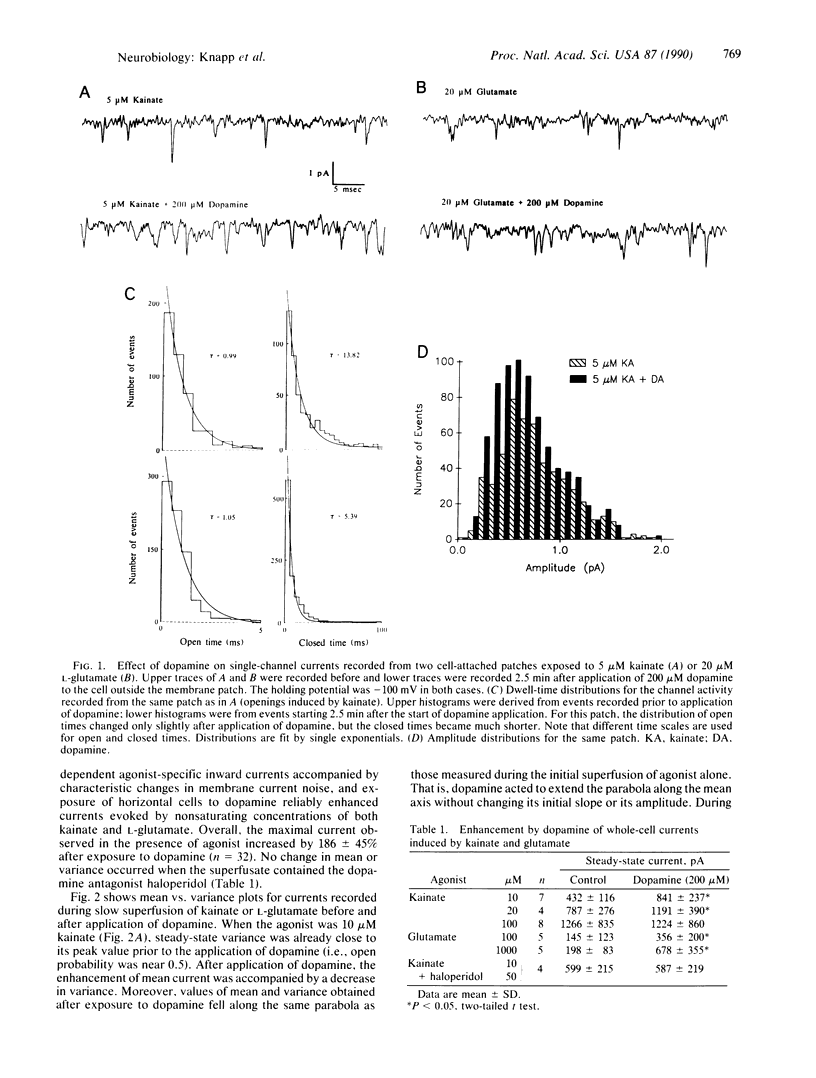
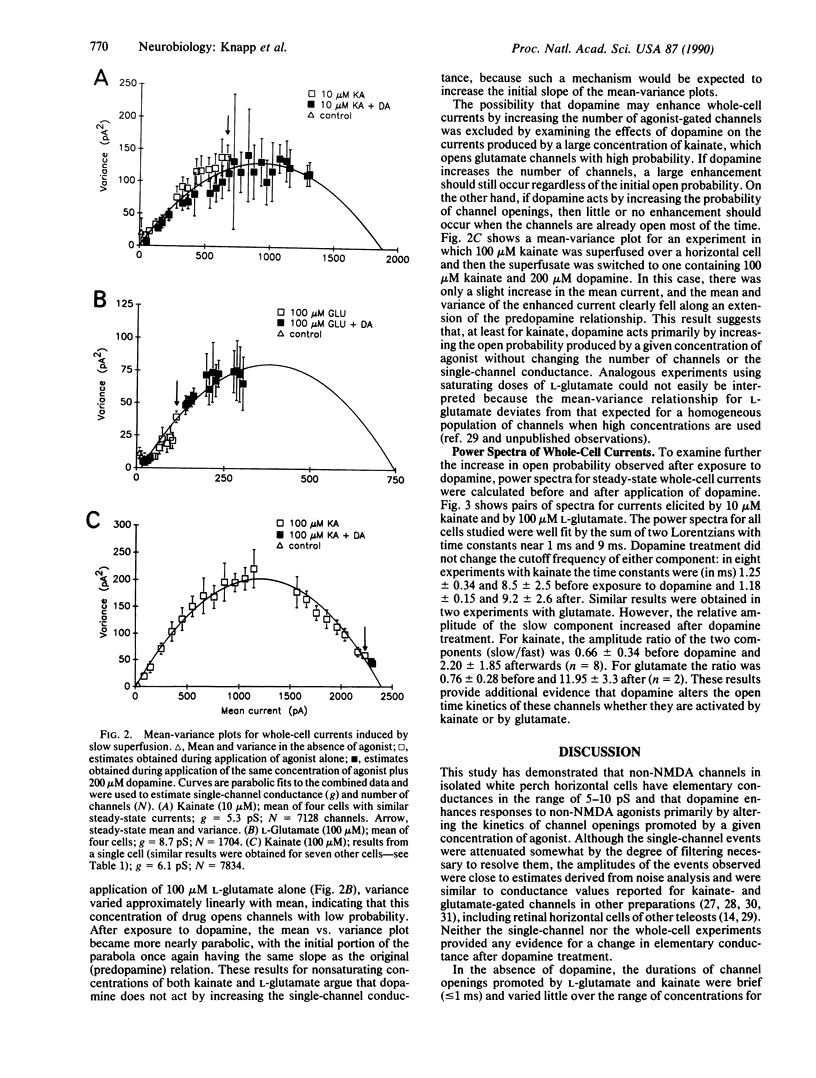
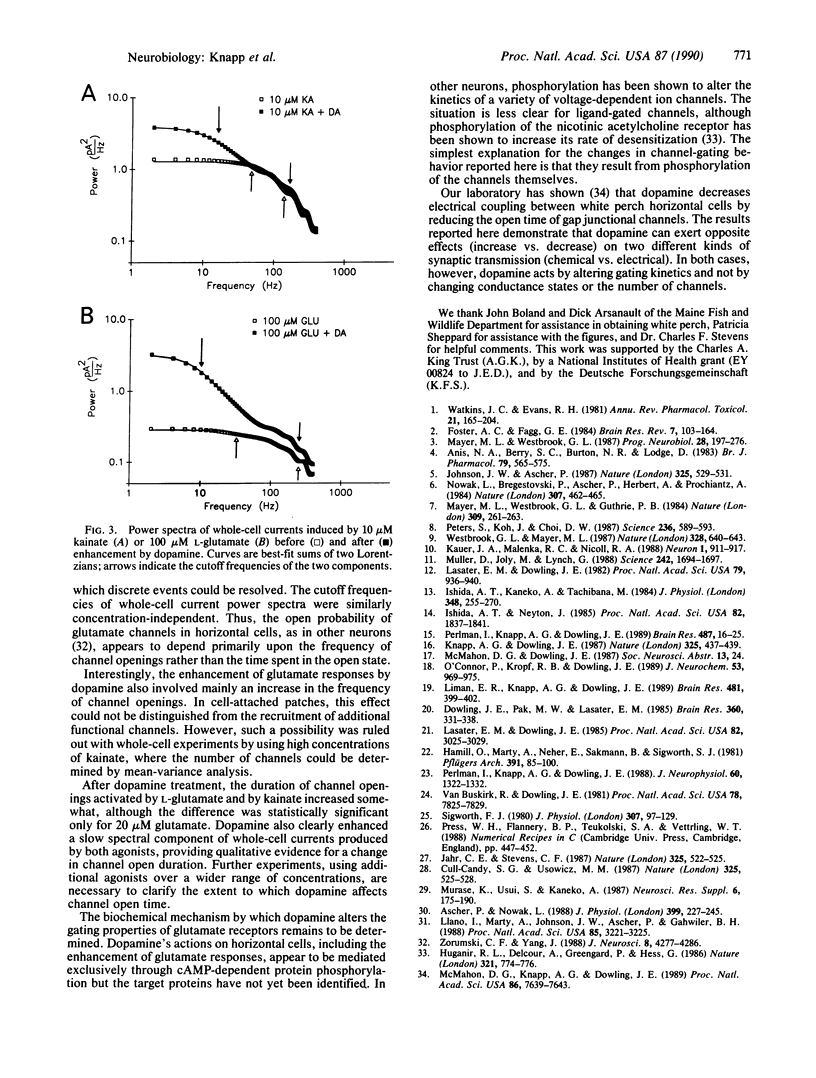
Abstract
Upon exposure to dopamine, cultured teleost retinal horizontal cells become more responsive to the putative photoreceptor neurotransmitter L-glutamate and to its analog kainate. We have recorded unitary and whole-cell currents to determine the mechanism by which dopamine enhances ion channels activated by these agents. In single-channel recordings from cell-attached patches with agonist in the patch pipette, the frequency of 5- to 10-pS unitary events, but not their amplitude, increased by as much as 150% after application of dopamine to the rest of the cell. The duration of channel openings also increased somewhat, by 20-30%. In whole-cell experiments, agonists with and without dopamine were applied to voltage-clamped horizontal cells by slow superfusion. Analysis of whole-cell current variance as a function of mean current indicated that dopamine increased the probability of channel opening for a give agonist concentration without changing the amount of current passed by an individual channel. For kainate, noise analysis additionally demonstrated that dopamine did not alter the number of functional channels. Dopamine also increased a slow spectral component of whole-cell currents elicited by kainate or glutamate, suggesting a change in the open-time kinetics of the channels. This effect was more pronounced for currents induced by glutamate than for those induced by kainate. We conclude that dopamine potentiates the activity of horizontal cell glutamate receptors by altering the kinetics of the ion channel to favor the open state.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anis N. A., Berry S. C., Burton N. R., Lodge D. The dissociative anaesthetics, ketamine and phencyclidine, selectively reduce excitation of central mammalian neurones by N-methyl-aspartate. Br J Pharmacol. 1983 Jun;79(2):565–575. doi: 10.1111/j.1476-5381.1983.tb11031.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ascher P., Nowak L. Quisqualate- and kainate-activated channels in mouse central neurones in culture. J Physiol. 1988 May;399:227–245. doi: 10.1113/jphysiol.1988.sp017077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cull-Candy S. G., Usowicz M. M. Multiple-conductance channels activated by excitatory amino acids in cerebellar neurons. Nature. 1987 Feb 5;325(6104):525–528. doi: 10.1038/325525a0. [DOI] [PubMed] [Google Scholar]
- Dowling J. E., Pak M. W., Lasater E. M. White perch horizontal cells in culture: methods, morphology and process growth. Brain Res. 1985 Dec 23;360(1-2):331–338. doi: 10.1016/0006-8993(85)91250-8. [DOI] [PubMed] [Google Scholar]
- Foster A. C., Fagg G. E. Acidic amino acid binding sites in mammalian neuronal membranes: their characteristics and relationship to synaptic receptors. Brain Res. 1984 May;319(2):103–164. doi: 10.1016/0165-0173(84)90020-1. [DOI] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Huganir R. L., Delcour A. H., Greengard P., Hess G. P. Phosphorylation of the nicotinic acetylcholine receptor regulates its rate of desensitization. Nature. 1986 Jun 19;321(6072):774–776. doi: 10.1038/321774a0. [DOI] [PubMed] [Google Scholar]
- Ishida A. T., Kaneko A., Tachibana M. Responses of solitary retinal horizontal cells from Carassius auratus to L-glutamate and related amino acids. J Physiol. 1984 Mar;348:255–270. doi: 10.1113/jphysiol.1984.sp015108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishida A. T., Neyton J. Quisqualate and L-glutamate inhibit retinal horizontal-cell responses to kainate. Proc Natl Acad Sci U S A. 1985 Mar;82(6):1837–1841. doi: 10.1073/pnas.82.6.1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahr C. E., Stevens C. F. Glutamate activates multiple single channel conductances in hippocampal neurons. Nature. 1987 Feb 5;325(6104):522–525. doi: 10.1038/325522a0. [DOI] [PubMed] [Google Scholar]
- Johnson J. W., Ascher P. Glycine potentiates the NMDA response in cultured mouse brain neurons. Nature. 1987 Feb 5;325(6104):529–531. doi: 10.1038/325529a0. [DOI] [PubMed] [Google Scholar]
- Kauer J. A., Malenka R. C., Nicoll R. A. A persistent postsynaptic modification mediates long-term potentiation in the hippocampus. Neuron. 1988 Dec;1(10):911–917. doi: 10.1016/0896-6273(88)90148-1. [DOI] [PubMed] [Google Scholar]
- Knapp A. G., Dowling J. E. Dopamine enhances excitatory amino acid-gated conductances in cultured retinal horizontal cells. 1987 Jan 29-Feb 4Nature. 325(6103):437–439. doi: 10.1038/325437a0. [DOI] [PubMed] [Google Scholar]
- Lasater E. M., Dowling J. E. Carp horizontal cells in culture respond selectively to L-glutamate and its agonists. Proc Natl Acad Sci U S A. 1982 Feb;79(3):936–940. doi: 10.1073/pnas.79.3.936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasater E. M., Dowling J. E. Dopamine decreases conductance of the electrical junctions between cultured retinal horizontal cells. Proc Natl Acad Sci U S A. 1985 May;82(9):3025–3029. doi: 10.1073/pnas.82.9.3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liman E. R., Knapp A. G., Dowling J. E. Enhancement of kainate-gated currents in retinal horizontal cells by cyclic AMP-dependent protein kinase. Brain Res. 1989 Mar 6;481(2):399–402. doi: 10.1016/0006-8993(89)90822-6. [DOI] [PubMed] [Google Scholar]
- Llano I., Marty A., Johnson J. W., Ascher P., Gähwiler B. H. Patch-clamp recording of amino acid-activated responses in "organotypic" slice cultures. Proc Natl Acad Sci U S A. 1988 May;85(9):3221–3225. doi: 10.1073/pnas.85.9.3221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer M. L., Westbrook G. L., Guthrie P. B. Voltage-dependent block by Mg2+ of NMDA responses in spinal cord neurones. Nature. 1984 May 17;309(5965):261–263. doi: 10.1038/309261a0. [DOI] [PubMed] [Google Scholar]
- Mayer M. L., Westbrook G. L. The physiology of excitatory amino acids in the vertebrate central nervous system. Prog Neurobiol. 1987;28(3):197–276. doi: 10.1016/0301-0082(87)90011-6. [DOI] [PubMed] [Google Scholar]
- McMahon D. G., Knapp A. G., Dowling J. E. Horizontal cell gap junctions: single-channel conductance and modulation by dopamine. Proc Natl Acad Sci U S A. 1989 Oct;86(19):7639–7643. doi: 10.1073/pnas.86.19.7639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller D., Joly M., Lynch G. Contributions of quisqualate and NMDA receptors to the induction and expression of LTP. Science. 1988 Dec 23;242(4886):1694–1697. doi: 10.1126/science.2904701. [DOI] [PubMed] [Google Scholar]
- Nowak L., Bregestovski P., Ascher P., Herbet A., Prochiantz A. Magnesium gates glutamate-activated channels in mouse central neurones. Nature. 1984 Feb 2;307(5950):462–465. doi: 10.1038/307462a0. [DOI] [PubMed] [Google Scholar]
- Perlman I., Knapp A. G., Dowling J. E. Local superfusion modifies the inward rectifying potassium conductance of isolated retinal horizontal cells. J Neurophysiol. 1988 Oct;60(4):1322–1332. doi: 10.1152/jn.1988.60.4.1322. [DOI] [PubMed] [Google Scholar]
- Perlman I., Knapp A. G., Dowling J. E. Responses of isolated white perch horizontal cells to changes in the concentration of photoreceptor transmitter agonists. Brain Res. 1989 May 15;487(1):16–25. doi: 10.1016/0006-8993(89)90935-9. [DOI] [PubMed] [Google Scholar]
- Peters S., Koh J., Choi D. W. Zinc selectively blocks the action of N-methyl-D-aspartate on cortical neurons. Science. 1987 May 1;236(4801):589–593. doi: 10.1126/science.2883728. [DOI] [PubMed] [Google Scholar]
- Sigworth F. J. The variance of sodium current fluctuations at the node of Ranvier. J Physiol. 1980 Oct;307:97–129. doi: 10.1113/jphysiol.1980.sp013426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Buskirk R., Dowling J. E. Isolated horizontal cells from carp retina demonstrate dopamine-dependent accumulation of cyclic AMP. Proc Natl Acad Sci U S A. 1981 Dec;78(12):7825–7829. doi: 10.1073/pnas.78.12.7825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins J. C., Evans R. H. Excitatory amino acid transmitters. Annu Rev Pharmacol Toxicol. 1981;21:165–204. doi: 10.1146/annurev.pa.21.040181.001121. [DOI] [PubMed] [Google Scholar]
- Westbrook G. L., Mayer M. L. Micromolar concentrations of Zn2+ antagonize NMDA and GABA responses of hippocampal neurons. Nature. 1987 Aug 13;328(6131):640–643. doi: 10.1038/328640a0. [DOI] [PubMed] [Google Scholar]
- Zorumski C. F., Yang J. AMPA, kainate, and quisqualate activate a common receptor-channel complex on embryonic chick motoneurons. J Neurosci. 1988 Nov;8(11):4277–4286. doi: 10.1523/JNEUROSCI.08-11-04277.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]



