Abstract
Background
Timing of birth is a major determinant of newborn health. African American women are at increased risk for early birth, particularly via the inflammatory pathway. Variants of the IL1RN) gene, which encode the interleukin-1 receptor antagonist (IL-1Ra) protein, are implicated in early birth. The biological pathways linking these variables remain unclear. Evidence also suggests inflammatory pathways differ by race; however, studies among African American women are lacking.
Objectives
We assessed whether an IL1RN variant was associated with timing of birth among African American women and whether this relationship was mediated by lower anti-inflammatory IL-1Ra production or related to a decrease in inhibition of proinflammatory IL-1β production.
Methods
A candidate gene study using a prospective cohort design was used. We collected blood samples at 28–32 weeks gestation among African American women experiencing an uncomplicated pregnancy (N = 89). IL1RN SNP rs2637988 was genotyped and lipopolysaccharide-stimulated IL-1Ra and IL-1β production quantified. Medical record review determined timing of birth.
Results
Women with GG genotype gave birth earlier than women with AA/AG genotypes (b* = 0.21, p = .04). There was no indirect effect of IL1RN SNP rs2637988 allele status on timing of birth through IL-1Ra production, as evidenced by a nonsignificant product of coefficients in mediational analyses (ab = .006, 95% CI [-0.05, 0.13]. Women with GG genotype demonstrated less inhibition of IL-1β production for a unit positive difference in IL-1Ra production than women with AA/AG genotypes (b* = 0.93, p = .03). Greater IL-1β production at 28–32 weeks of pregnancy was marginally associated with earlier birth (b* = 0.21, p = .05).
Discussion
Women with GG genotype may be at risk for earlier birth due to diminished IL-1β inhibition, allowing for initiation of a robust inflammatory response upon even mild immune challenge. Study of inflammatory contributions to early birth among African American women may be key to identifying potential prognostic markers of risk and targeted preventive interventions.
Keywords: African Americans, candidate gene analysis, cytokines, genetics, interleukin 1 receptor antagonist, pregnancy, prematurity
Timing of birth is a major determinant of health across the lifespan (Behrman & Stith Butler, 2007). Compared to those born full term (39–40 weeks), babies born early as well as late are at increased risk for morbidity and mortality (Matthews, MacDorman, & Thoma, 2015; Morken, Klungsoyr, & Skjaerven, 2014; Seikku et al., 2016). Early birth is particularly prevalent among African American women; U.S. rates of birth before full term reach 32.0% among White women compared to 40.4% among African American women (Hamilton, Martin, Osterman, Curtin, & Matthews, 2015). Improved methods to prevent early birth and eliminate racial disparities in early birth are sorely needed; however, the maternal biological underpinnings driving early birth, particularly among African American women, are not fully understood.
It has recently been appreciated that early birth is best characterized as a syndrome preceded by several major instigating biological pathways, including inflammation, hypothalamic-pituitary-adrenal axis activation, and decidual hemorrhage (Manuck et al., 2015; Myatt et al., 2012). African American women, specifically, appear to be most susceptible to early birth via the inflammatory pathway (Frey et al., 2016; Menon et al., 2009). Indeed, inflammatory responses are tightly regulated during pregnancy (Christian & Porter, 2014; Gillespie, Porter, & Christian, 2016) and, like the alternative instigating pathways, inflammation ultimately promotes the classical signs and symptoms of labor: cervical dilation, uterine contraction, and rupture of fetal membranes (Romero, Dey, & Fisher, 2014; Shynlova, Lee, Srikhajon, & Lye, 2013).
Given these data, and the estimated 24–34% heritability of timing of birth (Kistka et al., 2008; W. Wu et al., 2015), genetic variants with inflammatory implications have been of considerable interest in the study of early birth. Interestingly, genetic variants within the IL1RN gene, which encodes the anti-inflammatory interleukin (IL)-1 receptor antagonist (Ra) protein, have been more consistently implicated in early birth than genetic variants within the IL-1β gene, which directs production of proinflammatory IL-1β (Cui, Wang, Zhang, & Liu, 2015; Dolan et al., 2010; Jones et al., 2012; Yilmaz et al., 2012). However, among African American women, elevated peripheral and amniotic fluid IL-1β levels but not diminished IL-1Ra levels have been found among those experiencing preterm versus term labor (Brou et al., 2012; Gabay, Lamacchia, & Palmer, 2010; Menon, Williams, & Fortunato, 2007; Velez et al., 2008). Therefore, the biological processes linking IL1RN genetic variants to timing of birth, particularly among African American women, are not immediately clear. Further, the role that natural antagonists play in limiting pro-inflammatory activity in pregnancy is considerably understudied.
Among nonpregnant populations, IL1RN genetic variants are associated with risk for diseases marked by inflammation (Benke et al., 2011; He, Peng, Xiong, Xu, & Liu, 2015; Korthagen, van Moorsel, Kazemier, Ruven, & Grutters, 2012; X. Wu et al., 2013). As previously discussed, enhanced inflammation is also thought to play a large role in expediting the onset of labor. Among White samples, disease-associated IL1RN tagging polymorphisms (e.g., IL1RN single nucleotide polymorphism (SNP) rs2637988) appear to impact risk by lowering IL-1Ra expression (Korthagen et al., 2012). It is unclear whether this pathway holds in the development of early labor, particularly among African American women. Alternatively, IL1RN genetic variants may decrease the ability of IL-1Ra to competitively inhibit proinflammatory IL-1β production during an inflammatory immune response, with IL-1β ultimately influencing timing of birth. Further, IL1RN gene association studies have typically enrolled heterogeneous cohorts and analyzed data without racial stratification (e.g., Cui et al., 2015), highlighting a critical need to determine whether associations among IL1RN genetic variants and timing of birth hold among African American women.
To extend the current literature, we evaluated whether a tagging variant of the IL1RN gene, SNP rs2637988, was associated with timing of birth among generally healthy African American women. We also aimed to delineate the biological processes that may link the IL1RN variant to timing of birth. We evaluated whether the relationship between IL1RN SNP rs2637988 allele status and timing of birth was mediated by lower IL-1Ra production (Figure 1, Panel A). As an alternative hypothesis, we evaluated whether IL1RN SNP rs2637988 allele status affected the inhibition of IL-1β production by IL-1Ra during the inflammatory immune response, with IL-1β production, in turn, associated with timing of birth (Figure 1, Panel B).
FIGURE 1.
Conceptual diagrams of pathways linking interleukin-1 receptor antagonist (IL-1 RN) genetic variants with timing of birth. Panel A shows the indirect effect of IL1RN SNP rs2637988 allele status (X) on days gestation at birth (Y) through IL-1Ra production (M). Panel B shows the effect of IL-1Ra production (X) on IL-1β production (Y) conditional on IL1RN SNP rs2637988 allele status (Z), as well as the effect of IL-1β production (X) on days gestation at birth (Y). Boxes outlined with dashes list potentially confounding variables. IL = interleukin; Ra = receptor antagonist; SNP = single nucleotide polymorphism.
Methods
Study Design and Participants
African American women of non-Hispanic ethnicity aged 18–35 and carrying a single fetus pregnancy were recruited from two obstetrics/gynecology (OB/GYN) clinics, and the greater Columbus, Ohio community. Given the potential for error in estimation of due date according to last menstrual period alone or by ultrasound in later pregnancy, confirmation or determination of due date by ultrasound performed at ≤ 15 weeks gestation was required for study participation (American College of Obstetricians and Gynecologists, 2014). Women were excluded if they reported tobacco, alcohol, or illicit drug use beyond the first trimester, a chronic immune-impacting condition (e.g., diabetes, rheumatoid arthritis), regular use of medications with immune implications (e.g., corticosteroids, progesterone), or experienced a complication of pregnancy with known immune implications (i.e., gestational diabetes or preeclampsia; Chaiworapongsa, Chaemsaithong, Yeo, & Romero, 2014; Lekva, Norwitz, Aukrust, & Ueland, 2016). Women diagnosed with fetal anomaly were also not eligible for enrollment.
The protocol for the study was approved by the Institutional Review Boards of The Ohio State University and OhioHealth. Informed consent and HIPAA authorization were obtained from all participating women. Enrollment occurred from September 2013 to June 2015.
Following a prospective cohort design and a candidate gene approach, women attended a single study visit between 28 weeks 0 days and 32 weeks 6 days gestation, at which time they were not experiencing symptoms of preterm labor (N = 96). Women reporting current cold- or flu-like illness or antibiotic use prior to the visit were rescheduled at least seven days removed but still within the gestational age window. During the study visit, interviews were performed and blood was drawn. To reduce the influence of diurnal variation on immune function, venipuncture was standardized to occur between 11:00am and 4:00pm for all women (Curtis & Fagundes, 2016). After women gave birth, prenatal, labor and delivery, and newborn medical records were reviewed. This approach allowed establishment of a time sequence of the assessed mediational pathway, with inheritance of stable genetic attributes acquired prior to measurement of the inflammatory immune response, which also precedes the timing of birth outcome. Of the women enrolled, two were removed due to unsuccessful venipuncture, one was removed because she provided an incorrect estimated due date leading to blood sampling outside the study window, one woman was lost to follow-up, and three women developed preeclampsia following enrollment. Therefore, analyses were performed on a final sample of 89 women.
Variables and Measurement
Sample characteristics
Demographic characteristics were determined by self-report. Obstetric history, complications of the current pregnancy, and circumstances of labor and birth were extracted from the medical records.
DNA purification and allelic discrimination
K2EDTA-treated whole blood was stored in aliquots at −80°C until processed in batches. At this time, blood was quick-thawed in a 37° water bath and deoxyribonucleic acid (DNA) was extracted and purified using PCR DNA Column Kits according to manufacturer instructions (5 Prime, Gaithersburg, MD). DNA concentration and purity were determined using the Nanodrop 2000 (Thermo Scientific, Wilmington, DE) and samples were diluted to reach a final concentration of 20ηg of DNA per well. Allelic discrimination was performed, in duplicate, using probe-based TaqMan SNP Genotyping Assays and Master Mix (Applied Biosystems, Foster City, CA), amplification by polymerase chain reaction, and the CFX96 Touch Real-Time PCR Detection System (Bio-Rad, Hercules, CA). Allele calls were made by examining relative fluorescence units for each allele and required corresponding discrimination for each of the duplicate wells.
IL-1Ra and IL-1β production
IL-1Ra and IL-1β production were determined using a highly standardized stimulation protocol. Within one hour of collection, 50μl of heparinized whole blood was aliquoted under sterile conditions into 500μl lipopolysaccharide (LPS) solution; i.e., 500pg LPS from Salmonella abortus equi S-form (Enzo Life Sciences, Ann Arbor, MI) per ml RPMI medium (MP Biomedicals, Santa Ana, CA). This procedure was also repeated using RPMI medium alone to produce a control condition. Cultures were incubated at 37°C for four hours and centrifuged at 3000 RPM for five minutes. Supernatants were aspirated and stored in aliquots at −80°C until thawed in batches. IL-1Ra levels were determined using the Human IL-1Ra Platinum ELISA (ebiosciences, San Diego, CA) and PowerWave Spectrophotometer (BioTek, Winooski, VT) per manufacturer instructions. Intra- and inter-assay coefficients of variation were 9.4% and 17.2%, respectively. IL-1β levels were determined using the MSD Pro-inflammatory II 4-plex Immunoassay and Sector Imager 2400 per manufacturer instructions (Meso Scale Discovery, Gaithersburg, MD). Intra- and inter-assay coefficients of variation were 6.1% and 4.5%, respectively. For each analyte, production was calculated by subtracting the quantity produced in the control condition from the quantity produced in the LPS-stimulated condition. Undetectable control values were set at the lower limit of detection for the appropriate assay.
Timing of birth
Timing of birth was operationalized as days gestation at birth. Days gestation at birth was calculated by considering the estimated due date and actual date of birth, each extracted from the medical record. Estimated due dates were assigned by providers per usual care, with all participants assigned a due date per ultrasound determination or confirmation at ≤ 15 weeks gestation. For example, a birth occurring on the expected due date was assigned a value of 280 days (i.e., 40 weeks) gestation.
Statistical Analyses
Sample characteristics and descriptive statistics were examined according to mean and standard deviation (SD) or count and percentage as appropriate. For all analyses, IL1RN SNP rs2637988 allele status was dichotomized as lacking minor allele A (i.e., GG genotype) versus possessing minor allele A (i.e., AA/AG genotypes). Hardy-Weinberg equilibrium of the genetic data was assessed using χ2 tests (α = .05). Spearman rank order correlations among IL-1Ra production, IL-1β production, and days gestation at birth were examined. Potentially confounding variables were identified by examining bivariate associations among IL1RN SNP rs2637988 allele status, IL-1 Ra production, IL-1β production, days gestation at birth, and sample characteristics using Student’s t-tests, χ2 tests for independence, or Fisher’s exact tests with α at .05 and controlled as appropriate.
First, a linear regression model was fit to test the association between IL1RN SNP rs2637988 allele status (X) and days gestation at birth (Y; yi = b0 + b1xi + ei, where b0 is the intercept, ei is error in the estimation of yi and b1 is the coefficient assigned to the predictor in estimation of the outcome (H0: β1 = 0; H1: β1 ≠ 0). Due to non-normality and heteroskedasticity of error terms, days gestation at birth (Y) was transformed by reflecting the negatively skewed variable (i.e., − (days gestation) + 288 + 1) and taking the square root of the reflected value. X was coded as 0 = IL1RN SNP rs2637988 AA/AG and 1 = IL1RN SNP rs2637988 GG, so b1 gives the change in transformed value of days gestation at birth associated with the GG genotype.
Next, the framework and process of Hayes (2013) was applied to test the proposed mediational pathway. Linear regression models were used to evaluate the indirect effect of IL1RN SNP rs2637988 allele status (X) coded as described above, on days gestation at birth (Y), transformed as described above, through IL-1Ra production (M; conceptually depicted in Figure 1, Panel A). The effect of X on M was estimated (Mediational Model 1: mi = b0 + a1xi + ei) followed by the effect of M on Y controlling for X (Mediational Model 2: yi = b0 + c′1xi b1mi + ei). Here, a1, b1, and c1 are the coefficients assigned to each predictor in the estimation of the respective outcome. The indirect effect was quantified as the product of a1 and b1. Inference was achieved by constructing 10,000 bias-corrected bootstrap confidence intervals, with replacement, with support for a statistically significant indirect effect stemming from a confidence interval that did not contain zero (H0 for mediation: Ta1Tb1 = 0; H1 for mediation: Ta1Tb1 ≠ 0, where the subscript T indicates the true value of the coefficient).
The effect of IL-1Ra production (X) on IL-1β production (Y) conditional on IL1RN SNP rs2637988 allele status (Z; conceptually depicted in Figure 1, Panel B) was also estimated using a linear regression model (Conditional Model: yi = b0 + b1xi + b2zi + b3xizi + ei), where b1, b2, and b3 are the coefficients assigned to each predictor in the estimation of the outcome (H0 for moderation: β3 = 0; H1 for moderation: β3 ≠ 0). Due to non-normality and heteroskedasticity of error terms, IL-1β production (Y) was transformed by taking the square root of the positively skewed variable variable. Finally, a linear regression model was fit to test the association between IL-1β production (X) and the transformed value of days gestation at birth (Y; yi = b0 + b1xi + ei; H0: β1 = 0; H1: β1 ≠ 0).
Residual, leverage, and influence diagnostics were examined for each model. No observation had a Cook’s distance greater than 1, which corresponds to a distance beyond a 50% confidence interval between the vector of the estimated regression coefficient with and without the observation included in the model (Cook & Weisberg, 1982). SPSS 22.0 (New York, NY) was used to run the Hayes process to test the mediational pathway while STATA 12.0 (College Station, TX) was used for all other analyses.
Results
Sample Characteristics
Characteristics of the sample, descriptive statistics, and comparisons by IL1RN SNP rs2637988 allele status (i.e., GG genotype versus AA/AG genotypes) are summarized in Table 1. Mean days gestation at birth fell at 273.8 days (39 weeks 0.8 days (SD = 9.2 days; Mdn = 275 days; range: 239–288). Among the 89 women, 31 (34.8%) gave birth before full term (39 weeks) and 5 (5.6%) gave birth before term (37 weeks). A total of 36 (40.4%) women in our sample underwent induction or pre-labor cesarean section, which is slightly lower than typical U.S. rates (Hamilton et al., 2015; MacDorman, Declercq, & Zhang, 2010; Zhang et al., 2010). Induction or prelabor cesarean section was rare before full term (n = 7; 7.9%).
TABLE 1.
Sample Characteristics and Comparisons by IL1RN SNP rs2637988 Allele Status
| Characteristic | All (n = 89) | GG (n = 25) | AA or AG (n = 64) | pa | |||
|---|---|---|---|---|---|---|---|
|
|
|
|
|||||
| n | (%) | n | (%) | n | (%) | ||
| Marital status (unmarried/separated) | 67 | (75.3) | 19 | (76.0) | 48 | (75.0) | .92 |
| Maternal education (< bachelor’s) | 65 | (73.0) | 17 | (68.0) | 48 | (75.0) | .50 |
| Insurance (private not reported) | 59 | (66.3) | 15 | (60.0) | 44 | (68.7) | .43 |
| Parity (multiparous) | 61 | (68.5) | 17 | (68.0) | 44 | (68.8) | .95 |
| Birth before term (previous, no) | 81 | (91.0) | 23 | (92.0) | 58 | (90.6) | .60 |
| Contractions/ruptureb (yes) | 53 | (59.6) | 15 | (60.0) | 38 | (59.4) | .98 |
| Method of delivery (vaginal)c | 44 | (83.0) | 14 | (93.3) | 30 | (78.9) | .20 |
| Induction of labor (yes)b | 19 | (21.3) | 5 | (20.0) | 14 | (21.9) | .98 |
| Primary indication for inductiond | .63 | ||||||
| Elective (yes) | 7 | (36.8) | 2 | (40.0) | 5 | (35.7) | |
| Uncontrolled hypertension | 5 | (26.3) | 2 | (40.0) | 3 | (21.4) | |
| Oligohydramnios | 3 | (15.8) | 0 | (0.0) | 3 | (21.4) | |
| Small for gestational age | 1 | (5.3) | 0 | (0.0) | 1 | (7.1) | |
| Nonreassuring fetal wellbeing | 3 | (15.8) | 1 | (20.0) | 2 | (14.3) | |
| Method of deliverye (vaginal) | 16 | (84.2) | 4 | (80.0) | 12 | (85.7) | .62 |
| Prelabor C/S (yes)b | 17 | (19.1) | 5 | (20.0) | 12 | (18.8) | .98 |
| Primary indication for prelabor C/Sd | .53 | ||||||
| Elective (yes) | 12 | (70.6) | 4 | (80.0) | 8 | (66.7) | |
| Uncontrolled hypertension (yes) | 1 | (5.9) | 0 | (0.0) | 1 | (8.3) | |
| Oligohydramnios (yes) | 1 | (5.9) | 0 | (0.0) | 1 | (8.3) | |
| Nonreassuring fetal wellbeing (yes) | 1 | (5.9) | 0 | (0.0) | 1 | (8.3) | |
| Malpresentation (yes) | 1 | (5.9) | 0 | (0.0) | 1 | (8.3) | |
| History of shoulder dystocia (yes) | 1 | (5.9) | 1 | (20.0) | 0 | (0.0) | |
|
|
|
|
|||||
| M | (SD) | M | (SD) | M | (SD) | ||
|
|
|
|
|||||
| Maternal age (years) | 26.4 | (4.5) | 27.3 | (4.1) | 26.1 | (4.7) | .26 |
| IL-1Ra production (pg/ml) | 4873.4 | (1402.4) | 5160.1 | (1264.6) | 4761.4 | (1446.6) | .23 |
| IL-1β production (pg/ml) | 41.7 | (31.2) | 44.4 | (40.4) | 40.7 | (27.1) | .86 |
| Gestation length (days) | 273.8 | (9.2) | 271.2 | (8.3) | 274.8 | (9.4) | .06 |
Note. C/S = Cesarean section; IL = interleukin, Ra = receptor antagonist; SNP = single nucleotide polymorphism.
p-values are for comparisons of women with IL1RN SNP rs2637988 GG genotype versus IL1RN SNP rs2637988 AA/AG genotypes using Student’s t-tests, χ2 tests, or Fisher’s exact tests (when cells had fewer than five observations).
Reason for presentation to Labor and Delivery suite.
If presented with contractions/rupture.
Primary indications grouped into elective versus all other indications for group comparisons.
If presented for induction.
Among 89 participants, 64 possessed a minor allele genotype (AA or AG) and 25 lacked a minor allele genotype (GG). Major allele frequency was .522; minor allele frequency was .478. Observed allele frequencies were in Hardy-Weinberg equilibrium (p = .69).
IL-1Ra production and IL-1β production were positively associated (rS = .31, p < .05). Neither IL-1Ra production nor IL-1β production were significantly correlated with days gestation at birth (see Tables; Supplemental Digital Content 1).
Women with at least a bachelor’s degree had significantly higher IL-1Ra production (M = 5422, SD = 1303 versus M = 4671, SD = 1393, respectively; p = .02) and significantly higher IL-1β production (M = 53.8, SD = 38.2 versus M = 37.2, SD = 27.2, respectively, p = .03) than women without a bachelor’s degree. Women reporting private insurance also had significantly higher IL-1Ra production (M = 5425, SD = 1345 versus M = 4583, SD = 1357, respectively; p = .007) than women not reporting private insurance. Women with a history of birth before term gave birth 4.7 days earlier than women without a history of birth before term (M = 269.5, SD = 10.0 versus M = 274.2, SD = 9.1, respectively). This difference was not statistically significant in our sample (p = .21). However, there is a large literature supporting an association between timing of birth in prior pregnancy and current pregnancy (Iams, 2014). To minimize potential for confounding, all linear regression models controlled for maternal education and insurance status. We did not control for history of birth before term in models for which allele status served as predictor, as allele status is a stable attribute that may contribute to repeated early births. History of birth before term was included as a covariate to ensure that this variable was not unduly influencing results when examining the relationship between IL-1β production and timing of birth.
Allele status and timing of birth
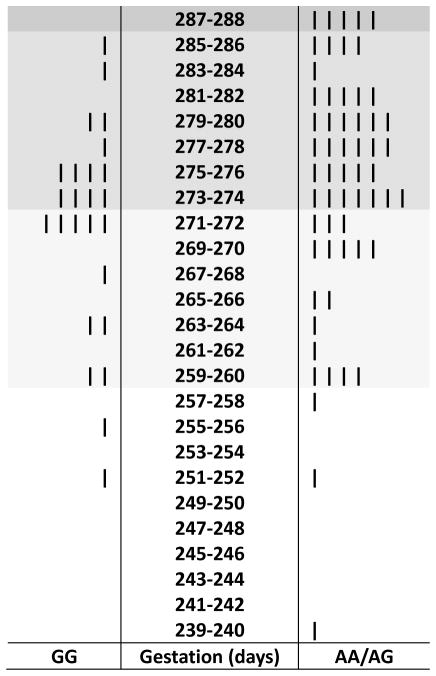
Women with GG genotype for IL1RN SNP rs2637988 gave birth significantly earlier than women with AA/AG genotypes (b* = 0.21, p = .04; i.e., the total effice of IL1RN SNP rs2637988 allele status on timing of birth). Mean timing of birth was 271.2 days or 38 weeks 5.2 days (SD = 8.3; Mdn = 273; range: 252–285) versus 274.8 days or 39 weeks 1.8 days (SD = 9.4 days; Mdn = 276.5; range: 239–288) for the GG and AA/AG groups, respectively (Figure 2).
FIGURE 2.
Back-to-back stem and leaf plot of timing of birth among women with IL1RN SNP rs2637988 GG genotype versus women with IL1RN SNP rs2637988 AA/AG genotypes. Early term, full term, and late term births are shaded. SNP = single nucleotide polymorphism.
IL-1Ra production
As shown in Table 2, there was no effect of allele status on IL-1Ra production, the assessed mediator (b* = 0.11, p = .31). While there was a significant direct effect of IL1RN SNP rs2637988 allele status in predicting timing of birth, controlling for IL-1Ra production (b* = 0.21, p = .05), there was no indirect effect of IL1RN SNP rs2637988 allele status on timing of birth through IL-1Ra production, as evidenced by a 95% bootstrapping confidence interval of the indirect effect that contained 0 (ab = .006; 95% CI [-0.052, 0.131]).
TABLE 2.
Mediation Analysis: Effect of IL1RN SNP rs2637988 Allele Status on Days Gestation at Birth Through IL-1Ra Production
| Predictor | M: IL-1Ra productiona | Y: Gestation at birthb, c | ||||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||
| Coeff. | (SE) | p | Coeff. | (SE) | p | |||
| X: IL1RN SNP rs2637988 allele status | a1 | 0.33 | (0.322) | .31 | c′1 | 0.56 | (0.280) | .05 |
| M: IL-1Ra production | --- | --- | --- | b1 | 0.02 | (0.094) | .85 | |
| Covariate: maternal education | 0.20 | (0.487) | .69 | 0.31 | (0.421) | .47 | ||
| Covariate: insurance status | -0.67 | (0.458) | .15 | 0.62 | (0.400) | .13 | ||
| Constant | b0 | 5.17 | (0.447) | < .001 | b0 | 2.98 | (0.620) | < .001 |
Note. n = 89. a1 = coefficient assigned to X in the estimation of M; b1= coefficient assigned to M in the estimation of Y; c′1 = coefficient assigned to X in the estimation of Y; Coeff. = coefficient; b0= intercept; IL = interleukin; M = mediator; Ra = receptor antagonist; SE = standard error; SNP = single nucleotide polymorphism; X = predictor.
R2 = .09, p = .04.
square root of reflected days gestation (i.e., -(days gestation) + 288 + 1).
R2 = .07, p = .18.
IL-1β production
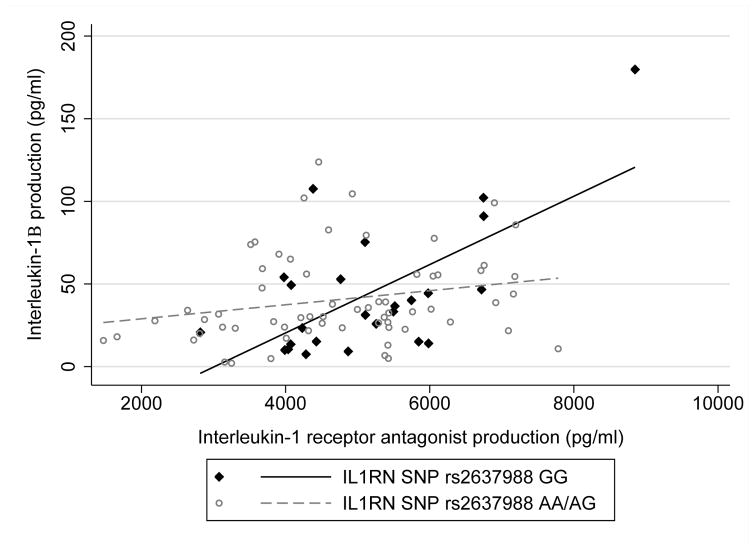
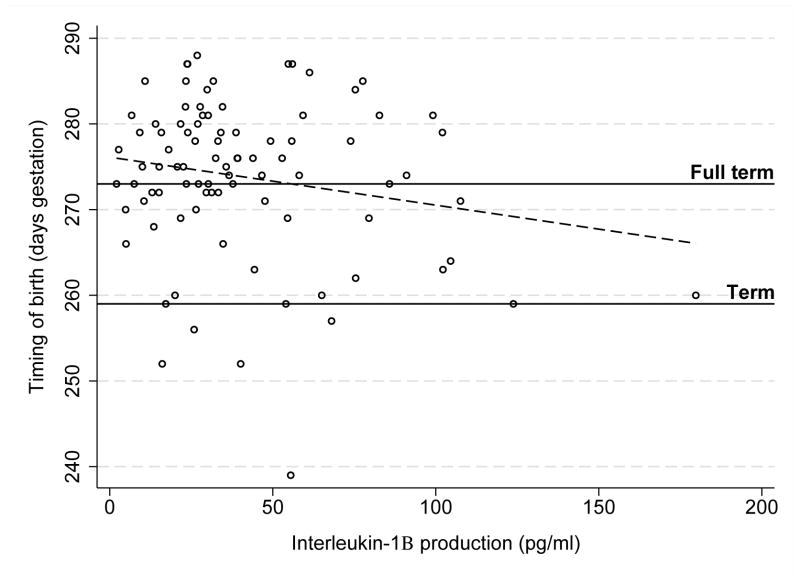
The relationship between IL-1Ra production and IL-1β production was conditional on IL1RN SNP rs2637988 allele status as evidenced by a significant interaction (b* = 0.93, p = .03). Specifically, women with GG genotype showed significantly less inhibition of IL-1β production for a unit positive difference in IL-1Ra production than women with AA/AG genotypes (Figure 3). To confirm that this finding was reflecting a difference in the relationship among IL-1Ra production and IL-1β production among women with GG versus AA/AG genotypes and not related to a main effect of allele status on IL-1β production, we also regressed IL-1β production onto allele status; no difference in IL-1β production was noted among women with GG versus AA/AG genotypes (b* = 0.004, p = .97). Finally, controlling for history of birth before term as well as maternal education and insurance status, greater IL-1β production was marginally associated with earlier birth (b* = 0.21, p = .05; Figure 4).
FIGURE 3.
IL1RN SNP rs2637988 allele status and inhibition of IL-1β production. Women with GG genotype showed less inhibition of IL-1β production for a unit positive difference in IL-1Ra production than women with AA/AG genotypes (b* = 0.93, p = .03). IL-1β production was normalized by square root transformation for analysis. SNP = single nucleotide polymorphism.
FIGURE 4.
IL-1β production and timing of birth. Greater IL-1β production was associated with marginally earlier birth (b* = 0.21, p = .05). Days gestation at birth was normalized by reflecting the variable (i.e., -(days gestation) + 288 + 1) followed by square root transformation for analysis.
Discussion
Among generally healthy African American women, allele status at IL1RN SNP rs2637988 was significantly associated with timing of birth. Women with GG genotype gave birth earlier, with mean timing of birth falling within the early term range (37–38 weeks) among the GG group and full term range (39–40 weeks) among the AA/AG group (Spong, 2013). This finding extends the current literature, which has generally linked IL1RN variants to timing of birth among racially heterogeneous cohorts and focused on more extreme timing of birth outcomes (e.g., birth before term) (Cui et al., 2015; Dolan et al., 2010; Jones et al., 2012). We provide novel evidence that the relationship between IL1RN SNP rs2637988 and timing of birth does not appear to be mediated by an overall decrease in IL-1Ra production upon immune challenge. In fact, women with GG genotype showed slightly, but not significantly, higher IL-1Ra production than women with AA/AG genotypes. This finding differs from that reported in a study of healthy adults in which GG genotype for this SNP was associated with lower mononuclear cell IL-1Ra mRNA (Korthagen et al., 2012). However, comparisons are difficult due to differences in populations under study and laboratory methodologies. Our mediation model also suggests that ex vivo production of IL-1Ra in pregnancy is a poor predictor of timing of birth. This is consistent with some (Brou et al., 2012; Genc et al., 2004), but not all (Heng, Di Quinzio, Permezel, Rice, & Georgiou, 2008; Ruiz et al., 2012), studies evaluating circulating cytokine concentrations within various maternal fluids during pregnancy and labor.
We also investigated a more complex potential pathway. We hypothesized that the IL1RN SNP rs2637988 risk variant may result in decreased inhibition of IL-1β production during the inflammatory immune response and greater IL-1β production, in turn, would predict earlier birth. Our findings lend support to this pathway. We measured total ex vivo IL-1Ra and IL-1β production following four hours of whole blood incubation with LPS, which aimed to mimic the in vivo conditions of a bacterially-induced inflammatory immune response while minimizing participant risk. Consistent with previous reports among pregnant and nonpregnant samples, IL-1Ra and IL-1β production covaried (Heng et al., 2014; Langereis et al., 2011). However, inhibition of IL-1β by IL-1Ra during the inflammatory immune response appeared to be compromised among women with GG genotype, which is important considering that greater IL-1β production went on to predict earlier birth. These findings are consistent with several studies suggesting that the manner in which IL-1Ra and IL-β interact are important in maintaining pregnancy. For example, a decrease in IL-1Ra levels has been reported alongside a rise in IL-1β levels sampled from cervicovaginal fluid as spontaneous term labor approaches (Heng et al., 2014). A diminished cervicovaginal IL-1Ra:IL-1β ratio in the second trimester has also been linked to increased risk for future birth before term (Genc et al., 2004).
While this study provides novel data regarding biological pathways associated with timing of birth among African American women, there are several important limitations. First, among our sample, the rate of birth prior to full term (39 weeks) was 34.8% compared to a national rate of 40.4% and the rate of birth prior to term (37 weeks) was 5.6% compared to a national rate of 13.2% (Hamilton et al., 2015). This difference is not surprising given this study’s exclusion of women with behaviors (e.g., tobacco use), chronic conditions (e.g., diabetes mellitus), and complications of pregnancy (e.g., gestational diabetes, preeclampsia) associated with inflammation but also strongly related to early birth. For example, the odds of birth before term are approximately 4.5 times greater during pregnancy complicated by preeclampsia versus normotensive pregnancy (Davies, Bell, & Bhattacharya, 2016). While this approach enhanced internal validity of findings, greater variability in the distribution of timing of birth would improve ability to detect relationships and confirm null findings. While data points of large influence were not present according to Cook and Weisberg’s definition (Cook’s d > 1; (Cook & Weisberg, 1982), replication also remains critical to strengthening generalizability and ensuring unique cases were not influencing results.
Further, 59.6% of study participants presented to labor and delivery following onset of contractions or rupture of membranes, while 40.4% presented to labor and delivery to undergo induction or pre-labor cesarean section. Clearly, the high rates of birth that are not preceded by labor processes decrease the ability to assess pathways to spontaneously initiated birth, the primary aim of the study. However, induction and pre-labor cesarean sections affect more than 50% of births in the U.S. and inclusion of these cases offers some benefit in terms of generalizability and hypothesis generation (Laughon et al., 2012; Zhang et al., 2010). It is certainly plausible that an impaired inflammatory immune response during pregnancy may contribute to the development of spontaneously initiated early birth as well as complications of pregnancy that increase risk for medically-indicated early birth. For example, while this study excluded women with preeclampsia due to known inflammatory features, a growing body of literature implicates inflammation in additional hypertensive disorders (Li et al., 2016; Tangerås et al., 2015). The role of IL1RN and IL-1β inhibition in the development of various complications of pregnancy with hypothesized immune components is worth exploring.
To summarize, the current study found that women with GG genotype of IL1RN SNP rs2637988 gave birth significantly earlier than women with AA/AG genotypes. This relationship was not mediated by IL-1Ra production; however, women with GG genotype showed decreased inhibition of IL-1β production. Greater IL-1β production, in turn, was marginally associated with earlier birth in this small, healthy sample. This work confirms IL1RN as a noteworthy gene involved in timing of birth among African American women and implicates impaired IL-1β inhibition during the inflammatory immune response as a potential mechanism underlying early birth. The molecular underpinnings linking IL1RN genetic variants to decreased IL-1β inhibition remain to be determined. Our findings may reflect linkage disequilibrium among the assessed tagging SNP and additional allele(s) of the IL-1 gene cluster or IL1RN genetic variants may result in, for example, allosteric switching or hydrogen bonding disruptions capable of transforming IL-1Ra from a competitive inhibitor to a partial proinflammatory agonist (Greenfeder et al., 1995; Hailey, Capraro, Barkho, & Jennings, 2013). These are interesting possibilities to consider in future mechanistic work.
It is also critical that future studies apply advances in mechanistic knowledge to the development of clinical applications, namely biological screening tools and preventive interventions. Considering that growing evidence characterizes early birth as a syndrome preceded by diverse instigating biological pathways (Esplin et al., 2015; Myatt et al., 2012), we propose that clinical tools be developed to predict and prevent the instigating pathway (e.g., inflammation) as opposed to the shared syndromic features (e.g., cervical dilation, uterine contraction, membrane rupture). By mimicking a potential in vivo response to immune challenge, our measure of ex vivo IL-1β production may have potential to predict future risk for inflammatory early birth among African American women not yet experiencing signs and symptoms of labor. Women known to be at risk for early birth via the inflammatory pathway may reap particular benefit from preventive interventions with anti-inflammatory properties, including progesterone and omega-3 fatty acid supplementation (Alvarez-Curto & Milligan, 2016; Flores-Espinosa et al., 2014). Indeed, decades of research in early birth prevention have strongly suggested that one size does not fit all. For example, progesterone supplementation is quite beneficial among women with a singleton pregnancy and prior birth before term but harmful among women with multiple gestation (O’Brien & Lewis, 2016). There is incredible opportunity for precision approaches to usher in an era of risk prediction and targeted prevention in the context of prenatal care. Clinical tools designed with racial differences in biological pathways in mind may also be key to eliminating racial disparities in birth outcomes.
Supplementary Material
Two brief tables providing Hardy-Weinberg Test of Equilibrium and Spearman Rank Correlations. .pdf
Acknowledgments
This work was supported by the National Institute of Nursing Research of the National Institutes of Health (Award Number F31NR01460; SLG); the National Center for Advancing Translational Sciences of the National Institutes of Health (Award Number UL1TR001070); Association of Women’s Health, Obstetric and Neonatal Nurses, Midwest Nursing Research Society; Sigma Theta Tau International Epsilon Chapter; Cola-Cola Critical Difference for Women and The Ohio State University Department of Women’s, Gender and Sexuality Studies; The Ohio State University Graduate School; and The Ohio State University Office of Diversity and Inclusion. The content is solely the responsibility of the authors and does not necessarily represent the official views of the funding agencies. The funding agencies had no role in study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the paper for publication. Portions of this paper were presented at the Midwest Nursing Research Society 40th Annual Research Conference, Milwaukee, WI, March, 2016 and documented as an Abstract of Distinction (Gillespie et al., 2016).
We appreciate the contributions of our Undergraduate Research Assistants, Amy Kole and Patricia Do, to data collection. We would like to thank our study participants and the staff at The Ohio State University Wexner Medical Center and Riverside Methodist Hospital.
Footnotes
The authors report no conflicts of interest.
Contributor Information
Shannon L. Gillespie, Postdoctoral Researcher, College of Nursing, The Ohio State University (OSU), Columbus, OH.
Jeremy L. Neal, Assistant Professor, School of Nursing, Vanderbilt University, Nashville, TN.
Lisa M. Christian, Associate Professor, Department of Psychiatry & Behavioral Health, The Institute for Behavioral Medicine Research, and Department of Obstetrics and Gynecology, Wexner Medical Center, Department of Psychology, The Ohio State University, Columbus, OH.
Laura A. Szalacha, Professor, College of Nursing, University of Arizona, Tucson, AZ.
Donna O. McCarthy, Professor, Marquette University, Milwaukee, WI.
Pamela J. Salsberry, Professor, Department of Health Behavior and Health Promotion, College of Public Health, The Ohio State University, Columbus, OH.
References
- Alvarez-Curto E, Milligan G. Metabolism meets immunity: The role of free fatty acid receptors in the immune system. Biochemical Pharmacology. 2016;114:3–13. doi: 10.1016/j.bcp.2016.03.017. [DOI] [PubMed] [Google Scholar]
- American College of Obstetricians and Gynecologists. Committee opinion no 611: Method for estimating due date. Obstetrics and Gynecology. 2014;124:863–866. doi: 10.1097/01.AOG.0000454932.15177.be. [DOI] [PubMed] [Google Scholar]
- Behrman RE, Stith Butler A, editors. Preterm birth: Causes, consequences, and prevention. Washington, D.C: National Academies Press; 2007. [PubMed] [Google Scholar]
- Benke KS, Carlson MC, Doan BQ, Walston JD, Xue QL, Reiner AP, … Fallin MD. The association of genetic variants in interleukin-1 genes with cognition: Findings from the cardiovascular health study. Experimental Gerontology. 2011;46:1010–1019. doi: 10.1016/j.exger.2011.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brou L, Almli LM, Pearce BD, Bhat G, Drobek CO, Fortunato S, Menon R. Dysregulated biomarkers induce distinct pathways in preterm birth. BJOG : An International Journal of Obstetrics and Gynaecology. 2012;119:458–473. doi: 10.1111/j.1471-0528.2011.03266.x. [DOI] [PubMed] [Google Scholar]
- Chaiworapongsa T, Chaemsaithong P, Yeo L, Romero R. Pre-eclampsia part 1: Current understanding of its pathophysiology. Nature Reviews Nephrology. 2014;10:466–480. doi: 10.1038/nrneph.2014.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christian LM, Porter K. Longitudinal changes in serum proinflammatory markers across pregnancy and postpartum: Effects of maternal body mass index. Cytokine. 2014;70:134–140. doi: 10.1016/j.cyto.2014.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook RD, Weisberg S. Residuals and influence in regression. New York, NY: Chapman and Hall; 1982. [Google Scholar]
- Cui J, Wang F, Zhang X, Liu L. Maternal and fetal IL1RN polymorphisms and the risk of preterm delivery: A meta-analysis. Journal of Maternal-Fetal & Neonatal Medicine. 2015;28:100–105. doi: 10.3109/14767058.2014.900040. [DOI] [PubMed] [Google Scholar]
- Curtis AM, Fagundes CT. Understanding the role of cellular molecular clocks in controlling the innate immune response. Methods in Molecular Biology. 2016;1390:301–316. doi: 10.1007/978-1-4939-3335-8_19. [DOI] [PubMed] [Google Scholar]
- Davies EL, Bell JS, Bhattacharya S. Preeclampsia and preterm delivery: A population-based case-control study. Hypertension in Pregnancy. 2016;35:510–519. doi: 10.1080/10641955.2016.1190846. [DOI] [PubMed] [Google Scholar]
- Dolan SM, Hollegaard MV, Merialdi M, Betran AP, Allen T, Abelow C, … Menon R. Synopsis of preterm birth genetic association studies: The preterm birth genetics knowledge base (PTBGene) Public Health Genomics. 2010;13:514–523. doi: 10.1159/000294202. [DOI] [PubMed] [Google Scholar]
- Esplin MS, Manuck TA, Varner MW, Christensen B, Biggio J, Bukowski R, … Ilekis J. Cluster analysis of spontaneous preterm birth phenotypes identifies potential associations among preterm birth mechanisms. American Journal of Obstetrics & Gynecology. 2015;213:429e1–429.e9. doi: 10.1016/j.ajog.2015.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores-Espinosa P, Pineda-Torres M, Vega-Sánchez R, Estrada-Gutiérrez G, Espejel-Nuñez A, Flores-Pliego A, … Zaga-Clavellina V. Progesterone elicits an inhibitory effect upon LPS-induced innate immune response in pre-labor human amniotic epithelium. American Journal of Reproductive Immunology. 2014;71:61–72. doi: 10.1111/aji.12163. [DOI] [PubMed] [Google Scholar]
- Frey HA, Stout MJ, Pearson LN, Tuuli MG, Cahill AG, Strauss JF, 3rd, … Macones GA. Genetic variation associated with preterm birth in African-American women. American Journal of Obstetrics & Gynecology. 2016;215:235.e1–235.e8. doi: 10.1016/j.ajog.2016.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabay C, Lamacchia C, Palmer G. IL-1 pathways in inflammation and human diseases. Nature Reviews Rheumatology. 2010;6:232–241. doi: 10.1038/nrrheum.2010.4. [DOI] [PubMed] [Google Scholar]
- Genc MR, Witkin SS, Delaney ML, Paraskevas LR, Tuomala RE, Norwitz ER, Onderdonk AB. A disproportionate increase in IL-1β over IL-1ra in the cervicovaginal secretions of pregnant women with altered vaginal microflora correlates with preterm birth. American Journal of Obstetrics & Gynecology. 2004;190:1191–1197. doi: 10.1016/j.ajog.2003.11.007. [DOI] [PubMed] [Google Scholar]
- Gillespie SL, Neal JL, Christian LM, Szalacha LA, McCarthy DO, Salsberry PJ. An IL1RN polymorphism predicts early birth among African American women. Western Journal of Nursing Research. 2016;38:1391–1392. doi: 10.1177/0193945916658201. [DOI] [PubMed] [Google Scholar]
- Gillespie SL, Porter K, Christian LM. Adaptation of the inflammatory immune response across pregnancy and postpartum in Black and White women. Journal of Reproductive Immunology. 2016;114:27–31. doi: 10.1016/j.jri.2016.02.001. doi:S0165-0378(15)30088-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenfeder SA, Varnell T, Powers G, Lombard-Gillooly K, Shuster D, McIntyre KW, … Ju G. Insertion of a structural domain of interleukin (IL)-1β confers agonist activity to the IL-1 receptor antagonist. Implications for IL-1 bioactivity. Journal of Biological Chemistry. 1995;270:22460–22466. doi: 10.1074/jbc.270.38.22460. [DOI] [PubMed] [Google Scholar]
- Hailey KL, Capraro DT, Barkho S, Jennings PA. Allosteric switching of agonist/antagonist activity by a single point mutation in the interluekin-1 receptor antagonist, IL-1Ra. Journal of Molecular Biology. 2013;425:2382–2392. doi: 10.1016/j.jmb.2013.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton BE, Martin JA, Osterman MJ, Curtin SC, Matthews TJ. Births: Final data for 2014. National Vital Statistics Reports. 2015;64(12):1–64. Retrieved from http://www.cdc.gov/nchs/data/nvsr/nvsr64/nvsr64_12.pdf. [PubMed] [Google Scholar]
- Hayes AF. Introduction to mediation, moderation, and conditional process analysis: A regression-based approach. New York, NY: Guilford; 2013. [Google Scholar]
- He Y, Peng S, Xiong W, Xu Y, Liu J. Association between polymorphism of interleukin-1 beta and interleukin-1 receptor antagonist gene and asthma risk: A meta-analysis. Scientific World Journal. 2015;2015 doi: 10.1155/2015/685684. Article 685684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heng YJ, Di Quinzio MK, Permezel M, Rice GE, Georgiou HM. Interleukin-1 receptor antagonist in human cervicovaginal fluid in term pregnancy and labor. American Journal of Obstetrics & Gynecology. 2008;199:656e1–656.e7. doi: 10.1016/j.ajog.2008.06.011. [DOI] [PubMed] [Google Scholar]
- Heng YJ, Liong S, Permezel M, Rice GE, Di Quinzio MK, Georgiou HM. The interplay of the interleukin 1 system in pregnancy and labor. Reproductive Sciences. 2014;21:122–130. doi: 10.1177/1933719113492204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iams JD. Prevention of preterm parturition [Clinical practice] New England Journal of Medicine. 2014;370:254–261. doi: 10.1056/NEJMcp1103640. [DOI] [PubMed] [Google Scholar]
- Jones NM, Holzman C, Tian Y, Witkin SS, Genc M, Friderici K, … Wirth J. Innate immune system gene polymorphisms in maternal and child genotype and risk of preterm delivery. Journal of Maternal-Fetal & Neonatal Medicine. 2012;25:240–247. doi: 10.3109/14767058.2011.569614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kistka ZA, DeFranco EA, Ligthart L, Willemsen G, Plunkett J, Muglia LJ, Boomsma DI. Heritability of parturition timing: An extended twin design analysis. American Journal of Obstetrics & Gynecology. 2008;199:43e1–43.e5. doi: 10.1016/j.ajog.2007.12.014. [DOI] [PubMed] [Google Scholar]
- Korthagen NM, van Moorsel CH, Kazemier KM, Ruven HJT, Grutters JC. IL1RN genetic variations and risk of IPF: A meta-analysis and mRNA expression study. Immunogenetics. 2012;64:371–377. doi: 10.1007/s00251-012-0604-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langereis JD, Oudijk EJD, Schweizer RC, Lammers JWJ, Koenderman L, Ulfman LH. Steroids induce a disequilibrium of secreted interleukin-1 receptor antagonist and interleukin-1β synthesis by human neutrophils. European Respiratory Journal. 2011;37:406–415. doi: 10.1183/09031936.00170409. [DOI] [PubMed] [Google Scholar]
- Laughon SK, Zhang J, Grewal J, Sundaram R, Beaver J, Reddy UM. Induction of labor in a contemporary obstetric cohort. American Journal of Obstetrics & Gynecology. 2012;206:486e1–486.e9. doi: 10.1016/j.ajog.2012.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lekva T, Norwitz ER, Aukrust P, Ueland T. Impact of systemic inflammation on the progression of gestational diabetes mellitus. Current Diabetes Reports. 2016;16:26. doi: 10.1007/s11892-016-0715-9. [DOI] [PubMed] [Google Scholar]
- Li Y, Wang Y, Ding X, Duan B, Li L, Wang X. Serum levels of TNF-α and IL-6 are associated with pregnancy-induced hypertension. Reproductive Sciences. 2016;10:1402–1408. doi: 10.1177/1933719116641760. doi:1933719116641760. [DOI] [PubMed] [Google Scholar]
- MacDorman MF, Declercq E, Zhang J. Obstetrical intervention and the singleton preterm birth rate in the United States from 1991–2006. American Journal of Public Health. 2010;100:2241–2247. doi: 10.2105/AJPH.2009.180570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manuck TA, Esplin MS, Biggio J, Bukowski R, Parry S, Zhang H … Eunice Kennedy Shriver National Institute of Child Health and Human Development Genomics and Proteomics Network for Preterm Birth Research. The phenotype of spontaneous preterm birth: Application of a clinical phenotyping tool. American Journal of Obstetrics & Gynecology. 2015;212:487e1–487.e11. doi: 10.1016/j.ajog.2015.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews TJ, MacDorman MF, Thoma ME. Infant mortality statistics from the 2013 period linked birth/infant death data set. National Vital Statistics Reports. 2015;64(9):1–30. Retrieved from http://www.cdc.gov/nchs/data/nvsr/nvsr64/nvsr64_09.pdf. [PubMed] [Google Scholar]
- Menon R, Pearce B, Velez DR, Merialdi M, Williams SM, Fortunato SJ, Thorsen P. Racial disparity in pathophysiologic pathways of preterm birth based on genetic variants. Reproductive Biology and Endocrinology. 2009;7:62. doi: 10.1186/1477-7827-7-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon R, Williams SM, Fortunato SJ. Amniotic fluid interleukin-1β and interleukin-8 concentrations: Racial disparity in preterm birth. Reproductive Sciences. 2007;14:253–259. doi: 10.1177/1933719107301336. [DOI] [PubMed] [Google Scholar]
- Morken NH, Klungsøyr K, Skjaerven R. Perinatal mortality by gestational week and size at birth in singleton pregnancies at and beyond term: A nationwide population-based cohort study. BMC Pregnancy and Childbirth. 2014;14:172. doi: 10.1186/1471-2393-14-172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myatt L, Eschenbach DA, Lye SJ, Mesiano S, Murtha AP, Williams SM … International Preterm Birth Collaborative Pathways and Systems Biology Working Groups. A standardized template for clinical studies in preterm birth. Reproductive Sciences. 2012;19:474–482. doi: 10.1177/1933719111426602. [DOI] [PubMed] [Google Scholar]
- O’Brien JM, Lewis DF. Prevention of preterm birth with vaginal progesterone or 17-alpha-hydroxyprogesterone caproate: A critical examination of efficacy and safety. American Journal of Obstetrics & Gynecology. 2016;214:45–56. doi: 10.1016/j.ajog.2015.10.934. [DOI] [PubMed] [Google Scholar]
- Romero R, Dey SK, Fisher SJ. Preterm labor: One syndrome, many causes. Science. 2014;345:760–765. doi: 10.1126/science.1251816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz RJ, Jallo N, Murphey C, Marti CN, Godbold E, Pickler RH. Second trimester maternal plasma levels of cytokines IL-1Ra, IL-6 and IL-10 and preterm birth. Journal of Perinatology. 2012;32:483–490. doi: 10.1038/jp.2011.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seikku L, Gissler M, Andersson S, Rahkonen P, Stefanovic V, Tikkanen M, … Rahkonen L. Asphyxia, neurologic morbidity, and perinatal mortality in early-term and postterm birth. Pediatrics. 2016;137:e20153334. doi: 10.1542/peds.2015-3334. [DOI] [PubMed] [Google Scholar]
- Shynlova O, Lee YH, Srikhajon K, Lye SJ. Physiologic uterine inflammation and labor onset: Integration of endocrine and mechanical signals. Reproductive Sciences. 2013;20:154–167. doi: 10.1177/1933719112446084. [DOI] [PubMed] [Google Scholar]
- Spong CY. Defining “term” pregnancy: Recommendations from the Defining “Term” Pregnancy Workgroup. JAMA. 2013;309:2445–2446. doi: 10.1001/jama.2013.6235. [DOI] [PubMed] [Google Scholar]
- Tangerås LH, Austdal M, Skråstad RB, Salvesen KÅ, Austgulen R, Bathen TF, Iversen AC. Distinct first trimester cytokine profiles for gestational hypertension and preeclampsia. Arteriosclerosis, Thrombosis, and Vascular Biology. 2015;35:2478–2485. doi: 10.1161/ATVBAHA.115.305817. [DOI] [PubMed] [Google Scholar]
- Velez DR, Fortunato SJ, Morgan N, Edwards TL, Lombardi SJ, Williams SM, Menon R. Patterns of cytokine profiles differ with pregnancy outcome and ethnicity. Human Reproduction. 2008;23:1902–1909. doi: 10.1093/humrep/den170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu W, Witherspoon DJ, Fraser A, Clark EAS, Rogers A, Stoddard GJ, … Jorde LB. The heritability of gestational age in a two-million member cohort: Implications for spontaneous preterm birth. Human Genetics. 2015;134:803–808. doi: 10.1007/s00439-015-1558-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X, Kondragunta V, Kornman KS, Wang HY, Duff GW, Renner JB, Jordan JM. IL-1 receptor antagonist gene as a predictive biomarker of progression of knee osteoarthritis in a population cohort. Osteoarthritis and Cartilage. 2013;21:930–938. doi: 10.1016/j.joca.2013.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yilmaz Y, Verdi H, Taneri A, Yazici AC, Ecevit AN, Karakaş NM, … Atac FB. Maternal-fetal proinflammatory cytokine gene polymorphism and preterm birth. DNA and Cell Biology. 2012;31:92–97. doi: 10.1089/dna.2010.1169. [DOI] [PubMed] [Google Scholar]
- Zhang J, Troendle J, Reddy UM, Laughon SK, Branch DW, Burkman R … Consortium on Safe Labor. Contemporary cesarean delivery practice in the United States. American Journal of Obstetrics & Gynecology. 2010;203:326e1–326.e10. doi: 10.1016/j.ajog.2010.06.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Two brief tables providing Hardy-Weinberg Test of Equilibrium and Spearman Rank Correlations. .pdf