sigma-Aldrich, St. Louis, MO, USA). The flow of helium as carrier gas was 1 mL/min. The injector temperature was 300 °C. Split/Splitless injection was used (splitless time 1 min). The column temperature was programmed as followed: isothermal 115 °C for 3 min, then heating up to 300 °C at a rate of 4 K/min, then isothermal 300 °C for 30 min. The ion source temperature was 250 °C and the transfer line temperature was 300 °C. The mass spectra were recorded in the electron impact (EI) ionization mode at 70 eV, m/z range 70–850. The chromatograms were recorded after 3 min solvent delay. The retention time tR was normalized to the second peak of the internal standard. The intensity was normalized to the weighted sample and the total intensity of both peaks of the internal standard.
4. Conclusions
The isolated skeleton of the marine demosponge I. basta was analyzed with respect to its amino acid composition. These investigations revealed that the skeleton of I. basta contains 17 proteinogenic amino acids as well as five different non-proteinogenic amino acids. Moreover, we detected nine halogenated amino acids, all of them are halogenated tyrosines.
In conclusion, we can state the following:
The composition of non-halogenated amino acids in I. basta is similar to that of A. cavernicola. Abundant amino acids such as glycine and hydroxyproline confirm the collagenous nature of the I. basta spongin in analogy to all other investigated sponges.
I. basta exhibits a similar variety of halogenated amino acids as already observed for A. cavernicola—in contrast to the Dictyoceratida sponges H. equina and S. officinalis obliqua. It is, therefore, tempting to speculate that this variety of halogenated amino acids is characteristic for the order Verongida.
The differences in amino acid composition in the sponge skeletons of I. basta and A. cavernicola clearly show that the spongin in the skeletons of Verongid sponges is similar, but also exhibits some characteristic differences.
Further investigations of the amino acid composition of other sponge samples should be performed in the future to include a larger set of different sponge species into this comparison.
Acknowledgments
We acknowledge support by the German Research Foundation and the Open Access Publication Funds of the TU Dresden. The authors gratefully acknowledge financial support from the German Research Foundation (DFG grant no. Br 1278/17-1). Thanks are due to Renate Schulze for the ICP-OES measurements as well as to Susanne Goldberg and Silvia Mühle for the SEM/EDX measurements.
Author Contributions
P.J.S. provided the sponge samples. S.U. and S.M. designed the GC-MS experiments. S.U. performed the isolation of the sponge skeletons, the Ba(OH)2 treatments and the experiments. S.U. and S.M. analyzed data resulting from experiments. E.B. supervised the scientific work. S.U., S.M., P.J.S. and E.B. wrote this manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Arrieta J.M., Arnaud-Haond S., Duarte C.M. What lies underneath: Conserving the oceans’ genetic resources. Proc. Natl. Acad. Sci. USA. 2010;107:18318–18324. doi: 10.1073/pnas.0911897107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.MarinLit, The Royal Society of Chemistry. [(accessed on 31 August 2016)]. Available online: http://pubs.rsc.org/marinlit/
- 3.Storch V., Welsch U. Kükenthal—Zoologisches Praktikum. 27th ed. Springer; Berlin/Heidelberg, Germany: 2014. [Google Scholar]
- 4.Müller W.E.G. Origin of Metazoa: Sponges as living fossils. Naturwissenschaften. 1998;85:11–25. doi: 10.1007/s001140050444. [DOI] [PubMed] [Google Scholar]
- 5.Li C.-W., Chen J.-Y., Hua T.-E. Precambrian sponges with cellular structures. Science. 1998;279:879–882. doi: 10.1126/science.279.5352.879. [DOI] [PubMed] [Google Scholar]
- 6.Bergquist P.R. Sponges. University of California Press; Berkley, CA, USA: 1978. [Google Scholar]
- 7.Reiswig H.M. Particle feeding in natural populations of three marine demosponges. Biol. Bull. 1971;141:568–591. doi: 10.2307/1540270. [DOI] [Google Scholar]
- 8.Rohde S., Schupp P.J. Allocation of chemical and structural defenses in the sponge Melophlus sarasinorum. J. Exp. Mar. Biol. Ecol. 2011;399:76–83. doi: 10.1016/j.jembe.2011.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rohde S., Nietzer S., Schupp P.J. Prevalence and mechanisms of dynamic chemical defenses in tropical sponges. PLoS ONE. 2015;10:e0132236. doi: 10.1371/journal.pone.0132236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thoms C., Schupp P.J. Chemical Defense Strategies in Sponges: A Review. In: Custódio M.R., Lôbo-Hajdu G., Hajdu E., Muricy G., editors. Porifera Research: Biodiversity, Innovation and Sustainability. Museu Nacional; Rio de Janeiro, Brazil: 2007. pp. 627–637. Série Livros 28. [Google Scholar]
- 11.Thoms C., Schupp P.J. Activated chemical defenses in marine sponges: A case study on Aplysinella rhax. J. Chem. Ecol. 2008;34:1242–1252. doi: 10.1007/s10886-008-9518-z. [DOI] [PubMed] [Google Scholar]
- 12.Thoms C., Ebel R., Proksch P. Activated chemical defense in Aplysina sponges revisited. J. Chem. Ecol. 2006;32:97–123. doi: 10.1007/s10886-006-9355-x. [DOI] [PubMed] [Google Scholar]
- 13.Proksch P., Putz A., Ortlepp S., Kjer J., Bayer M. Bioactive natural products from marine sponges and fungal endophytes. Phytochem. Rev. 2010;9:475–489. doi: 10.1007/s11101-010-9178-9. [DOI] [Google Scholar]
- 14.Wehner R., Gehring W.J. Zoologie. 24th ed. Georg Thieme Verlag; Stuttgart, Germany: 2007. pp. 698–699. [Google Scholar]
- 15.Gazave E., Lapébie P., Renard E., Vacelet J., Rocher C., Ereskovsky A.V., Lavrov D.V., Borchiellini C. Molecular Phylogeny Restores the Supra-Generic Subdivision of Homoscleromorph Sponges (Porifera, Homoscleromorpha) PLoS ONE. 2010;5:e14290. doi: 10.1371/journal.pone.0014290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gazave E., Lapébie P., Ereskovsky A.V., Vacelet J., Renard E., Cárdenas P., Borchiellini C. No longer Demospongiae: Homoscleromorpha formal nomination as a fourth class of Porifera. Hydrobiologia. 2012;687:3–10. doi: 10.1007/s10750-011-0842-x. [DOI] [Google Scholar]
- 17.Van Soest R.W.M., Boury-Esnault N., Vacelet J., Dohrmann M., Erpenbeck D., de Voogd N.J., Santodomingo N., Vanhoorne B., Kelly M., Hooper J.N.A. Global diversity of sponges (Porifera) PLoS ONE. 2012;7:e35105. doi: 10.1371/journal.pone.0035105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bergquist P.R., Cook S.D.C. Order Verongida. In: Hooper J.N.A., van Soest R.W.M., editors. Systema Porifera: A Guide to the Classification of Sponges. Kluwer Academic/Plenum Publishers; New York, NY, USA: 2002. pp. 1081–1096. [Google Scholar]
- 19.Ehrlich H., Maldonado M., Spindler K.D., Eckert C., Hanke T., Born R., Goebel C., Simon P., Heinemann S., Worch H. First evidence of chitin as a component of the skeletal fibers of marine sponges. Part I. Verongidae (Demospongia: Porifera) J. Exp. Zool. Part B Mol. Dev. Evol. 2007;308:347–356. doi: 10.1002/jez.b.21156. [DOI] [PubMed] [Google Scholar]
- 20.Brunner E., Ehrlich H., Schupp P., Hedrich R., Hunoldt S., Kammer M., Machill S., Paasch S., Bazhenov V.V., Kurek D.V., et al. Chitin-based scaffolds are an integral part of the skeleton of the marine demosponge Ianthella basta. J. Struct. Biol. 2009;168:539–547. doi: 10.1016/j.jsb.2009.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Uriz M.J., Turon X., Becerro M.A., Agell G. Siliceous spicules and skeleton frameworks in sponges: Origin, diversity, ultrastructural patterns, and biological functions. Microsc. Res. Tech. 2003;62:279–299. doi: 10.1002/jemt.10395. [DOI] [PubMed] [Google Scholar]
- 22.Ehrlich H. Spongin. In: Gorb S.N., editor. Biological Materials of Marine Origin—Invertebrates, Biologically-Inspired Systems (Book 1) Springer; Dordrecht, The Netherlands: 2010. pp. 245–256. [Google Scholar]
- 23.Junqua S., Robert L., Garrone R., de Ceccatty M.P., Vacelet J. Biochemical and morphological studies on collagens of horny sponges. Ircinia filaments compared to spongines. Connect. Tissue Res. 1974;2:193–203. doi: 10.3109/03008207409152244. [DOI] [PubMed] [Google Scholar]
- 24.Exposito J.-Y., Cluzel C., Garrone R., Lethias C. Evolution of collagens. Anat. Rec. 2002;268:302–316. doi: 10.1002/ar.10162. [DOI] [PubMed] [Google Scholar]
- 25.Römpp Online: Spongin. [(accessed on 25 August 2016)]. Available online: https://roempp.thieme.de.
- 26.Ehrlich H., Ilan M., Maldonado M., Muricy G., Bavestrello G., Kljajic Z., Carballo J.L., Schiaparelli S., Ereskovsky A., Schupp P., et al. Three-dimensional chitin-based scaffolds from Verongida sponges (Demospongiae: Porifera). Part I. Isolation and identification of chitin. Int. J. Biol. Macromol. 2010;47:132–140. doi: 10.1016/j.ijbiomac.2010.05.007. [DOI] [PubMed] [Google Scholar]
- 27.Hackman R.H. Studies on chitin IV the occurrence of complexes in which chitin and protein are covalently linked. Aust. J. Biol. Sci. 1960;13:568–577. doi: 10.1071/BI9600568. [DOI] [Google Scholar]
- 28.Blackwell J., Weih M.A. Structure of chitin-protein complexes: Ovipositor of the ichneumon fly Megarhyssa. J. Mol. Biol. 1980;137:49–60. doi: 10.1016/0022-2836(80)90156-4. [DOI] [PubMed] [Google Scholar]
- 29.Blackwell J., Germinario L.T., Weih M.A. Chitin-Protein Complexes: Ordered Biopolymer Composites. In: Carraher C.E. Jr., Gebelein C.G., editors. Biological Activities of Polymers, ACS Symposium Series. American Chemical Society; Washington, DC, USA: 1982. pp. 149–162. [Google Scholar]
- 30.Greve H., Kehraus S., Krick A., Kelter G., Maier A., Fiebig H.H., Wright A.D., König G.M. Cytotoxic Bastadin 24 from the australian sponge Ianthella quadrangulata. J. Nat. Prod. 2008;71:309–312. doi: 10.1021/np070373e. [DOI] [PubMed] [Google Scholar]
- 31.Calcul L., Inman W.D., Morris A.A., Tenney K., Ratnam J., McKerrow J.H., Valeriote F.A., Crews P. Additional insights on the bastadins: Isolation of analogues from the sponge Ianthella cf. reticulata and exploration of the oxime configuration. J. Nat. Prod. 2010;73:365–372. doi: 10.1021/np9005986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Niemann H., Lin W.H., Müller W.E.G., Kubbutat M., Lai D.W., Proksch P. Trimeric hemibastadin congener from the marine sponge Ianthella basta. J. Nat. Prod. 2013;76:121–125. doi: 10.1021/np300764u. [DOI] [PubMed] [Google Scholar]
- 33.Ortlepp S., Sjörgren M., Dahlsröm M., Weber H., Ebel R., Edrada R., Thoms C., Schupp P., Bohlin L., Proksch P. Anti-fouling activity of bromotyrosine-derived sponge metabolites and synthetic analogues. Mar. Biotechnol. 2007;9:776–785. doi: 10.1007/s10126-007-9029-x. [DOI] [PubMed] [Google Scholar]
- 34.Ciminello P., Fattorusso E., Forino M., Magno S., Pansini M. Chemistry of verongida Sponges VIII—Bromocompounds from the Mediterranean sponges Aplysina aerophoba and Aplysina cavernicola. Tetrahedron. 1997;53:6565–6572. doi: 10.1016/S0040-4020(97)00311-6. [DOI] [Google Scholar]
- 35.Tymiak A.A., Rinehart L.R., Jr. Biosynthesis of dibromotyrosine-derived antimicrobial compounds by the marine sponge Aplysina fistularis (Verongia aurea) J. Am. Chem. Soc. 1981;103:6763–6765. doi: 10.1021/ja00412a047. [DOI] [Google Scholar]
- 36.Thomson J.E., Barrow K.D., Faulkner D.J. Localization of two brominated metabolites, aerothionin and homoaerothionin, in spherulous cells of the marine sponge Aplysina fistularis (=Verongia thiona) Acta Zool. 1981;64:199–210. doi: 10.1111/j.1463-6395.1983.tb00801.x. [DOI] [Google Scholar]
- 37.Turon X., Becerro M.A., Uriz M.J. Distribution of brominated compounds within the sponge Aplysina aerophoba: Coupling of X-ray microanalysis with cryofixation techniques. Cell Tissue Res. 2000;301:311–322. doi: 10.1007/s004410000233. [DOI] [PubMed] [Google Scholar]
- 38.Tabudravu J.N., Eijsink V.G.H., Gooday G.W., Jaspars M., Komander D., Legg M., Synstad B., van Aalten D.M.F. Psammaplin A, a chitinase inhibitor isolated from the fijian marine sponge Aplysinella rhax. Bioorg. Med. Chem. 2002;10:1123–1128. doi: 10.1016/S0968-0896(01)00372-8. [DOI] [PubMed] [Google Scholar]
- 39.Saper J., White W.E. Amino-acid composition of sclero-protein of the sponge Hippospongia equina. Nature. 1958;181:285–286. doi: 10.1038/181285b0. [DOI] [Google Scholar]
- 40.Low E.M. Halogenated amino acids of the bath sponge. J. Mar. Res. 1951;10:239–245. [Google Scholar]
- 41.Ueberlein S., Machill S., Niemann H., Proksch P., Brunner E. The skeletal amino acid composition of the marine demosponge Aplysina cavernicola. Mar. Drugs. 2014;12:4417–4438. doi: 10.3390/md12084417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bergquist P.R., Kelly-Borges M. Systematics and biogeography of the genus Ianthella (Demospongiae: Verongida: Ianthellida) in the South-West Pacific. Beagle Rec. North. Territ. Mus. Arts Sci. 1995;12:151–176. [Google Scholar]
- 43.Erwin P.M., Thacker R.W. Phylogenetic analyses of marine sponges within the order Verongida: A comparison of morphological and molecular data. Invertebr. Biol. 2007;126:220–234. doi: 10.1111/j.1744-7410.2007.00092.x. [DOI] [Google Scholar]
- 44.Kunze K., Niemann H., Ueberlein S., Schulze R., Ehrlich H., Brunner E., Proksch P., van Pée K.-H. Brominated skeletal components of the marine demosponges Aplysina cavernicola and Ianthella basta: Analytical and biochemical investigations. Mar. Drugs. 2013;11:1271–1287. doi: 10.3390/md11041271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rohde S., Schupp P.J. Growth and regeneration of the elephant ear sponge Ianthella basta (Porifera) Hydrobiologia. 2012;687:219–226. doi: 10.1007/s10750-011-0774-5. [DOI] [Google Scholar]
- 46.Campbell F.L. The detection and estimation of insect chitin; and the irrelation of “chitinization” to hardness and pigmentation of the cuticula of the american cockroach, periplaneta americana L. Ann. Entomol. Soc. Am. 1929;22:401–426. doi: 10.1093/aesa/22.3.401. [DOI] [Google Scholar]
- 47.Monsigny M., Petit C., Roche A.-C. Colorimetric determination of neutral sugars by a resorcinol sulfuric acid micromethod. Anal. Biochem. 1988;175:525–530. doi: 10.1016/0003-2697(88)90578-7. [DOI] [PubMed] [Google Scholar]
- 48.Ehrlich H., Simon P., Carrillo-Cabrera W., Bazhenov V.V., Botting J.P., Ilan M., Ereskovsky A.V., Muricy G., Worch H., Mensch A., et al. Insights into chemistry of biological materials: Newly discovered silica-Aragonite-Chitin biocomposites in demosponges. Chem. Mater. 2010;22:1462–1471. doi: 10.1021/cm9026607. [DOI] [Google Scholar]
- 49.North M. Principles and Applications of Stereochemistry. CRC Press; Cheltenham, UK: 1998. pp. 75–77. [Google Scholar]
- 50.Pretsch E., Bühlmann P., Badertscher M. Spektroskopische Daten zur Strukturaufklärung Organischer Verbindungen. Springer; Heidelberg, Germany: 2010. 5. Auflage. [Google Scholar]
- 51.Hunt S., Breuer S.W. Isolation of a new naturally occurring halogenated amino acid: Monochloro-monobromotyrosine. Biochim. Biophys. Acta Gen. Subj. 1971;252:401–404. doi: 10.1016/0304-4165(71)90021-3. [DOI] [PubMed] [Google Scholar]
- 52.Horton H.R., Moran L.A., Scrimgeour K.G., Perry M.D., Rawn J.D. Biochemie. Pearson Studium Verlag; München, Germany: 2008. [Google Scholar]
- 53.Čmelik S. Über einen Farbstoff von Protein-Natur aus dem Schwamme Aplysina aerophoba Nardo. Hoppe-Seyler´s Z. Physiol. Chem. 1952;289:218–220. doi: 10.1515/bchm2.1952.289.4.218. [DOI] [PubMed] [Google Scholar]