Abstract
Objective
To summarize the epidemiology and outcomes of children with multiple organ dysfunction syndrome (MODS), as part of the Eunice Kennedy Shriver National Institute of Child Health and Human Development MODS Workshop (March 26–27, 2015).
Data Sources
Literature review, research data, and expert opinion
Study Selection
Not applicable
Data Extraction
Moderated by an experienced expert from the field, issues relevant to the epidemiology and outcomes of children with MODS were presented, discussed and debated with a focus on identifying knowledge gaps and research priorities.
Data Synthesis
Summary of presentations and discussion supported and supplemented by relevant literature.
Conclusions
A full understanding the epidemiology and outcome of MODS in children is limited by inconsistent definitions and populations studied. Nonetheless, pediatric MODS is common among PICU patients, occurring in up to 57% depending on the population studied; sepsis remains its leading cause. Pediatric MODS leads to considerable short-term morbidity and mortality. Long-term outcomes of MODS in children have not been well studied; however, studies of adults and children with other critical illnesses suggest that the risk of long-term adverse sequelae is high. Characterization of the long-term outcomes of pediatric MODS is crucial to identify opportunities for improved treatment and recovery strategies that will improve the quality of life of critically ill children and their families. The Workshop identified important knowledge gaps and research priorities intended to promote the development of standard definitions and the identification of modifiable factors related to its occurrence and outcome.
Keywords: Multiple organ dysfunction syndrome, pediatric, epidemiology, health care outcomes
Definition of Multiple Organ Dysfunction Syndrome (MODS)
The concept of a syndrome involving the simultaneous dysfunction of multiple organs first appeared in 1975; it was suggested by Dr. Baue a surgeon.(1) The syndrome has had rich nomenclature through the years, including multiple organ failure (MOF), multiple-organ-system failure (MOSF), multiple system organ failure (MSOF), hypermetabolism organ failure complex, and nonbacteremic clinical sepsis. The long list of names for the syndrome complicates the task of performing a literature search. However, multiple organ dysfunction syndrome (MODS) became the standard name of this condition in the 1990s. Given the temporal occurrence of MODS during the illness course, several modifying adjectives have been used in its description, including primary vs. secondary MODS, early vs. late MODS, new MODS, and progressive or sequential MODS. The inherent difficulty in separating many of these terms has been acknowledged.(2) For example, primary MODS is defined as two simultaneous dysfunctional organs within a week of PICU admission and without subsequent additional organ dysfunction. However, some patients develop additional organ dysfunction during and/or after the first week of PICU admission. These patients might be best categorized as having “progressive MODS,” a recently proposed term that will subsequently be discussed later in sections on New and Progressive MODS.
MODS is a syndrome. By definition, a syndrome is a group of symptoms and signs that consistently occur together or a condition characterized by a set of associated symptoms and signs. The symptoms and signs usually have a common mechanism, and a syndrome has predictable outcomes. Pediatric MODS occurs as a result of multiple potential triggers during the course of childhood critical illness. It is characterized by the simultaneous failure or dysfunction of organs or organ systems, including the respiratory, cardiovascular, neurological, renal, hematological and hepatic systems.(3) It is common in patients admitted to pediatric intensive care units (PICUs), occurring in nearly 20% of children on admission in the largest study to date.(4) MODS commonly involves a severe, dysregulated, systemic inflammatory process, which occurs in approximately 95% of primary MODS.(2) This systemic inflammatory response syndrome (SIRS) can be caused by any condition (e.g. infection, trauma, shock, etc.) that can trigger a systemic inflammatory process. MODS increases the risk of death(4–6) and poor functional outcomes.(4)
Currently, MODS cannot be considered as a disease. A “disease” is defined as a disorder that produces specific symptoms/signs and is not simply a direct result of a physical injury. Typically, the etiology of a disease is well defined. As more is learned about the etiology of a syndrome, it can become a disease. For example, Down syndrome is now named trisomy 21, and it is diagnosed by a karyotype demonstrating three copies of chromosome number 21. Similarly, homocysteinemia is differentiated from Marfan syndrome by a homocysteine blood level measurement > 15 μmol/L. There is currently no pathognomonic test for MODS; it remains a clinical diagnosis.
MODS diagnostic criteria: past and present
Defining any medical condition in the absence of a “gold standard” diagnostic test is challenging. The challenges in defining a syndrome such as MODS are even greater, as its causation is multifactorial and the underlying biology is incompletely understood. Nonetheless, its study is enhanced by refinement of definitions and diagnostic criteria as new knowledge permits. The first set of MODS diagnostic criteria in children were suggested by Wilkinson and colleagues in 1987.(7) The list was changed in 1996 by Proulx(2) (Table 1), in which MODS was defined as the simultaneous dysfunction of at least two organ systems. The organs and systems considered were respiratory, cardiovascular, neurological, hematological, renal, hepatic, and gastrointestinal. In 2005, an international pediatric sepsis consensus conference developed a new set of diagnostic criteria (Table 2).(8)
Table 1. Diagnostic criteria of multiple organ dysfunction syndrome (MODS) according to Proulx et al. (2).
MODS is defined as the concurrent dysfunction of two or more organ systems. Each organ failure or dysfunction is defined by meeting one or more criteria within each system. Non-survivors are considered to have all organs failing on date of death.
Respiratory dysfunction.
|
|
|
Cardiovascular dysfunction.
|
|
|
Hematological dysfunction.
|
|
|
Neurological dysfunction.
|
|
|
Hepatic dysfunction.
|
|
|
Gastrointestinal (GI) dysfunction.
|
|
|
Renal dysfunction.
|
Table 2. Diagnostic criteria of multiple organ dysfunction syndrome (MODS) according to Goldstein et al (8).
MODS is defined as the concurrent dysfunction of two or more systems. Each organ failure or dysfunction is defined by meeting one or more criteria of each organ system.
(Age-specific ranges for physiologic and laboratory variables at the end of this table.)
| Respiratory dysfunction.§ |
|---|
|
|
|
|
Cardiovascular dysfunction. Despite administration of intravenous fluid bolus ≥ 40 mL/kg in 1 hr:
|
|
|
Hematological dysfunction.
|
|
|
Neurological dysfunction.
|
|
|
Hepatic dysfunction.
|
|
|
Renal dysfunction.
|
Proven need assumes O2 requirement was tested by decreasing flow with subsequent increase in flow if required.
ALT = alanine transaminase (SGPT)
In postoperative patients, this requirement can be met if the patient has developed an acute inflammatory or infectious process in the lungs that prevents him or her from being extubated.
Acute respiratory distress syndrome (ARDS) by these criteria must include a PaO2/FiO2 ratio ≤ 200 mm Hg, bilateral infiltrates, acute onset, and no evidence of left heart failure. Acute lung injury (ALI) is defined identically except the PaO2/FiO2 ratio is 201–300 mm Hg.
BP = Blood pressure
SD = Standard deviation
Despite their strengths, both sets of diagnostic criteria have weaknesses. For example, the number of organ systems that were considered is arbitrary; seven organ systems were considered in 1996, while only six were considered in 2005. Additionally, there are also problems with respect to the individual criteria within each organ system. For example, Goldstein(8) stated that respiratory dysfunction could only be diagnosed if any of the following criteria are met:
PaO2/FiO2 < 300 mmHg in the absence of cyanotic heart disease or preexisting lung disease or
PaCO2 > 65 mmHg or 20 mmHg over baseline PaCO2 or
Proven need† for > 0.50 FiO2 to maintain saturation ≥ 92% or
Need for non-elective invasive or non-invasive mechanical ventilation
This list of diagnostic criteria has not been scientifically validated, and whether these four criteria each have the same diagnostic importance remains to be determined. Moreover, some criteria for other organs are not adjusted to age (e.g., hemoglobin level, white blood cell (WBC) count, serum urea nitrogen (BUN), and creatinine). Another problem is that we only need to observe two simultaneous dysfunctional organs to diagnose MODS, regardless of the pathophysiology of the organ dysfunction. This may not always be optimal. Finally, the same diagnostic importance, the same weight, is given to organ systems with different associated mortality risks. Although that is acceptable as diagnostic criteria, data suggest that individual organ system failures may be associated with different risks. For example, Leteurtre demonstrated that the risk of death is at least two times higher with neurological dysfunction than it is with respiratory dysfunction.(9)
Revisiting MODS diagnostic criteria: epidemiological impact
Thus, efforts to improve MODS diagnostic criteria appear well justified. However, revisiting the MODS diagnostic criteria may have significant impact on the perception of MODS epidemiology. In a small prospective study conducted in 2010, Villeneuve and colleagues(10) compared the ability of Proulx’s and Goldstein’s MODS diagnostic criteria to predict ICU mortality.(2, 8) The sensitivity to predict mortality was 68.1% for the former vs. 85.4% for the latter set of criteria (p=0.39), while specificity was 81.5% vs. 65.1% (p<0.001). The prevalence of MODS at PICU entry was 15.5% vs. 30.7%, while the incidence of new MODS (onset after ICU admission) was 22.3% vs. 38.6%. Similarly, Weiss et al reported a 7-fold difference in the incidence of severe sepsis when using two different sets of diagnostic criteria.(11) As a result, comparing incidence rates in different studies using different diagnostic criteria may be misleading. Clearly, diagnostic criteria are not interchangeable. One cannot change the diagnostic criteria of a syndrome without changing its capacity to predict outcomes (sensitivity, specificity) and its epidemiology (incidence, prevalence, clinical impact, etc.).
Diagnostic criteria of new and progressive MODS
It would be difficult to carry out randomized controlled trials (RCTs) in a general PICU population today using mortality as the primary outcome measure because the mortality rate is so low (approximately 3–6%).(4, 6, 12) Similarly, it would be difficult to conduct an RCT in PICU patients using MODS as the primary outcome measure because MODS is commonly present at, or soon after, PICU admission. Proulx reported that 78% of MODS occurs during the first day in the PICU.(2) Patients who have the primary outcome prior to randomization cannot be enrolled into an RCT.
New or progressive MODS (NPMODS) may be a better outcome measure. NPMODS is defined as the proportion of patients who develop new or progressive MODS (or who die) within a given time period (e.g., from the time between randomization and PICU discharge). New MODS can occur in patients with no or single organ dysfunction at time zero (e.g., PICU entry or randomization) and is defined as the development of two or more concurrent organ dysfunctions at any time during the study period. Patients with MODS at time zero can develop progressive MODS, defined as the development of at least one additional concurrent organ dysfunction at any time during the study period.
At Sainte-Justine Hospital, new and progressive MODS occurred in 13.1% and 10.5% of 842 consecutive admissions in 2010.(13) Therefore, considering progressive in addition to new MODS as outcomes in a pediatric RCT almost doubles the number of outcome events. Thus, the incidence rate of NPMODS in PICU patients is sufficient to be used as a primary outcome in RCTs. As a result, NPMODS was chosen as the primary outcome measure of the Transfusion Requirements in the PICU (TRIPICU)(14) and the “Age of Blood in Children in PICU” (ABC-PICU, NCT01977547) RCTs and as a secondary outcome in the Heart and Lung Failure – Pediatric Insulin Titration trial (HALF-PINT, NCT01565941).
All-cause and disease-specific incidence, prevalence, and risk factors
The importance of the numerator (case definition) and denominator (defining the population at risk)
Accurate and consistent MODS case identification enhances the ability to compare observational studies, to determine the appropriate sample size for clinical research studies, and to monitor epidemiological trends. The incidence of a disease or condition refers to the proportion of a group initially free of the condition that develops the condition over a given time period, while the prevalence is the proportion of a group possessing a clinical condition at a given point in time. Incidence can be measured in terms of incidence density, the number of new cases/total person-time at risk; or incidence rate, the number of new cases/total number of people at risk over time. On the other hand, prevalence studies can be either point prevalence studies, with prevalence measured at the time of a study, or period prevalence studies, measuring cases present during a specific time period. Accurate identification of the numerator and denominator populations is crucial. In defining the cases (numerator), it is important to ensure only true cases are identified. In terms of the denominator, it is important to clearly identify the population at risk that is of interest. For example, should all susceptible patients be in the denominator population, or should the denominator population be restricted to children with specific diseases such as sepsis? These questions are integral to accurate and consistent determination of MODS incidence and prevalence.
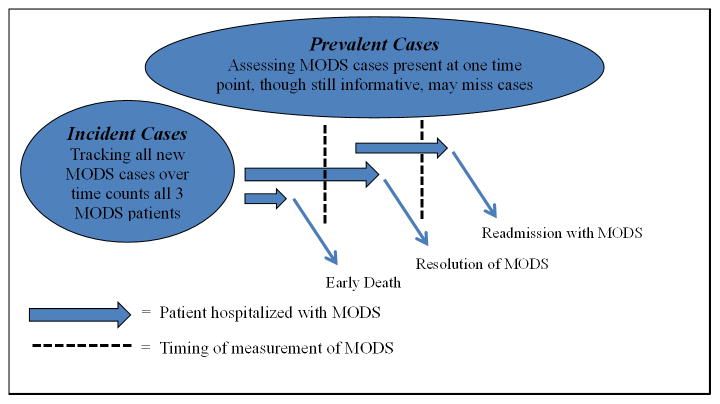
Given that MODS occurs nearly exclusively during receipt of care among hospitalized children, notably within the PICU, the majority of the pediatric MODS studies are incidence studies. The benefit of these incidence studies lies in their longitudinal time course, unlike prevalence studies which are largely cross-sectional in nature. The determination of the timing of study during the course of hospitalization is crucial to ensuring the accurate determination of MODS incidence and prevalence (Figure 1).
Figure 1. The importance of timing in the determination of incident and prevalent cases of multiple organ dysfunction syndrome (MODS).
The timing of the study of MODS during the course of hospitalization is crucial to the accurate determination of its incidence and prevalence. If MODS is assessed continuously over time, then all new cases will be identified. However, if MODS is only assessed at a single time point, then MODS that results in death prior to that single time point (early death) will be missed. Similarly, MODS that resolves prior to the single time point assessment will also be missed.
All-cause and disease-specific epidemiology of pediatric MODS
The reported incidence rates of pediatric MODS in the general PICU setting across all diagnoses ranged between 6% and 57% over 28 years of studies (Table 3). As noted above, a significant portion of this variation in incidence is attributable to the variation in: definitions of MODS, the populations studied, and the timing of the assessment for MODS.(4, 6, 9, 15–20) Sepsis appears to be the leading cause of pediatric MODS in the published literature(2, 7, 21)(5, 22, 23) with 17 to 73% of children with sepsis also having MODS (with varying definitions of sepsis and time course contributing to the wide range of rates) (Table 4). The only point prevalence study of pediatric MODS was recently published and found that two thirds of children had MODS on the day of diagnosis of severe sepsis.(23) Other diagnoses associated with MODS are displayed in Table 5, with significant variation in definitions and incidence rates for MODS within diagnostic strata.(24–31)
Table 3.
All-cause incidence of multiple organ dysfunction syndrome.
| First Author | Year of Publication |
Study Location |
Study Period |
Type of Study | MODS Definition |
Numerator Population |
Denominator Population |
Incidence Rate (%) |
Noteworthy (*) |
|---|---|---|---|---|---|---|---|---|---|
| Wilkinson(15) | 1986 | United States | 1980–1982 | Cohort | Wilkinson- MOSF* | 226 | 831 | 27 | MOSF was measured on Day 1 only. |
| Proulx(16) | 1994 | Canada | 1990–1991 | Chart Review | Wilkinson-MOSF | 85 | 777 | 11 | |
| Tan(17) | 1998 | Singapore | 1997 | Prospective Cohort | Wilkinson-MODS* | 16 | 283 | 6 | MODS was defined as dysfunction in ≥ 3 organ systems. |
| Leteurtre(18) | 1999 | France & Canada | 1/1997–5/1997 | Prospective Cohort | PEMOD-PELOD | 269 | 594 | 45% using PELOD | 43% incidence rate using PEMOD |
| Tantalean(19) | 2003 | Peru | 8/1996–1/1997 | Prospective Cohort | Wilkinson-MODS | 156 | 276* | 57 | 7 readmits counted in the denominator population |
| Leteurtre(9) | 2003 | France, Canada & Switzerland | 1998–2000 | Prospective Cohort | PELOD | 965 | 1,806 | 53 | Daily organ dysfunction scores were estimated for the first time. |
| Khilnani(20) | 2006 | India | 1998–2003 | Prospective Cohort | OFI | 298 | 1,722 | 17 | |
| Typpo(4) | 2009 | United States | 2004–2005 | Retrospective Cohort | IPSCC | 8303* | 44,693 | 19 | MODS was assessed on Day 1 only. |
| Leteurtre(6) | 2013 | France & Belgium | 2006–2007 | Prospective Cohort | PELOD | 965 | 3,671 | 57 |
IPSCC = International pediatric sepsis consensus conference, MODS = Multiple organ dysfunction syndrome, MOSF = Multiple organ system failure, OFI = Organ failure index, PEMOD = Pediatric multiple organ dysfunction score, PELOD = Pediatric logistic organ dysfunction score
Table 4.
Incidence and prevalence of multiple organ dysfunction syndrome from sepsis.
| First Author | Year of Publication |
Location(s) of Study |
Period of Study |
Type of Study |
MODS Definition |
Numerator Population |
Denominator Population |
Incidence Rate (%) |
Noteworthy (*) |
|---|---|---|---|---|---|---|---|---|---|
| Wilkinson(7) | 1987 | United States | 1984–1985 | Prospective Cohort | Wilkinson | 177 | 726 | 24 | |
| Proulx(2) | 1996 | Canada | 1991–1992 | Prospective Cohort | Wilkinson-MODS | 191 | 1,058 | 18* | Incidence density used to estimate probability of MODS |
| Goh(21) | 1999 | Malaysia | 06/1995–12/1996 | Prospective Cohort* | OFI* | 84 | 495 | 17 | Only final 6 months of study design were prospective in nature. MODS Index self-created |
| Kutko(22) | 2003 | United States | 1998–1999 | Chart Review | Wilkinson-MOSF | 70 | 96* | 73 | Denominator population was the number of episodes (96) that occurred among 80 patients |
| Leclerc(5) | 2005 | France & Canada | 1/1997–5/1997 | Prospective Cohort | PELOD | 269 | 593 | 45* | Incidence density used to estimate probability of MODS |
| Weiss(23) | 2015 | Multinational | 2013–2014 (5 days) | Point Prevalence | Wilkinson-MOSF | 380* | 567 | Not Applicable | Numerator calculated by author based on reported prevalence of 67% on the day of severe sepsis diagnosis New or Progressive MODS occurred in 30% of study population |
MODS = Multiple organ dysfunction syndrome, MOSF = Multiple organ system failure, OFI = Organ failure index, PELOD = Pediatric logistic organ dysfunction score
Table 5.
Incidence of multiple organ dysfunction syndrome from non-sepsis conditions.
| First Author | Disease/ Condition |
Year of Publication |
Location(s) of Study |
Period of Study |
Type of Study | MODS Definition |
Numerator Population |
Denominator Population |
Incidence Rate (%) |
Noteworthy(*) |
|---|---|---|---|---|---|---|---|---|---|---|
| Seghaye(24) | Congenital Heart Disease | 1993 | Germany | 1985–1989 | Retrospective | Arbitrary-MOSF | 13 | 460 | 4 | |
| Calkins(25) | Trauma | 2002 | United States | 1998–2000 | Retrospective | Denver MOF Score* | 6 | 200 | 3 | Not validated in children, measured beyond 48 hours after injury |
| Andruszkow(26) | Trauma | 2014 | Germany | 2005–2008 | Prospective Cohort | MODS** | 7 | 59 | 12 | Adult-specific scoring system |
| Kraft(27) | Burn | 2014 | United States | 8 years (actual years not reported) | Prospective Cohort | Denver 2 MOF Score | 157 | 821 | 19 | |
| Jeschke(28) | Burn | 2015 | United States | 8 years (actual years not reported) | Prospective Cohort | Denver 2 MOF Score | 60 | 226 | 27 | |
| Feickert(29) | Liver Transplant | 2001 | Germany | 1981–1993 | Retrospective | Wilkinson-MOSF | 31 | 114 | 27 | |
| Jacobe(30) | Bone Marrow Transplant | 2003 | United Kingdom | 1994–1998 | Retrospective | Wilkinson-MOSF*** | 10 | 57 | 18 | MOSF defined as failure of more than 3 organ systems |
| Lamas(31) | Bone Marrow Transplant | 2003 | Spain | 1991–2000 | Retrospective | Wilkinson-MOSF | 44 | 49 | 90 |
MODS = multiple organ dysfunction syndrome, MOF = Multiple organ failure, MOSF = Multiple organ system failure
Risk factors for MODS
Several risk factors for MODS have been identified and include severe hypoxemia, cardiorespiratory arrest, shock, trauma, acute pancreatitis, gut malperfusion, acute leukemia, solid organ or hematopoietic stem cell transplantation, hemophagocytic lymphohistiocytosis, thrombotic microangiopathy, envenomation, and sepsis.(31) Notably, age is also a risk factor. Neonates and infants are more likely to be afflicted with MODS than older children.(4, 32) Modifiable process-of-care factors associated with MODS include delayed treatment of the primary insult and iatrogenic injury.(33)
In sum, an accurate understanding of the incidence of MODS requires that its nomenclature is clear and uniformly applied. Measures of frequency vary significantly by primary condition and can be affected by the timing of the MODS diagnosis. Furthermore, overlap in etiopathogenetic mechanisms requires caution in case identification. Finally, the structure and processes of health care delivery might influence the occurrence and progression of MODS.
Mortality
Intuitively, the greater the number of organ systems failing, the higher the likelihood of patient mortality. This was demonstrated in two large studies that evaluated the epidemiology and outcome of progressive organ dysfunction in critically ill children. The early validation study of the Pediatric Logistic Organ Dysfunction (PELOD) Score, which included 1,806 patients in seven PICUs in three countries, found that the in-hospital mortality rate for progressive MODS ranged from <1% for single organ system failure up to 50% with six organ systems failing.(9) In the development and validation of the Pediatric Multiple Organ Dysfunction Score (P-MODS), investigators prospectively studied 6,456 patients in a large, tertiary United States PICU.(34) They noted mortality rates <5% in patients with little to no organ dysfunction and mortality >80% in children with the most severe and highest number of organ systems failing. Although data suggested that for every additional dysfunctional organ system the odds of death increased by 2.25,(35) data from Leclerc et al demonstrated that this relationship is nonlinear, and the hazard ratios for death increase over 50-fold when organ system dysfunction occurs in the setting of septic shock.(5)
Similarly, other patient and care factors affect MODS-associated mortality. For example, delay in antibiotic administration leads to an exponential increase in the risk of mortality in infection-related MODS.(33) Other factors associated with increased risk of mortality from MODS include younger age (just as they have a higher incidence rate, infants also tend to have a higher mortality rate than older children)(32, 36) and the timing of the development of MODS (patients who developed MODS a week or more after hospital admission had mortality rates 2.5 times higher than those who developed MODS earlier in their hospitalization).(2) Hall and colleagues demonstrated that immune paralysis is far more common in MODS non-survivors than in survivors(37) and increases the risk of PICU mortality by nearly six-fold.(38) Survival after severe trauma and burn-related injury is highly associated with the extent and severity of concomitant MODS.(27) Thus, reports of MODS-associated mortality have varied widely depending on the study population (Figure 2).
Figure 2. Mortality after multiple organ dysfunction syndrome (MODS) reported in individual studies.
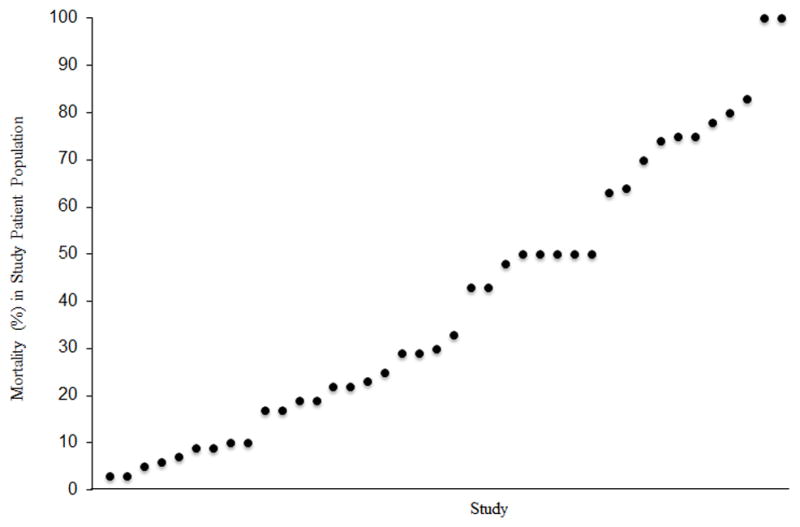
Each point in the graph represents the MODS-associated mortality reported in an individual study. Mortality varies markedly according to study size, inclusion criteria, and the therapeutic modality evaluated.(2, 5, 9, 16, 34, 37–40, 43, 75, 104–109)
Unfortunately, much of the data on outcome after MODS are short-term, and the lasting effects of MODS in children are not known. Extrapolating from cohort studies of childhood survivors of septic shock, hospital readmission and late death occur in this population at much higher rates than in the general pediatric population.(39) In their retrospective cohort study of 7,183 children with severe sepsis in Washington State between 1990 and 2004, Czaja reported that 6.8% died during their admission or within 28 days of discharge, and an additional 434 deaths (6.5% of initial survivors) occurred after hospital discharge. The median time to death was one year (intra-quartile range 1 month to 12 years).(39) Interestingly, 88% of the late deaths occurred in children who had at least one readmission after discharge from the initial sepsis hospitalization. Perhaps not surprising, many of the deaths were related to pre-existing chronic conditions, the most common of which was cancer.(39) The relationship between poor long-term survival after MODS in children with an underlying diagnosis of cancer has been well described.(40–42) The effect of emerging therapies on these outcomes, such as plasma exchange or immune-modulation for pediatric severe sepsis and MODS, remains unknown and to be determined.(43, 44)
Health-related quality of life, functional status, and costs
Studies of survivors of critical illness in childhood have repeatedly demonstrated long-term medical, psychological, and functional deficits after PICU discharge.(45–52) The long-term negative effects of critical illness and its care in children are undoubtedly more common than studies using only global outcome measures suggest, as specific sequelae, such as subtle but important changes in cognitive function, may go undiagnosed and untreated.(53–56)
Health-related quality of life (HRQL)
HRQL involves the subjective assessment of physical, mental, and social factors related to health conditions that affect quality of life. The impact of a health condition upon HRQL is influenced by biological, environmental, and individual factors.(57) The assessment of HRQL in children is difficult and controversial, particularly given the dynamics of child development, the influence of the family on a child’s quality of life, and the need to use proxy reports for young children (Figure 3). Therefore, many standardized measures of HRQL in children are heavily weighted toward assessment of physical function. Critical illness may affect multiple domains of functioning and may have a profound impact on quality of life,(55, 58–60) with impaired HRQL reported in 16–73% of PICU survivors from 3 months to 6 years after discharge.(61) However, there is a remarkable paucity of data on the impact of MODS itself on quality of life.(62) While the specific relationship between long-term problems and in-hospital organ dysfunction is not yet clear, studies have found that the severity and duration of symptoms (post-discharge) are related to the severity of the hospital course, suggesting that presence and severity of MODS may have a profound effect on HRQL.(63–66)
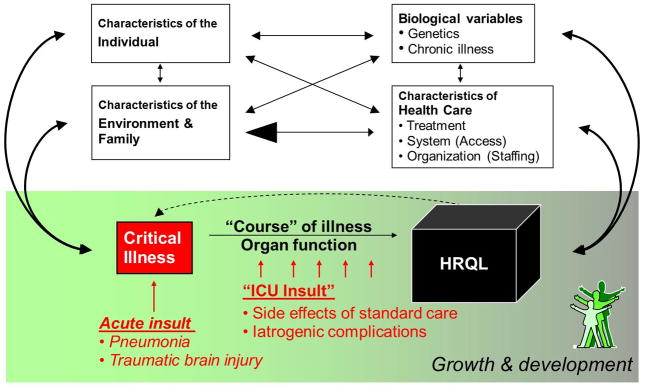
Figure 3. Influences on health-related quality of life (HRQL) after pediatric critical illness.
HRQL is affected by multiple factors, including individual psychological, biological, and environmental characteristics. In pediatric critical illness, additional factors are influential including the background capacity for growth and development, characteristics of the health care system, the illness or insult leading to critical illness, and the course of the illness (including both its natural history, response to therapy, and insults related to ICU care). These relationships are multi-directional. For example, a child’s HRQL can affect chronic illness status, individual psychological health, and the family of the child, which can in turn, affect the risk of subsequent episodes of critical illness. Modified from Wilson and Cleary, 1995.(57)
Functional status
The relationship between acute organ failure and physical function has been studied more extensively, although only in the short term. Typpo and colleagues studied more than 20,000 PICU admissions and found that an increase in the number of failing organs was associated with a greater decline in physical function at hospital discharge and higher rates of severe disability.(4) Overall, previously healthy children tended to have a greater decline in function compared to those with underlying disease, and a subset of patients actually improved at discharge compared to baseline (e.g., children with congenital heart disease or recipients of solid organ transplantation).(67) Likewise, among children with severe sepsis in the RESOLVE trial of activated protein C, worsening organ failure was associated with a greater decline in function at 28 days after enrollment (37% of subjects were still hospitalized at the time of assessment).(68) The longer-term consequences of MODS itself are not known. However, in a general PICU population, more than a third of children had fair or poor function 3 to 24 months after ICU discharge;(69) 35% had a decline in overall health status from baseline to 12 months after discharge;(58) and more than 40% of surviving previously normally functioning children suffered a decrease in function at 2 years post-discharge.(70) While most children improve over time, one study in a general PICU population found that some patients with only mild impairment at 1 month were worse at 6 months post-discharge.(50)
Costs
In general, critical care is expensive, accounting for up to one third of US hospital costs and up to 1% of the US gross domestic product.(71–74) Pediatric severe sepsis alone is associated with $4.8 billion in US hospital costs annually.(75) Long-term morbidity and the impact on the family after PICU hospitalization suggest that the economic effects of MODS may be extensive. However, the medical costs, resource use, post-discharge costs, and family financial impact of MODS are poorly understood.
Psychiatric, Cognitive, and Academic Outcomes after Critical Illness
Long term outcomes following pediatric critical illness are not fully characterized, and the post-discharge implications of MODS that develop during critical illness have not been investigated. Outcomes research to date has largely focused on specific diseases (e.g., meningococcemia, sepsis, traumatic brain injury) and interventions (e.g., extracorporeal life support, cardiac surgery). Patients have typically been evaluated at a single point in time from small, single center cohorts that are representative of critical care management from more than a decade ago.(76, 77) Due to the inherent challenges of longitudinal studies, high rates of loss to follow up are common. Additionally, and with rare exception, these studies rely on non-validated measurement tools and caregiver telephone interviews to evaluate post-discharge outcomes.(76) The limitations of these approaches have been well documented.(78, 79) However, despite these investigational challenges, the data generated consistently demonstrate that morbidity following pediatric critical illness can be substantial.(76, 77, 80)
Studies that characterize post-discharge cognitive and academic outcomes for general PICU patient cohorts are rare. Conversely, the risk of cognitive impairment following adult critical illness has been well established.(81, 82) Cognitive morbidity has also been documented for survivors of specific conditions such as sepsis and meningococcemia.(80, 83–85) PICU populations represent a heterogeneous group of children transferred from diverse healthcare systems throughout each PICU’s referral base. The time, expense, and logistical challenges of following these patients after discharge limit the feasibility of performing long term cognitive and academic assessments. There are a few studies that have attempted to evaluate cognitive outcomes despite the investigational challenges. Two of these investigations utilized formal neuropsychiatric testing to assess cognitive function, rather than caregiver interviews and/or questionnaires. Both studies found that PICU survivors scored significantly lower on memory and attention tasks than healthy controls.(66, 80) One of these studies included the only assessment of PICU academic outcomes to date. Teachers completed questionnaires and reported a greater decline in academic performance for the PICU survivors compared to controls.(80)
Slightly more research has been performed to investigate psychiatric morbidity surrounding pediatric critical illness. Critical illness represents an ongoing, repetitive, traumatic event that places PICU survivors and their families at high risk for psychopathology.(86) Critically ill children endure a multitude of stressors that include invasive painful procedures, separation from family, exposure to other critically ill and dying children, strangers performing intrusive tasks, altered levels of consciousness, impaired coping skills, elevations in noise and light levels, and sleep loss.(87–89) The development of post-traumatic stress symptoms and disorder (PTSD) in adult ICU survivors has been well described, and there is growing evidence that PTSD also occurs in children.(90) Depending on the study, post-traumatic stress symptoms or PTSD is present in as many as one third of PICU survivors.(90) It is likely that many of these children had MODS, although specific studies assessing MODS have not been conducted. Risk factors associated with increased psychiatric morbidity include delusional memories of the PICU experience, perception of a “threat to life”, parental psychopathology, and the presence of patient psychiatric symptoms at hospital discharge.(46, 49, 64, 66, 91–93)
A growing body of evidence demonstrates that parental psychiatric disturbances after PICU admission are common. In one study, parental levels of anxiety and depression were double the community rate at 12 months post-discharge.(94) Post-traumatic stress symptoms can develop and increase over time, even if they were originally absent at hospital discharge.(95) Specific PICU-related factors that increase parental stress include alterations in the caregiver role, changes in the child’s appearance, communication difficulties with healthcare staff, and emergent unplanned admissions.(96–98)
Preventing and mitigating psychiatric morbidity surrounding MODS and all pediatric critical illness represents a potentially high yield area of intervention for enhancing long-term patient and family quality of life. Studies of congenital heart disease patients have demonstrated that emotional and behavioral well-being affects overall quality of life more than physical functional level.(99) Mental health problems increase suicide risk, and they impact family relationships, social roles and adaptation, school function, and overall development.(90, 100–102) Surprisingly, the more easily quantifiable features of the ICU experience such as the length of stay, the admitting diagnosis, or the severity of illness are not consistently associated with higher levels of psychiatric morbidity.(49) In other words, the subjective experience of the critical illness event may have a stronger influence on parental and child distress than the medical characteristics of the ICU admission.(94) How something is experienced appears to be more important than what is experienced. These findings suggest that interventions aimed at reframing a child’s subjective experience of the PICU event could positively influence long term quality of life.
In summary, a paucity of investigations has evaluated the relationships between specific characteristics of the PICU clinical course including MODS and post-discharge outcomes. Nonetheless, the evidence to date suggests that psychiatric, cognitive, and academic morbidity following pediatric critical illness can be profound. Children with MODS have a high risk for acute morbidity and mortality.(4) Further investigation is required to evaluate the long term consequences of MODS. These pre-requisite steps may yield opportunities for prevention, early intervention, and ongoing care of these children to optimize long term potential despite the intrusion of pediatric critical illness into their lives.
Age-specific vital signs and laboratory parameters.
(lower values for heart rate, leukocyte count, and systolic blood pressure are for the 5th percentile and upper values are for 95th percentile).
| Age-Groupa | Heart rate (beats/min)b,c | Respiratory rate (breaths/min)d | Leukocyte count (leukocytes × 103/mm3)b,c | Systolic blood pressure (mmHg)b,c,e,f | |
|---|---|---|---|---|---|
| Tachycardia | Bradycardia | ||||
| 0 day–1 week | >180 | <100 | >50 | >34 | <59 |
| 1 week–1 month | >180 | <100 | >40 | >19.5 or <5 | <75 |
| 1 month–1 year | >180 | <90 | >34 | >17.5 or <5 | <75 |
| 2–5 years | >140 | NA | >22 | >15.5 or <6 | <74 |
| 6–12 years | >130 | NA | >18 | >13.5 or < 4.5 | <83 |
| 13– < 18 years | >110 | NA | >14 | >11 or < 4.5 | <90 |
Modified from: Parker MM. Pathophysiology of cardiovascular dysfunction in septic shock. New Horiz.1998;6:130–138.
Modified from: Rudolph CD, Rudolph AM (Eds). Rudolph’s Pediatrics – 21st Edition. The McGraw-Hill Companies Inc. New York, NY. 2002.
Table 6.
Identified Knowledge Gaps and Potential Opportunities for Study
|
Acknowledgments
We thank the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) and their Office of Science Policy, Analysis and Communications for their support of this Workshop.
Footnotes
Disclaimer: This information or content and conclusions are those of the authors and should not be construed as the official position or policy of, nor should any endorsements be inferred by, the National Institutes of Health, the US Department of Health and Human Services, or the US government.
Proven need assumes that the oxygen requirement was tested by decreasing flow with subsequent increase in flow if required.
Contributor Information
R. Scott Watson, Center for Child Health, Behavior, and Development, Seattle Children’s Research Institute, Division of Critical Care Medicine, Department of Pediatrics, University of Washington, Seattle, WA, USA.
Sheri S. Crow, Division of Pediatric Critical Care Medicine, Department of Pediatrics and Adolescent Medicine, Mayo Clinic College of Medicine, Mayo Eugenio Litta Children’s Hospital, Rochester, MN, USA.
Mary E. Hartman, Division of Critical Care Medicine, Department of Pediatrics, Washington University School of Medicine, St. Louis, MO, USA.
Jacques Lacroix, Division of Pediatric Critical Care Medicine, Department of Pediatrics, Sainte-Justine Hospital, Université de Montréal, Montreal, QC, Canada.
Folafoluwa O. Odetola, Division of Pediatric Critical Care, Child Health Evaluation and Research Unit, Department of Pediatrics and Communicable Diseases, University of Michigan, Ann Arbor, MI, USA.
Reference List
- 1.Baue AE. Multiple, progressive, or sequential systems failure. A syndrome of the 1970s. Arch Surg. 1975;110:779–781. doi: 10.1001/archsurg.1975.01360130011001. [DOI] [PubMed] [Google Scholar]
- 2.Proulx F, Fayon M, Farrell CA, Lacroix J, Gauthier M. Epidemiology of sepsis and multiple organ dysfunction syndrome in children. Chest. 1996;109:1033–1037. doi: 10.1378/chest.109.4.1033. [DOI] [PubMed] [Google Scholar]
- 3.Desmet L, Lacroix J. Transfusion in pediatrics. Crit Care Clin. 2004;20:299–311. doi: 10.1016/S0749-0704(03)00113-1. [DOI] [PubMed] [Google Scholar]
- 4.Typpo KV, Petersen NJ, Hallman DM, Markovitz BP, Mariscalco MM. Day 1 multiple organ dysfunction syndrome is associated with poor functional outcome and mortality in the pediatric intensive care unit. Pediatr Crit Care Med. 2009;10:562–570. doi: 10.1097/PCC.0b013e3181a64be1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Leclerc F, Leteurtre S, Duhamel A, et al. Cumulative influence of organ dysfunctions and septic state on mortality of critically ill children. Am J Respir Crit Care Med. 2005;171:348–353. doi: 10.1164/rccm.200405-630OC. [DOI] [PubMed] [Google Scholar]
- 6.Leteurtre S, Duhamel A, Salleron J, Grandbastien B, Lacroix J, Leclerc F. PELOD-2. an update of the PEdiatric logistic organ dysfunction score. Crit Care Med. 2013;41:1761–1773. doi: 10.1097/CCM.0b013e31828a2bbd. [DOI] [PubMed] [Google Scholar]
- 7.Wilkinson JD, Pollack MM, Glass NL, Kanter RK, Katz RW, Steinhart CM. Mortality associated with multiple organ system failure and sepsis in pediatric intensive care unit. J Pediatr. 1987;111:324–328. doi: 10.1016/s0022-3476(87)80448-1. [DOI] [PubMed] [Google Scholar]
- 8.Goldstein B, Giroir B, Randolph A. International pediatric sepsis consensus conference: definitions for sepsis and organ dysfunction in pediatrics. Pediatr Crit Care Med. 2005;6:2–8. doi: 10.1097/01.PCC.0000149131.72248.E6. [DOI] [PubMed] [Google Scholar]
- 9.Leteurtre S, Martinot A, Duhamel A, et al. Validation of the paediatric logistic organ dysfunction (PELOD) score: prospective, observational, multicentre study. Lancet. 2003;362:192–197. doi: 10.1016/S0140-6736(03)13908-6. [DOI] [PubMed] [Google Scholar]
- 10.Villeneuve A, Lacroix J, Proulx F, Ducruet T, Poitras N. Multiple organ dysfunction syndrome in critically ill children: value of two sets of diagnostic criteria [abstract]Villeneuve A, Lacroix J, Proulx F, Ducruet T, Poitras N. Crit Care Med (Suppl) 2011;39:A114. [Google Scholar]
- 11.Weiss SL, Fitzgerald JC, Faustino EV, et al. Understanding the global epidemiology of pediatric critical illness: the power, pitfalls, and practicalities of point prevalence studies. Pediatr Crit Care Med. 2014;15:660–666. doi: 10.1097/PCC.0000000000000156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pollack MM, Holubkov R, Funai T, et al. Simultaneous Prediction of New Morbidity, Mortality, and Survival Without New Morbidity From Pediatric Intensive Care: A New Paradigm for Outcomes Assessment. Crit Care Med. 2015;43:1699–1709. doi: 10.1097/CCM.0000000000001081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Demaret P, Tucci M, Karam O, Trottier H, Ducruet T, Lacroix J. Clinical Outcomes Associated With RBC Transfusions in Critically Ill Children: A 1-Year Prospective Study. Pediatr Crit Care Med. 2015;16:505–514. doi: 10.1097/PCC.0000000000000423. [DOI] [PubMed] [Google Scholar]
- 14.Lacroix J, Hebert PC, Hutchison JS, et al. Transfusion strategies for patients in pediatric intensive care units. N Engl J Med. 2007;356:1609–1619. doi: 10.1056/NEJMoa066240. [DOI] [PubMed] [Google Scholar]
- 15.Wilkinson JD, Pollack MM, Ruttimann UE, Glass NL, Yeh TS. Outcome of pediatric patients with multiple organ system failure [see comments] Crit Care Med. 1986;14:271–274. doi: 10.1097/00003246-198604000-00002. [DOI] [PubMed] [Google Scholar]
- 16.Proulx F, Gauthier M, Nadeau D, Lacroix J, Farrell CA. Timing and predictors of death in pediatric patients with multiple organ system failure. Crit Care Med. 1994;22:1025–1031. doi: 10.1097/00003246-199406000-00023. [DOI] [PubMed] [Google Scholar]
- 17.Tan GH, Tan TH, Goh DY, Yap HK. Risk factors for predicting mortality in a paediatric intensive care unit. Ann Acad Med Singapore. 1998;27:813–818. [PubMed] [Google Scholar]
- 18.Leteurtre S, Martinot A, Duhamel A, et al. Development of a pediatric multiple organ dysfunction score: use of two strategies. Med Decis Making. 1999;19:399–410. doi: 10.1177/0272989X9901900408. [DOI] [PubMed] [Google Scholar]
- 19.Tantalean JA, Leon RJ, Santos AA, Sanchez E. Multiple organ dysfunction syndrome in children. Pediatr Crit Care Med. 2003;4:181–185. doi: 10.1097/01.PCC.0000059421.13161.88. [DOI] [PubMed] [Google Scholar]
- 20.Khilnani P, Sarma D, Zimmerman J. Epidemiology and peculiarities of pediatric multiple organ dysfunction syndrome in New Delhi, India. Intensive Care Med. 2006;32:1856–1862. doi: 10.1007/s00134-006-0373-5. [DOI] [PubMed] [Google Scholar]
- 21.Goh A, Lum L. Sepsis, severe sepsis and septic shock in paediatric multiple organ dysfunction syndrome. J Paediatr Child Health. 1999;35:488–492. doi: 10.1046/j.1440-1754.1999.355409.x. [DOI] [PubMed] [Google Scholar]
- 22.Kutko MC, Calarco MP, Flaherty MB, et al. Mortality rates in pediatric septic shock with and without multiple organ system failure. Pediatr Crit Care Med. 2003;4:333–337. doi: 10.1097/01.PCC.0000074266.10576.9B. [DOI] [PubMed] [Google Scholar]
- 23.Weiss SL, Fitzgerald JC, Pappachan J, et al. Global epidemiology of pediatric severe sepsis: the sepsis prevalence, outcomes, and therapies study. Am J Respir Crit Care Med. 2015;191:1147–1157. doi: 10.1164/rccm.201412-2323OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Seghaye MC, Engelhardt W, Grabitz RG, et al. Multiple system organ failure after open heart surgery in infants and children. Thorac Cardiovasc Surg. 1993;41:49–53. doi: 10.1055/s-2007-1013820. [DOI] [PubMed] [Google Scholar]
- 25.Calkins CM, Bensard DD, Moore EE, et al. The injured child is resistant to multiple organ failure: a different inflammatory response? J Trauma. 2002;53:1058–1063. doi: 10.1097/00005373-200212000-00005. [DOI] [PubMed] [Google Scholar]
- 26.Andruszkow H, Fischer J, Sasse M, et al. Interleukin-6 as inflammatory marker referring to multiple organ dysfunction syndrome in severely injured children. Scand J Trauma Resusc Emerg Med. 2014;22:16. doi: 10.1186/1757-7241-22-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kraft R, Herndon DN, Finnerty CC, Shahrokhi S, Jeschke MG. Occurrence of multiorgan dysfunction in pediatric burn patients: incidence and clinical outcome. Ann Surg. 2014;259:381–387. doi: 10.1097/SLA.0b013e31828c4d04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jeschke MG, Pinto R, Kraft R, et al. Morbidity and survival probability in burn patients in modern burn care. Crit Care Med. 2015;43:808–815. doi: 10.1097/CCM.0000000000000790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Feickert HJ, Schepers AK, Rodeck B, Geerlings H, Hoyer PF. Incidence, impact on survival, and risk factors for multi-organ system failure in children following liver transplantation. Pediatr Transplant. 2001;5:266–273. doi: 10.1034/j.1399-3046.2001.005004266.x. [DOI] [PubMed] [Google Scholar]
- 30.Jacobe SJ, Hassan A, Veys P, Mok Q. Outcome of children requiring admission to an intensive care unit after bone marrow transplantation. Crit Care Med. 2003;31:1299–1305. doi: 10.1097/01.CCM.0000060011.88230.C8. [DOI] [PubMed] [Google Scholar]
- 31.Lamas A, Otheo E, Ros P, et al. Prognosis of child recipients of hematopoietic stem cell transplantation requiring intensive care. Intensive Care Med. 2003;29:91–96. doi: 10.1007/s00134-002-1549-2. [DOI] [PubMed] [Google Scholar]
- 32.Bestati N, Leteurtre S, Duhamel A, et al. Differences in organ dysfunctions between neonates and older children: a prospective, observational, multicenter study. Crit Care. 2010;14:R202. doi: 10.1186/cc9323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Weiss SL, Fitzgerald JC, Balamuth F, et al. Delayed antimicrobial therapy increases mortality and organ dysfunction duration in pediatric sepsis. Crit Care Med. 2014;42:2409–2417. doi: 10.1097/CCM.0000000000000509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Graciano AL, Balko JA, Rahn DS, Ahmad N, Giroir BP. The Pediatric Multiple Organ Dysfunction Score (P-MODS): development and validation of an objective scale to measure the severity of multiple organ dysfunction in critically ill children. Crit Care Med. 2005;33:1484–1491. doi: 10.1097/01.ccm.0000170943.23633.47. [DOI] [PubMed] [Google Scholar]
- 35.Odetola FO, Gebremariam A, Freed GL. Patient and hospital correlates of clinical outcomes and resource utilization in severe pediatric sepsis. Pediatrics. 2007;119:487–494. doi: 10.1542/peds.2006-2353. [DOI] [PubMed] [Google Scholar]
- 36.Weiss SL, Fitzgerald JC, Pappachan J, et al. Global epidemiology of pediatric severe sepsis: the sepsis prevalence, outcomes, and therapies study. American Journal of Respiratory and Critical Care Medicine. 2015;191:1147–1157. doi: 10.1164/rccm.201412-2323OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Marquardt DJ, Knatz NL, Wetterau LA, Wewers MD, Hall MW. Failure to recover somatotropic axis function is associated with mortality from pediatric sepsis-induced multiple organ dysfunction syndrome. Ped Crit Care Med. 2010;11:18–25. doi: 10.1097/PCC.0b013e3181b06046. [DOI] [PubMed] [Google Scholar]
- 38.Hall MW, Knatz NL, Vetterly C, et al. Immunoparalysis and nosocomial infection in children with multiple organ dysfunction syndrome. Intensive Care Med. 2011;37:525–532. doi: 10.1007/s00134-010-2088-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Czaja AS, Zimmerman JJ, Nathens AB. Readmission and late mortality after pediatric severe sepsis. Pediatrics. 2009;123:849–857. doi: 10.1542/peds.2008-0856. [DOI] [PubMed] [Google Scholar]
- 40.Fiser RT, West NK, Bush AJ, Sillos EM, Schmidt JE, Tamburro RF. Outcome of severe sepsis in pediatric oncology patients. Pediatr Crit Care Med. 2005;6:531–536. doi: 10.1097/01.pcc.0000165560.90814.59. [DOI] [PubMed] [Google Scholar]
- 41.Tamburro RF, Barfield RC, Shaffer ML, et al. Changes in outcomes (1996–2004) for pediatric oncology and hematopoietic stem cell transplant patients requiring invasive mechanical ventilation. Pediatr Crit Care Med. 2008;9:270–277. doi: 10.1097/PCC.0b013e31816c7260. [DOI] [PubMed] [Google Scholar]
- 42.Hagen SA, Craig DM, Martin PL, et al. Mechanically ventilated pediatric stem cell transplant recipients: effect of cord blood transplant and organ dysfunction on outcome. Pediatr Crit Care Med. 2003;4:206–213. doi: 10.1097/01.PCC.0000043293.83440.79. [DOI] [PubMed] [Google Scholar]
- 43.Qu L, Kiss JE, Dargo G, Carcillo JA. Outcomes of previously healthy pediatric patients with fulminant sepsis-induced multisystem organ failure receiving therapeutic plasma exchange. Journal of Clinical Apheresis. 2011;26:208–213. doi: 10.1002/jca.20296. [DOI] [PubMed] [Google Scholar]
- 44.Nguyen TC, Han YY, Kiss JE, et al. Intensive plasma exchange increases a disintegrin and metalloprotease with thrombospondin motifs-13 activity and reverses organ dysfunction in children with thrombocytopenia-associated multiple organ failure. Crit Care Med. 2008;36:2878–2887. doi: 10.1097/ccm.0b013e318186aa49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jones C, Griffiths RD, Slater T, Benjamin KS, Wilson S. Significant cognitive dysfunction in non-delirious patients identified during and persisting following critical illness. Intensive Care Med. 2006;32:923–926. doi: 10.1007/s00134-006-0112-y. [DOI] [PubMed] [Google Scholar]
- 46.Bronner MB, Knoester H, Bos AP, Last BF, Grootenhuis MA. Posttraumatic stress disorder (PTSD) in children after paediatric intensive care treatment compared to children who survived a major fire disaster. Child Adolesc Psychiatry Ment Health. 2008;2:9. doi: 10.1186/1753-2000-2-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Knoester H, Bronner MB, Bos AP, Grootenhuis MA. Quality of life in children three and nine months after discharge from a paediatric intensive care unit: a prospective cohort study. Health Qual Life Outcomes. 2008;6:21. doi: 10.1186/1477-7525-6-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Buysse CM, Raat H, Hazelzet JA, et al. Long-term health-related quality of life in survivors of meningococcal septic shock in childhood and their parents. Qual Life Res. 2007;16:1567–1576. doi: 10.1007/s11136-007-9271-8. [DOI] [PubMed] [Google Scholar]
- 49.Davydow DS, Richardson LP, Zatzick DF, Katon WJ. Psychiatric morbidity in pediatric critical illness survivors: a comprehensive review of the literature. Arch Pediatr Adolesc Med. 2010;164:377–385. doi: 10.1001/archpediatrics.2010.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fiser DH, Long N, Roberson PK, Hefley G, Zolten K, Brodie-Fowler M. Relationship of pediatric overall performance category and pediatric cerebral performance category scores at pediatric intensive care unit discharge with outcome measures collected at hospital discharge and 1- and 6-month follow-up assessments. Crit Care Med. 2000;28:2616–2620. doi: 10.1097/00003246-200007000-00072. [DOI] [PubMed] [Google Scholar]
- 51.Hanekamp MN, Mazer P, van der Cammen-van Zijp MH, et al. Follow-up of newborns treated with extracorporeal membrane oxygenation: a nationwide evaluation at 5 years of age. Crit Care. 2006;10:R127. doi: 10.1186/cc5039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wagner K, Risnes I, Berntsen T, et al. Clinical and psychosocial follow-up study of children treated with extracorporeal membrane oxygenation. Ann Thorac Surg. 2007;84:1349–1355. doi: 10.1016/j.athoracsur.2007.05.019. [DOI] [PubMed] [Google Scholar]
- 53.Robertson CMT, Joffe AR, Moore AJ, Watt JM. Neurodevelopmental outcome of young pediatric intensive care survivors of serious brain injury. Ped Crit Care Med. 2002;3:345–350. doi: 10.1097/00130478-200210000-00003. [DOI] [PubMed] [Google Scholar]
- 54.Fiser DH. Outcome evaluations as measures of quality in pediatric intensive care [review] Pediatr Clin North Am. 1994;41:1423–1438. doi: 10.1016/s0031-3955(16)38880-0. [DOI] [PubMed] [Google Scholar]
- 55.Limperopoulos C, Majnemer A, Shevell MI, et al. Functional limitations in young children with congenital heart defects after cardiac surgery. Pediatrics. 2001;108:1325–1331. doi: 10.1542/peds.108.6.1325. [DOI] [PubMed] [Google Scholar]
- 56.Hopkins RO. Does critical illness and intensive care unit treatment contribute to neurocognitive and functional morbidity in pediatric patients? [comment] Jornal de Pediatria. 2007;83:488–490. doi: 10.2223/JPED.1729. [DOI] [PubMed] [Google Scholar]
- 57.Wilson IB, Cleary PD. Linking clinical variables with health-related quality of life. A conceptual model of patient outcomes. JAMA. 1995;273:59–65. [PubMed] [Google Scholar]
- 58.Gemke RJ, Bonsel GJ, van Vught AJ. Long-term survival and state of health after paediatric intensive care. Arch Dis Child. 1995;73:196–201. doi: 10.1136/adc.73.3.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.de Keizer NF, Bonsel GJ, Gemke RJ. Health status prediction in critically ill children: a pilot study introducing Standardized Health Ratios. Qual Life Res. 1997;6:192–199. doi: 10.1023/a:1026450403009. [DOI] [PubMed] [Google Scholar]
- 60.Gemke RJ, Bonsel GJ. Reliability and validity of a comprehensive health status measure in a heterogeneous population of children admitted to intensive care. J Clin Epidemiol. 1996;49:327–333. doi: 10.1016/0895-4356(95)00528-5. [DOI] [PubMed] [Google Scholar]
- 61.Colville GA, Pierce CM. Children’s self-reported quality of life after intensive care treatment. Pediatr Crit Care Med. 2013;14:e85–e92. doi: 10.1097/PCC.0b013e3182712997. [DOI] [PubMed] [Google Scholar]
- 62.Aspesberro F, Mangione-Smith R, Zimmerman JJ. Health-related quality of life following pediatric critical illness. Intensive Care Med. 2015;41:1235–1246. doi: 10.1007/s00134-015-3780-7. [DOI] [PubMed] [Google Scholar]
- 63.Jones S, Rantell K, Stevens K, et al. Outcome at 6 months after admission for pediatric intensive care: a report of a national study of pediatric intensive care units in the United kingdom. Pediatrics. 2006;118:2101–2108. doi: 10.1542/peds.2006-1455. [DOI] [PubMed] [Google Scholar]
- 64.Colville G, Kerry S, Pierce C. Children’s factual and delusional memories of intensive care. Am J Respir Crit Care Med. 2008;177:976–982. doi: 10.1164/rccm.200706-857OC. [DOI] [PubMed] [Google Scholar]
- 65.Rennick JE, Rashotte J. Psychological outcomes in children following pediatric intensive care unit hospitalization: a systematic review of the research. J Child Health Care. 2009;13:128–149. doi: 10.1177/1367493509102472. [DOI] [PubMed] [Google Scholar]
- 66.Elison S, Shears D, Nadel S, Sahakian B, Garralda ME. Neuropsychological function in children following admission to paediatric intensive care: a pilot investigation. Intensive Care Med. 2008;34:1289–1293. doi: 10.1007/s00134-008-1093-9. [DOI] [PubMed] [Google Scholar]
- 67.Typpo KV, Petersen NJ, Petersen LA, Mariscalco MM. Children with chronic illness return to their baseline functional status after organ dysfunction on the first day of admission in the pediatric intensive care unit. J Pediatr. 2010;157:108–113. doi: 10.1016/j.jpeds.2009.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Farris RW, Weiss NS, Zimmerman JJ. Functional outcomes in pediatric severe sepsis: further analysis of the researching severe sepsis and organ dysfunction in children: a global perspective trial. Pediatr Crit Care Med. 2013;14:835–842. doi: 10.1097/PCC.0b013e3182a551c8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Morrison AL, Gillis J, O’Connell AJ, Schell DN, Dossetor DN, Mellis C. Quality of life of survivors of pediatric intensive care. Ped Crit Care Med. 2002;3:1–5. doi: 10.1097/00130478-200201000-00001. [DOI] [PubMed] [Google Scholar]
- 70.Taylor A, Butt W, Ciardulli M. The functional outcome and quality of life of children after admission to an intensive care unit. Intensive Care Med. 2003;29:795–800. doi: 10.1007/s00134-003-1690-6. [DOI] [PubMed] [Google Scholar]
- 71.Halpern NA, Bettes L, Greenstein R. Federal and nationwide intensive care units and healthcare costs: 1986–1992. Crit Care Med. 1994;22:2001–2007. [PubMed] [Google Scholar]
- 72.Halpern NA, Pastores SM, Greenstein RJ. Critical care medicine in the United States 1985–2000: An analysis of bed numbers, use, and costs. Crit Care Med. 2004;32:1254–1259. doi: 10.1097/01.ccm.0000128577.31689.4c. [DOI] [PubMed] [Google Scholar]
- 73.Jacobs P, Noseworthy TW. National estimates of intensive care utilization and costs: Canada and the United States. Crit Care Med. 1990;18:1282–1286. doi: 10.1097/00003246-199011000-00020. [DOI] [PubMed] [Google Scholar]
- 74.Cooper LM, Linde-Zwirble WT. Medicare intensive care unit use: Analysis of incidence, cost, and payment. Crit Care Med. 2004;32:2247–2253. doi: 10.1097/01.ccm.0000146301.47334.bd. [DOI] [PubMed] [Google Scholar]
- 75.Hartman ME, Linde-Zwirble WT, Angus DC, Watson RS. Trends in the epidemiology of pediatric severe sepsis*. Pediatr Crit Care Med. 2013;14:686–693. doi: 10.1097/PCC.0b013e3182917fad. [DOI] [PubMed] [Google Scholar]
- 76.Knoester H, Grootenhuis MA, Bos AP. Outcome of paediatric intensive care survivors. Eur J Pediatr. 2007;166:1119–1128. doi: 10.1007/s00431-007-0573-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Namachivayam P, Taylor A, Montague T, et al. Long-stay children in intensive care: long-term functional outcome and quality of life from a 20-yr institutional study. Pediatr Crit Care Med. 2012;13:520–528. doi: 10.1097/PCC.0b013e31824fb989. [DOI] [PubMed] [Google Scholar]
- 78.Ellert U, Ravens-Sieberer U, Erhart M, Kurth BM. Determinants of agreement between self-reported and parent-assessed quality of life for children in Germany-results of the German Health Interview and Examination Survey for Children and Adolescents (KiGGS) Health Qual Life Outcomes. 2011;9:102. doi: 10.1186/1477-7525-9-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Varni JW, Limbers CA, Burwinkle TM. Parent proxy-report of their children’s health-related quality of life: an analysis of 13,878 parents’ reliability and validity across age subgroups using the PedsQL 4. 0 Generic Core Scales. Health Qual Life Outcomes. 2007;5:2. doi: 10.1186/1477-7525-5-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Als LC, Nadel S, Cooper M, Pierce CM, Sahakian BJ, Garralda ME. Neuropsychologic function three to six months following admission to the PICU with meningoencephalitis, sepsis, and other disorders: a prospective study of school-aged children. Crit Care Med. 2013;41:1094–1103. doi: 10.1097/CCM.0b013e318275d032. [DOI] [PubMed] [Google Scholar]
- 81.Hopkins RO, Jackson JC. Long-term neurocognitive function after critical illness. Chest. 2006;130:869–878. doi: 10.1378/chest.130.3.869. [DOI] [PubMed] [Google Scholar]
- 82.Jackson JC, Mitchell N, Hopkins RO. Cognitive functioning, mental health, and quality of life in ICU survivors: an overview. Crit Care Clin. 2009;25:615–28. x. doi: 10.1016/j.ccc.2009.04.005. [DOI] [PubMed] [Google Scholar]
- 83.Bronner MB, Knoester H, Sol JJ, Bos AP, Heymans HS, Grootenhuis MA. An explorative study on quality of life and psychological and cognitive function in pediatric survivors of septic shock. Pediatr Crit Care Med. 2009;10:636–642. doi: 10.1097/PCC.0b013e3181ae5c1a. [DOI] [PubMed] [Google Scholar]
- 84.Vermunt LC, Buysse CM, Joosten KF, et al. Survivors of septic shock caused by Neisseria meningitidis in childhood: psychosocial outcomes in young adulthood. Pediatr Crit Care Med. 2011;12:e302–e309. doi: 10.1097/PCC.0b013e3182192d7f. [DOI] [PubMed] [Google Scholar]
- 85.Vermunt LC, Buysse CM, Aarsen FK, et al. Long-term cognitive functioning in children and adolescents who survived septic shock caused by Neisseria meningitidis. Br J Clin Psychol. 2009;48:195–208. doi: 10.1348/014466508X391094. [DOI] [PubMed] [Google Scholar]
- 86.Davidson JE, Jones C, Bienvenu OJ. Family response to critical illness: postintensive care syndrome-family. Crit Care Med. 2012;40:618–624. doi: 10.1097/CCM.0b013e318236ebf9. [DOI] [PubMed] [Google Scholar]
- 87.Al-Samsam RH, Cullen P. Sleep and adverse environmental factors in sedated mechanically ventilated pediatric intensive care patients. Pediatr Crit Care Med. 2005;6:562–567. doi: 10.1097/01.pcc.0000165561.40986.a6. [DOI] [PubMed] [Google Scholar]
- 88.Corser NC. Sleep of 1- and 2-year-old children in intensive care. Issues Compr Pediatr Nurs. 1996;19:17–31. doi: 10.3109/01460869609026852. [DOI] [PubMed] [Google Scholar]
- 89.Cureton-Lane RA, Fontaine DK. Sleep in the pediatric ICU: an empirical investigation. Am J Crit Care. 1997;6:56–63. [PubMed] [Google Scholar]
- 90.Nelson LP, Gold JI. Posttraumatic stress disorder in children and their parents following admission to the pediatric intensive care unit: a review. Pediatr Crit Care Med. 2012;13:338–347. doi: 10.1097/PCC.0b013e3182196a8f. [DOI] [PubMed] [Google Scholar]
- 91.Shears D, Nadel S, Gledhill J, Garralda ME. Short-term psychiatric adjustment of children and their parents following meningococcal disease. Pediatr Crit Care Med. 2005;6:39–43. doi: 10.1097/01.PCC.0000144705.81825.EE. [DOI] [PubMed] [Google Scholar]
- 92.Saxe GN, Stoddard F, Hall E, et al. Pathways to PTSD, part I: Children with burns. Am J Psychiatry. 2005;162:1299–1304. doi: 10.1176/appi.ajp.162.7.1299. [DOI] [PubMed] [Google Scholar]
- 93.Rees G, Gledhill J, Garralda ME, Nadel S. Psychiatric outcome following paediatric intensive care unit (PICU) admission: a cohort study. Intensive Care Med. 2004;30:1607–1614. doi: 10.1007/s00134-004-2310-9. [DOI] [PubMed] [Google Scholar]
- 94.Colville G, Pierce C. Patterns of post-traumatic stress symptoms in families after paediatric intensive care. Intensive Care Med. 2012;38:1523–1531. doi: 10.1007/s00134-012-2612-2. [DOI] [PubMed] [Google Scholar]
- 95.Carty J, O’Donnell ML, Creamer M. Delayed-onset PTSD: a prospective study of injury survivors. J Affect Disord. 2006;90:257–261. doi: 10.1016/j.jad.2005.11.011. [DOI] [PubMed] [Google Scholar]
- 96.Bronner MB, Knoester H, Bos AP, Last BF, Grootenhuis MA. Follow-up after paediatric intensive care treatment: parental posttraumatic stress. Acta Paediatr. 2008;97:181–186. doi: 10.1111/j.1651-2227.2007.00600.x. [DOI] [PubMed] [Google Scholar]
- 97.Bronner MB, Peek N, Knoester H, Bos AP, Last BF, Grootenhuis MA. Course and predictors of posttraumatic stress disorder in parents after pediatric intensive care treatment of their child. J Pediatr Psychol. 2010;35:966–974. doi: 10.1093/jpepsy/jsq004. [DOI] [PubMed] [Google Scholar]
- 98.Balluffi A, Kassam-Adams N, Kazak A, Tucker M, Dominguez T, Helfaer M. Traumatic stress in parents of children admitted to the pediatric intensive care unit. Pediatr Crit Care Med. 2004;5:547–553. doi: 10.1097/01.PCC.0000137354.19807.44. [DOI] [PubMed] [Google Scholar]
- 99.Kamphuis M, Ottenkamp J, Vliegen HW, et al. Health related quality of life and health status in adult survivors with previously operated complex congenital heart disease. Heart. 2002;87:356–362. doi: 10.1136/heart.87.4.356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Zatzick DF, Jurkovich GJ, Fan MY, et al. Association between posttraumatic stress and depressive symptoms and functional outcomes in adolescents followed up longitudinally after injury hospitalization. Arch Pediatr Adolesc Med. 2008;162:642–648. doi: 10.1001/archpedi.162.7.642. [DOI] [PubMed] [Google Scholar]
- 101.Weissman MM, Wolk S, Goldstein RB, et al. Depressed adolescents grown up. JAMA. 1999;281:1707–1713. doi: 10.1001/jama.281.18.1707. [DOI] [PubMed] [Google Scholar]
- 102.Pine DS, Cohen JA. Trauma in children and adolescents: risk and treatment of psychiatric sequelae. Biol Psychiatry. 2002;51:519–531. doi: 10.1016/s0006-3223(01)01352-x. [DOI] [PubMed] [Google Scholar]
- 103.Shudy M, de Almeida ML, Ly S, et al. Impact of pediatric critical illness and injury on families: a systematic literature review. Pediatrics. 2006;118(Suppl 3):S203–S218. doi: 10.1542/peds.2006-0951B. [DOI] [PubMed] [Google Scholar]
- 104.Ceneviva G, Paschall JA, Maffei F, Carcillo JA. Hemodynamic support in fluid-refractory pediatric septic shock. Pediatrics. 1998;102:e19. doi: 10.1542/peds.102.2.e19. [DOI] [PubMed] [Google Scholar]
- 105.Goldstein SL, Somers MJ, Baum MA, et al. Pediatric patients with multi-organ dysfunction syndrome receiving continuous renal replacement therapy. Kidney Int. 2005;67:653–658. doi: 10.1111/j.1523-1755.2005.67121.x. [DOI] [PubMed] [Google Scholar]
- 106.Jodele S, Laskin BL, Goebel J, et al. Does early initiation of therapeutic plasma exchange improve outcome in pediatric stem cell transplant-associated thrombotic microangiopathy? Transfusion. 2013;53:661–667. doi: 10.1111/j.1537-2995.2012.03776.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Leteurtre S, Duhamel A, Grandbastien B, et al. Daily estimation of the severity of multiple organ dysfunction syndrome in critically ill children. CMAJ. 2010;182:1181–1187. doi: 10.1503/cmaj.081715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Nadel S, Goldstein B, Williams MD, et al. Drotrecogin alfa (activated) in children with severe sepsis: a multicentre phase III randomised controlled trial. Lancet. 2007;369:836–843. doi: 10.1016/S0140-6736(07)60411-5. [DOI] [PubMed] [Google Scholar]
- 109.Nguyen TC, Carcillo JA. Therapeutic plasma exchange as a strategy to reverse multiple organ dysfunction syndrome in patients receiving extracorporeal life support. Ped Crit Care Med. 2015;16:383–385. doi: 10.1097/PCC.0000000000000372. [DOI] [PMC free article] [PubMed] [Google Scholar]