Abstract
The Aedes aegypti mosquito is an efficient vector for the transmission of Zika, chikungunya, and dengue viruses, causing major epidemics and a significant social and economic burden throughout the tropics and subtropics. The primary means of preventing these diseases is household-level mosquito control. However, relatively little is known about the economic burden of Ae. aegypti control in resource-limited communities. We surveyed residents from 40 households in a high-risk community at the urban periphery in the city of Machala, Ecuador, on dengue perceptions, vector control interventions, household expenditures, and factors influencing purchasing decisions. The results of this study show that households spend a monthly median of US$2.00, or 1.90% (range: 0.00%, 9.21%) of their family income on Ae. aegypti control interventions. Households reported employing, on average, five different mosquito control and dengue prevention interventions, including aerosols, liquid sprays, repellents, mosquito coils, and unimpregnated bed nets. We found that effectiveness and cost were the most important factors that influence people’s decisions to purchase a mosquito control product. Our findings will inform the development and deployment of new Ae. aegypti control interventions by the public health and private sectors, and add to prior studies that have focused on the economic burden of dengue-like illness.
Keywords: Aedes aegypti, mosquito control, economic cost, dengue fever, KAP, Ecuador
1. Introduction
Febrile illnesses transmitted by the Aedes aegypti mosquito—such as Zika, dengue, and chikungunya—present a rapidly increasing public health problem in tropical and subtropical regions, where a large proportion of the world’s population are at risk of disease [1]. Low-income countries are particularly vulnerable to the social and economic impacts of these emerging epidemics due to limited resources in the public health sector and at the household-level to prevent and manage the disease [2]. Prior studies estimate that the economic burden of dengue infections in the Americas costs US$2.1 billion per year on average (in 2010 US dollars), with a range of US$1–4 billion [3]. Initial estimates of the short-term economic impact of the Zika virus epidemic for 2016 in the Latin American and the Caribbean region are US$3.5 billion [4]. Control of Ae. aegypti mosquitoes through chemical and biological targeting and management of larval habitat (e.g., containers with water) remains the principal means of preventing and controlling dengue, chikungunya, and Zika outbreaks. However, relatively little is known about the economic burden of and barriers to household-level mosquito control in resource-limited communities.
In Ecuador, febrile illnesses transmitted by Ae. aegypti have replaced malaria as the most prevalent-mosquito borne diseases. Over a five year period (2010 to 2014), 72,060 cases of dengue were reported in the country, as compared to 1138 cases of malaria [5]. Dengue is hyper-endemic in Ecuador’s lowland coastal region, where new cases present every year, with peak transmission during the rainy season (February to May), and sporadic transmission the rest of the year. The first cases of locally acquired chikungunya were reported in Ecuador at the end of 2014, resulting in over 33,000 cases in 2015. The first cases of Zika were reported in the country on 7 January 2016, and to date (6 October 2016) 2695 suspected cases of Zika have been reported [6]. The primary vectors for these diseases are Ae. aegypti and Ae. albopictus; however, Ae. albopictus has not been reported in Ecuador. Ongoing surveillance studies in Machala indicate that the true burden of these vector-borne diseases is much higher than reported due to a high proportion of asymptomatic or mild cases and limited access to laboratory diagnostics (Stewart-Ibarra et al. in prep).
Previous studies in Machala indicate that local social-ecological conditions (e.g., poor housing conditions, piped water infrastructure, demographics of the heads of households) influenced Ae. aegypti abundance and the presence of dengue [7,8,9]. Community members in two proximate urban areas specified economic factors as a key barrier to dengue prevention, including low household income, employment, type of housing, the cost of water storage (e.g., cisterns), and the cost of vector control [9]. However, no prior studies have estimated the economic burden of Ae. aegypti control in these communities.
In 2015, the Ministry of Health (MoH) in Ecuador began a transition from a vertical vector control program to a horizontal decentralized program with integrated management strategies in Ae. aegypti endemic regions. The MoH typically conducts focal fumigation in and around the households of individuals with dengue, chikungunya, or Zika. In these areas, environmental fogging is conducted from trucks, and household visits are performed by MoH field workers to eliminate larval habitats by overturning containers with water and treating stored water with larvicides (Bti and temefos). The government bears the burden for costs of dengue prevention and vector control, thus the interventions are free from the perspective of the household. However, in the peripheral areas of Machala, public health personnel are not able to effectively implement these vector control strategies due to limited resources. Therefore, in the absence of effective interventions by government institutions, it has become imperative that families invest household resources to protect themselves against dengue fever and other mosquito-borne diseases.
Although dengue is an important vector-borne disease, the literature regarding the costs and benefits of control options is relatively sparse [10]. Previous research has found that dengue fever poses a financial burden to households, not only in terms of the direct costs of dengue (e.g., hospitalization, treatment), but also indirect costs (e.g., loss of productivity, emotional stress) [11,12,13,14,15]. Studies have also evaluated the costs of malaria prevention at the household level [16,17,18] and documented the type and costs of mosquito control measures from malaria and dengue-endemic regions [19,20]. While these studies help to highlight the types and costs of products used in malaria endemic regions, few have gone further to examine a household’s reasons for buying mosquito control products [17,19]. Moreover, at the household level, protective measures to prevent Anopheles-transmitted malaria and Ae. aegypti-transmitted illnesses are different due to differences in vector behavior. For example, bed nets for night use are effective against Anopheles species, but largely ineffective against the day-biting Ae. aegypti mosquito.
The aim of this study was to investigate the economic and socio-behavioral factors influencing household-level Ae. aegypti control in the city of Machala, a dengue-endemic area with high Ae. aegypti densities. This study was conducted in 2015, during the emergence of the chikungunya epidemic and one year prior to the emergence of Zika fever in the city. The study does not aim to address government costs of public health interventions (dengue prevention and control) or costs to the healthcare system for individual for cases of dengue fever. We aimed to assess the mosquito control products available in local markets, the costs incurred by households on dengue prevention and mosquito control, and the factors that influenced a household’s decision to purchase a mosquito control product. This evidence is intended to be an additional input for researchers and decision-makers examining the costs and feasibility of proposed vector control interventions.
2. Methods
2.1. Study Site and Study Population
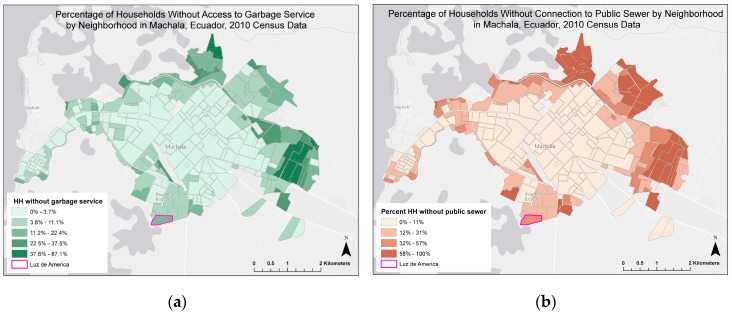
Machala, Ecuador (population of approximately 246,000) is the capital city of El Oro Province and is a major port on the Pacific Coast, located 70 km north of the Peruvian border [21]. Machala is typical of mid-sized cities in Latin America that experienced rapid, unplanned growth from 1960 to 1980, resulting in uneven access to piped water, garbage collection, and paved roads in the urban periphery (Figure 1) [9]. We conducted this investigation in a low-income urban area located at the southernmost edge of the city, Luz de America (population of 1368, approximately 267 households). Population estimates were derived from vector control activities that transpired in the area from 2012 to 2013. The neighborhood was bordered by mixed commercial and residential buildings to the north and east, and mangroves and abandoned shrimp ponds to the southwest. Streets were unpaved and most households had access to sewerage, garbage collection, and piped water inside the home. Many households (43%) reported daily or weekly interruptions in the piped water supply, and thus, stored water in cisterns or elevated tanks (80%).
Figure 1.
Location of study site in Machala, Ecuador. Luz de America indicated with neighborhood level census information from 2010 data: (a) Percentage of households without access to garbage service (21.2% for Luz de America) and (b) without connection to public sewerage system (37.7% for Luz de America). Data source: Instituto nacional de estadistica y censos (INEC). Neighborhood map source: National Institute of Meteorology and Hydrology of Ecuador (INAMHI).
From 2014 to 2016, during the peak dengue transmission season, the average Ae. aegypti Household Index (percentage of houses infested with larvae and/or pupae), was 14.78 and the average Breteau Index (number of positive containers per 100 houses inspected) was 21.40, per MoH records. Common Ae. aegypti larval habitat in this community included standing water in puddles, 55 gallon drums for water storage, tires, and discarded containers in and around the household patio. This neighborhood was selected because the MoH identified the community as a high-risk area for dengue, and because the MoH was not conducting any regular Ae. aegypti control interventions in the community during the study period.
This study was conducted from March 2015 to August 2015, in conjunction with a pilot field trial of a novel intervention to reduce the indoor density of Ae. aegypti populations. Forty households were selected based on (a) their willingness to participate in this study, and (b) that they did not own an air conditioning unit.
2.2. Research Methods
The study was conducted in accordance with the Declaration of Helsinki and the protocol was reviewed and approved by the Institutional Review Boards at SUNY Upstate Medical University (project identification code: 2014-0), Syracuse University, University of Colorado, and the Central University of Ecuador. Heads of household (>18 years) were consented by trained field technicians, and signed an informed consent form prior to study start.
2.3. Product Surveys
To determine the types and costs of Ae. aegypti control products available for sale to the Luz de America community, we sampled 10 neighborhood stores (within one kilometer from the neighborhood limits) and three supermarkets, representative of the five large supermarkets in the city. Additionally, the primary outdoor market in central Machala was surveyed for items that could not be found at the other stores. At all stores, we documented the product name, size, active ingredient, availability, and price of the different mosquito control products. At the neighborhood stores and outdoor market, we asked store owners for the prices of products, whereas at the supermarkets, the prices listed on the tag below the products were documented. The products were categorized into repellents, aerosols, mosquito coils, liquid sprays, and other categories as needed. We took photos of the products and printed them on flashcards for use during the household surveys.
2.4. Household Surveys
From March to June 2015 we surveyed the head of household or the responsible adult who resided in the home during the day in a face-to-face interview that solicited information about household demographics, dengue knowledge and perceptions, vector control and water storage practices, and barriers to employing prevention practices. This survey was a modification of an instrument that was used previously in a dengue surveillance study in Machala by project investigators [8,9].
Following the completion of the household surveys, we designed a semi-structured questionnaire to further explore the economic themes that emerged. We surveyed 38 of the same 40 households during the second half of the study period (July to August 2015). Two households were lost to follow up because the residents had moved. The information solicited included direct quantitative questions on household expenditures and income. For comparison, we consulted the latest data available on household income from Ecuador’s National Institute of Statistics and Census (INEC, by its Spanish acronym). As economic data specific to Machala was not available, we investigated national average wages from the most recent national survey in 2011–2012. Survey respondents were also asked open-ended questions regarding mosquito control and dengue prevention practices and the costs incurred. Using the flashcards from the store product surveys, survey respondents were asked to identify specific products purchased and the factors that influenced their decision to purchase the product. The survey instrument was piloted prior to study start. The survey instrument in English and Spanish can be found in Supplementary Materials.
Two trained local field workers conducted the interviews. The semi-structured questionnaire was audio recorded and later transcribed into Spanish text by project technicians. For the open-ended question regarding factors that influence the decision to purchase a product, we used standard qualitative theme analysis to identify emergent themes and the most common responses from the transcripts [22]. To estimate the relative importance of each response, we created a database of codes in excel, and tabulated the number of households from which each response emerged. Coding in Spanish was manually conducted by NH and cross-validated by AMSI.
2.5. Analysis
A priori, we identified five variables (home ownership, prior chikungunya or dengue infection, stability of job, household income below minimum wage, gender) that we thought would influence mosquito control expenditures and ran independent sample t-tests to evaluate the evidence that the associated population means were significantly different. We also compared total number of strategies employed to expenditures on mosquito control using Pearson parametric correlation tests. Finally, we categorized number of mosquito control strategies employed by households into high/moderate or low and compared the means using a two-sample t-test in the two groups to the response no difficulties (1 = responded “no difficulties”, 0 = other response) to mosquito control. All analysis was done in R version 3.3.1 [23].
3. Results
3.1. Household Demographics
Thirty percent (n = 12) of the heads of households were female and a majority had either completed primary (43%) or secondary (55%) education (Table 1). One-quarter of respondents (n = 10) rented their home. The median number of persons living per household was five. Many of the households had access to municipal sewerage (83%) and piped water inside the home (65%). However, 43% (n = 17) reported daily or weekly interruptions in the piped water supply. Eighty percent (n = 32) of households store water in cisterns or elevated tanks. The water stored is non-drinking water used for cooking, washing dishes/clothes, showering, cleaning the home, etc.
Table 1.
Socio-demographic information from survey respondents (n = 40).
| Socio-Demographics | % Households (n) |
|---|---|
| Female head of household | 30% (12) |
| House is rented | 25% (10) |
| Head of household has only primary education | 43% (17) |
| Head of household has secondary education | 55% (22) |
| Head of household is currently employed | 85% (34) |
| Employed and earns less than the minimum wage (n = 34) | 41% (14) |
| Employed and job is unstable (n = 34) | 38% (13) |
| Water access & storage | |
| Access to municipal sewerage | 83% (33) |
| Piped water inside the house | 65% (26) |
| Daily or weekly interruptions in the piped water supply | 43% (17) |
| Water stored in cisterns or elevated tanks | 80% (32) |
| Housing Condition | |
| Have screens on all windows and doors | 20% (8) |
| Patio condition is good (n = 29) | 17% (5) |
| General housing condition is good | 15% (6) |
| Knowledge & perceptions | |
| Someone in the family with prior dengue or chikungunya infection | 60% (24) |
| Knowledge that dengue is transmitted by mosquitoes | 90% (36) |
| Consider dengue to be a serious problem in the community | 88% (35) |
| Dengue is one of the three most important health problems in the community (open-ended response). | 65% (26) |
| Primary challenge to vector control (only one response selected) | |
| Economic limitations | 38% (15) |
| Lack of information | 10% (4) |
| Lack of time | 20% (8) |
| No difficulties | 32% (13) |
In the community where we conducted our studies, median weekly family income was US$107.50 (range: US$50.00, 250.00). Eighty-five percent (n = 34) of the heads of households are employed, however 38% (n = 13) of this employed group have a job that is unstable and 41% (n = 14) reported that the head of household earns less than the minimum wage, US$350 per month. In comparison, the average national monthly income for households in Ecuador was US$709.
Knowledge and Perceptions of Vector Control
Most survey respondents (88%) reported that dengue was a serious problem in their communities (Table 1). Approximately two-thirds reported that someone in their family had been ill from dengue or chikungunya, and through open-ended responses, they identified dengue as one of the top three health concerns in their community. Ninety percent were aware that dengue was transmitted by mosquitoes. Economic limitations were identified by survey respondents as the most common challenge to employing vector control strategies, reported by 38% of respondents, followed by no difficulties (32%) as the second most common answer. In our statistical analysis, we found that the mean in the high/moderate utilizers of mosquito control activities that reported no difficulties (M = 0.167) was significantly lower than the mean in the low utilizer group (M = 0.456) with a p-value < 0.05.
3.2. Household Mosquito Control and Dengue Prevention Interventions
Survey respondents in the semi-structured survey reported employing a median of five different mosquito control and dengue prevention strategies in their household (range: 0, 10) including chemical control, elimination of larval habitat, mosquito avoidance behaviors, and mosquito repellent strategies (Table 2) when asked the open-ended question, “what strategies do you use to prevent dengue transmission in your home and reduce the number of mosquito bites?”. The most commonly reported mosquito-control practice was the use of bed-nets, however, almost all respondents reported only using bed-nets at night. Likewise, households reported closing doors and windows to prevent mosquitoes from entering the home only in the evening hours.
Table 2.
Mosquito control and dengue prevention strategies reported by survey respondents (n = 38).
| Mosquito Control and Dengue Prevention Strategies | % Households (n) |
|---|---|
| Sleep under bed-net | 92% (35) |
| Close windows and doors | 61% (23) |
| Cover tanks with water/do not let water accumulate outside | 55%(21) |
| Fumigation | 53% (20) |
| Burn plants for smoke | 50% (20) |
| Eliminate trash | 50% (19) |
| General cleaning | 45% (17) |
| Apply repellent | 32% (12) |
| Use liquid larvicide provided by the MoH * | 29% (11) |
| Apply diesel to floors | 26% (10) |
| Cut vegetation | 24% (9) |
| Use other insecticides | 24% (9) |
* Bacillus thuringiensis subspecies israelensis (Bti) was being used by the MoH (Ministry of Health) at the time of the study. Bti contains spores that produce toxins that specifically target the larvae of the mosquito. When resources are adequate, the MoH administers liquid larvicides at no cost to the household.
3.3. Store Product Surveys
Surveys of small neighborhood stores showed that all 10 stores sold at least one type of mosquito control product, defined here as any product that kills or repels mosquitoes. Five different mosquito control products were found; the most common product was Dragon (active ingredient: tetramethrin) liquid insecticide 450 mL (median price: US$2.50). The three large supermarkets had far more variety and availability of mosquito control products (Table 3). The most abundant types of product available were liquid insecticides and mosquito coils. The only items used by households and not found in neighborhood stores or supermarkets were bed-nets and Palosanto (Bursera graveolens), a type of wood burned to create smoke, which acts as a spatial repellent against mosquitos. These two items were only available at the central outdoor markets. The price of an untreated bed net ranged from US$9.00 to US$35.00 (median: US$13.50) depending on material and size. The price of Palosanto was about US$1.00 for a small bag, which contained about five small sticks.
Table 3.
Mosquito control and dengue prevention products available in markets in Machala in 2015.
| Type of Product | Product Brand | Description (Available Products) | Main Active Ingredient | Median Price per Unit or per mL ($US) | Price Range per Item ($US) |
|---|---|---|---|---|---|
| Repellent | Detan | Lotion 60 mL, 120 mL | Diethyl Toluamide “deet” | 0.02 | 2.09–2.99 |
| OFF! | Spray 127 mL | Diethyl Toluamide “deet” | 0.05 | 6.59–6.59 | |
| Liquid insecticides | Dragon | Liquid 230 mL, 450 mL, 950 mL, 475 mL w/spray nozzle | Tetramethrin 0.46% | 0.01 | 1.34–4.39 |
| Torvi | Liquid 230 mL, 500 mL, 1000 mL, 630 mL w/spray nozzle | Cypermethrin 0.25% | 0.01 | 1.26–4.05 | |
| Flit-Kit | 750 mL w/ spray nozzle | Cypermethrin | 0.01 | 3.49–3.49 | |
| Mosquito coils | Incienso | 10 spiral units | D-Allethrin 0.20% | 0.10 | 0.99–0.99 |
| Baygon | 6 double spiral units | D-Allethrin 0.20% | 0.18 | 1.00–1.19 | |
| Aguila | 10 spiral units | Dimefluthrin 0.02% | 0.10 | 0.90–0.98 | |
| Lanju | 10 spiral units | Dimefluthrin 0.03% | 0.09 | 0.90–0.95 | |
| Aerosol sprays | Sapolio | Spray 235 mL | D-Tetramethrin 0.15% | 0.01 | 3.00–3.85 |
| Raid | Spray 235 mL, 360 mL | D-Tetramethrin 0.35% | 0.01 | 3.20–4.81 | |
| PIX | Spray 300 mL | Chlorpyrifos 0.5% | 0.01 | 3.03–3.03 | |
| Rodasol | Spray 400 mL | Bioallethrin 0.2% | 0.01 | 4.24–4.39 | |
| Equipment | Mosquito Racket | Rechargable racket | 4.49 | 3.99–4.99 | |
| Dragon | Insecticide pump | 2.69 | 2.39–2.99 | ||
| (Bed-net) | Untreated bed-net, varying sizes and styles | 13.50 | 9.00–35.00 | ||
| Other | Palosanto | Small bag of five sticks | 0.20 | 0.75–1.25 |
3.4. Household Expenditures
We found that households typically spent US$2.00 on mosquito control per week representing 1.90% (range: 0.00%, 9.21%) of median weekly income (Table 4). After accounting for food expenditures, the average household was left with US$27.50 weekly (US$3.93 daily), for use for housing, water, electricity, transport, communication, recreation, health, etc.
Table 4.
Weekly household expenditures reported by survey respondents (n = 38).
| Measures | Median (US$) | Min (US$) | Max (US$) | % of Weekly Income |
|---|---|---|---|---|
| Income | 107.50 | 50.00 | 250.00 | 100.0% |
| Food expenditures | 80.00 | 30.00 | 120.00 | 74.0% |
| Mosquito control expenditures | 2.00 | 0.00 | 9.21 | 1.9% |
3.5. Factors Influencing Decision to Purchase a Product
From the analysis of coded open-ended responses, we found that the effectiveness of a product, low cost, and ease of use/application were the most important factors that influence people’s decisions to purchase a mosquito control product (Table 5). Other important factors, reported by one-third of households, included minimal health effects and a recommendation by a friend or family member. For effectiveness, respondents determined the success of a product either by seeing dead mosquitos on the ground or experiencing fewer mosquito bites. Several respondents reported purchasing a product because a friend/family member has recommended the product or out of curiosity, but they only continued to purchase the product if they experienced good results. The following quotes are representative of responses to “what factors influence your decision to purchase a mosquito control product?”
Table 5.
Summary of open-ended responses regarding factors that influence the decision to purchase a mosquito-control product (n = 38).
| Factors | % Households (n) |
|---|---|
| Effective product | 42% (16) |
| Low cost | 39% (15) |
| Easy to use/apply | 34% (13) |
| Minimal effects on health | 29% (11) |
| Recommended by a friend/family member | 29% (11) |
| Easy access in my neighborhood | 21% (8) |
| Accustomed to use/always have used the product | 11% (4) |
| Heard about the product on TV/news/radio | 8% (3) |
| Product kills mosquitoes (vs repel) | 8% (3) |
| The product lasts for a long time | 8% (3) |
| Co-benefits of use, i.e., product kills other pests also | 5% (2) |
Example Quote 1.
“I am not accustomed to buying the same item. If I see something new I like to try it to see how it works. If it is efficient and works then I will buy it again.”
Example Quote 2.
“I buy incense (mosquito coils) because it was effective for my mom…the moment it is turned on the mosquitos disappear. It is very effective.”
The second and third most important factors on whether to purchase a product were the cost and ease of use/application, respectively. Whereas low cost of a product saves money, ease of use of a product saves time. Some liquid spray insecticides are sold with the spray nozzle attached to be used immediately; another option is to buy the fumigation pump, a method used by households that mix diesel with liquid insecticide.
Health concerns, or minimal effects on health, were identified as an important factor by 29% of households. Some of the perceived harmful effects were allergies, breathing problems, irritation of the throat, and harm to the lungs. Products that generated smoke and aerosolized insecticides were identified as the strategies that households had most frequently discontinued. Health concerns were the predominant reasons for stopping these practices. Several households reported that, even though they understood the health concerns associated with use of insecticides, they continued to fumigate, but changed their fumigation practices. For example, respondents would fumigate and leave the home for 10-15 minutes and/or wait to do so when children were at school.
Example Quote 3.
“After I fumigate, I leave the home. I know not to enter. If I do, I will fall, just like the mosquitos.”
3.6. Predictors of Monthly Expenditures
The strongest predictor of mosquito control and dengue prevention expenditures was whether the occupants of the household owned (Mean = US$9.90) or rented (M = US$5.11) their home (p = 0.058, Table 6). We found a slight increase in expenditures in households that self-reported that someone in the family had fallen ill from dengue or chikungunya (M = US$9.71) compared to households with no prior infections (M = US$8.14), although the difference was not statistically significant (p = 0.590). We found that households where the head of household had a stable job spent more on mosquito control and dengue prevention (M = US$9.71) than those with an unstable job (M = US$7.44), although the difference was not statistically significant (p = 0.406). We found no association between monthly mosquito control and dengue prevention expenditures and household income above/below minimum wage (p = 0.872) and gender of the head of the household (p = 0.884). In correlation analysis, we found a moderate positive association between total number of strategies employed and expenditures on mosquito control and dengue prevention strategies (p = 0.000).
Table 6.
Bi-variate associates of hypothesized predictors of mosquito control and dengue prevention expenditures.
| Measure | n | Mean (US$) | p-Value |
|---|---|---|---|
| Home ownership | 40 | Own = 9.90 Rent = 5.11 | 0.058 1 |
| Someone in the household with prior chikungunya or dengue infection | 38 | Yes = 9.71 No = 8.14 | 0.590 |
| Stability of job | 40 | Stable = 9.71 Unstable = 7.44 | 0.406 |
| Household income above/below minimum wage | 38 | Above = 9.00 Below = 10.86 | 0.872 |
| Gender | 40 | Male = 8.56 Female = 9.00 | 0.884 |
| Total strategies employed 2 | 40 | r = 0.571 | 0.000 1 |
1 Significant at p = 0.1 level; 2 Pearson parametric correlation test.
4. Discussion
As countries throughout the tropics and subtropics face the rising epidemics of Ae. aegypti-transmitted febrile illnesses—such as dengue, chikungunya, and Zika fever—socio-economic research on Ae. aegypti control is urgently needed to inform the development and implementation of novel mosquito control strategies. Novel Ae. aegypti control technologies are promising (e.g., sterile insect methods via Wolbachia infected mosquitoes and genetically modified mosquitoes) [24,25,26]. However, the effectiveness of their implementation depends largely on community perceptions of risk, perceptions of the relative effectiveness of these strategies, and the economic and behavioral barriers to implementation at the household-level [9,27,28,29,30,31,32].
The burden of Ae. aegypti febrile illness is a major public health priority in Machala, as reflected by community perceptions of risk and prevalence. The results of this study indicate that households in low-income communities spend more than 10% of their family discretionary income on interventions related to mosquito-borne disease. Discretionary income, or income that is left for spending after paying for household necessities (e.g., food and shelter) was calculated based on national results from INEC data. A typical household in Ecuador spends about 8% of household income on housing, water, electricity, gas, and other fuels. In Luz de America, a family with the median weekly income (US$107.50) can be expected to spend about US$8.50 on housing, water, electricity and gas. After accounting for median self-reported expenditures on food (US$80.00) households are left with US$19.00 weekly on discretionary income. Of this amount, the US$2.00 on mosquito control and dengue prevention strategies represents more than 10% of discretionary income.
Households in this study employed five mosquito control interventions to reduce the burden of Ae. aegypti transmitted illness. Our findings are consistent with a previous study in Machala both in terms of the number and diversity of strategies employed by households to prevent dengue fever [9]. However, there is little empirical evidence that these interventions effectively prevent disease. Indeed, a majority of these households self-reported that someone in their family had been ill with dengue or chikungunya. Novel interventions that are both low cost and effective at reducing the population of Ae. aegypti are urgently needed. This study can help guide the development of novel mosquito control strategies for robust markets, such as Ecuador, and help governments decide how to spend on vector control interventions, by comparing the economic burden to households to the cost of other type of interventions [33]. Innovative control strategies are promising, such as lethal oviposition traps, use of transgenic Ae. aegypti that prevents larvae from developing into adulthood, and attractive toxic sugar baits [34,35,36,37]. The success of these strategies will rest on common themes of economic and behavioral barriers that can impede or assist in implementation at the household-level.
We found that economic limitations were the most important barrier to household-level vector control followed by the response “no difficulties.” Home ownership and stability of the job of the head of the household, proxies of poverty, were associated with expenditures on mosquito control and dengue prevention. Our findings support reports by prior studies on malaria prevention, which found that prior to the mass distribution of free insecticide-treated mosquito nets, that achieved great equity in coverage, access to bed nets was largely dependent upon wealth [17,38,39,40]. In low-income communities, financial subsidies for mosquito control interventions will be a key strategy to promote use. Households that reported no challenges to vector control were more likely to employ fewer strategies to vector control. Combining our narrative, we interpret this to mean that the ease of access in the neighborhoods, variety of products available, and the low cost of certain products may make it very easy, and with no reported difficulties, to carry out mosquito control strategies in the household, especially in households that report employing very few mosquito control strategies.
We identified key factors that influence people’s decisions to purchase a mosquito control product, with important implications for interventions. For effective Ae. aegpyti control to take place in this area, interventions must be simultaneously effective, low cost, and easy to use. In our study, many respondents had stopped using insecticide sprays and products that produce smoke because of health concerns. While supermarkets sold a range of products (repellents, aerosols, mosquito coils, liquid sprays, and equipment), the easiest to find in neighborhood stores were tetramethrin-based products. Cypermethrin and tetramethrin, active ingredients used in popular insecticide sprays, are classified as possible human carcinogens [41,42]. Prolonged exposure to smoke from mosquito coils may have harmful effects on the lungs or mutagenic effects [43,44]. Families have to make difficult decisions, balancing the health effects of certain products against the health effects of diseases transmitted by Aedes mosquitoes.
The survey results indicate that the community knowledge was very high on the transmission of dengue, and community perceptions were very high regarding the severity, importance, and risk of dengue fever. Focus groups conducted in Puerto Rico found that participants with a previous dengue diagnosis were more concerned about risk of the disease and recommended using repellent more often than their counterparts without a previous dengue diagnosis [45]. Additional studies in Thailand and Malaysia have documented a direct link between knowledge on dengue prevention and prevention practices [46,47]. More specific questions on community knowledge of dengue epidemiology and vector bionomics would be a primary interest in Machala because of an increasing trend of Ae. aegypti-transmitted illnesses in this highly populated urban environment. In South America, continued evaluation of people’s knowledge, perceptions, and practice are of great importance to improve integrated control measures and organize health education programs to resolve the dengue problem.
This study provides an important perspective on Ae. aegypti control, despite the small sample size of the study population. We chose our study site in Machala because this was an area where the MoH had stopped the systematic fumigation and larviciding of homes due to resource limitations. This community was typical of low-income communities in the urban periphery in coastal Ecuador, where the political-institutional and community-household determinants that result in high densities of Ae. aegypti are intimately connected with poverty. For example, risk factors such as inadequate access to municipal public services and utilities (e.g., garbage collection, sewerage, piped water) are prevalent in economically disadvantaged areas and can lead to an increase in Ae. aegypti densities. Previous work in Machala found that residents in the urban periphery felt neglected by government institutions [9]. In our study population, residents have taken it upon themselves to prevent dengue fever and other mosquito-borne diseases in their family. However, for most of the residents, mosquito control expenditures represented a substantial proportion of their income, and individual household protection may not be effective at preventing disease. Innovative vector control interventions that focus on community mobilization can yield more cost-effective solutions [29,33,48]. Community-based pilot dengue control programs by the MoH in Machala have been found to effectively reduce densities of Ae. aegypti [49,50,51]; however, the sustainability and scalability of these interventions has been challenged due to the recent restructuring of the MoH and elimination of the national vector control program.
A limitation of this study was determining the period during which people used the different mosquito control products. Future studies should include a specific time period when asking, “which of these products do you purchase?” We chose not to report the percentage of household’s responses to that question because recall periods could bias the results. For smaller consumption items, appropriate recall periods are typically two and four weeks in economic household surveys [52]. In this study, surveys were conducted at one time point in one community. Future studies would ideally repeat the questionnaires over a period of one year, to capture seasonal variation in consumer behavior, and in a range of communities representing different income levels. Since our interviews took place at the end of the rainy season, the responses by study participants may underestimate true expenditures during the peak of dengue transmission.
Previous dengue or chikungunya infection was self-reported by respondents. We did not differentiate between dengue or chikungunya affected households. With the rise of chikungunya and Zika throughout Latin America, the distinction between Ae. aegypti-transmitted illnesses is an important step in order to measure and compare the perception of risk for each of the diseases and attribute the adoption of vector control strategies to the respective disease. It is well-known that acceptable risk is lower for unknown and unfamiliar activities [53]. Zika’s newness and unfamiliarity may have important implications for perception of risk and the adoption of preventative strategies.
A strength of this study was the validation of product prices from different sources. The prices self-reported by residents were found to be very similar to prices documented during the product surveys. These results provide important information about the types of products used by households and relative amounts spent per product.
5. Conclusions
The results of this study demonstrate that households in the urban periphery spend a considerable proportion of discretionary household income to prevent mosquito-borne diseases. These findings also show a robust and healthy market for commercial mosquito control products, even among the poorest of households in Machala, Ecuador.
Dengue has a high economic impact, but the true economic cost of the disease is unknown. A 2015 literature review from the Costing Dengue Working Group in the Americas found only a few economic studies in the region [54]. The published literature is lacking in quantity and quality. The cost studies are many times unclear and not representative of the total economic costs of dengue, particularly the prevention costs to households [55]. The importance of determining the economic burden of dengue prevention at the household level is so that it can be used for cost-effectiveness analyses to provide important information about efficient resource allocation [56].
This study addresses the need for economic research specific to dengue prevention at the household level to ensure informed decision making on the various options for controlling and preventing Ae. aegypti transmitted diseases. The findings from this study contribute to a body of research on the types and costs of interventions used by households that face a high burden of Ae. aegypti-transmitted illnesses, such as dengue, chikungunya, and Zika. We also identified the barriers and factors that influence a household’s decision to purchase a mosquito control product. This information is important to understand the role of these interventions in prevention of mosquito-borne diseases and economic burden imposed on households.
Helping community members to protect themselves against dengue fever and other vector borne diseases is a critical public health objective. Interventions that are low cost, effective, easy to use, and pose minimal health risks to families will have the greatest chance of widespread use. Even with vaccines available in the future, vector control is likely to remain a key means of prevention of Ae. aegypti-transmitted illnesses. With the rise of chikungunya and Zika fever, more work must be done to identify interventions that efficiently reduce Ae. aegypti densities, especially in vulnerable communities that face the greatest social and economic burden.
Acknowledgments
We would like to thank the community members of Luz de America for their participation in this study. We would also like to thank colleagues at the Ministry of Health in Machala for facilitating and supporting this research. This study was supported in part by the Deployed Warfighters Protection Program Grant (W911QY-15-1-0005) and a fellowship from the Rotary Club International to Naveed Heydari.
Supplementary Materials
The following are available online at www.mdpi.com/1660-4601/14/2/196/s1, Survey instrument in English and Spanish.
Author Contributions
Naveed Heydari and Anna M. Stewart-Ibarra conceived and designed the study. Naveed Heydari, Prissila Fernandez, Jefferson Adrian, and Efraín Beltrán Ayala piloted the study and conducted primary data collection. Naveed Heydari, Anna M. Stewart-Ibarra, and David A. Larsen contributed analysis tools and analyzed the data. Naveed Heydari wrote the manuscript. All co-authors reviewed and approved the final manuscript.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- 1.Bhatt S., Gething P.W., Brady O.J., Messina J.P., Farlow A.W., Moyes C.L., Drake J.M., Brownstein J.S., Hoen A.G., Sankoh O., et al. The global distribution and burden of dengue. Nature. 2013;496:504–507. doi: 10.1038/nature12060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gubler D.J., Clark G.G. Dengue/Dengue Hemorrhagic Fever: The Emergence of a Global Health Problem. Emerg. Infect. Dis. 1995;1:55–57. doi: 10.3201/eid0102.952004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Suaya J.A., Shepard D.S., Siqueira J.B., Martelli C.T., Lum L.C., Tan L.H., Kongsin S., Jiamton S., Garrido F., Montoya R., et al. Cost of dengue cases in eight countries in the Americas and Asia: A prospective study. Am. J. Trop. Med. Hyg. 2009;80:846–855. [PubMed] [Google Scholar]
- 4.World Bank. The Short-Term Economic Costs of Zika in Latin America and the Caribbean. [(accessed on 20 November 2016)]. Available online: http://pubdocs.worldbank.org/en/410321455758564708/The-short-term-economic-costs-of-Zika-in-LCR-final-doc-autores-feb-18.pdf.
- 5.Dirección Nacional de Vigilancia Epidemiológica. [(accessed on 20 November 2016)];Anuario Epidemiológico. 1994–2014. Available online: www.salud.gob.ec/direccion-nacional-de-vigilancia-epidemiologica/
- 6.Zika Cases and Congenital Syndrome Associated with Zika Virus Reported by Countries and Territories in the Americas, 2015–2016. [(accessed on 15 February 2017)]. Available online: http://www.google.com.hk/url?sa=t&rct=j&q=&esrc=s&source=web&cd=3&ved=0ahUKEwj3wNm7spHSAhVCkJQKHU08Au8QFggoMAI&url=http%3A%2F%2Fwww.paho.org%2Fhq%2Findex.php%3Foption%3Dcom_docman%26task%3Ddoc_view%26Itemid%3D270%26gid%3D37070%26lang%3Den&usg=AFQjCNFdD9qX3DrrzBfxr4dEev208s89-A&sig2=b0HO5keznfahblQaWoGaIA&cad=rja.
- 7.Stewart Ibarra A.M., Munoz A.G., Ryan S.J., Borbor M.J., Ayala E.B., Finkelstein J.L., Mejia R., Ordonez T., Coronel G.C.R., Rivero K. Spatiotemporal clustering, climate periodicity, and social-ecological risk factors for dengue during an outbreak in Machala, Ecuador, in 2010. BMC Infect. Dis. 2014;14:610. doi: 10.1186/s12879-014-0610-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stewart Ibarra A.M., Ryan S.J., Beltrán E., Mejía R., Silva M., Muñoz Á. Dengue Vector Dynamics (Aedes aegypti) Influenced by Climate and Social Factors in Ecuador: Implications for Targeted Control. PLoS ONE. 2013;8:e78263. doi: 10.1371/journal.pone.0078263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stewart Ibarra A.M., Luzadis V.A., Cordova M.J.B., Silva M., Ordoñez T., Ayala E.B., Ryan S.J. A social-ecological analysis of community perceptions of dengue fever and Aedes aegypti in Machala, Ecuador. BMC Public Health. 2014;14:1135. doi: 10.1186/1471-2458-14-1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Beatty M.E., Beutels P., Meltzer M.I., Shepard D.S., Hombach J., Hutubessy R., Dessis D., Coudeville L., Dervaux B., Wichmann O., et al. Health economics of dengue: A systematic literature review and expert panel’s assessment. Am. J. Trop. Med. Hyg. 2011;84:473–488. doi: 10.4269/ajtmh.2011.10-0521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Harving M.L., Rönsholt F.F. The economic impact of dengue hemorrhagic fever on family level in Southern Vietnam. Dan. Med. Bull. 2007;54:170–172. [PubMed] [Google Scholar]
- 12.Khun S., Manderson L. Health seeking and access to care for children with suspected dengue in Cambodia: An ethnographic study. BMC Public Health. 2007;7:262. doi: 10.1186/1471-2458-7-262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huy R., Wichmann O., Beatty M., Ngan C., Duong S., Margolis H.S., Vong S. Cost of dengue and other febrile illnesses to households in rural Cambodia: A prospective community-based case-control study. BMC Public Health. 2009;9:155. doi: 10.1186/1471-2458-9-155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Clark D.V., Mammen M.P., Nisalak A., Puthimethee V., Endy T.P. Economic Impact of Dengue Fever/Dengue Hemorrhagic Fever in Thailand at the Family and Population Levels. Am J. Trop. Med. Hyg. 2005;72:786–791. [PubMed] [Google Scholar]
- 15.Van Damme W., Van Leemput L., Por I., Hardeman W., Meessen B. Out-of-pocket health expenditure and debt in poor households: Evidence from Cambodia. Trop. Med. Int. Health. 2004;9:273–280. doi: 10.1046/j.1365-3156.2003.01194.x. [DOI] [PubMed] [Google Scholar]
- 16.Guiguemdé T.R., Dao F., Curtis V., Traoré A., Sondo B., Testa J., Ouédraogo J.B. Household expenditure on malaria prevention and treatment for families in the town of Bobo-Dioulasso, Burkina Faso. Trans. R. Soc. Trop. Med. Hyg. 1994;88:285–287. doi: 10.1016/0035-9203(94)90079-5. [DOI] [PubMed] [Google Scholar]
- 17.Wiseman V., McElroy B., Conteh L., Stevens W. Malaria prevention in the Gambia: Patterns of expenditure and determinants of demand at the household level. Trop. Med. Int. Health. 2006;11:419–431. doi: 10.1111/j.1365-3156.2006.01586.x. [DOI] [PubMed] [Google Scholar]
- 18.Okrah J., Traoré C., Palé A., Sommerfeld J., Müller O. Community factors associated with malaria prevention by mosquito nets: An exploratory study in rural Burkina Faso. Trop. Med. Int. Health. 2002;7:240–248. doi: 10.1046/j.1365-3156.2002.00856.x. [DOI] [PubMed] [Google Scholar]
- 19.Snehalatha K.S., Ramaiah K.D., Vijay Kumar K.N., Das P.K. The mosquito problem and type and costs of personal protection measures used in rural and urban communities in Pondicherry region, South India. Acta Trop. 2003;88:3–9. doi: 10.1016/S0001-706X(03)00155-4. [DOI] [PubMed] [Google Scholar]
- 20.Mulla M.S., Thavara U., Tawatsin A., Kong-Ngamsuk W., Chompoosri J. Mosquito burden and impact on the poor: Measures and costs for personal protection in some communities in Thailand. J. Am. Mosq. Control Assoc. 2001;17:153–159. [PubMed] [Google Scholar]
- 21.Población y Demografía. [(accessed on 15 February 2017)]. Available online: http://www.monografias.com/trabajos94/poblacion-y-demografia/poblacion-y-demografia.shtml.
- 22.Bogdan R., Biklen S.K. Qualitative Research for Education: An Introduction to Theories and Methods. 5th ed. Pearson; London, UK: 2006. [Google Scholar]
- 23.R Core Team . R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing; Vienna, Austria: 2013. [Google Scholar]
- 24.Alphey L. Genetic Control of Mosquitoes. Annu. Rev. Entomol. 2014;59:205–224. doi: 10.1146/annurev-ento-011613-162002. [DOI] [PubMed] [Google Scholar]
- 25.Araújo H.R.C., Carvalho D.O., Ioshino R.S., Costa-da-Silva A.L., Capurro M.L. Aedes aegypti Control Strategies in Brazil: Incorporation of New Technologies to Overcome the Persistence of Dengue Epidemics. Insects. 2015;6:576–594. doi: 10.3390/insects6020576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McGraw E.A., O’Neill S.L. Beyond insecticides: New thinking on an ancient problem. Nat. Rev. Microbiol. 2013;11:181–193. doi: 10.1038/nrmicro2968. [DOI] [PubMed] [Google Scholar]
- 27.Parks W., Lloyd L. Planning Social Mobilization and Communication for Dengue Fever Prevention and Control. World Health Organization; Geneva, Switzerland: 2004. [Google Scholar]
- 28.Gubler D.J., Clark G.G. Community involvement in the control of Aedes aegypti. Acta Trop. 1996;61:169–179. doi: 10.1016/0001-706X(95)00103-L. [DOI] [PubMed] [Google Scholar]
- 29.Hanh T.T., Hill P.S., Kay B.H., Quy T.M. Development of a framework for evaluating the sustainability of community-based dengue control projects. Am. J. Trop. Med. Hyg. 2009;80:312–318. [PubMed] [Google Scholar]
- 30.Okorie P.N., Marshall J.M., Akpa O.M., Ademowo O.G. Perceptions and recommendations by scientists for a potential release of genetically modified mosquitoes in Nigeria. Malar. J. 2014;13:154. doi: 10.1186/1475-2875-13-154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Amin L., Hashim H. Factors influencing stakeholders attitudes toward genetically modified Aedes mosquito. Sci. Eng. Ethics. 2014;21:655–681. doi: 10.1007/s11948-014-9557-5. [DOI] [PubMed] [Google Scholar]
- 32.Marshall J.M., Touré M.B., Traore M.M., Famenini S., Taylor C.E. Perspectives of people in Mali toward genetically-modified mosquitoes for malaria control. Malar. J. 2010;9:128. doi: 10.1186/1475-2875-9-128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Alfonso-Sierra E., Basso C., Beltran-Ayala E., Mitchell-Foster K., Quintero J., Cortes S., Manrique-Saide P., Guillermo-May G., Caprara A., Carvalho de Lima E., et al. Innovative dengue vector control interventions in Latin America: What do they cost? Pathog. Glob. Health. 2016;7724:1–11. doi: 10.1080/20477724.2016.1142057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Johnson B.J., Ritchie S.A., Fonseca D.M. The State of the Art of Lethal Oviposition Trap-Based Mass Interventions for Arboviral Control. Insects. 2017;8:5. doi: 10.3390/insects8010005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Paz-Soldan V.A., Yukich J., Soonthorndhada A., Giron M., Apperson C.S., Ponnusamy L., Schal C., Morrison A.C., Keating J., Wesson D.M. Design and Testing of Novel Lethal Ovitrap to Reduce Populations of Aedes Mosquitoes: Community-Based Participatory Research between Industry, Academia and Communities in Peru and Thailand. PLoS ONE. 2016;11:e0160386. doi: 10.1371/journal.pone.0160386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.De Andrade P.P., Aragão F.J., Colli W., Dellagostin O.A., Finardi-Filho F., Hirata M.H., de Castro Lira-Neto A., de Melo M.A., Nepomuceno A.L., da Nóbrega F.G., et al. Use of transgenic Aedes aegyptiin Brazil: Risk perception and assessment. Bull. World Health Organ. 2016;94:766–771. doi: 10.2471/BLT.16.173377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Scott-Fiorenzano J.M., Fulcher A.P., Seeger K.E., Allan S.A., Kline D.L., Koehler P.G., Müller G.C., Xue R.D. Evaluations of dual attractant toxic sugar baits for surveillance and control of Aedes aegypti and Aedes albopictus in Florida. Parasites Vectors. 2017;10:9. doi: 10.1186/s13071-016-1937-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Steketee R.W., Eisele T.P. Is the scale up of malaria intervention coverage also achieving equity? PLoS ONE. 2009;4:e8409. doi: 10.1371/journal.pone.0008409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rashed S., Johnson H., Dongier P., Moreau R., Lee C., Lambert J., Schaefer C., Hospital M., Dimensions S., Sante C. Permethrin-Impregnated Bed nets in a Malarious Area II. Determ. Febr. Epis. Cost Treat. 2000;62:181–186. [Google Scholar]
- 40.Nuwaha F. People’s perception of malaria in Mbarara, Uganda. Trop. Med. Int. Health. 2002;7:462–470. doi: 10.1046/j.1365-3156.2002.00877.x. [DOI] [PubMed] [Google Scholar]
- 41.Toxnet: Cypermethrin. [(accessed on 1 May 2016)]; Available online: https://toxnet.nlm.nih.gov/cgi-bin/sis/search/a?dbs+hsdb:@term+@DOCNO+6600.
- 42.Toxnet: Tetramethrin. [(accessed on 1 May 2016)]; Available online: https://toxnet.nlm.nih.gov/cgi-bin/sis/search/a?dbs+hsdb:@term+@DOCNO+6738.
- 43.Liu W.K., Ng T.B., Wong C.C. Biochemical and cellular changes in bronchoalveolar lavaged samples from rats after inhalation of mosquito-coil smoke. Toxicol. Lett. 1989;45:121–132. doi: 10.1016/0378-4274(89)90001-5. [DOI] [PubMed] [Google Scholar]
- 44.Matsumoto K., Tamakawa K., Takahashi Y., Seki T., Tsunoda A. Effect of several factors on indoor air pollution—Mutagenicity of mosquito coil smoke. EISEI KAGAKU. 1989;35:237–240. doi: 10.1248/jhs1956.35.237. [DOI] [Google Scholar]
- 45.Pérez-Guerra C.L., Zielinski-Gutierrez E., Vargas-Torres D., Clark G.G. Community beliefs and practices about dengue in Puerto Rico. Rev. Panam. Salud Pública. 2009;25:218–226. doi: 10.1590/S1020-49892009000300005. [DOI] [PubMed] [Google Scholar]
- 46.Al-Dubai S.A., Ganasegeran K., Mohanad Rahman A., Alshagga M.A., Saif-Ali R. Factors affecting dengue fever knowledge, attitudes and practices among selected urban, semi-urban and rural communities in Malaysia. Southeast Asian J. Trop. Med. Public Health. 2013;44:37–49. [PubMed] [Google Scholar]
- 47.Koenraadt C.J., Tuiten W., Sithiprasasna R., Kijchalao U., Jones J.W., Scott T.W. Dengue knowledge and practices and their impact on Aedes aegypti populations in Kamphaeng Phet, Thailand. Am. J. Trop. Med. Hyg. 2006;74:692–700. [PubMed] [Google Scholar]
- 48.Parks W., Lloyd L., Nathan M.B., Hosein E., Odugleh A., Clark G.G., Gubler D.J., Prasittisuk C., Palmer K., San Martin J.L., et al. International experiences in social mobilization and communication for dengue prevention and control. Dengue Bull. 2004;28:S1–S7. [Google Scholar]
- 49.Breilh J., Spiegel J., Wilches A.A., Mitchell-foster K., Delgado J.A. Integrating participatory community mobilization processes to improve dengue prevention: An eco-bio-social scaling up of local success in Machala, Ecuador. Trans. R. Soc. Trop. Med. Hyg. 2015;109:126–133. doi: 10.1093/trstmh/tru209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Quintero J., Brochero H., Manrique-Saide P., Barrera-Pérez M., Basso C., Romero S., Caprara A., Cunha J.C.D.L., Beltrán-Ayala E., Mitchell-Foster K., et al. Ecological, biological and social dimensions of dengue vector breeding in five urban settings of Latin America: A multi-country study. BMC Infect. Dis. 2014;14:38. doi: 10.1186/1471-2334-14-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mitchell-Foster K. Ph.D. Thesis. University of British Columbia; Vancouver, BC, Canada: Oct 21, 2013. Interdisciplinary Knowledge Translation and Evaluation Strategies for Participatory Dengue Prevention in Machala, Ecuador. [Google Scholar]
- 52.Deaton A. The Analysis of Household Surveys. The John Hopkins University Press; Baltimore, MD, USA: 2000. [Google Scholar]
- 53.Fischhoff B., Slovic P., Lichtenstein S., Read S., Combs B. How safe is safe enough? A psychometric study of attitudes towards technological risks and benefits. Policy Sci. 1978;9:127–152. doi: 10.1007/BF00143739. [DOI] [Google Scholar]
- 54.Constenla D., Garcia C., Lefcourt N. Assessing the economics of dengue: Results from a systematic review of the literature and expert survey. Pharmacoeconomics. 2015;33:1107–1135. doi: 10.1007/s40273-015-0294-7. [DOI] [PubMed] [Google Scholar]
- 55.Constenla D., Armien B., Arredondo J., Carabali M., Carrasquilla G., Castro R., Durand L., Durán-Arenas L., García M.E., Gallegos R.V., et al. Costing dengue fever cases and outbreaks: Recommendations from a costing dengue working group in the Americas. Value Health Reg. Issues. 2015;8:80–91. doi: 10.1016/j.vhri.2015.06.001. [DOI] [PubMed] [Google Scholar]
- 56.Drummond M.F., Sculpher M.J., Claxton K., Stoddart G.L., Torrance G.W. Methods for the Economic Evaluation of Health Care Programmes. Oxford University Press; Oxford, UK: 2015. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.