Abstract
Background: Though increasing evidence supports association between gaseous air pollution and stroke, it remains unclear whether the effects differ in season, sex and age. The aim of this study was to examine the associations of gaseous air pollution with stroke admissions in Beijing, 2013–2014 in different subgroups. Methods: Case-crossover design and conditional logistic regression were used to perform the analyses. We examined the exposure-response relationship between air pollution and stroke. Stratified analyses were performed in different seasons, sex, and age groups. Results: There were 147,624 stroke admissions during the study period. In the whole study period, percent changes of stroke admissions were 0.82% (95% CI: 0.52% to 1.13%) and 0.73% (95% CI: 0.44% to 1.03%) per 10 μg/m3 increase in the same day conentration of nitrogen dioxide (NO2) and sulfur dioxide (SO2). The positive associations were higher in warm seasons and with patients >65 years (p < 0.05). Contrary effects of carbon monoxide (CO) and ozone on stroke admissions were observed in different seasons. Conclusions: NO2 and SO2 were positively associated with stroke admissions, with stronger effects in warm seasons and with patients >65 years. The associations of CO and ozone with stroke admissions differed across seasons.
Keywords: stroke, air pollution, hospital admission, exposure-response relationship
1. Introduction
Due to increasing motorized traffic and industrial and agricultural activities, concentrations of air pollutants increased substantially in recent decades [1,2,3]. Stroke is a leading cause of death and disability globally, which accounts for five million deaths each year representing nearly 10% of all deaths, and 44 million disability-adjusted life-years are lost annually due to stroke [4]. In China, stroke prevalence estimates in 2013 were statistically greater than those reported in China three decades ago. The age-standardized prevalence, incidence and mortality rates were 1114.8 per 100,000 people, 246.8 and 114.8 per 100,000 person-years, respectively [5]. Therefore, effects of air pollution on stroke should be explored given the great stroke burden in terms of mortality and disability worldwide for primary prevention efforts.
Although exposure to air pollution has been associated with an increase in mortality and morbidity of stroke, these studies were mainly focused on particulate matters (PM) and air pollutants from fossil fuel combustion [6,7,8,9]. For carbon monoxide (CO) and ozone, studies reported inconsistent results [10,11,12,13]. For example, Tian et al. (2015) found that low environmental CO was associated with reduced risk of daily stroke admissions [10] and Männistö et al. (2015) observed decreased odds of stroke events with exposure to ozone [12]. These findings suggest that there may be a non-linear exposure-response relationship which was not well researched. Besides, it remains unclear how air pollution influences stroke in different seasons with varied concentrations.
It is important to understand the characteristics of individuals who are at increased risk of adverse stroke events due to air pollution. Some subpopulations may be particularly sensitive to PM exposure, such as elderly subjects, diabetic patients, and individuals with known coronary artery disease [14,15]. However, prior findings about the modifying effects of characteristics of individuals for gaseous air pollution remain inconsistent. For example, Kan et al. (2008) found that females and the elderly were more vulnerable to nitrogen dioxide (NO2), sulfur dioxide (SO2), and ozone [16]. Zheng et al. (2013) also found elderly people had higher estimates for cerebrovascular admissions for NO2 and SO2 than the younger [17]. However, other studies suggest opposite results or no modifying effects of age and sex [8,18].
The aim of this study was to examine the associations of gaseous air pollutants with stroke admissions and their exposure-response relationships, using a case-crossover design, and to determine whether the associations differed in season, sex, and age, in order to capture the susceptible subpopulations.
2. Materials and Methods
2.1. Air Pollution and Health Data
NO2, SO2, CO, ozone, and particulate matter that is 2.5 µm or less in diameter (PM2.5) measurements in Beijing, China during 1 January 2013 to 31 December 2014 were retrieved from the Centre of City Environmental Protection Monitoring Website Platform of Beijing. The District-level 24-h mean concentrations of air pollutants were used as metrics of exposures. Meteorological data in 16 districts of Beijing were obtained from the Chinese Meteorological Bureau.
We obtained disease data from the medical record database for cardiovascular and cerebrovascular diseases in Beijing. The medical record database contains all the hospitals that have the capability to diagnose and treat cardiovascular and cerebrovascular disease in Beijing. Hospital admissions for stroke (International Classification of Diseases, 10th revision, ICD10: I61-63) and demographic characteristics, including sex and age, between 1 January 2013 to 31 December 2014 were retrieved from the database.
The protocol of this study was approved by the School of Public Health, Capital Medical University (SPHCMU) Institutional Review Board (IRB00009511). Informed consent was not required for this study because all health data were analyzed at the aggregated level, no individual record information for patients was involved and no patients were contacted.
2.2. Statistical Analysis
A bidirectional case-crossover design was used to examine the associations of gaseous air pollutants and stroke admissions. The case-crossover design is a variant of the matched case control study and consists of only cases, which serve as their own controls in the analysis. This design can control the effects of day of week, season, and slowly varying confounders. In this study, case period was defined as the day of hospital admission. Control periods were chosen in a two-week window before and after the case period for the same days of the week as the hospital admission [19]. Conditional logistic regression was used to perform the analyses.
Temperature, relative humidity, and holidays were controlled as previous studies have shown that these variables are the potential confounding factors [20,21]. We used natural cubic splines with three degrees of freedom (df) to include the effects of temperature and relative humidity on stroke on the day of hospital admission. The public holidays were controlled through binary variable (coded as public holiday = 1 and no public holiday = 0) in the model. All results were from models including temperature, relative humidity, and holidays.
We graphically examined the exposure-response relationship of air pollutants with stroke admissions. We applied natural cubic splines with 3 df for NO2, SO2 and ozone, and 4 df for CO to model the relationship. Two-pollutant models adjusting the other gaseous air pollutants were fitted to check the robustness of our results.
Percent change and 95% confidence interval (CI) in stroke admissions associated with a 10.0 μg/m3 increase in daily concentration of NO2, SO2 and ozone, and a 1.0 mg/m3 increase in daily concentration of CO were estimated on the same day (lag0), the previous day (lag1), the two preceding days (lag2), and three days moving average (lag0–2).
Stratified analyses were performed to examine whether the associations differed in season (warm season: May to October and cold season: November to April), sex (men and women) and age (>65 years and ≤65 years). Two-pollutant models adjusting PM2.5 were fitted to check the modifying effect of PM2.5 on associations of gaseous air pollutants and stroke admissions. We tested the statistical significance of subgroup differences through Z test [10].
Sensitivity analyses were performed to check the robustness of the results. We used a different method to choose controls, i.e., time-stratified case-crossover design. Degrees of freedom were changed for meteorological variables. We also used temperature and relative humidity lagged by up to two weeks to control the potential lagged effects of meteorological variables.
The conditional logistic regression was performed using the PHREG procedure in SAS statistical software (Version 9.2; SAS Institute, Inc., Cary, NC, USA). We used the Akaike’s Information Criterion to choose the df for the splines and determine the goodness of the model fit. All statistical tests were two-sided, and p-values less than 0.05 were considered statistically significant.
3. Results
There were 147,624 stroke admissions in 2013–2014 that formed the basis for this study. On average there were 205.1 hospital admissions per day for stroke, including 125.7 for men and 119.2 for patients >65 years (Table 1).
Table 1.
Summary statistics of stroke admissions in Beijing, China, 2013–2014.
| Count/Day | Mean | SD | Median | IQR | Minimum | Maximum |
|---|---|---|---|---|---|---|
| All days | ||||||
| All | 205.1 | 62.8 | 212.0 | 113.0 | 81.0 | 375.0 |
| Men | 125.7 | 40.1 | 128.0 | 70.0 | 42.0 | 225.0 |
| Women | 79.4 | 24.5 | 82.0 | 42.0 | 28.0 | 150.0 |
| Age > 65 | 119.2 | 37.9 | 126.0 | 69.0 | 41.0 | 215.0 |
| Age ≤ 65 | 85.8 | 26.7 | 85.0 | 43.0 | 27.0 | 179.0 |
| Warm days | ||||||
| All | 206.6 | 64.4 | 210.0 | 118.5 | 81.0 | 375.0 |
| Men | 127.2 | 41.5 | 129.0 | 75.0 | 51.0 | 225.0 |
| Women | 79.4 | 24.7 | 81.5 | 42.5 | 28.0 | 150.0 |
| Age > 65 | 120.5 | 39.5 | 126.5 | 72.0 | 45.0 | 208.0 |
| Age ≤ 65 | 86.1 | 26.7 | 86.0 | 44.5 | 36.0 | 179.0 |
| Cold days | ||||||
| All | 203.6 | 61.2 | 213.5 | 108.0 | 81.0 | 340.0 |
| Men | 124.1 | 38.6 | 128.0 | 65.0 | 42.0 | 218.0 |
| Women | 79.5 | 24.4 | 82.5 | 41.0 | 30.0 | 134.0 |
| Age > 65 | 118.0 | 36.3 | 126.0 | 64.0 | 41.0 | 215.0 |
| Age ≤ 65 | 85.6 | 26.8 | 85.0 | 43.0 | 27.0 | 152.0 |
SD, standard deviation; IQR, interquartile range.
Summary statistics of air pollutants and meteorological variables for all days in Beijing, China, 2013–2014 are presented in Table 2. The means (standard deviation, SD) of air pollutants and meteorological variables were 52.5 (28.1) µg/m3 for NO2, 24.5 (24.8) µg/m3 for SO2, 1.7 (1.2) mg/m3 for CO, 117.5 (73.2) µg/m3 for ozone, 89.8 (73.2) µg/m3 for PM2.5, 11.9 (11.2) °C for temperature, and 56.1 (17.5) % for relative humidity, respectively. On warm days, the means (SD) of NO2, SO2, CO, and ozone were 44.9 (21.5) µg/m3, 11.4 (11.0) µg/m3, 1.2 (0.6) mg/m3, and 163.7 (69.1) µg/m3, respectively. On cold days, the corresponding values were 60.1 (31.2) µg/m3, 43.7 (38.7) µg/m3, 2.2 (1.4) mg/m3, and 70.6 (39.9) µg/m3, respectively.
Table 2.
Summary statistics of air pollutants and meteorological variables in Beijing, China, 2013–2014.
| Variables | Mean | SD | Med | IQR | Min | Max |
|---|---|---|---|---|---|---|
| All days | ||||||
| NO2 (μg/m3) | 52.5 | 28.1 | 48.3 | 36.3 | 1.0 | 196.8 |
| SO2 (μg/m3) | 24.5 | 24.8 | 14.8 | 27.1 | 1.0 | 197.1 |
| CO (mg/m3) | 1.7 | 1.2 | 1.3 | 1.0 | 0.0 | 11.5 |
| Ozone (μg/m3) | 117.5 | 73.2 | 99.0 | 102.5 | 0.0 | 703.0 |
| PM2.5 (μg/m3) | 89.8 | 73.2 | 71.4 | 82.0 | 4.0 | 685.5 |
| Temperature (°C) | 11.9 | 11.2 | 13.5 | 20.7 | −13.3 | 31.1 |
| Relative humidity (%) | 56.1 | 17.5 | 56.5 | 27.2 | 12.8 | 96.0 |
| Warm season | ||||||
| NO2 (μg/m3) | 44.9 | 21.5 | 42.4 | 58.0 | 1.0 | 159.9 |
| SO2 (μg/m3) | 11.4 | 11.0 | 8.0 | 14.0 | 1.0 | 197.1 |
| CO (mg/m3) | 1.2 | 0.6 | 1.0 | 1.5 | 0.0 | 9.0 |
| Ozone (μg/m3) | 163.7 | 69.1 | 160.0 | 101.1 | 2.0 | 703.0 |
| PM2.5 (μg/m3) | 79.8 | 57.7 | 66.6 | 70.3 | 4.0 | 373.0 |
| Temperature (°C) | 20.9 | 5.5 | 22.0 | 7.7 | 5.0 | 31.1 |
| Relative humidity (%) | 64.1 | 15.2 | 65.3 | 21.1 | 18.4 | 94.3 |
| Cold season | ||||||
| NO2 (μg/m3) | 60.1 | 31.2 | 55.7 | 79.0 | 1.0 | 196.8 |
| SO2 (μg/m3) | 43.7 | 38.7 | 34.0 | 57.5 | 2.0 | 537.0 |
| CO (mg/m3) | 2.2 | 1.4 | 2.0 | 3.0 | 0.1 | 11.5 |
| Ozone (μg/m3) | 70.6 | 39.9 | 67.0 | 44.2 | 0.0 | 331.0 |
| PM2.5 (μg/m3) | 100.6 | 84.8 | 80.0 | 92.9 | 4.3 | 685.5 |
| Temperature (°C) | 2.5 | 7.1 | 1.3 | 10.5 | −13.3 | 20.9 |
| Relative humidity (%) | 47.8 | 15.7 | 46.8 | 22.0 | 12.8 | 96.0 |
SD, standard deviation; Med, median; IQR, interquartile range; Min, minimum value; Max, maximum value; NO2, nitrogen dioxide; SO2, sulfur dioxide; CO, carbon monoxide; PM2.5, particulate matter that is 2.5 µm or less in diameter.
Spearman correlation coefficients for correlations among the exposure variables are presented in Table 3. NO2, SO2, and CO were positively correlated with each other (correlation coefficient, r = 0.58 to 0.68, p < 0.001) and negatively correlated with ozone (r = −0.43 to –0.29, p < 0.001). Correlations between temperature and NO2, SO2, and CO were negative (r = −0.29 to –0.42, p < 0.001). Correlation between temperature and ozone was positive (r = 0.79, p < 0.001).
Table 3.
Spearman correlation coefficients among the exposure variables.
| Variable | NO2 | SO2 | CO | Ozone | PM2.5 | T | RH |
|---|---|---|---|---|---|---|---|
| NO2 | 1.00 | 0.58 * | 0.61 * | −0.29 * | 0.61 * | −0.24 * | 0.13 * |
| SO2 | - | 1.00 | 0.68 * | −0.46 * | 0.48 * | −0.60 * | −0.21 * |
| CO | - | - | 1.00 | −0.43 * | 0.68 * | −0.42 * | 0.18 * |
| Ozone | - | - | - | 1.00 | −0.09 * | 0.79 * | 0.15 * |
| PM2.5 | - | - | - | - | 1.00 | −0.05 * | 0.40 * |
| T | - | - | - | - | - | 1.00 | 0.36 * |
| RH | - | - | - | - | - | - | 1.00 |
NO2, nitrogen dioxide; SO2, sulfur dioxide; CO, carbon monoxide; PM2.5, particulate matter that is 2.5 µm or less in diameter; T, temperature; RH, relative humidity. * p < 0.001.
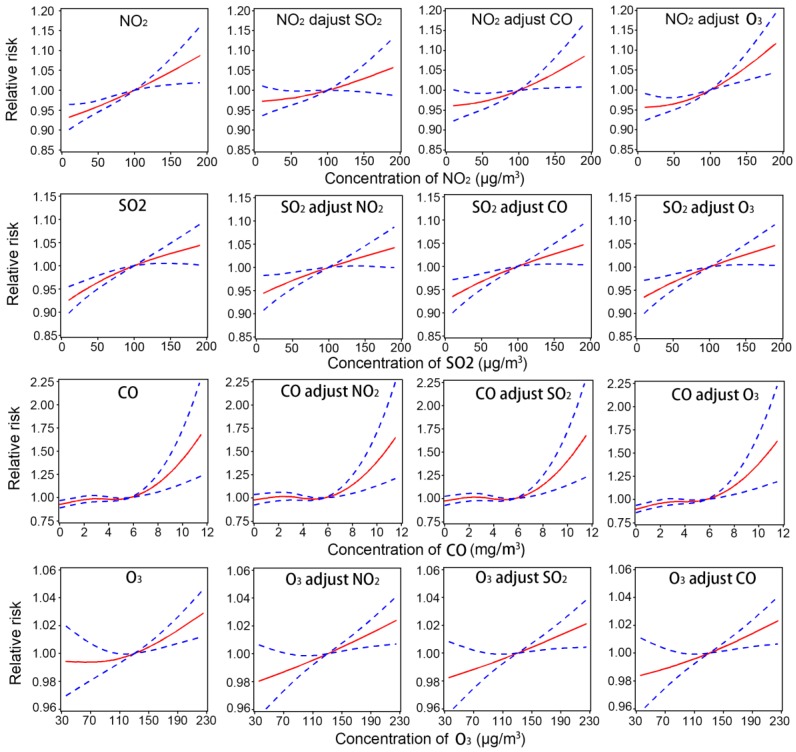
Exposure-response relationships of stroke admissions with air pollutants in different pollutant models are shown in Figure 1. The exposure-response curves suggest an approximately linear rise in stroke risk with daily changes in NO2, SO2, and ozone concentrations in both single- and two-pollutant models. For CO, there was a linear increase in stroke risk at higher CO concentrations, with a threshold of around 5 mg/m3 for a 24-h average exposure.
Figure 1.
Exposure-response relationships of stroke admissions with air pollutants on the concurrent day in different pollutant models.
Percent changes (95% CI) in hospital admissions for stroke associated with a 10.0 μg/m3 increase in NO2, SO2, and ozone, and a 1.0 mg/m3 increase in CO are shown in Table 4.
Table 4.
Percent change (95% CI) in hospital admissions for stroke associated with a 10.0 μg/m3 increase in NO2, SO2, and ozone, and 1.0 mg/m3 increase in CO stratified by season.
| Variables | All Days | Season | Test for Difference between Seasons | ||
|---|---|---|---|---|---|
| Warm Season | Cold Season | Z Value | p Value | ||
| NO2 | |||||
| Lag0 | 0.82 (0.52 to 1.13) # | 2.35 (1.76 to 2.94) # | 0.40 (0.02 to 0.78) * | 5.426 | <0.001 |
| Lag1 | 0.39 (0.11 to 0.67) * | 1.80 (1.22 to 2.37) # | −0.13 (−0.46 to 0.20) | 5.735 | <0.001 |
| Lag2 | −0.14 (−0.40 to 0.12) | 0.34 (−0.22 to 0.90) | −0.08 (−0.38 to 0.23) | 1.281 | 0.200 |
| 3 days average | 0.22 (−0.18 to 0.61) | 2.24 (1.44 to 3.05) # | −0.37 (−0.84 to 0.10) | 5.516 | <0.001 |
| SO2 | |||||
| Lag0 | 0.73 (0.44 to 1.03) # | 2.38 (1.45 to 3.32) # | 0.67 (0.35 to 1.00) # | 3.406 | 0.001 |
| Lag1 | 0.40 (0.11 to 0.68) * | 1.20 (0.28 to 2.12) * | 0.34 (0.04 to 0.64) * | 1.741 | 0.082 |
| Lag2 | 0.16 (−0.10 to 0.42) | 0.43 (−0.45 to 1.33) | 0.22 (−0.05 to 0.49) | 0.451 | 0.652 |
| 3 days average | 0.39 (−0.03 to 0.81) | 0.32 (−0.91 to 1.56) | 0.36 (−0.10 to 0.82) | −0.065 | 0.948 |
| CO | |||||
| Lag0 | 1.35 (0.60 to 2.11) # | −1.86 (−3.70 to 0.01) | 2.07 (1.20 to 2.95) # | −3.714 | <0.001 |
| Lag1 | 0.34 (−0.33 to 1.01) | −4.26 (−5.98 to −2.51) # | 0.78 (0.04 to 1.52) * | −5.143 | <0.001 |
| Lag2 | −0.40 (−1.03 to 0.23) | −5.87 (−7.51 to −4.20) # | −0.25 (−0.94 to 0.45) | −6.016 | <0.001 |
| 3 days average | −0.01 (−0.95 to 0.95) | −7.95 (−10.34 to −5.49) # | 0.56 (−0.50 to 1.64) | −6.103 | <0.001 |
| Ozone | |||||
| Lag0 | 0.23 (0.08 to 0.37) * | 0.78 (0.62 to 0.95) # | −2.06 (−2.39 to −1.73) # | 14.946 | <0.001 |
| Lag1 | 0.22 (0.09 to 0.36) * | 0.60 (0.44 to 0.76) # | −1.48 (−1.78 to −1.18) # | 11.862 | <0.001 |
| Lag2 | 0.03 (−0.11 to 0.16) | 0.36 (0.20 to 0.51) # | −1.44 (−1.73 to −1.15) # | 10.556 | <0.001 |
| 3 days average | 0.20 (0.00 to 0.40) | 0.67 (0.44 to 0.91) # | −1.90 (−2.35 to −1.45) # | 9.833 | <0.001 |
CI, confidence interval; NO2, nitrogen dioxide; SO2, sulfur dioxide; CO, carbon monoxide. * p < 0.05; # p < 0.001.
In the whole study period, NO2 was positively associated with stroke admissions on the same day (0.82%, 95% CI: 0.52% to 1.13%) and the previous day (0.39%, 95% CI: 0.11% to 0.67%). When stratified by season, stroke admissions were positively associated with the same day, previous day, and three days moving average concentration of NO2 in warm season (p < 0.05). However, in cold seasons, the positive association was only observed on the same day (0.40%, 95% CI: 0.02% to 0.78%). The associations were higher in warm days than in cold days at lag0 (Z = 5.426, p < 0.001), lag1 (Z = 5.735, p < 0.001), and lag0–2 day (Z = 5.516, p < 0.001).
Similar with NO2, SO2 was positively associated with stroke admissions at lag0 (0.73%, 95% CI: 0.44% to 1.03%) and lag1 (0.40%, 95% CI: 0.11% to 0.68%) day in the whole study period. In both warm and cold seasons, positive associations with SO2 were observed on the same day and the previous day (p < 0.05), with statistically significant higher values in warm seasons at lag0 day (Z = 3.406, p = 0.001).
For CO, associations with stroke admissions were different when stratified by seasons. In cold seasons, stroke admissions were positively associated with the same day (2.47%, 95% CI: 1.54% to 3.41%) and the previous day (1.04%, 95% CI: 0.25% to 1.84%) concentration of CO. However, in warm seasons, CO was negatively associated with stroke admissions at lag1 (−4.26%, 95% CI: −5.98% to −2.51%), lag2 (−5.87%, 95% CI: −7.51% to −4.20%) and lag0–2 (−7.95%, 95% CI: −10.34% to −5.49%) day.
In addition, a 10 µg/m3 increase in the same day and the previous day concentration of ozone corresponded to a 0.23% (95% CI: 0.08% to 0.37%) and 0.22% (95% CI: 0.09% to 0.36%) increase in stroke admissions in the whole study period. However, the associations were different when stratified by season. The ambient ozone was positively associated with stroke admissions in warm season, and negatively associated with stroke admissions in cold season (p < 0.05).
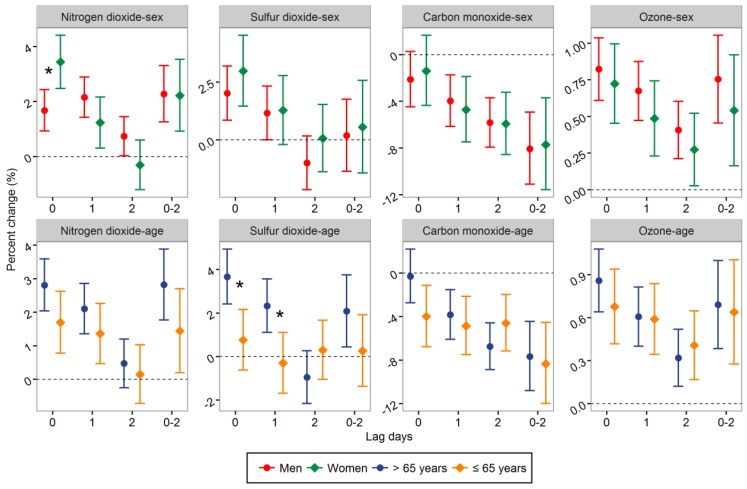
Percent changes in stroke admissions stratified by sex and age were calculated in different seasons. In warm seasons, positive associations of stroke admissions with NO2 and SO2 appeared to be stronger in patients >65 years, with statistically significant higher values for patients >65 years at lag0 (Z = 3.021, p = 0.003) and lag1 day (Z = 2.766, p = 0.006) for SO2 (Figure 2). The positive association of stroke admissions with the same day concentration of NO2 was significantly higher in female patients (Z = −2.830, p = 0.005). However, the results were inconsistent in the other lag structures.
Figure 2.
Percent change (95% CI) in hospital admissions for stroke associated with a 10.0 μg/m3 increase in NO2, SO2, and ozone, and 1.0 mg/m3 increase in CO stratified by sex and age in warm seasons. * The difference between two subgroups was statistically significant (p < 0.05).
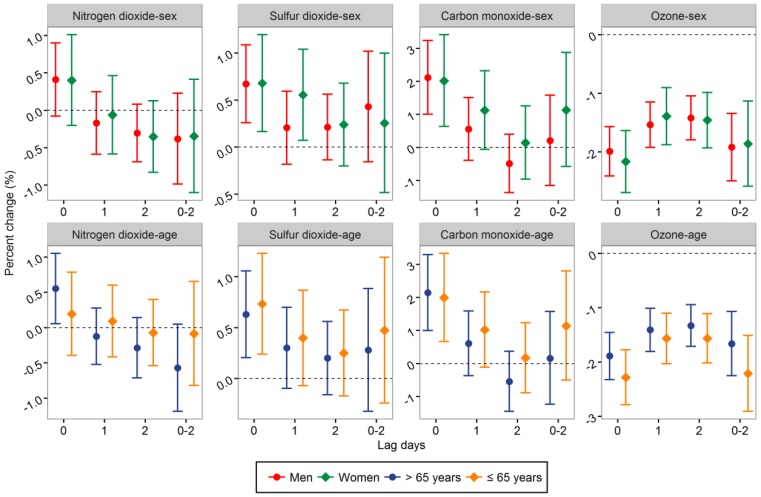
No statistically significant differences were found in different sex and age subgroups in cold seasons (Figure 3).
Figure 3.
Percent change (95% CI) in hospital admissions for stroke associated with a 10.0 μg/m3 increase in NO2, SO2, and ozone, and 1.0 mg/m3 increase in CO stratified by sex and age in cold seasons.
Two-pollutant models adjusting PM2.5 were performed in both warm and cold seasons (Table 5 and Table 6). In warm seasons, the positive associations of stroke admissions with NO2, SO2, and ozone were weaker in two-pollutant model than in single-pollutant model. The differences were statistically significant for NO2 at lag1 (Z = 2.530, p = 0.011) and lag2 day (Z = 2.078, p = 0.038). The negative associations of stroke admissions with CO became stronger after adjusting PM2.5. The differences were statistically significant at lag0 (Z = 2.738, p = 0.006) and lag0–2 day (Z = 2.194, p = 0.028).
Table 5.
Percent change (95% CI) in stroke admissions associated with a 10.0 μg/m3 increase in NO2, SO2, and ozone, and 1.0 mg/m3 increase in CO after adjusting PM2.5 in warm season.
| Variables | Single-Pollutant Model | Model Adjusting PM2.5 | Z Value * | p Value * |
|---|---|---|---|---|
| NO2 | ||||
| Lag0 | 2.35 (1.76 to 2.94) | 1.63 (0.97 to 2.30) | 1.572 | 0.116 |
| Lag1 | 1.80 (1.22 to 2.37) | 0.70 (0.08 to 1.33) | 2.530 | 0.011 |
| Lag2 | 0.34 (−0.22 to 0.90) | −0.51 (−1.08 to 0.06) | 2.078 | 0.038 |
| 3 days average | 2.24 (1.44 to 3.05) | 1.07 (0.17 to 1.97) | 1.907 | 0.056 |
| SO2 | ||||
| Lag0 | 2.38 (1.45 to 3.32) | 1.91 (0.89 to 2.93) | 0.666 | 0.505 |
| Lag1 | 1.20 (0.28 to 2.12) | 0.56 (−0.39 to 1.51) | 0.946 | 0.344 |
| Lag2 | 0.43 (−0.45 to 1.33) | −3.04 (−3.98 to −2.09) | 0.866 | 0.386 |
| 3 days average | 0.32 (−0.91 to 1.56) | −1.00 (−2.28 to 0.30) | 1.438 | 0.150 |
| CO | ||||
| Lag0 | −1.86 (−3.70 to 0.01) | −5.68 (−7.65 to −3.66) | 2.738 | 0.006 |
| Lag1 | −4.26 (−5.98 to −2.51) | −6.73 (−8.48 to −4.96) | 1.957 | 0.050 |
| Lag2 | −5.87 (−7.51 to −4.20) | −5.90 (−7.57 to −4.21) | 0.028 | 0.978 |
| 3 days average | −7.95 (−10.34 to −5.49) | −11.79 (−14.19 to −9.32) | 2.194 | 0.028 |
| Ozone | ||||
| Lag0 | 0.78 (0.62 to 0.95) | 0.71 (0.53 to 0.89) | 0.573 | 0.567 |
| Lag1 | 0.60 (0.44 to 0.76) | 0.41 (0.24 to 0.58) | 1.631 | 0.103 |
| Lag2 | 0.36 (0.20 to 0.51) | 0.21 (0.06 to 0.37) | 1.257 | 0.209 |
| 3 days average | 0.67 (0.44 to 0.91) | 0.42 (0.17 to 0.67) | 1.444 | 0.149 |
CI, confidence interval; NO2, nitrogen dioxide; SO2, sulfur dioxide; CO, carbon monoxide; PM2.5, particulate matter that is 2.5 µm or less in diameter. * Test for difference between models.
Table 6.
Percent change (95% CI) in stroke admissions associated with a 10.0 μg/m3 increase in NO2, SO2, and ozone, and 1.0 mg/m3 increase in CO after adjusting PM2.5 in cold seasons.
| Variables | Single-Pollutant Model | Model Adjusting PM2.5 | Z Value * | p Value * |
|---|---|---|---|---|
| NO2 | ||||
| Lag0 | 0.40 (0.02 to 0.78) | 0.72 (0.12 to 1.31) | −0.870 | 0.385 |
| Lag1 | −0.13 (−0.46 to 0.20) | −0.23 (−0.60 to 0.14) | 0.407 | 0.684 |
| Lag2 | −0.08 (−0.38 to 0.23) | −0.38 (−0.69 to −0.06) | 0.232 | 0.816 |
| 3 days average | −0.37 (−0.84 to 0.10) | −0.65 (−1.23 to −0.07) | 0.738 | 0.460 |
| SO2 | ||||
| Lag0 | 0.67 (0.35 to 1.00) | 0.76 (0.39 to 1.12) | −0.336 | 0.737 |
| Lag1 | 0.34 (0.04 to 0.64) | 0.39 (0.05 to 0.74) | −0.224 | 0.823 |
| Lag2 | 0.22 (−0.05 to 0.49) | 0.03 (−0.26 to 0.33) | 0.913 | 0.361 |
| 3 days average | 0.36 (−0.10 to 0.82) | 0.38 (−0.12 to 0.89) | −0.072 | 0.943 |
| CO | ||||
| Lag0 | 2.07 (1.20 to 2.95) | 4.13 (2.79 to 5.49) | −2.520 | 0.012 |
| Lag1 | 0.78 (0.04 to 1.52) | 0.69 (−0.11 to 1.51) | 0.146 | 0.884 |
| Lag2 | −0.25 (−0.94 to 0.45) | −0.62 (−1.33 to 0.10) | 0.736 | 0.461 |
| 3 days average | 0.56 (−0.50 to 1.64) | 0.77 (−0.50 to 2.05) | −0.242 | 0.809 |
| Ozone | ||||
| Lag0 | −2.06 (−2.39 to −1.73) | −2.23 (−2.57 to −1.90) | 0.732 | 0.464 |
| Lag1 | −1.48 (−1.78 to −1.18) | −1.54 (−1.85 to −1.22) | 0.265 | 0.791 |
| Lag2 | −1.44 (−1.73 to −1.15) | −1.32 (−1.63 to −1.02) | −0.547 | 0.584 |
| 3 days average | −1.90 (−2.35 to −1.45) | −2.12 (−2.58 to −1.65) | 0.652 | 0.514 |
CI, confidence interval; NO2, nitrogen dioxide; SO2, sulfur dioxide; CO, carbon monoxide; PM2.5, particulate matter that is 2.5 µm or less in diameter. * Test for difference between models.
In cold seasons, two-pollutant models show that adjustment for PM2.5 had no obvious impact on the associations of stroke admissions with NO2, SO2, and ozone. The positive association of stroke admissions with CO at lag0 day was higher after adjusting PM2.5 (Z = −2.520, p = 0.012).
Sensitivity analyses show that changing the method of choosing controls, the degrees of freedom and the lag days of temperature and relative humidity did not change the main findings (Table 7).
Table 7.
Percent change (95% CI) in stroke admissions associated with a 10.0 μg/m3 increase in NO2, SO2, and ozone, and 1.0 mg/m3 increase in CO in sensitivity analyses.
| Variables | Sensitivity Analysis I * | Sensitivity Analysis II # | Sensitivity Analysis III † |
|---|---|---|---|
| NO2 | |||
| Lag0 | 0.77 (0.45 to 1.09) | 0.82 (0.52 to 1.13) | 1.14 (0.87 to 1.40) |
| Lag1 | 0.38 (0.10 to 0.66) | 0.39 (0.11 to 0.67) | 0.75 (0.49 to 1.02) |
| Lag2 | −0.17 (−0.44 to 0.10) | −0.14 (−0.41 to 0.12) | 0.08 (−0.19 to 0.34) |
| 3 days average | 0.42 (0.02 to 0.83) | 0.20 (−0.20 to 0.60) | 0.91 (0.56 to 1.27) |
| SO2 | |||
| Lag0 | 0.38 (0.07 to 0.69) | 0.72 (0.43 to 1.02) | 1.11 (0.83 to 1.39) |
| Lag1 | 0.20 (−0.09 to 0.50) | 0.40 (0.11 to 0.68) | 0.76 (0.49 to 1.04) |
| Lag2 | −0.18 (−0.45 to 0.09) | 0.16 (−0.10 to 0.42) | 0.35 (0.08 to 0.61) |
| 3 days average | −0.02 (−0.46 to 0.43) | 0.36 (−0.06 to 0.78) | 1.11 (0.70 to 1.51) |
| CO | |||
| Lag0 | 1.19 (0.42 to 1.98) | 1.32 (0.56 to 2.10) | 2.36 (1.68 to 3.04) |
| Lag1 | 0.41 (−0.28 to 1.10) | 0.35 (−0.33 to 1.03) | 1.33 (0.65 to 2.01) |
| Lag2 | −0.25 (−0.90 to 0.40) | −0.43 (−1.07 to 0.22) | −0.09 (−0.75 to 0.58) |
| 3 days average | 0.43 (−0.55 to 1.43) | −0.13 (−1.10 to 0.84) | 2.01 (1.07 to 2.95) |
| Ozone | |||
| Lag0 | 0.29 (0.14 to 0.44) | 0.24 (0.09 to 0.38) | 0.23 (0.10 to 0.36) |
| Lag1 | 0.16 (0.02 to 0.30) | 0.23 (0.09 to 0.37) | 0.24 (0.11 to 0.37) |
| Lag2 | −0.07 (−0.21 to 0.07) | 0.03 (−0.10 to 0.17) | 0.07 (−0.06 to 0.20) |
| 3 days average | 0.28 (0.08 to 0.49) | 0.22 (0.02 to 0.42) | 0.12 (−0.06 to 0.31) |
CI, confidence interval; NO2, nitrogen dioxide; SO2, sulfur dioxide; CO, carbon monoxide. * Using time-stratified case-crossover design. # Changing the temperature and relative humidity degrees of freedom to four instead of three. † Adjusting temperature and relative humidity lagged by up to two weeks.
4. Discussion
This study suggests an approximately linear exposure-response relationship of stroke with NO2 and SO2, and they were positively associated with stroke admissions. The results were consistent with previous findings worldwide and supported by epidemiological and experimental studies [8,18,22,23,24]. The putative biological mechanisms linking air pollution to cardiovascular diseases involve direct effects on the cardiovascular system, blood, and lung receptors, and/or indirect effects mediated through pulmonary oxidative stress and inflammatory responses [23,24,25].
Some studies examined the seasonal difference in effects of NO2 and SO2 on stroke but generated inconsistent results [8,17,26]. For example, Wichmann and Voyi (2012) reported positive associations of cerebrovascular mortality with NO2 in warm season [8], whereas Xiang et al. (2013) observed opposite results [26]. We found that the positive associations of NO2 and SO2 with stroke admissions were higher in warm seasons. Combined effects of high air pollution and temperature levels, and the varied ventilation conditions across seasons may explain the seasonal difference [6].
The subgroup analyses show that the positive associations of stroke admissions with NO2 and SO2 appearing to be stronger in patients >65 years in warm seasons, consistent with previous studies [16,17]. The evidence for interaction between air pollution and comorbid factors on stroke risk is growing [27,28]. Potentially, preexisting respiratory or cardiovascular conditions are more prevalent for the elderly than the younger. The vulnerable condition in the elderly could impact the effects of air pollutants on stroke [29]. Another possible theory behind the difference is that recurrent stroke is more common for older patients. Previous studies suggest that short-term trigger effects of air pollution on cardiovascular events are factors primarily for patients with a history of cardiovascular disease [30,31]. Furthermore, the proportion of stroke subtype may be different between two age groups, and the association of air pollution with stroke may differ across stroke subtype [32]. Thus, analyses including information on types of previous stroke diseases and comorbid factors might help to understand potential pathways in future studies.
There were two recent meta-analyses examining the acute effects of air pollution on stroke, and both reported a significantly positive association between ambient CO exposure and the same day stroke hospitalizations [33,34]. However, Tian et al. (2015) found that low environmental CO was associated with reduced risk of daily stroke hospitalizations [10]. In this study, an almost linear exposure-response relationship of stroke admissions at higher concentrations of CO was observed with a threshold value.
When stratified by season, negative associations between stroke admissions and CO in warm season with lower CO levels were observed. These findings suggest that ambient CO at low levels may have no or beneficial effects on stroke. Furthermore, the anti-inflammatory and beneficial neuroprotective effects of CO under certain circumstances have been suggested by recent experimental studies [35,36]. Therefore, the short-term beneficial effects of low environmental CO against stroke are biologically plausible.
Although several epidemiological studies have examined the association of ambient ozone with stroke, they reported inconsistent results. Some studies reported statistically significant and positive associations [37,38], whereas others presented no or negative associations [12,39,40]. This study found that exposure to ambient ozone was positively associated with stroke admissions in warm season, but negatively associated with stroke admissions in cold season, similar with the results reported by Villeneuve et al. (2012) [41].
Some studies stated that the negative association of ozone with stroke might be attributed to its negative association with other air pollutants, which were more strongly associated with cardiovascular events [12]. Furthermore, one meta-analysis reported a positive association between stroke and ambient ozone exposure only in a subgroup of Asia area [34]. They stated that the observed geographic differences of the associations may reflect a shape difference of exposure-response function for stroke between low and high pollution settings. In this study, daily mean concentration of ozone was lower in cold season. The seasonal difference may be attributed to the difference in ozone levels. Notably, ozone was highly correlated with temperature in this study. This relationship together with a misspecified temperature model might also contribute to the difference between seasons.
Previous studies suggest that PM2.5 may increase the risk of stroke admissions and mortality [6,7]. It is necessary to check the modification effects of PM2.5 on the associations of gaseous pollutants and stroke admissions. Two-pollutant models show that PM2.5 had no obvious impact on the associations of stroke admissions with SO2 and ozone [17,42]. For NO2, the effects diminished after adjustment for PM2.5 in warm season [43]. However, the directions of effect estimates did not change. Therefore, effects of NO2, SO2, and ozone on stroke admissions in single-pollutant models were reliable. In this study, the negative associations of CO with stroke admissions in warm season and positive associations in cold season both became stronger [44]. The effects of CO on stroke may be underestimated.
There are several strengths of this study. First, the effect estimates in case-crossover design are probably not due to confounding by age, gender, smoking, underlying chronic disease, or other individual-level characteristics. Moreover, the medical record database contains all hospitals with the capability to diagnose and treat cardiovascular and cerebrovascular disease in Beijing and provides a large number of cases. District-daily-level air pollutants and the large range of concentrations used in this study can provide better control of measurement error than use of data from one monitoring site and small range of concentrations.
This study has several potential limitations. First, possible misclassification of diseases may exist related to the unrecognized occurrence of stroke and non-hospitalized individuals, with the onset of stroke symptoms in the days prior to hospital admissions. Furthermore, the use of district-level concentrations rather than individual exposure may lead to measurement error. Given the ecological design of this study, caution should be exercised in inferring cause-effect relations, especially for ozone, which is possibly non-linearly correlated with stroke.
5. Conclusions
NO2 and SO2 were positively associated with stroke admissions, with stronger effects in warm seasons, and in patients >65 years. This study also suggests an approximately linear exposure-response relationship of stroke admissions at higher CO levels, with a threshold effect. The associations of CO and ozone with stroke admissions differed by season.
Acknowledgments
We sincerely thank those who participated in data collection and management.
Author Contributions
Fangfang Huang participated in the data collection, performed data analysis, and drafted the manuscript. Yanxia Luo designed the study’s analytic strategy, participated in the interpretation of data, and edited the manuscript. Peng Tan and Qin Xu participated in the data collection and management. Lixin Tao, Jin Guo and Feng Zhang assisted in study design and data analysis. Xueqin Xie and Xiuhua Guo developed the study design, participated in data analysis, led the data interpretation, and edited the manuscript. All authors read and approved the final version of the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Brauer M., Freedman G., Frostad J., van Donkelaar A., Martin R.V., Dentener F., van Dingenen R., Estep K., Amini H., Apte J.S., et al. Ambient air pollution exposure estimation for the Global Burden of Disease 2013. Environ. Sci. Technol. 2016;50:79–88. doi: 10.1021/acs.est.5b03709. [DOI] [PubMed] [Google Scholar]
- 2.Wang Y., Ying Q., Hu J., Zhang H. Spatial and temporal variations of six criteria air pollutants in 31 provincial capital cities in China during 2013–2014. Environ. Int. 2014;73:413–422. doi: 10.1016/j.envint.2014.08.016. [DOI] [PubMed] [Google Scholar]
- 3.Huang F., Li X., Wang C., Xu Q., Wang W., Luo Y., Tao L., Gao Q., Guo J., Chen S., et al. PM2.5 spatiotemporal variations and the relationship with meteorological factors during 2013–2014 in Beijing, China. PLoS ONE. 2015;10:e0141642. doi: 10.1371/journal.pone.0141642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Feigin V.L., Lawes C.M., Bennett D.A., Barker-Collo S.L., Parag V. Worldwide stroke incidence and early case fatality reported in 56 population-based studies: A systematic review. Lancet Neurol. 2009;8:355–369. doi: 10.1016/S1474-4422(09)70025-0. [DOI] [PubMed] [Google Scholar]
- 5.Wang W., Jiang B., Sun H., Ru X., Sun D., Wang L., Jiang Y., Li Y., Wang Y., Chen Z., et al. Prevalence, incidence and mortality of stroke in China: Results from a nationwide population-based survey of 480,687 adults. Circulation. 2017 doi: 10.1161/CIRCULATIONAHA.116.025250. [DOI] [PubMed] [Google Scholar]
- 6.Huang F., Luo Y., Guo Y., Tao L., Xu Q., Wang C., Wang A., Li X., Guo J., Yan A., et al. Particulate matter and hospital admissions for stroke in Beijing, China: Modification effects by ambient temperature. J. Am. Heart Assoc. 2016;5 doi: 10.1161/JAHA.116.003437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lin H., Tao J., Du Y., Liu T., Qian Z., Tian L., Di Q., Zeng W., Xiao J., Guo L., et al. Differentiating the effects of characteristics of PM pollution on mortality from ischemic and hemorrhagic strokes. Int. J. Hyg. Environ. Health. 2016;219:204–211. doi: 10.1016/j.ijheh.2015.11.002. [DOI] [PubMed] [Google Scholar]
- 8.Wichmann J., Voyi K. Ambient air pollution exposure and respiratory, cardiovascular and cerebrovascular mortality in Cape Town, South Africa: 2001–2006. Int. J. Environ. Res. Public Health. 2012;9:3978–4016. doi: 10.3390/ijerph9113978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Feigin V.L., Roth G.A., Naghavi M., Parmar P., Krishnamurthi R., Chugh S., Mensah G.A., Norrving B., Shiue I., Ng M., et al. Global burden of stroke and risk factors in 188 countries, during 1990–2013: A systematic analysis for the Global Burden of Disease Study 2013. Lancet Neurol. 2016;15:913–924. doi: 10.1016/S1474-4422(16)30073-4. [DOI] [PubMed] [Google Scholar]
- 10.Tian L., Qiu H., Pun V.C., Ho K.F., Chan C.S., Yu I.T. Carbon monoxide and stroke: A time series study of ambient air pollution and emergency hospitalizations. Int. J. Cardiol. 2015;201:4–9. doi: 10.1016/j.ijcard.2015.07.099. [DOI] [PubMed] [Google Scholar]
- 11.Bell M.L., Peng R.D., Dominici F., Samet J.M. Emergency hospital admissions for cardiovascular diseases and ambient levels of carbon monoxide: Results for 126 United States urban counties, 1999–2005. Circulation. 2009;120:949–955. doi: 10.1161/CIRCULATIONAHA.109.851113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Männistö T., Mendola P., Laughon Grantz K., Leishear K., Sundaram R., Sherman S., Ying Q., Liu D. Acute and recent air pollution exposure and cardiovascular events at labour and delivery. Heart. 2015;101:1491–1498. doi: 10.1136/heartjnl-2014-307366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carlsen H.K., Forsberg B., Meister K., Gislason T., Oudin A. Ozone is associated with cardiopulmonary and stroke emergency hospital visits in Reykjavik, Iceland 2003–2009. Environ. Health. 2013;12 doi: 10.1186/1476-069X-12-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Martinelli N., Olivieri O., Girelli D. Air particulate matter and cardiovascular disease: A narrative review. Eur. J. Intern. Med. 2013;24:295–302. doi: 10.1016/j.ejim.2013.04.001. [DOI] [PubMed] [Google Scholar]
- 15.Brook R.D., Rajagopalan S., Pope C.A., 3rd, Brook J.R., Bhatnagar A., Diez-Roux A.V., Holguin F., Hong Y., Luepker R.V., Mittleman M.A., et al. Particulate matter air pollution and cardiovascular disease: An update to the scientific statement from the American Heart Association. Circulation. 2010;121:2331–2378. doi: 10.1161/CIR.0b013e3181dbece1. [DOI] [PubMed] [Google Scholar]
- 16.Kan H., London S.J., Chen G., Zhang Y., Song G., Zhao N., Jiang L., Chen B. Season, sex, age, and education as modifiers of the effects of outdoor air pollution on daily mortality in Shanghai, China: The Public Health and Air Pollution in Asia (PAPA) Study. Environ. Health Perspect. 2008;116:1183–1188. doi: 10.1289/ehp.10851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zheng S., Wang M., Wang S., Tao Y., Shang K. Short-term effects of gaseous pollutants and particulate matter on daily hospital admissions for cardio-cerebrovascular disease in Lanzhou: Evidence from a heavily polluted city in China. Int. J. Environ. Res. Public Health. 2013;10:462–477. doi: 10.3390/ijerph10020462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Qian Y., Zhu M., Cai B., Yang Q., Kan H., Song G., Jin W., Han M., Wang C. Epidemiological evidence on association between ambient air pollution and stroke mortality. J. Epidemiol. Community Health. 2013;67:635–640. doi: 10.1136/jech-2012-201096. [DOI] [PubMed] [Google Scholar]
- 19.Carracedo-Martinez E., Taracido M., Tobias A., Saez M., Figueiras A. Case-crossover analysis of air pollution health effects: A systematic review of methodology and application. Environ. Health Perspect. 2010;118:1173–1182. doi: 10.1289/ehp.0901485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen R., Wang C., Meng X., Chen H., Thach T.Q., Wong C.M., Kan H. Both low and high temperature may increase the risk of stroke mortality. Neurology. 2013;81:1064–1070. doi: 10.1212/WNL.0b013e3182a4a43c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Low R.B., Bielory L., Qureshi A.I., Dunn V., Stuhlmiller D.F., Dickey D.A. The relation of stroke admissions to recent weather, airborne allergens, air pollution, seasons, upper respiratory infections, and asthma incidence, 11 September, 2001, and day of the week. Stroke. 2006;37:951–957. doi: 10.1161/01.STR.0000214681.94680.66. [DOI] [PubMed] [Google Scholar]
- 22.Chen R., Zhang Y., Yang C., Zhao Z., Xu X., Kan H. Acute effect of ambient air pollution on stroke mortality in the China air pollution and health effects study. Stroke. 2013;44:954–960. doi: 10.1161/STROKEAHA.111.673442. [DOI] [PubMed] [Google Scholar]
- 23.Lundbäck M., Mills N.L., Lucking A., Barath S., Donaldson K., Newby D.E., Sandström T., Blomberg A. Experimental exposure to diesel exhaust increases arterial stiffness in man. Part. Fibre Toxicol. 2009;6 doi: 10.1186/1743-8977-6-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lucking A.J., Lundback M., Mills N.L., Faratian D., Barath S.L., Pourazar J., Cassee F.R., Donaldson K., Boon N.A., Badimon J.J., et al. Diesel exhaust inhalation increases thrombus formation in man. Eur. Heart J. 2008;29:3043–3051. doi: 10.1093/eurheartj/ehn464. [DOI] [PubMed] [Google Scholar]
- 25.Brook R.D., Franklin B., Cascio W., Hong Y., Howard G., Lipsett M., Luepker R., Mittleman M., Samet J., Smith S.C., Jr., et al. Air pollution and cardiovascular disease: A statement for healthcare professionals from the Expert Panel on Population and Prevention Science of the American Heart Association. Circulation. 2004;109:2655–2671. doi: 10.1161/01.CIR.0000128587.30041.C8. [DOI] [PubMed] [Google Scholar]
- 26.Xiang H., Mertz K.J., Arena V.C., Brink L.L., Xu X., Bi Y., Talbott E.O. Estimation of short-term effects of air pollution on stroke hospital admissions in Wuhan, China. PLoS ONE. 2013;8:e0061168. doi: 10.1371/journal.pone.0061168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Oudin A., Strömberg U., Jakobsson K., Stroh E., Lindgren A.G., Norrving B., Pessah-Rasmussen H., Engström G., Björk J. Hospital admissions for ischemic stroke: Does long-term exposure to air pollution interact with major risk factors? Cerebrovasc. Dis. 2011;31:284–293. doi: 10.1159/000322600. [DOI] [PubMed] [Google Scholar]
- 28.O'Donnell M.J., Fang J., Mittleman M.A., Kapral M.K., Wellenius G.A. Investigators of the Registry of Canadian Stroke Network. Fine particulate air pollution (PM2.5) and the risk of acute ischemic stroke. Epidemiology. 2011;22:422–431. doi: 10.1097/EDE.0b013e3182126580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zanobetti A., Schwartz J., Gold D. Are there sensitive subgroups for the effects of airborne particles? Environ. Health Perspect. 2000;108:841–845. doi: 10.1289/ehp.00108841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Oudin A., Forsberg B., Jakobsson K. Air pollution and stroke. Epidemiology. 2012;23:505–506. doi: 10.1097/EDE.0b013e31824ea667. [DOI] [PubMed] [Google Scholar]
- 31.Pope C.A., Muhlestein J.B., May H.T., Renlund D.G., Anderson J.L., Horne B.D. Ischemic heart disease events triggered by short-term exposure to fine particulate air pollution. Circulation. 2006;114:2443–2448. doi: 10.1161/CIRCULATIONAHA.106.636977. [DOI] [PubMed] [Google Scholar]
- 32.Oudin A., Strömberg U., Jakobsson K., Stroh E., Björk J. Estimation of short-term effects of air pollution on stroke hospital admissions in southern Sweden. Neuroepidemiology. 2010;34:131–142. doi: 10.1159/000274807. [DOI] [PubMed] [Google Scholar]
- 33.Shah A.S., Lee K.K., McAllister D.A., Hunter A., Nair H., Whiteley W., Langrish J.P., Newby D.E., Mills N.L. Short term exposure to air pollution and stroke: Systematic review and meta-analysis. BMJ. 2015;350 doi: 10.1136/bmj.h1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yang W.S., Wang X., Deng Q., Fan W.Y., Wang W.Y. An evidence-based appraisal of global association between air pollution and risk of stroke. Int. J. Cardiol. 2014;175:307–313. doi: 10.1016/j.ijcard.2014.05.044. [DOI] [PubMed] [Google Scholar]
- 35.Wang B., Cao W., Biswal S., Doré S. Carbon monoxide-activated Nrf2 pathway leads to protection against permanent focal cerebral ischemia. Stroke. 2011;42:2605–2610. doi: 10.1161/STROKEAHA.110.607101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yabluchanskiy A., Sawle P., Homer-Vanniasinkam S., Green C.J., Foresti R., Motterlini R. CORM-3, a carbon monoxide-releasing molecule, alters the inflammatory response and reduces brain damage in a rat model of hemorrhagic stroke. Crit. Care Med. 2012;40:544–552. doi: 10.1097/CCM.0b013e31822f0d64. [DOI] [PubMed] [Google Scholar]
- 37.Xu X., Sun Y., Ha S., Talbott E.O., Lissaker C.T. Association between ozone exposure and onset of stroke in Allegheny County, Pennsylvania, USA, 1994–2000. Neuroepidemiology. 2013;41:2–6. doi: 10.1159/000345138. [DOI] [PubMed] [Google Scholar]
- 38.Henrotin J.B., Zeller M., Lorgis L., Cottin Y., Giroud M., Béjot Y. Evidence of the role of short-term exposure to ozone on ischaemic cerebral and cardiac events: The Dijon Vascular Project (DIVA) Heart. 2010;96:1990–1996. doi: 10.1136/hrt.2010.200337. [DOI] [PubMed] [Google Scholar]
- 39.Rodopoulou S., Samoli E., Chalbot M.C., Kavouras I.G. Air pollution and cardiovascular and respiratory emergency visits in Central Arkansas: A time-series analysis. Sci. Total Environ. 2015;536:872–879. doi: 10.1016/j.scitotenv.2015.06.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lisabeth L.D., Escobar J.D., Dvonch J.T., Sánchez B.N., Majersik J.J., Brown D.L., Smith M.A., Morgenstern L.B. Ambient air pollution and risk for ischemic stroke and transient ischemic attack. Ann. Neurol. 2008;64:53–59. doi: 10.1002/ana.21403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Villeneuve P.J., Johnson J.Y., Pasichnyk D., Lowes J., Kirkland S., Rowe B.H. Short-term effects of ambient air pollution on stroke: Who is most vulnerable? Sci. Total Environ. 2012;430:193–201. doi: 10.1016/j.scitotenv.2012.05.002. [DOI] [PubMed] [Google Scholar]
- 42.Henrotin J.B., Besancenot J.P., Bejot Y., Giroud M. Short-term effects of ozone air pollution on ischaemic stroke occurrence: A case-crossover analysis from a 10-year population-based study in Dijon, France. Occup. Environ. Med. 2007;64:439–445. doi: 10.1136/oem.2006.029306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bedada G.B., Smith C.J., Tyrrell P.J., Hirst A.A., Agius R. Short-term effects of ambient particulates and gaseous pollutants on the incidence of transient ischaemic attack and minor stroke: A case-crossover study. Environ. Health. 2012;11 doi: 10.1186/1476-069X-11-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tian L., Qiu H., Pun V.C., Lin H., Ge E., Chan J.C., Louie P.K., Ho K.F., Yu I.T. Ambient carbon monoxide associated with reduced risk of hospital admissions for respiratory tract infections. Am. J. Respir. Crit. Care Med. 2013;188:1240–1245. doi: 10.1164/rccm.201304-0676OC. [DOI] [PubMed] [Google Scholar]