Abstract
Two new sesquiterpenes, microsphaeropsisin B (1) and C (2), and two new de-O-methyllasiodiplodins, (3R, 7R)-7-hydroxy-de-O-methyllasiodiplodin (4) and (3R)-5-oxo-de-O-methyllasiodiplodin (5), together with one new natural product (6) and twelve known compounds (3, 7–17), were isolated from the co-cultivation of mangrove endophytic fungus Trichoderma sp. 307 and aquatic pathogenic bacterium Acinetobacter johnsonii B2. Their structures, including absolute configurations, were elucidated by extensive analysis of spectroscopic data, electronic circular dichroism, Mo2(AcO)4-induced circular dichroism, and comparison with reported data. All of the isolated compounds were tested for their α-glucosidase inhibitory activity and cytotoxicity. New compounds 4 and 5 exhibited potent α-glucosidase inhibitory activity with IC50 values of 25.8 and 54.6 µM, respectively, which were more potent than the positive control (acarbose, IC50 = 703.8 µM). The good results of the tested bioactivity allowed us to explore α-glucosidase inhibitors in lasiodiplodins.
Keywords: co-cultivation, mangrove endophytic fungus, sesquiterpene, lasiodiplodin, α-glucosidase inhibitor
1. Introduction
Seven examples of marine bioactive compounds or derivatives were approved by the U.S. Food and Drug Administration or in clinical trials, such as salinosporamide A, plitidepsin, bryostatin 1, cytarabine, vidarabine, eribulin mesylate, and trabectidin (ET-743) [1]. Microorganisms from the mangrove environment produce a multitude of novel and biologically-active natural products [2,3]. According to genomic studies, numerous microorganisms have far greater potential to produce specialized metabolites than was thought from classic bioactivity screens. However, dozens of fungal gene clusters may be silent under standard laboratory growth conditions, which lead to the fact that some secondary metabolites pathways cannot be expressed. Therefore, certain groups of fungi have the potential to produce even more structurally-diverse secondary metabolites if the fungal cryptic biosynthetic pathways are activated [4]. Similar studies were reported for the genomes of filamentous fungi, such as Aspergillus spp. [5]. Microbial interspecies competition can have dramatic effects on small molecules, which were produced to defend the habitat or as chemical signals, and may be different from their single-species counterparts [6,7]. Consequently, microorganism co-culture, which is the cultivation of two or more microorganisms in one culture vessel, and a potent way to activate the silent gene clusters and enhance chemical diversity for drug discovery, has aroused great concern in natural product research [8]. A variety of studies have explored the induction of fungal metabolites in fungal and bacterial co-cultures [9,10,11,12], as well as in fungal co-cultures [13,14,15,16].
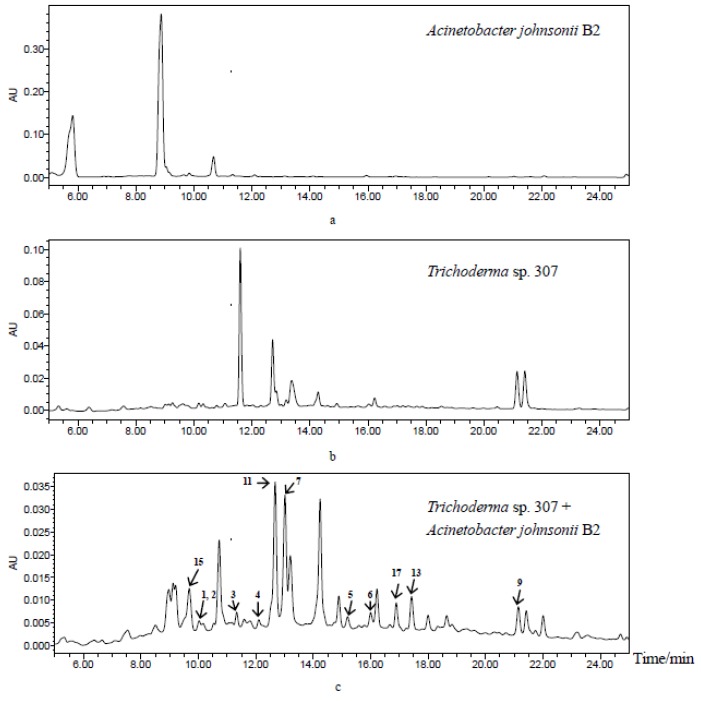
In continuing the search for novel and bioactive natural products from mangrove endophytic fungi [17,18,19,20], we recently turned to our interest in microorganism co-culture in order to obtain new bioactive compounds. After analyzing the high-performance liquid chromatography (HPLC) profiles of the co-cultivation extracts of 616 strains of mangrove endophytic fungi and Acinetobacter johnsonii B2, together with their monoculture extracts, we found that the co-cultivation of Trichoderma sp. 307 and Acinetobacter johnsonii B2 led to the production of different metabolites to those produced in pure-cultivating of fungal and bacterial controls (Figure 1). As a result, we have discovered two new furan-type isoeremophilane sesquiterpenes (1–2), three new de-O-methyllasiodiplodins (4–6, including one new natural product), along with twelve known molecules (3, 7–17). Herein, the isolation, structure elucidation, biological evaluation, and a brief discussion on the structure–activity relationship (SAR) of compounds 1–17 are reported.
Figure 1.
HPLC profiles of secondary metabolites from Acinetobacter johnsonii B2 (a), Trichoderma sp. 307 (b) and co-cultivation of two microorganisms (c) from up to down (detection wavelength: 254 nm).
2. Results and Discussion
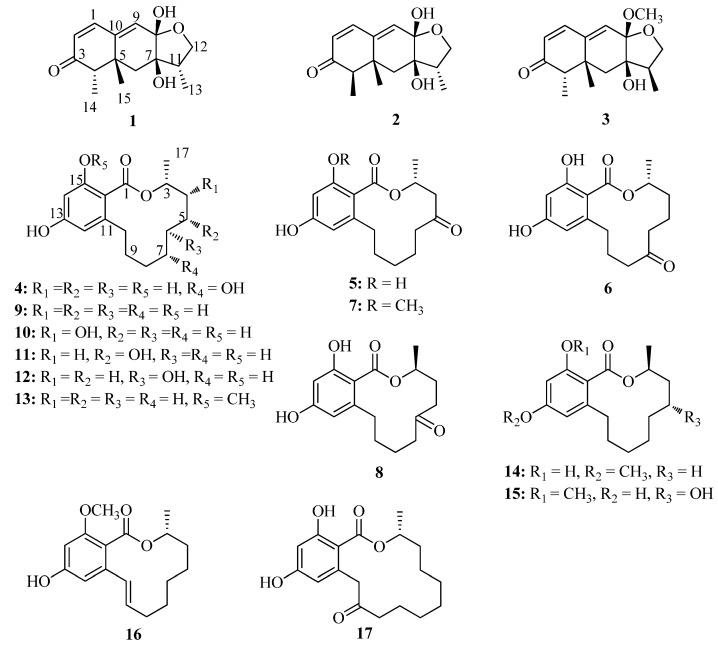
The mangrove endophytic fungus Trichoderma sp. 307 was co-cultured with an aquatic pathogenic bacterium named Acinetobacter johnsonii B2 on solid rice medium at 28 °C for 29 days. The CHCl3 extract of the fermentation was repeatedly fractionated and purified to obtain compounds 1–17 (Figure 2).
Figure 2.
Structures of compounds 1–17.
Compound 1 (1.8 mg) was obtained as a white powder. Its molecular formula C15H20O4 was deduced from the high resolution electrospray ionization mass spectroscopy (HRESIMS) peak at m/z 265.1438 [M + H]+ (calculated for C15H21O4, 265.1434), implying six degrees of unsaturation. The infrared radiation (IR) spectrum suggested the presence of hydroxy (3175 and 3355 cm−1) and conjugated carbonyl (1665 cm−1) groups. The 13C nuclear magnetic resonance (NMR) and distortionless enhancement by polarization transfer (DEPT) spectroscopic data (Table 1) revealed carbon signals for three methyl groups (δC 28.0, 14.8, and 9.2), two methylenes (δC 71.8 and 34.6), five methines (δC 146.6, 135.3, 126.3, 55.0, and 43.9), and five quaternary carbons, including one carbonyl group (δC 206.8), one ketal carbon (δC 100.5), one oxygenated carbon (δC 77.8), and two quaternary carbons (δC 139.4 and 39.9). The presence of one carbonyl group and two double bonds was attributable to three degrees of unsaturation, and the remaining three degrees of unsaturation indicated the existence of the tricyclic ring system in 1. The 1H NMR and heteronuclear single-quantum correlation (HSQC) spectra of 1 (Table 1) displayed signals for three methyls [δH 1.35 (H-15, s); 1.01 (H-13, d, J = 7.2 Hz); 0.95 (H-14, d, J = 7.3 Hz)], one oxygen-bearing methylene [δH 4.07 (H-12a, dd, J = 8.8, 8.3 Hz); 3.34 (H-12b, dd, J = 8.8, 6.4 Hz)], one methylene [δH 1.79 (H-6a, d, J = 14.1 Hz); 1.44 (H-6b, d, J = 14.1 Hz)], three olefinic methines [δH 7.04 (H-1, d, J = 9.8 Hz); 6.10 (H-9, s); 5.84 (H-2, d, J = 9.8 Hz)], and two methine groups [δH 2.58 (H-11, ddq, J = 8.3, 7.2, 6.4 Hz); 2.24 (H-4, q, J = 7.3 Hz)].
Table 1.
1H (400 MHz) and 13C (100 MHz) NMR data of 1 and 2.
| Position | 1 a | 2 a | ||
|---|---|---|---|---|
| δC | δH (J in Hz) | δC | δH (J in Hz) | |
| 1 | 146.6 | 7.04 (d, J = 9.8) | 146.1 | 7.01 (d, J = 9.8) |
| 2 | 126.3 | 5.84 (d, J = 9.8) | 128.4 | 5.93 (d, J = 9.8) |
| 3 | 206.8 | 203.4 | ||
| 4 | 55.0 | 2.24 (q, J = 7.3) | 54.4 | 2.24 (q, J = 6.9) |
| 5 | 39.9 | 40.4 | ||
| 6a | 34.6 | 1.79 (d, J = 14.1) | 38.9 | 1.87 (d, J = 14.0) |
| 6b | 1.44 (d, J = 14.1) | 1.51 (d, J = 14.0) | ||
| 7 | 77.8 | 77.6 | ||
| 8 | 100.5 | 100.3 | ||
| 9 | 135.3 | 6.10 (s) | 132.2 | 5.94 (s) |
| 10 | 139.4 | 142.4 | ||
| 11 | 43.9 | 2.58 (ddq, J = 8.3, 7.2, 6.4) | 43.9 | 2.54 (ddq, J = 7.8, 6.9, 6.4) |
| 12a | 71.8 | 4.07 (dd, J = 8.8, 8.3) | 71.6 | 4.04 (dd, J = 8.3, 7.8) |
| 12b | 3.34 (dd, J = 8.8, 6.4) | 3.31 (dd, J = 8.3, 6.4) | ||
| 13 | 9.2 | 1.01 (d, J = 7.2) | 9.2 | 1.01 (d, J = 6.9) |
| 14 | 14.8 | 0.95 (d, J = 7.3) | 7.5 | 1.08 (d, J = 6.9) |
| 15 | 28.0 | 1.35 (s) | 20.9 | 1.16 (s) |
a Measured in MeOH-d4.
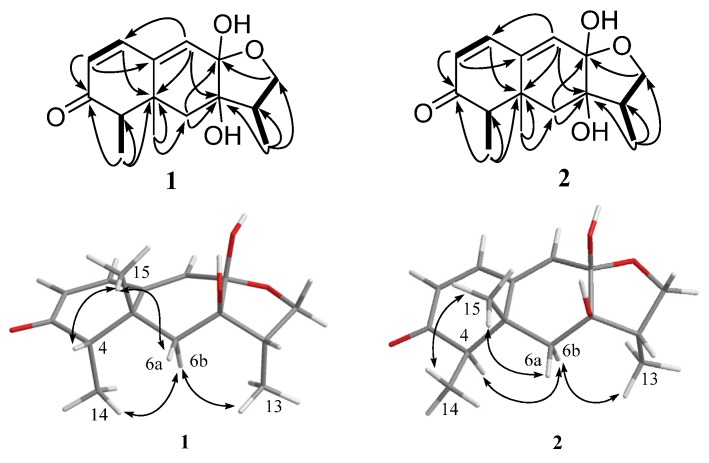
According to the 1H-1H correlation spectroscopy (COSY) spectrum, there were three independent spin systems of H-1/H-2, H-4/H-14, and H-12/H-11/H-13 (Figure 3). The ultra violet (UV) maximum at 284 nm revealed that the carbonyl group (δC 206.8, C-3) and the double bonds (δC 146.6, C-1; 126.3, C-2) were conjugated, which was confirmed by the heteronuclear multiple bond correlation (HMBC) correlations from olefinic protons H-1, and H-2 to C-3. The observed HMBC correlations (Figure 3) from H-1 to C-5, from H-2 to C-10, from H-6 to C-5 and C-8, from H-9 to C-1, C-5, and C-7, from H-14 to C-3, C-4, and C-5, and from H-15 to C-5, C-6, and C-10 illustrated the existence of a naphthalenone moiety with two methyl groups at C-4 and C-5, respectively. In addition, the HMBC correlations from H-11 to C-12 and C-13, H-12 to C-8, as well as H-13 to C-7, C-11, and C-12 were observed, which indicated the presence of a furan ring. As evidenced by the 13C NMR chemical shift (δC 100.5), C-8 was determined to be a hemiacetal carbon and was further connected to C-12 via the oxygen atom, which revealed the presence of a furan hemiacetal moiety. In the light of the NMR data (δC 77.8, C-7), the position of another hydroxyl group was assigned at C-7. Therefore, the planar structure of 1 was established.
Figure 3.
Key HMBC (black arrows), 1H-1H COSY (bold lines), and NOESY (double arrows) correlations of compounds 1 and 2.
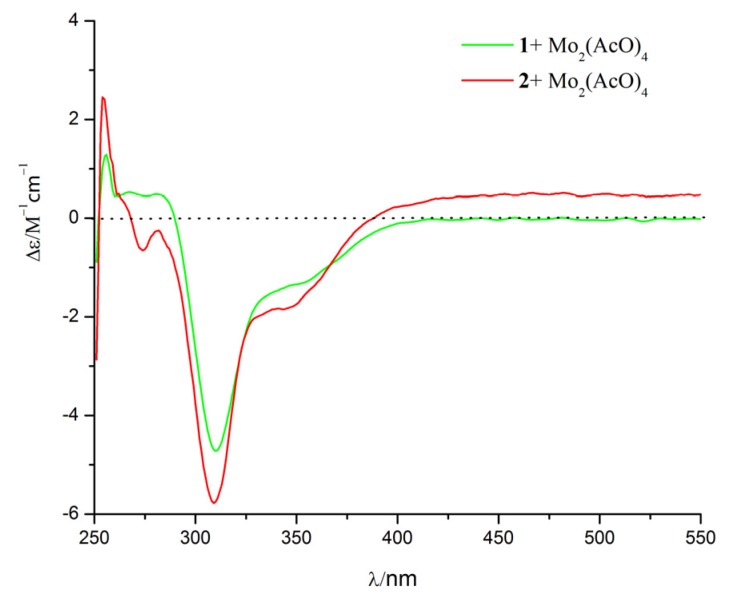
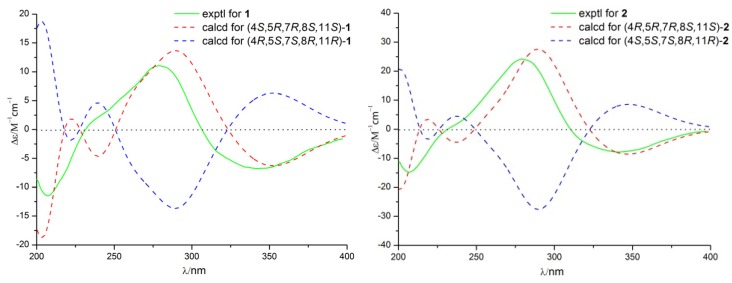
The relative configuration of 1 was established by nuclear Overhauser enhancement spectroscopy (NOESY) experiment (Figure 3). The NOESY correlations of H-15 with H-4 and H-6a, and of H-6b with H-13 and H-14, revealed that H-4, H-6a, and H-15 were on the same plane of the ring system, whereas H-13 and H-14 were on the opposite side. The absolute configurations of the 7,8-diol moieties were determined by Snatzke’s method [21,22,23]. Negative Cotton effects at 310 and 400 nm in the Mo2(AcO)4-induced circular dichroism (CD) spectrum (Figure 4) suggested the 7R and 8S configurations. To support the above deduction, the theoretical electronic circular dichroism (ECD) spectrum was calculated. The calculated ECD spectrum of 1 matched well with the experimental one (Figure 5), which indicated the (4S, 5R, 7R, 8S, 11S)-configuration of 1. Thus, compound 1 was a new furan-type isoeremophilane sesquiterpene, for which we suggest the trivial name microsphaeropsisin B.
Figure 4.
Mo2(AcO)4-induced CD spectra of compounds 1 and 2.
Figure 5.
Calculated and experimental ECD spectra of compounds 1 and 2.
Compound 2 (3 mg) was obtained as a white powder. The molecular formula was C15H20O4, corresponding to six degrees of unsaturation, based on the HRESIMS peak at m/z 265.1437 [M + H]+ (calculated for C15H21O4, 265.1434). The IR spectrum indicated the presence of hydroxy (3185 and 3365 cm−1) and conjugated carbonyl (1670 cm−1) groups. The 1H and 13C NMR spectra together with HSQC correlations of 2 revealed the signals for one carbonyl group (δC 203.4), one ketal carbon (δC 100.3), one oxygenated carbon (δC 77.6), two quaternary carbons (δC 142.4 and 40.4), five methines (δC 146.1, 132.2, 128.4, 54.4 and 43.9), two methylenes (δC 71.6 and 38.9), and three methyl groups (δC 20.9, 9.2 and 7.5). A detailed comparison of the NMR data with those for compound 1 showed the presence of the same furan-type isoeremophilane sesquiterpene framework, except for the different chemical shifts of H-6, H-14, H-15, and C-6, C-10, C-14, and C-15 (Table 1). The deduction was supported by the COSY correlations from H-1 to H-2, from H-4 to H-14 and from H-11 to H-12 and H-13, along with the observed HMBC correlations from H-1 to C-3, C-5, C-9, and C-10, from H-2 to C-4 and C-10, from H-4 to C-3, C-6, C-10, and C-14, from H-6 to C-5, C-7, C-8, C-10 and C-15, from H-9 to C-1, C-5, and C-7, from H-11 to C-7, from H-12 to C-8 and C-11, from H-13 to C-7, C-11, and C-12, from H-14 to C-3, C-4, and C-5, as well as from H-15 to C-4, C-6, and C-10 (Figure 3). In addition, compound 2 and 1 had the same mass unit. Thus, we tentatively supposed that compounds 1 and 2 were a pair of epimers, which was confirmed by the increase of chemical shifts for C-6 and C-10, and the decrease of chemical shifts for C-14 and C-15, as well as the NOESY spectroscopic data analysis. The NOESY correlations of H-6a with H-15, and of H-15 with H-14 (Figure 3) suggested their syn-orientation, whereas the correlation of H-6b with H-4 and H-13 indicated these two protons were on the opposite face of the molecule. The absolute configurations of the 7,8-diol groups in 2 was also established by Snatzke’s method. In the Mo2(AcO)4-induced CD spectrum (Figure 4), negative Cotton effects at 309 and 398 nm supported the 7R and 8S configurations. According to a comparison of the calculated ECD spectrum with the experimental data (Figure 5), the absolute configuration of 2 was assigned as 4R, 5R, 7R, 8S, 11S. Therefore, the gross structure of 2 was identified as shown, named as microsphaeropsisin C.
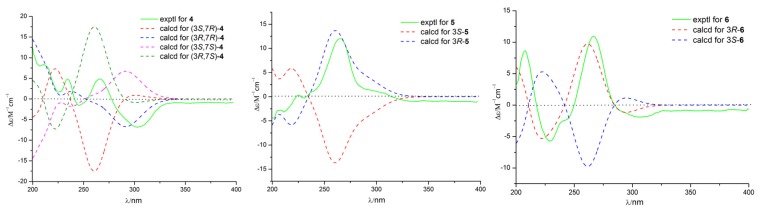
Compound 4 (2.3 mg) was obtained as a white powder and gave a molecular formula of C16H22O5 by HRESIMS (m/z 293.1389 [M − H]−, calculated for C16H21O5, 293.1389), with six degrees of unsaturation. The 1H and 13C NMR spectra of 4 were similar to those of de-O-methyllasiodiplodin [24], which suggested that compound 4 was a de-O-methyllasiodiplodin analogue. The major difference in the 1H NMR spectrum of 4 in comparison with that of de-O-methyllasiodiplodin was the presence of an oxygenated methine proton for H-7 that was shifted downfield to δH 4.27 (rather than one methylene protons at δH 1.42 and 1.60 in de-O-methyllasiodiplodin). The downfield shifts observed for C-7 in the 13C NMR spectrum also indicated the presence of a hydroxy group at C-7 (δC 68.0) in 4 instead of a methylene group at C-7 (δC 21.1) in de-O-methyllasiodiplodin. The position of the hydroxyl group at C-7 in 4 was further supported on the basis of COSY correlations from H-3 to H-4 and H-17, from H-6 to H-7 and from H-9 to H-10, along with HMBC correlations from H-4 to C-17, from H-5 to C-4, C-6 and C-17, from H-6 to C-7, from H-7 to C-6, from H-8 to C-9, and from H9 to C-6, C-7 and C-8 (Figure 6). In order to determine the absolute configuration of 4, the theoretical ECD spectrum was calculated. As a result, the calculated curve of (3R, 7R)-4 matched well with the experimental one (Figure 7). Hence, the structure of 4 was assigned as (3R, 7R)-7-hydroxy-de-O-methyllasiodiplodin.
Figure 6.
Key HMBC (black arrows) and 1H-1H COSY (bold lines) correlations of compounds 4, 5, and 6.
Figure 7.
Calculated and experimental ECD spectra of compounds 4, 5, and 6.
Compound 5 (2.3 mg) was obtained as a white powder with specific optical rotation of (c 0.025, MeOH). Its molecular formula was determined as C16H20O5 based on HRESIMS (m/z 291.1232 [M − H]−, calculated for C16H19O5, 291.1232), with seven degrees of unsaturation. Its 1H NMR and 13C NMR data (Table 2) bore good resemblance to those of 5-oxolasiodiplodin [25], except for the presence of a chelated hydroxyl proton (δH 11.97) and the absence of the 1H and 13C signals of the methoxy group (δH/C 3.75/55.7) in 5. Accordingly, the structure of 5 was proposed as 5-oxo-de-O-methyllasiodiplodin, which was confirmed by the HMBC correlations (Figure 6) from the chelated hydroxyl proton 15-OH to C-15. The absolute configuration at C-3 was determined as 3R by comparing the calculated ECD spectrum with the experimental one. As a result, the experimental ECD spectrum of 5 showed excellent accordance with (3R)-5 (Figure 7). Thus, the structure and absolute configuration of 5 were identified as shown in Figure 1, named as (3R)-5-oxo-de-O-methyllasiodiplodin.
Table 2.
1H (400 MHz) and 13C (100 MHz) NMR data of 4–6.
| Position | 4 a | 5 b | 6 b | |||
|---|---|---|---|---|---|---|
| δC | δH (J in Hz) | δC | δH (J in Hz) | δC | δH (J in Hz) | |
| 1 | 172.0 | 171.0 | 171.8 | |||
| 3 | 73.7 | 5.37 (m) | 69.3 | 5.69 (ddq, J = 10.8, 6.3, 2.3) | 74.9 | 5.28 (ddq, J = 12.8, 6.3, 3.3) |
| 4 | 31.4 | 1.94 (m) | 46.1 | 3.31 (dd, J = 16.4, 10.8) | 31.7 | 1.94 (dd, J = 12.8, 7.7) |
| 1.65 (m) | 2.39 (dd, J = 16.4, 2.3) | 1.84 (dd, J = 7.7, 3.3) | ||||
| 5 | 30.2 | 1.74 (m) | 210.7 | 19.2 | 2.08 (m) | |
| 1.28 (m) | 1.76 (m) | |||||
| 6 | 32.5 | 2.12 (m) | 46.1 | 2.61 (m) | 38.6 | 2.64 (m) |
| 1.83 (m) | 2.30 (m) | 2.55 (m) | ||||
| 7 | 68.0 | 4.27 (m) | 21.8 | 2.01 (m) | 211.4 | |
| 1.86 (m) | ||||||
| 8 | 24.1 | 1.92 (m) | 29.9 | 1.48 (m) | 42.1 | 2.44 (m) |
| 1.67 (m) | 1.25 (m) | 2.35 (m) | ||||
| 9 | 31.1 | 2.06 (m) | 30.3 | 1.66 (m) | 28.9 | 2.20 (m) |
| 1.76 (m) | 1.57 (m) | 1.75 (m) | ||||
| 10 | 33.7 | 3.22 (m) | 36.8 | 3.45 (m) | 33.6 | 3.14 (m) |
| 2.59 (m) | 2.11 (m) | 2.61 (m) | ||||
| 11 | 148.3 | 149.0 | 148.0 | |||
| 12 | 111.9 | 6.74 (d, J = 2.4) | 111.1 | 6.17 (d, J = 2.6) | 110.8 | 6.24 (d, J = 2.6) |
| 13 | 163.9 | 160.5 | 160.5 | |||
| 14 | 102.3 | 6.84 (d, J = 2.4) | 101.8 | 6.26 (d, J = 2.6) | 102.0 | 6.30 (d, J = 2.6) |
| 15 | 164.9 | 166.1 | 165.8 | |||
| 16 | 107.6 | 104.8 | 106.0 | |||
| 17 | 18.2 | 1.38 (d, J = 6.3) | 19.9 | 1.44 (d, J = 6.3) | 19.6 | 1.35 (d, J = 6.3) |
| 15-OH | 12.42 (s) | 11.97 (s) | 12.05(s) | |||
a Measured in pyridine-d5; b Measured in CDCl3.
Compound 6 (2.6 mg) was isolated as colorless needles, and its molecular formula was determined to be C16H20O5 with seven degrees of unsaturation. Comparison of the 1D NMR data of 6 (Table 2) with those of 5 showed close similarity with some minor variations for the chemical shifts of C-3 through C-9, along with the change from the methylene group C-7 in de-O-methyllasiodiplodin to a carbonyl group at δC 211.4 in 6. It was deduced that the position of the ketone carbonyl in the alkyl ring was changed. The deduction was supported by HMBC correlations from H-5, H-6, and H-8 to the ketone carbonyl C-7 (δC 211.4) (Figure 6). The 3R-configuration of 6 was determined by the comparison of the calculated ECD spectrum with the experimental one (Figure 7). To the best of our knowledge, compound 6 is reported here as a new natural product and was named as (3R)-7-oxo-de-O-methyllasiodiplodin.
The structures of compounds 3, 7–17 were assigned by comparison of their spectroscopic data (1D and 2D NMR, MS) and optical rotations with published values. Therefore, the known compounds 3, 7–17 were identified as microsphaeropsisin (3) (3.2 mg) [26], (3R)-5-oxolasiodiplodin (7) (67.9 mg) [25], (3S)-6-oxo-de-O-methyllasiodiplodin (8) (3.8 mg) [27], (3R)-de-O-methyllasiodiplodin (9) (74.3 mg) [24], (3R,4R)-4-hydroxy-de-O-methyllasiodiplodin (10) (71.8 mg) [28], (3R,5R)-5-hydroxy-de-O-methyllasiodiplodin (11) (93.1 mg) [29], (3R,6R)-6-hydroxy-de-O-methyllasiodiplodin (12) (3.5 mg) [29], (3R)-lasiodiplodin (13) (29 mg) [30], (3S)-ozoroalide (14) (15 mg) [31], (3S,5R)-5-hydroxylasiodiplodin (15) (16.8 mg) [25], (E)-9-etheno-lasiodiplodin (16) (15.9 mg) [27], and (3R)-nordinone (17) (18 mg) [32], respectively.
α-Glucosidase inhibitors are helpful to prevent deterioration of Type 2 diabetes and for the treatment of the disease in the early stage, which can delay the liberation of glucose from food and retard glucose absorption, thus lowering the postprandial blood glucose level [33]. Some lasiodiplodins with α-glucosidase inhibitory activity had been reported [34], so the α-glucosidase inhibitory effects of the isolated compounds were evaluated. As a result (Table 3), compounds 4, 5, 8, 9, and 10 exhibited potent α-glucosidase inhibitory activity with IC50 of 25.8, 54.6, 64.2, 48.9, and 60.3 µM, respectively, which were much better than acarbose (IC50 of 703.8 µM) as a positive control. Compounds 16 and 17 revealed seven-fold better inhibitory effects (IC50 of 101.3 and 105.7 μM, respectively) than acarbose. Compounds 2, 6, 7, and 14 showed moderate inhibitory activity against α-glucosidase with IC50 values of 188.7, 178.5, 176.8, and 198.1 μM, respectively. The other molecules were inactive with IC50 values more than 200 μM. The results indicated that the configuration at C-5 in compounds 1 and 2 might affect α-glucosidase inhibitory activity. Moreover, the methoxy group at C-15 in the lasiodiplodin derivatives decreased the activity (5 vs. 7 and 9 vs. 13). For compounds 4, 10, 11, and 12, compounds 4 and 10 showed potent α-glucosidase inhibitory effects, whereas 11 and 12 were inactive, which attested that the position of the hydroxyl group had a significant impact on the activity. Similarly, according to the different activities of compounds 5, 6, and 8, the position of the carbonyl moiety also exercised a great influence on the α-glucosidase inhibitory effects. In addition, the C-9-C-10 double bond of compound 16 was essential for the activity (13 vs. 16).
Table 3.
α-Glucosidase inhibitory activities a.
| Compounds | IC50 (µM) | Compounds | IC50 (µM) |
|---|---|---|---|
| 1 | >200 | 10 | 60.3 ± 0.7 |
| 2 | 188.7 ± 1.2 | 11 | >200 |
| 3 | >200 | 12 | >200 |
| 4 | 25.8 ± 0.2 | 13 | >200 |
| 5 | 54.6 ± 0.5 | 14 | 198.1 ± 1.5 |
| 6 | 178.5 ± 1.1 | 15 | ˃200 |
| 7 | 176.8 ± 1.4 | 16 | 101.3 ± 0.9 |
| 8 | 64.2 ± 0.5 | 17 | 105.7 ± 1.0 |
| 9 | 48.9 ± 0.4 | Acarbose b | 703.8 ± 2.2 |
a IC50 values are shown as mean ± SD from two independent experiments; b Positive control.
All isolates were also evaluated for their cytotoxic activity against rat pituitary adenoma GH3 cell lines and rat prolactinoma MMQ cell lines by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-2H-tetrazolium bromide (MTT) method. Compound 9 exhibited more potent cytotoxicity against GH3 and MMQ cell lines with IC50 values of 6.44 and 6.58 μM, respectively, while the cytotoxicity against rat normal pituitary cells (RPC) as positive control with IC50 of 6.94 µM. Compound 17 displayed moderate cytotoxicity with IC50 values of 12.33 and 10.13 μM, respectively, which was ten-fold better than RPC cell lines with an IC50 value of 100.03 μM. Compound 8 was less active with IC50 values of 21.42 and 13.59 μM, respectively, which was seven-fold better than RPC cells with IC50 of 142.8 µM as positive control. However, the rest of compounds showed no cytotoxicity against the two cell lines with IC50 values more than 50 μM. The above consequences revealed that methylation of 13-OH or 15-OH in lasiodiplodins resulted in diminished cytotoxicity and some compounds had selective activity against rat normal cells and cancer cells. Moreover, the position of the carbonyl group and hydroxyl moiety might play a significant role in the cytotoxicity. The SAR analysis was also confirmed by our previous studies [35,36].
3. Materials and Methods
3.1. General Experimental Procedures
Optical rotations were recorded using MCP 200 Polarimeter (Anton Paar GmbH, Graz, Austria). Optical density (OD) values were read on a Multiskan Spectrum Microplate Reader (Thermo Scientific Inc., Shanghai, China). CD spectra were acquired on a Chirascan Spectrometer (Applied Photophysics Ltd., Surrey, UK). IR spectra were carried out on a Nicolet Nexus 670 spectrophotometer, in KBr discs. NMR spectra were obtained on a Bruker AVANCE 400 (Bruker Co. Ltd., Zurich, Switzerland). Thin-layer chromatography (TLC) was carried out on pre-coated silica gel GF-254 plates (Qingdao Haiyang Chemical Co., Ltd., Qingdao, China) and column chromatography (CC) was performed over silica gel (Qingdao Haiyang Chemical Co., Ltd., Qingdao, China, 200–300 mesh) on a Sephadex LH-20 (GE healthcare, Buckinghamshire, UK). Semi-preparative HPLC was performed on a Waters 1525 system using a semi-preparative Ultimate XB-C18 column (5 μm, 21.2 mm × 250 mm; Welch) coupled with a Waters 2998 photodiode array detector (Waters Corp., Milford, MA, USA). ESIMS data were measured on a Thermo LCQ DECA XP plus mass spectrometer (Thermo Scientific, Waltham, MA, USA). All reagents and solvents were of commercial quality.
3.2. Fungal and Bacterial Material
Strain 307, identified as Trichoderma sp. (GenBank accession number: KX816009), was isolated from the stem bark of Clerodendrum inerme, collected in Zhanjiang Mangrove National Nature Reserve in Guangdong Province, China. A voucher specimen (registration number: 307) has been deposited at the Institute of Marine Biological Natural Products, School of Marine Sciences, Sun Yat-sen University, China.
Bacterium B2, identified as Acinetobacter johnsonii (GenBank accession number: KY077679), was isolated from an aquaculture pond at the Maoming Experimental Station in Guangdong (Guanli Marine Organisms LLC.). A voucher specimen (registration number: B2) has been deposited at the Institute of Marine Biological Natural Products, School of Marine Sciences, Sun Yat-sen University, China.
3.3. Co-Cultivation, Extraction, and Isolation
Strain 307 was cultured for one week at 28 °C in five Petri dishes (i.d. 90 mm) containing 25 mL of potato dextrose agar medium. In order to obtain the mycelial suspension, the agar-supporting mycelia were cut and transferred to two 1000 mL Erlenmeyer flasks containing 500 mL of potato dextrose broth and then incubated at 28 °C for four days on a rotary shaker at 150 rpm. The bacterium B2 was cultured in a 1000 mL Erlenmeyer flask containing 500 mL of lysogeny broth at 37 °C for 24 h on a rotary shaker at 150 rpm. Then, 5 mL of the fungal seed broth and 1 mL of the bacterial seed broth were added into rice medium (94 bottles of 1000 mL Erlenmeyer flasks, each containing 50 g of rice, 100 mL distilled water), and incubated at 28 °C for 28 days under static conditions and daylight. Following incubation, the mycelia and solid rice medium were extracted three times with MeOH. The MeOH solution was concentrated under reduced pressure to afford the MeOH solution, which was extracted three times with CHCl3 to give 42.6 g of crude extract. The extract was then separated into 11 fractions (Fr. 1–Fr. 11) by column chromatography over silica gel eluted by a gradient of petroleum ether/EtOAc from 100:0 to 0:100 and EtOAc/MeOH from 100:0 to 0:100. Fr. 2 (319.4 mg) was applied to Sephadex LH-20 (CH2Cl2/MeOH v/v, 1:1) to yield compound 9 (74.3 mg). Fr. 3 (1355.1 mg) was divided into six fractions (Fr. 3.1 to Fr. 3.6) by CC over silica gel eluting with CHCl3/MeOH (v/v, 99:1), and afforded compound 14 (15 mg). Fr. 3.2 (232.1 mg) was further purified by semipreparative HPLC with 70% MeOH-H2O to obtain 17 (18 mg). Fr. 3.3 (301 mg) was subsequently separated on aSephadex LH-20 CC and eluted with CH2Cl2/MeOH (v/v, 1:1) to yield 13 (29 mg). Fr. 3.4 (428.2 mg) was chromatographed on silica gel (petroleum ether/EtOAc v/v, 2:8) to give subfraction Fr. 3.4.5, which was further purified by HPLC with 60% CH3CN-H2O to afford 5 (2.3 mg), and 16 (15.9 mg). Fr. 3.5 (12 mg) was separated by HPLC with 75% MeOH-H2O for 8 (3.8 mg) and 6 (2.6 mg). Fr. 3.6 (203.5 mg) was fractionated on a Sephadex LH-20 CC (CH2Cl2/MeOH v/v, 1:1) and further purified using HPLC eluted with 75% MeOH-H2O to give 10 (71.8 mg). Fr. 4 (651 mg) was applied to the Sephadex LH-20 CC eluted with CH2Cl2/MeOH (v/v, 1:1) to give subfractions Fr. 4.1 and Fr. 4.2. Fr. 4.1 (401.8 mg) was then purified by HPLC (70% MeOH-H2O) to yield 7 (67.9 mg). Fr. 4.2 (160.9 mg) was further purified using HPLC with 68% MeOH-H2O to obtain 11 (93.1 mg). Fr. 5 (463.2 mg) was subjected to silica gel CC, eluted with petroleum ether/EtOAc (v/v, 100:0 to 0:100), to obtain subfractions Fr. 5.1-6. Fr. 5.4 (52.1 mg) was purified by HPLC with 60% MeOH-H2O to yield 12 (3.5 mg) and Fr. 5.4.1 (11.7 mg), which was further purified using HPLC (75% MeOH-H2O) to obtain 3 (3.2 mg). Fr. 5.5 (72.6 mg) was purified by repeated HPLC with 70% MeOH-H2O to yield 4 (2.3 mg). Fr. 6 (113.9 mg) was separated into three subfractions (Fr. 6.1-3) by silica gel CC using a stepwise gradient eluting with mixtures of petroleum ether/EtOAc (v/v, 100:0 to 0:100). Fr. 6.2 (38.9 mg) was subsequently purified by HPLC eluting with 60% MeOH-H2O to give 1 (1.8 mg) and 15 (16.8 mg). Fr. 6.3 (50.2 mg) was purified by HPLC (60% MeOH-H2O) to yield 3 mg of 2.
Microsphaeropsisin B (1), white powder; (c 0.100, MeOH); UV (MeOH) λmax (log ε) 284 (3.69) nm; ECD (MeOH) λmax (Δε) 203 (−18.7), 222 (1.9), 240 (−4.6), 290 (13.7), 352 (−6.3) nm; IR (KBr) λmax 3355, 3175, 1665 cm−1; 1H NMR (400 MHz, MeOH-d4) and 13CNMR (100 MHz, MeOH-d4) see Table 1; ESIMS m/z 265.1 [M + H]+; HRESIMS m/z 265.1438 [M + H]+ (calculated for C15H21O4, 265.1434).
Microsphaeropsisin C (2), white powder; (c 0.025, MeOH); UV (MeOH) λmax (log ε) 283 (3.79) nm; ECD (MeOH) λmax (Δε) 206 (−18.7), 225 (2.7), 240 (−0.8), 288 (21.1), 353 (−8.3) nm; IR (KBr) λmax 3365, 3185, 1670 cm−1; 1H NMR (400 MHz, MeOH-d4) and 13C NMR (100 MHz, MeOH-d4) see Table 1; ESIMS m/z 263.1 [M − H]−; HRESIMS m/z 265.1437 [M + H]+ (calculated for C15H21O4, 265.1434).
(3R, 7R)-7-hydroxy-de-O-methyllasiodiplodin (4), white powder; (c 0.033, MeOH); ECD (MeOH) λmax (Δε) 228 (0.9), 238 (1.9), 292 (−6.7) nm; 1H NMR (400 MHz, pyridine-d5) and 13C NMR (100 MHz, pyridine-d5) see Table 2; ESIMS m/z 293.0 [M − H]−; HRESIMS m/z 293.1389 [M − H]−, calculated for C16H21O5, 293.1389).
(3R)-5-oxo-de-O-methyllasiodiplodin (5), white powder; (c 0.025, MeOH); ECD (MeOH) λmax (Δε) 207 (−3.6), 219 (−5.8), 260 (13.7) nm; 1H NMR (400 MHz, CDCl3) and 13C NMR (100 MHz, CDCl3) see Table 2; HRESIMS m/z 291.1232 [M − H]−, calculated for C16H19O5, 291.1232).
(3R)-7-oxo-de-O-methyllasiodiplodin (6), colorless needles; (c 0.020, CHCl3); ECD (MeOH) λmax (Δε) 223 (−5.3), 263 (9.7) nm; 1H NMR (400 MHz, CDCl3) and 13C NMR (100 MHz, CDCl3) see Table 2; HRESIMS m/z 291.1231 [M − H]−, calculated for C16H19O5, 291.1232).
3.4. Calculation of ECD Spectra
The molecular Dreiding force field was run with Spartan 14 software (Wavefunction Inc., Irvine, CA, USA). The time-dependent density functional theory (TDDFT) calculations were carried out with Gaussian 05 (Gaussian, Wallingford, CT, USA). The energy-minimized conformers were generated and optimized at the B3LYP/6-31G (d) level. The integral equation formalism variant polarizable continuum model (IEF-PCM) solvent model for MeOH was used. The ECD spectra were calculated at the RB3LYP/6-311++G (2d, p) level by SpecDis 3.0 (University of Würzburg, Würzburg, Germany) and OriginPro 8.5 (OriginLab, Ltd., Northampton, MA, USA) based on the final optimized structures. All calculations were performed by the high-performance grid computing platform at Sun Yat-Sen University.
3.5. α-Glucosidase Inhibitory Activity Assay
The assay of α-glucosidase inhibitory activity was carried out according to the reported method, with minor modifications [33]. All of the assays were performed under 0.01 M potassium phosphate buffer (pH 7). Enzyme solutions were prepared to give 2.0 Units/mL in 2 mL buffer solution. Test samples were dissolved in dimethyl sulphoxide (DMSO) to give an initial concentration of 4000 μM/L. One-hundred fifty-five microliters of phosphate buffer, 10 μL of test samples, and 10 μL of diluted enzyme solution were mixed in each well of a 96-well microtiter plate. After 20 min incubation at 37 °C, 25 μL of substrate (p-nitrophenyl-α-d-glucopyranoside, 1.5 mg/mL) was added to each well to begin the enzymatic reaction. The reaction was monitored spectrophotometrically by measuring the absorbance at 410 nm for a 1 min interval. Acarbose was used as a positive control. Calculations were performed according to the following equation: the inhibition rates (%) = [(B − S)/B] × 100% (B represents the OD value in the assay medium with DMSO, S represents the OD value in the assay medium with test samples or acarbose). All measurements were done in triplicate from two independent experiments. The reported IC50 was the average value of two independent experiments.
3.6. Cytotoxicity Assay
The cytotoxic activities against rat pituitary adenoma MMQ and GH3 cell lines were evaluated by MTT assay following the previous process [36,37]. Briefly, MMQ and GH3 cell lines were seeded in 96-well plates (Corning, New York, NY, USA) at a density of 5 × 104 cells per well. Then, 10 μL of MTT reagent was added to each well and incubated at 37 °C with 5% CO2 for 4 h. Subsequently, 100 μL of acidified isopropyl alcohol was added. Then the OD value was measured at 450 nm using a microplate reader and the cell proliferation rate relative to the control was calculated. The IC50 was analyzed by SPSS 13.0 (SPSS, Chicago, IL, USA). Rat normal pituitary cells were used as a positive control.
3.7. HPLC Profiles Conditions
The detection was operated on a Waters 1525 system coupled with a Waters 2998 photodiode array detector (Waters Corp., Milford, MA, USA). The samples were eluted from an analytical Ultimate XB-C18 column (5 μm, 4.6 × 250 mm; Welch) at a flow rate of 1.0 mL/min using the following binary gradient with solvent A consisting of 15% acetonitrile/85% H2O and solvent B consisting of 15% H2O/85% acetonitrile: 0–22 min, 100%A–100%B; 22–27 min, 100%B; 27–35 min, 100%B–100%A. The detection wavelength was from 200 to 700 nm. The temperature was maintained at 25 °C, and the injection volume was 30 µL.
4. Conclusions
A chemical investigation of the co-cultivation of Trichoderma sp. 307 and Acinetobacter johnsonii B2 led to the isolation of two new sesquiterpenes (1–2), two new de-O-methyllasiodiplodins (4–5), one new natural product (6), along with twelve known molecules (3, 7–17). To the best of our knowledge, compounds 1 and 2 were two unusual furan-type isoeremophilane sesquiterpenes. It is reasonable to deem that these compounds were produced by the fungus instead of the bacterium during the fungal-bacterial co-culture on the basis of their structures, their known fungal origins and their HPLC profiles. The α-glucosidase inhibitory effects and cytotoxicity of these isolated compounds were also estimated. The new compounds 4 and 5 revealed more potent inhibitory activity against α-glucosidase than the clinical α-glucosidase inhibitor acarbose, which allowed us to explore α-glucosidase inhibitors in lasiodiplodins.
Acknowledgments
The work was financially supported by National Natural Science Foundation of China (41606167 and 21272286), China’s Marine Commonweal Research Project (201305017) and Special Financial Fund of Innovative Development of Marine Economic Demonstration Project (GD2012-D01-001). The authors thank Yongjun Lu from School of Life Sciences and Biomedical Center, Sun Yat-Sen University for providing strain 307 and Zhijian Huang from School of Marine Sciences, Sun Yat-Sen University for providing strain B2.
Abbreviations
The following abbreviations are used in this manuscript:
| ECD | Electronic Circular Dichroism |
| MTT | 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-2H-tetrazolium bromide |
| HPLC | High Performance Liquid Chromatography |
Supplementary Materials
Supplementary materials relating to this article is available online at www.mdpi.com/1660-3397/15/2/35/s1. Figure S1: The HRESIMS spectrum of compound 1, Figure S2: The 13C NMR spectrum of compound 1, Figure S3: The 1H NMR spectrum of compound 1, Figure S4: The HSQC spectrum of compound 1, Figure S5: The 1H-1H COSY spectrum of compound 1, Figure S6: The HMBC spectrum of compound 1, Figure S7: The NOESY spectrum of compound 1, Figure S8: The HRESIMS spectrum of compound 2, Figure S9: The 13C NMR spectrum of compound 2, Figure S10: The 1H NMR spectrum of compound 2, Figure S11: The HSQC spectrum of compound 2, Figure S12: The 1H-1H COSY spectrum of compound 2, Figure S13: The HMBC spectrum of compound 2, Figure S14: The NOESY spectrum of compound 2, Figure S15: The HRESIMS spectrum of compound 4, Figure S16: The 13C NMR spectrum of compound 4, Figure S17: The 1H NMR spectrum of compound 4, Figure S18: The HSQC spectrum of compound 4, Figure S19: The 1H-1H COSY spectrum of compound 4, Figure S20: The HMBC spectrum of compound 4, Figure S21: The NOESY spectrum of compound 4, Figure S22: The HRESIMS spectrum of compound 5, Figure S23: The 13C NMR spectrum of compound 5, Figure S24: The 1H NMR spectrum of compound 5, Figure S25: The HSQC spectrum of compound 5, Figure S26: The 1H-1H COSY spectrum of compound 5, Figure S27: The HMBC spectrum of compound 5, Figure S28: The NOESY spectrum of compound 5, Figure S29: The HRESIMS spectrum of compound 6, Figure S30: The 13C NMR spectrum of compound 6, Figure S31: The 1H NMR spectrum of compound 6, Figure S32: The HSQC spectrum of compound 6, Figure S33: The 1H-1H COSY spectrum of compound 6, Figure S34: The HMBC spectrum of compound 6, Figure S35: The NOESY spectrum of compound 6.
Author Contributions
Lan Liu and Jing Li designed the whole experiment and contributed to manuscript preparation. Yongcheng Lin mainly analyzed the data and checked the error on structural identification. Shah Iram Niaz and Dilfaraz Khan researched data. Zhen Wang and Yonghong Zhu took part in the cytotoxicity assay. Haiyun Zhou took part in the MS analysis. Liuhong Zhang wrote the manuscript and took part in the extraction, isolation and α-glucosidase inhibitory activity assay.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Rocha-Martin J., Harrington C., Dobson A.D.W., O’Gara F. Emerging strategies and integrated systems microbiology technologies for biodiscovery of marine bioactive compounds. Mar. Drugs. 2014;12:3516–3559. doi: 10.3390/md12063516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liu H.J., Chen S.H., Liu W.Y., Liu Y.Y., Huang X.S., She Z.G. Polyketides with immunosuppressive activities from mangrove endophytic fungus Penicillium sp. ZJ-SY2. Mar. Drugs. 2016;14:217. doi: 10.3390/md14120217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jin L.M., Quan C.H., Hou X.Y., Fan S.D. Potential pharmacological resources: Natural bioactive compounds from marine-derived fungi. Mar. Drugs. 2016;14:76. doi: 10.3390/md14040076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rutledge P.J., Challis G.L. Discovery of microbial natural products by activation of silent biosynthetic gene clusters. Nat. Rev. Microbiol. 2015;13:509–523. doi: 10.1038/nrmicro3496. [DOI] [PubMed] [Google Scholar]
- 5.Keller N.P., Turner G., Bennett J.W. Fungal secondary metabolism—From biochemistry to genomics. Nat. Rev. Microbiol. 2005;3:937–947. doi: 10.1038/nrmicro1286. [DOI] [PubMed] [Google Scholar]
- 6.Adnani N., Vazquez-Rivera E., Adibhatla S.N., Ellis G.A., Braun D.R., Bugni T.S. Investigation of interspecies interactions within marine micromonosporaceae using an improved co-culture approach. Mar. Drugs. 2015;13:6082–6098. doi: 10.3390/md13106082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schroeckh V., Scherlach K., Nützmann H.W., Shelest E., Schmidt-Heck W., Schuemann J., Martin K., Hertweck C., Brakhage A.A. Intimate bacterial-fungal interaction triggers biosynthesis of archetypal polyketides in Aspergillus nidulans. Proc. Natl. Acad. Sci. USA. 2009;106:14558–14563. doi: 10.1073/pnas.0901870106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bertrand S., Bohni N., Schnee S., Schumpp O., Gindro K., Wolfender J.L. Metabolite induction via microorganism co-culture: A potential way to enhance chemical diversity for drug discovery. Biotechnol. Adv. 2014;32:1180–1204. doi: 10.1016/j.biotechadv.2014.03.001. [DOI] [PubMed] [Google Scholar]
- 9.Ola A.R., Thomy D., Lai D., Brötz-Oesterhelt H., Proksch P. Inducing secondary metabolite production by the endophytic fungus Fusarium tricinctum through coculture with Bacillus subtilis. J. Nat. Prod. 2013;76:2094–2099. doi: 10.1021/np400589h. [DOI] [PubMed] [Google Scholar]
- 10.Rateb M.E., Hallyburton I., Houssen W.E., Bull A.T., Goodfellow M., Santhanam R., Jaspars M., Ebel R. Induction of diverse secondary metabolites in Aspergillus fumigatus by microbial co-culture. RSC Adv. 2013;3:14444–14450. doi: 10.1039/c3ra42378f. [DOI] [Google Scholar]
- 11.Whitt J., Shipley S.M., Newman D.J., Zuck K.M. Tetramic acid analogues produced by coculture of Saccharopolyspora erythraea with Fusarium pallidoroseum. J. Nat. Prod. 2014;77:173–177. doi: 10.1021/np400761g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang Y., Wang L.P., Zhuang Y.B., Kong F.D., Zhang C.X., Zhu W.M. Phenolic polyketides from the co-cultivation of marine-derived Penicillium sp. WC-29–5 and Streptomyces fradiae 007. Mar. Drugs. 2014;12:2079–2088. doi: 10.3390/md12042079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bertrand S., Schumpp O., Bohni N., Monod M., Gindro K., Wolfender J.L. De novo production of metabolites by fungal co-culture of Trichophyton rubrum and Bionectria ochroleuca. J. Nat. Prod. 2013;76:1157–1165. doi: 10.1021/np400258f. [DOI] [PubMed] [Google Scholar]
- 14.Huang S., Ding W.J., Li C.Y., Cox D.G. Two new cyclopeptides from the co-culture broth of two marine mangrove fungi and their antifungal activity. Pharmacogn. Mag. 2014;10:410–414. doi: 10.4103/0973-1296.141781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhu F., Chen G.Y., Wu J.S., Pan J.H. Structure revision and cytotoxic activity of marinamide and its methyl ester, novel alkaloids produced by co-cultures of two marine-derived mangrove endophytic fungi. Nat. Prod. Res. 2013;27:1960–1964. doi: 10.1080/14786419.2013.800980. [DOI] [PubMed] [Google Scholar]
- 16.Kossuga M.H., Ferreira A.G., Sette L.D., Berlinck R.G. Two polyketides from a co-culture of two marine-derived fungal strains. Nat. Prod. Commun. 2013;8:721–724. [Google Scholar]
- 17.Li H.X., Huang H.B., Shao C.L., Huang H.R., Jiang J.Y., Zhu X., Lin Y.Y., Liu L., Lu Y.J., Li M.F., et al. Cytotoxic norsesquiterpene peroxides from the endophytic fungus Talaromyces flavus isolated from the mangrove plant Sonneratia apetala. J. Nat. Prod. 2011;74:1230–1235. doi: 10.1021/np200164k. [DOI] [PubMed] [Google Scholar]
- 18.Song Y.X., Qiao L.T., Wang J.J., Zeng H.M., She Z.G., Miao C.D., Hong K., Gu Y.C., Liu L., Lin Y.C. Two new meroterpenes from the mangrove endophytic fungus Aspergillus sp. 085241B. Helv. Chim. Acta. 2011;94:1875–1880. doi: 10.1002/hlca.201100111. [DOI] [Google Scholar]
- 19.Ma Y.H., Li J., Huang M.X., Liu L., Wang J., Lin Y.C. Six new polyketide decalin compounds from mangrove endophytic fungus Penicillium aurantiogriseum 328#. Mar. Drugs. 2015;13:6306–6318. doi: 10.3390/md13106306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huang M.X., Li J., Liu L., Yin S., Wang J., Lin Y.C. Phomopsichin A–D; four new chromone derivatives from mangrove endophytic fungus Phomopsis sp. 33#. Mar. Drugs. 2016;14:215. doi: 10.3390/md14110215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Di Bari L., Pescitelli G., Pratelli C., Pini D., Salvadori P. Determination of absolute configuration of acyclic 1,2-diols with Mo2(OAc)4. 1. Snatzke’s method revisited. J. Org. Chem. 2001;66:4819–4825. doi: 10.1021/jo010136v. [DOI] [PubMed] [Google Scholar]
- 22.Górecki M., Jablonska E., Kruszewska A., Suszczynska A., Urbanczyk-Lipkowska Z., Gerards M., Morzycki J.W., Szczepek W.J., Frelek J. Practical method for the absolute configuration assignment of tert/tert 1,2-diols using their complexes with Mo2(OAc)4. J. Org. Chem. 2007;72:2906–2916. doi: 10.1021/jo062445x. [DOI] [PubMed] [Google Scholar]
- 23.Liu J., Du D., Si Y.K., Lv H.J., Wu X.F., Li Y., Liu Y.Y., Yu S.S. Application of dimolybdenum reagent Mo2(OAc)4 for determination of the absolute configurations of vic-diols. Chin. J. Org. Chem. 2010;30:1270–1278. [Google Scholar]
- 24.Rudiyansyah, Garson M.J. Secondary metabolites from the wood bark of Durio zibethinus and Durio kutejensis. J. Nat. Prod. 2006;69:1218–1221. doi: 10.1021/np050553t. [DOI] [PubMed] [Google Scholar]
- 25.Matsuura H., Nakamori K., Omer E.A., Hatakeyama C., Yoshihara T., Ichihara A. Three lasiodiplodins from Lasiodiplodia theobromae IFO 31059. Phytochemistry. 1998;49:579–584. doi: 10.1016/S0031-9422(98)00267-2. [DOI] [Google Scholar]
- 26.Höller U., König G.M., Wright A.D. Three new metabolites from marine-derived fungi of the genera Coniothyrium and Microsphaeropsis. J. Nat. Prod. 1999;62:114–118. doi: 10.1021/np980341e. [DOI] [PubMed] [Google Scholar]
- 27.Yang R.Y., Li C.Y., Lin Y.C., Peng G.T., She Z.G., Zhou S.N. Lactones from a brown alga endophytic fungus (No. ZZF36) from the South China Sea and their antimicrobial activities. Bioorg. Med. Chem. Lett. 2006;16:4205–4208. doi: 10.1016/j.bmcl.2006.05.081. [DOI] [PubMed] [Google Scholar]
- 28.Shao T.M., Zheng C.J., Han C.R., Chen G.Y., Dai C.Y., Song X.P., Zhang J.C., Chen W.H. Lactones from Ficus auriculata and their effects on the proliferation function of primary mouse osteoblasts in vitro. Bioorg. Med. Chem. Lett. 2014;24:3952–3955. doi: 10.1016/j.bmcl.2014.06.035. [DOI] [PubMed] [Google Scholar]
- 29.Yang Q., Asai M., Matsuura H., Yoshihara T. Potato micro-tuber inducing hydroxylasiodiplodins from Lasiodiplodia theobromae. Phytochemistry. 2000;54:489–494. doi: 10.1016/S0031-9422(00)00156-4. [DOI] [PubMed] [Google Scholar]
- 30.Leet K.H., Hayashi N., Okano M., Hall I.H., Wu R.Y., Mcphailti A.T. Lasiodiplodin, a potent antileukemic macrolide from Euphorbia splendens. Phytochemistry. 1982;21:1119–1121. doi: 10.1016/S0031-9422(00)82428-0. [DOI] [Google Scholar]
- 31.Abreu P.J., Liu Y. Ozoroalide, a new macrolide from Ozoroa insignis. Fitoterapia. 2007;78:388–389. doi: 10.1016/j.fitote.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 32.Aver W.A., Peña-Rodriguez L. Minor metabolites of Monocillium nordinii. Phytochemistry. 1987;26:1353–1355. doi: 10.1016/S0031-9422(00)81811-7. [DOI] [Google Scholar]
- 33.Chen S.H., Liu Y.Y., Liu Z.M., Cai R.L., Lu Y.J., Huang X.S., She Z.G. Isocoumarins and benzofurans from the mangrove endophytic fungus Talaromyces amestolkiae possess α-glucosidase inhibitory and antibacterial activities. RSC Adv. 2016;6:26412–26420. doi: 10.1039/C6RA02566H. [DOI] [Google Scholar]
- 34.Chen S.H., Liu Z.M., Li H.X., Xia G.P., Lu Y.J., He L., Huang S.D., She Z.G. β-Resorcylic acid derivatives with α-glucosidase inhibitory activity from Lasiodiplodia sp. ZJ-HQ1, an endophytic fungus in the medicinal plant Acanthus ilicifolius. Phytochem. Lett. 2015;13:141–146. doi: 10.1016/j.phytol.2015.05.019. [DOI] [Google Scholar]
- 35.Li J., Xue Y.Y., Yuan J., Lu Y.J., Zhu X., Lin Y.C., Liu L. Lasiodiplodins from mangrove endophytic fungus Lasiodiplodia sp. 318#. Nat. Prod. Res. 2016;30:755–760. doi: 10.1080/14786419.2015.1062762. [DOI] [PubMed] [Google Scholar]
- 36.Huang J.G., Xu J.Y., Wang Z., Khan D., Niaz S.I., Zhu Y.H., Lin Y.C., Li J., Liu L. New lasiodiplodins from mangrove endophytic fungus Lasiodiplodia sp. 318#. Nat. Prod. Res. 2016;31:326–332. doi: 10.1080/14786419.2016.1239096. [DOI] [PubMed] [Google Scholar]
- 37.Wang X., Tan T., Mao Z.G., Lei N., Wang Z.M., Hu B., Chen Z.Y., She Z.G., Zhu Y.H., Wang H.J. The marine metabolite SZ-685C induces apoptosis in primary human nonfunctioning pituitary adenoma cells by inhibition of the Akt pathway in vitro. Mar. Drugs. 2015;13:1569–1580. doi: 10.3390/md13031569. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.