Abstract
Introduction
Recent reports of long-term survival after wild-type (WT) pig-to-monkey corneal xenotransplantation are encouraging. We experienced the rapid development of retrocorneal membranes, a rare complication after corneal allotransplantation (though seen in infants and young children). The original specific aim of the study was to determine the factors associated with successful (young) pig corneal transplantation in monkeys. However, when it was obvious that retrocorneal membranes rapidly developed, our aims became to determine the factors involved in its development after both WT and GE pig corneal xenotransplantation and to investigate the characteristics of the retrocorneal membrane.
Methods
Rhesus monkeys were recipients of penetrating keratoplasty using WT and GE pigs (n=2, respectively, 1–3 months-old). Local/systemic steroids were administered for 3 months. Grafts were evaluated by slit-lamp for corneal transparency, edema, and neovascularization. Hematoxylin and eosin, Masson trichrome staining, and immunohistochemical analysis were performed. Gal staining was also carried out to distinguish the origin of the membrane.
Results
All penetrating keratoplasty recipients developed fibrous retrocorneal membranes in the early post-transplantation period, regardless of whether the graft was from a WT or GE pig. There were no features of rejection, with no cell infiltrate in the graft or anterior chamber during the 3 months follow-up. There was no difference in the clinical course between the two groups (WT or GE corneas). Immunohistochemistry indicated that the retrocorneal membranes were CK-negative, α-SMA-positive, and vimentin-positive, suggesting that they were of fibrous (keratocytic) origin. Also, the membrane was Gal positive, suggesting that it is derived from pig cornea.
Conclusions
Following pig-to-monkey corneal xenotransplantation, we report that retrocorneal membranes are derived from donor pig keratocytes. Prevention of retrocorneal membranes will be necessary to achieve successful corneal xenotransplantation.
Keywords: Cornea, porcine; Monkey; Penetrating keratoplasty; Pig; Retrocorneal membrane; Xenotransplantation
Introduction
In view of the shortage of deceased human corneas, many attempts have been made to find alternative sources for corneal transplantation. Porcine corneas are one possibility (1–4). Previously, Pan et al., showed that corneas from Wuzhishan miniature pigs survived for 6 months in rhesus monkeys treated with local corticosteroids (5). More recently, Choi et al. reported that, with full immunosuppressive therapy using CD40–CD154 costimulatory pathway blockade, survival of wild-type (WT) pig corneas extended to >2 years in Chinese rhesus monkeys (6,7).
Corneas from genetically-engineered (GE) pigs, such as α1,3-galactosyltransferase gene-knockout pigs expressing a human complement-regulatory protein (GTKO/CD46 pigs), have been reported to have immunologic benefit over WT pig corneas in vitro. They would be expected to provide longer-term graft survival compared to corneas from WT pigs (8,9) as preformed anti-Gal-α1,3-Gal antibodies and complement activation play roles in graft rejection (7,10,11).
In our several cases of pig-to-monkey corneal xenotransplantation (12), we experienced the rapid development of a retrocorneal membrane, which is a rare complication after corneal allotransplantation, though it is seen in infants and young children. The original specific aim of the study was to determine the factors associated with successful (young) pig corneal transplantation in monkeys. However, when it was obvious that retrocorneal membranes rapidly developed, our aims became to determine the factors involved in its development after both WT and GE pig corneal xenotransplantation and to investigate the characteristics of the retrocorneal membrane.
Materials and Methods
All procedures corresponded to the ARVO Statement Regarding the Use of Animals in Ophthalmic and Vision Research and the University of Pittsburgh Institutional Animal Care and Use Committee guidance for laboratory animals (IACUC # 12070448). The 4 transplants were carried out between January 11, 2013 and June 26, 2014.
Animals
Four rhesus monkeys (3–4 years-old) were used as recipients (Alpha Genesis, Yamassee, SC). Donor corneas were prepared from Large White (genetically-unmodified) pigs (Wally Whippo, Enon Vally, PA; n=2) and GTKO/CD46 pigs on a Large White background (Revivicor, Blacksburg, VA; n=2). To match corneal thickness between donors and recipients, donor pigs were used at 1 to 3 month-old.
After euthanizing the pigs, corneal endothelial cell (CEC) density and corneal thickness were measured using confocal microscopy (Confoscan 3, Nidek Technologies, Fremont, CA) and a pachymeter (Tomey, Nagoya, Japan), respectively. Information on the corneas of the donors and recipients can be found in Table 1.
Table 1.
Corneal thickness and corneal endothelial cell density of donor pigs and recipient monkeys
| Donor pig | Age (months) |
Corneal thickness (μm) |
CECs density (/mm2) |
Recipient monkey# |
Age (months) |
Corneal thickness (µm) |
|---|---|---|---|---|---|---|
| WT | 1.5 | 644 | 3861 | M168 | 39 | 538 |
| 1 | 567 | 4825 | M169 | 48 | 445 | |
| GTKO/CD46 | 3 | 688 | 4040 | M87 | 35 | 480 |
| 3 | 703 | 3701 | M88 | 37 | 469 |
CECs = corneal endothelial cells
Pig-to-monkey penetrating keratoplasty
The donor corneoscleral tissues were dissected out and stored in Optisol GS solution (Bausch & Lomb, Rochester, NY) for 2–3 days before transplantation. Full-thickness corneal transplantation (penetrating keratoplasty) was carried out, as previously described (5). Briefly, the donor cornea was excised using a 6.5mm-diameter Barron corneal punch (Katena Products, Denville, NJ). The central cornea was excised from the recipient’s eye using a 6mm-diameter Hessburg-Barron vacuum trephine (Jedmed, St. Louis, MO). The grafts was placed and secured with 16 interrupted 10-0 nylon sutures (Ethicon, Somerville, NJ). Triamcinolone 20mg/0.5mL (Kenalog-40®, Bristol-Myers Squibb, New York, NY) was injected subconjunctivally, and the eyelids were closed with a 6-0 black silk suture. After 3 or 4 days, the tarsorrhaphy was opened to inspect the eye.
Postoperative treatment
All monkeys received topical tobramycin/dexamethasone eye ointment (Tobradex®, Alcon, Fort Worth, TX) ×2–3 weekly. Enrofloxacin 5mg/kg (Baytryl® 100, Leverkusen, Germany) was administered once a day intramuscularly for 3 days. Triamcinolone 20mg/0.5mL (Bristol-Myers Squibb) was injected subconjunctivally on post-operative days 0, 4, 7, and then every two weeks for 3 months. Methylprednisolone (Solu-medrol, Pfizer, New York, NY) was injected intramuscularly at an initial dose of 2mg/kg/day, tapered over 12 weeks, and discontinued at a final dose of 0.25mg/kg.
Clinical evaluation of the grafts
After sedation of the monkeys, the grafts were examined by portable slit-lamp biomicroscopy (Keeler Ophthalmic Instruments, Broomall, PA) to evaluate graft clarity, edema, and neovascularization. To evaluate graft rejection, a scoring system (providing a ‘rejection index’) was used (Table 2), as previously described by Pan et al (5). In this scoring system, if the sum of the scores of the 3 grades is greater than 6 for 2 consecutive weeks, the graft is considered to be rejected. Corneal thickness was measured with a pachymeter.
Table 2.
Rejection index (scoring system) evaluated by slit lamp
| Grade | Criteria |
|---|---|
| Clarity | |
| 0 | Clear cornea |
| 1 | Slight haze |
| 2 | Increased haze, but anterior chamber structures still clear |
| 3 | Advanced haze with difficult view of anterior chamber |
| 4 | Opaque cornea without view of anterior chamber |
| Edema | |
| 0 | No stromal or epithelial edema |
| 1 | Slight stromal edema |
| 2 | Diffuse stromal edema |
| 3 | Diffuse stromal edema with microcystic edema of epithelium |
| 4 | Bullous keratopathy |
| Neovascularization | |
| 0 | No vascularization |
| 1 | Vascularization of the host peripheral cornea |
| 2 | Vascularization to the corneal wound or sutures |
| 3 | Vascularization of the peripheral graft |
| 4 | Vascularization of the entire graft |
Recipients were electively euthanized after 3 months with an intravenous injection of pentobarbital (Beuthanasia-d, Merk Animal Health, Madison, NJ), and the enucleated eyes were examined by confocal microscopy to visualize the endothelial cells and stromal keratocytes, as previously described by Lee et al (13).
Immunohistochemistry
The naïve (non-grafted) and grafted corneas from each recipient were fixed with 10% formalin, stained with hematoxylin-eosin (H&E) and Masson trichrome, and then examined by light microscopy (Nikon, Elgin, IL). For immunohistochemical staining, anti-cytokeratin antibody (mouse monoclonal IgG2a, clone CAM5.2, BD Biosciences, San Jose, CA), and anti-α-smooth muscle actin (α-SMA) antibody (mouse monoclonal, IgG, clone1A4, Roche), and anti-vimentin antibody (mouse monoclonal IgG, clone V9, Roche, Basel, Switzerland) were used, as previously described (14). In addition, anti-CD166 (mouse monoclonal IgG1k, clone 3A6, BD Biosciences), anti-CD44 (rat IgG2b, clone IM7, Biolegend, San Diego, CA), and anti-lumican (rabbit monoclonal IgG, clone EPR8898, Abcam, Cambridge, UK) antibodies were used to further investigate the origin of the retrocorneal membrane (15,16). The naïve (non-grafted) eye of each animal was used as a control to determine the baseline immunohistochemical staining characteristics of the cellular layers of the normal cornea (14). The immunohistochemical characteristics of normal cornea and retrocorneal membrane are described in Table 3. To differentiate whether the retrocorneal membrane was derived from recipient monkey or donor pig tissue, staining for Gal was performed, as previously described (9).
Table 3.
Immunohistochemical characteristics of normal cornea and of retrocorneal membrane
| Tissue | Cytokeratin Cam5.2a |
α-SMA | Vimentin | CD166 | CD44 | Lumican |
|---|---|---|---|---|---|---|
| Normal cornea | ||||||
| Epithelium | − | − | +b | − | +b | − |
| Stromal keratocytes | − | − | + | − | − | + |
| Endothelium | − | − | + | +c | −d | − |
| Retrocorneal membrane | ||||||
| Epithelial cell derived | − | − | − | − | − | − |
| Fibroblastic keratocyte derived | − | + | + | − | + | + |
| Endothelial cell derived (Fibroblastic transformation) |
+ | + | + | − | + | (no data) |
− nonstaining; + positive staining; SMA =smooth muscle actin
Cam5.2 recognizes cytokeratin8.
Focal staining of the basal epithelial layer in half of the specimens.
Become negative once the endothelial cells transform to fibroblastic phenotype
Become positive once the endothelial cells transform to fibroblastic phenotype
Results
Development of a retrocorneal membrane after penetrating keratoplasty
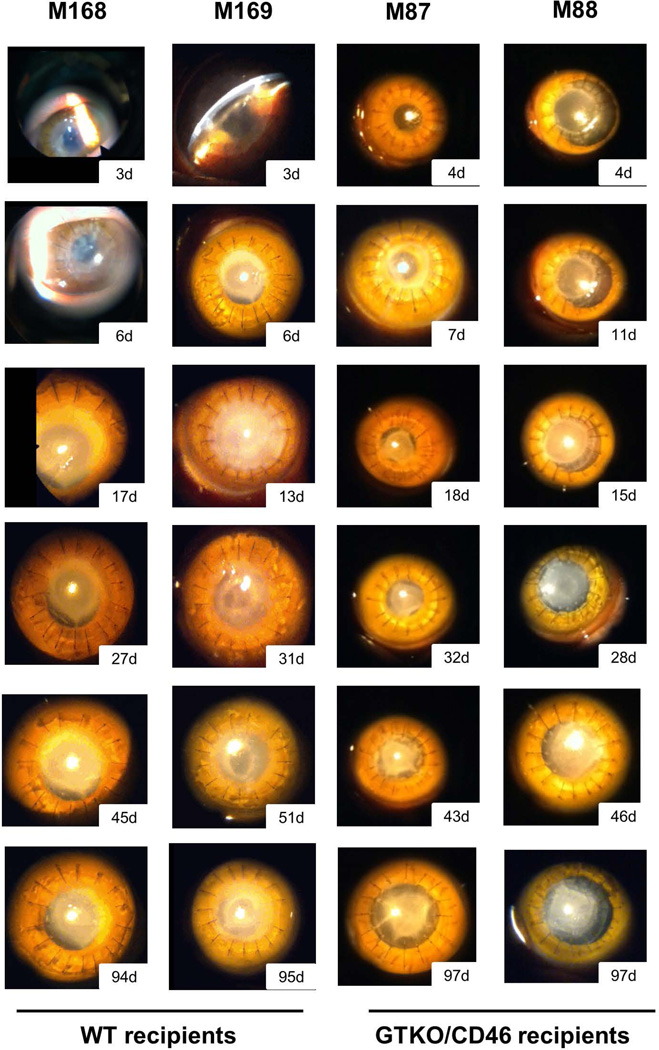
Recipient monkeys remained healthy without weight loss or infection throughout the period of follow-up. However, all recipients developed a retrocorneal membrane within 3 to 11 days, which greatly reduced graft transparency (Figure 1). Retrocorneal membranes were found in all recipients regardless of the source of the corneal graft (WT or GTKO/CD46 pig), and were not responsive to local/systemic corticosteroid therapy.
Figure 1. Clinical evaluation of corneal grafts after transplantation.
Pig corneal grafts after transplantation into monkeys were evaluated with slit-lamp biomicroscopy (magnification ×16). Retrocorneal membranes were found in all recipients regardless of donor pig type (WT or GTKO/CD46) at early time-points after transplantation (post-operative days 11, 7, 11, and 3, respectively, from left to right).
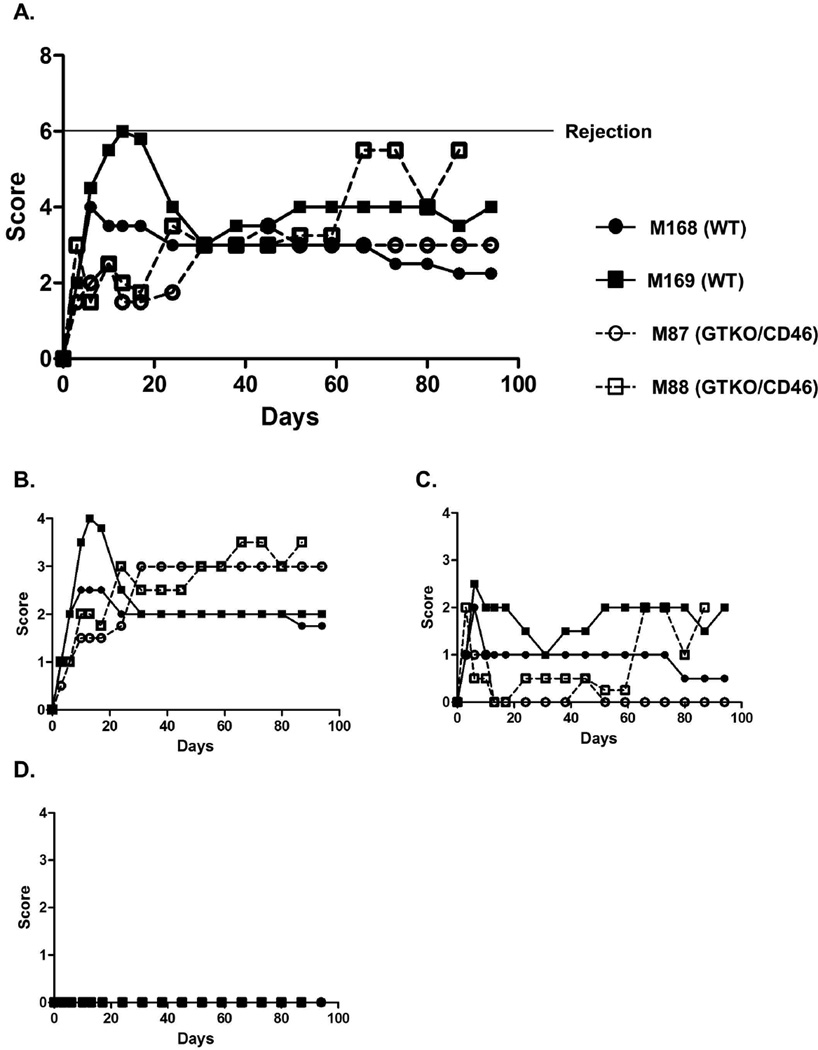
The rejection index was determined by the same corneal specialist ophthalmologist every week (Figure 2). None of the recipients satisfied the criteria for rejection (i.e., with a score >6 out of 12 for 2 consecutive weeks), but, although no persistent severe edema (score 0–1/4) or neovascularization (score 0/4) was present, graft clarity was greatly reduced due to the presence of the retrocorneal membrane (score of 2–3.5/4). Weekly attempts to measure corneal thickness with a pachymeter were made, but proved difficult because of the existence of the retrocorneal membrane.
Figure 2. Rejection index (score) of corneal grafts.
(A) Total rejection index (total score). Corneal xenografts after transplantation were evaluated, focusing on (B) graft clarity, (C) edema, and (D) neovascularization, based on a scoring system (Table 2) (introduced by Pan et al). The grafts are considered to be rejected if the total index (the sum of the scores of the 3 grades) is >6 for 2 consecutive weeks. Although graft clarity was greatly reduced due to the presence of a retrocorneal membrane, persistent severe stromal edema or neovascularization did not develop.
Results of confoscan
After euthanasia, both eyes were excised from the recipient monkey, and both the host (non-grafted) and grafted corneas were evaluated using confoscan. The host (non-grafted) cornea showed normal CEC morphology and density, with the characteristic mosaic appearance, without any activation of stromal keratocytes (data not shown). In contrast, the pig CEC layer was not visible due to the presence of the retrocorneal membrane, which appeared to be a homogeneous, highly-reflective membranous structure. Some activated keratocytes were observed in the stroma or near the membrane. Surrounding the graft, the native monkey CECs were enlarged compared to those in the host (non-grafted) eye, possibly a result of a compensatory increase in size after CEC injury (data not shown). CEC density in the native (non-transplanted) cornea of M88 was 3,683 cells/mm2, whereas in the monkey cornea surrounding the pig graft the CEC density was only 1,805 cells/mm2, a reduction in cell density of approximately 50% (possibly related to the surgical procedure and a low-grade inflammatory response). All 4 recipients showed similar confoscan results.
Immunohistochemistry
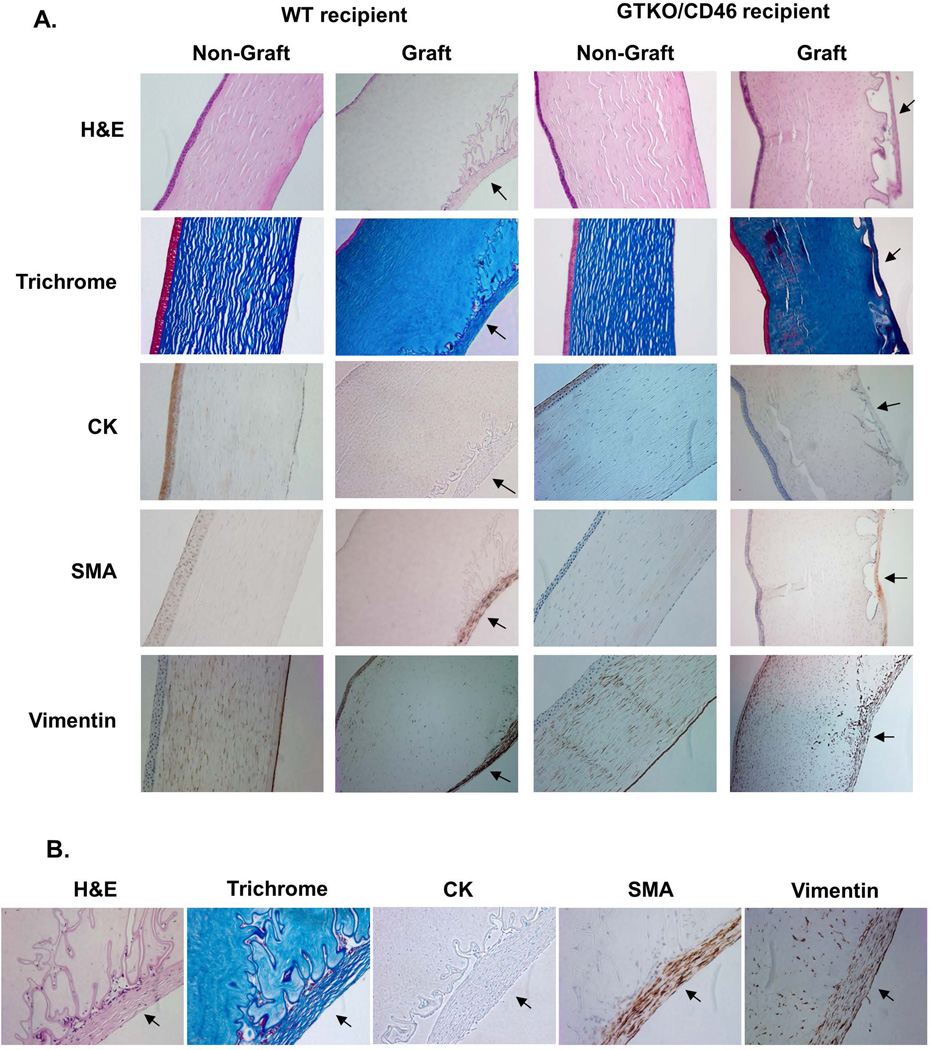
To distinguish the origin of the retrocorneal membrane, the corneal grafts were stained with various antibodies (Figure 3). Non-grafted corneas were used as controls, and all showed a normal structure with intact CECs. All pig grafts displayed a moderately thick collagenous membrane with some cellular components, strongly positive for trichrome (collagen) staining (Figure 3A, B). The membrane was located internal to the undulating Descemet’s membrane, with parts of the retrocorneal membrane directly attached to the CECs (Descemet’s membrane). No CECs were identified between the membrane and the corneal stroma. In contrast, there were in places gaps between the CECs and the retrocorneal membrane, with some CECs being trapped in these spaces. No inflammatory cell infiltrate was observed in the grafts.
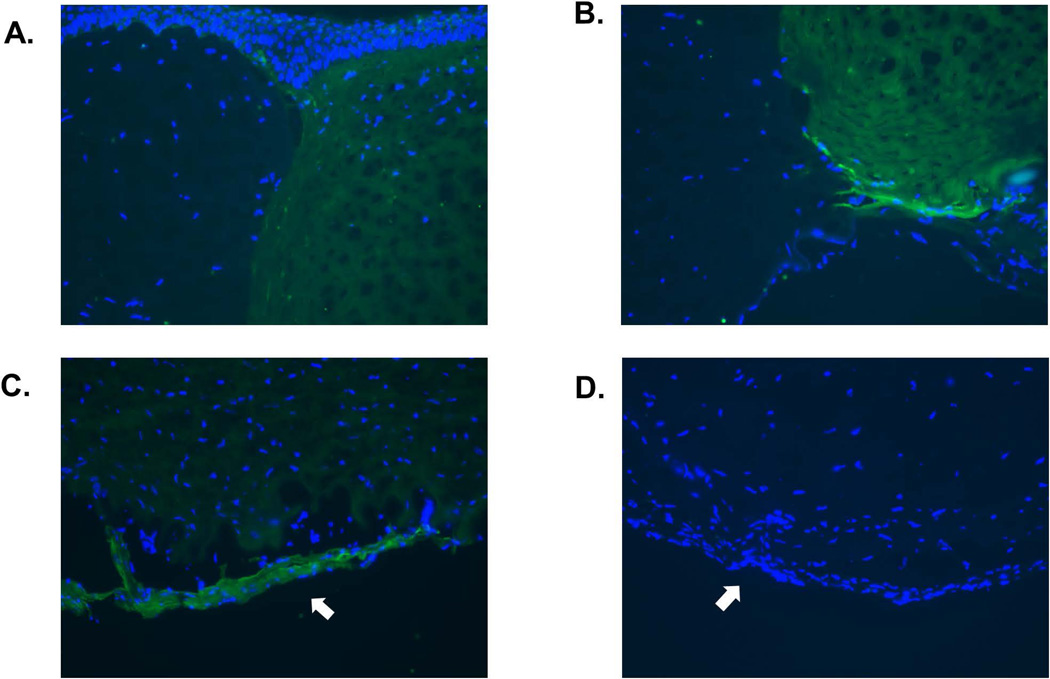
Figure 3. Immunohistochemical staining of pig corneal grafts 3 months after transplantation.
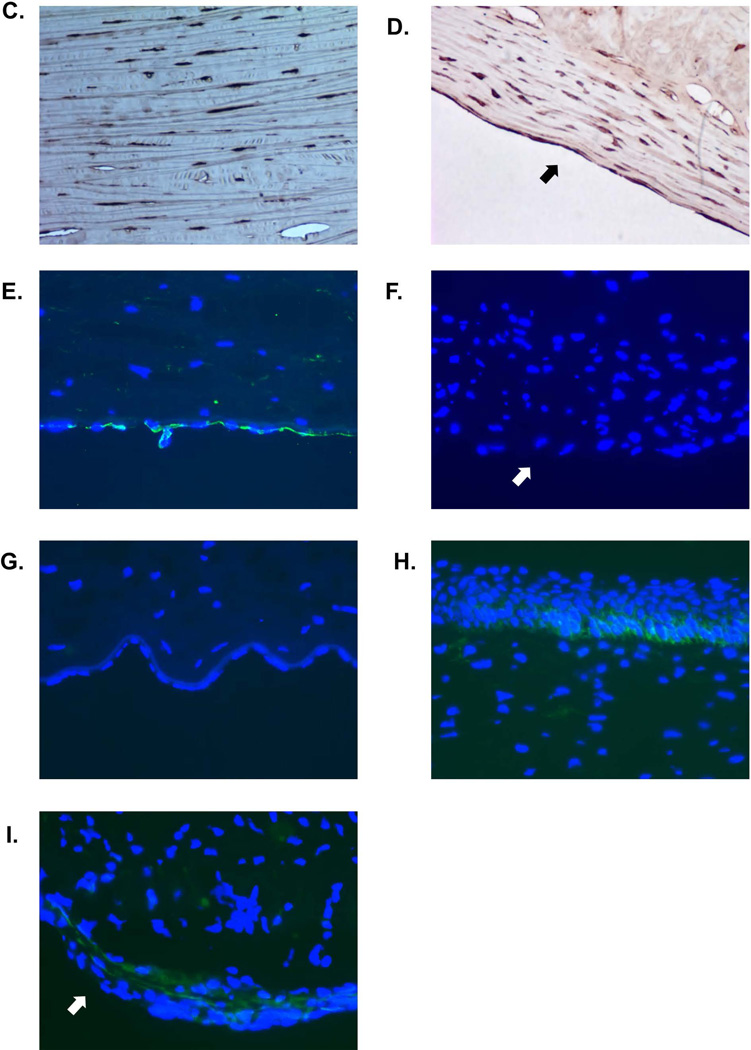
(A) Xenografts were stained with hematoxylin-eosin (H&E) and Masson trichrome to define the structures. They were also stained for cytokeratin (CK), α-smooth muscle (α–SMA), and vimentin. Non-grafted corneas were stained as controls. All non-grafted corneas showed normal structure with intact CECs. In contrast, all grafted corneas displayed moderately thick collagenous membranes with some cellular components, strongly positive for collagen (second row), in relation to the undulating Descemet’s membrane (arrows). Parts of the retrocorneal membrane were directly attached to CECs, and parts were divided by a space between the CECs and membrane, with a few CECs trapped in this space. No inflammatory cell infiltrate was observed. Healthy CECs are negative for CK and α–SMA, and positive for vimentin. Retrocorneal membranes (arrows) were negative for CK, but positive for both α–SMA and vimentin, indicating that the retrocorneal membranes were derived from stromal keratocytes. (Representative of 2 samples from each group, magnification ×100). (B) Higher magnification of retrocorneal membranes (magnification ×200). (C, D) Lumican staining (dark brown) of normal stromal keratocytes (C) and retrocorneal membrane (arrow, D). The cellular component of the retrocorneal membrane was positive for lumican. (E,F) CD166 staining (green) of normal endothelial cells (E) and retrocorneal membrane (arrow, F). Normal endothelial cells were positive for CD166, but retrocorneal membrane was negative. (G–I) CD44 staining (green) of normal endothelium (G), normal epithelium (H), and retrocorneal membrane (arrow, I). Staining for CD44 was positive in normal endothelial cells and in the basal layer of epithelium. The retrocorneal membrane was also positive for CD44. (Blue = cell nuclei, magnification ×400)
Healthy CECs in the host (non-grafted) cornea were negative for CK and α–SMA and positive for vimentin. The retrocorneal membranes, however, proved negative for CK but positive for both α–SMA and vimentin (Figure 3A, B), indicating that they were derived from stromal keratocytes (14). All pig grafts, regardless of their source (i.e., WT or GTKO/CD46) showed similar results. To confirm that retrocorneal membranes are keratocytic in origin, the tissues were stained with a keratocyte marker, lumican (Figure 3C, D). Normal keratocytes showed positivity, as did those in the retrocorneal membrane. To exclude the possibility that the membrane originated from the endothelium, tissues were stained for CD166 and CD44. The retrocorneal membrane was proven to be negative for CD166 (Figure 3E, F), and positive for CD44 (Figure 3G–I), indicating that it originated from stromal keratocytes.
To distinguish whether the membranes were derived from recipient or donor tissue, staining for Gal was carried out. A distinct difference was documented between Gal-positive tissues (in WT pig grafts) and Gal-negative tissues (in monkey cornea) (Figure 4). WT pig corneas expressed Gal in the stroma as well as on the endothelial cells (9), and the retrocorneal membrane was also positive for Gal. This indicated that the origin of the retrocorneal membrane was the pig corneal graft. Retrocorneal membranes in monkeys that received GTKO/CD46 pig corneal grafts showed no expression of Gal (as expected due to the lack of Gal expression in the graft). Therefore, we cannot definitively determine the origin of the retrocorneal membrane after GTKO/CD46 pig corneal transplantation, but the data from the WT grafts strongly suggest that all retrocorneal membranes originated from donor tissues.
Figure 4. Gal staining of pig corneal grafts 3 months after transplantation.
(A,B) Gal-staining (green) of the anterior and posterior cornea of a WT pig graft. Recipient tissue (left) was negative for Gal, while the donor pig graft (right) was positive. (C) The retrocorneal membrane (arrow) from a WT pig graft showed positivity for Gal. (D) The retrocorneal membrane (arrow) from a GTKO/CD46 pig graft did not show positivity for Gal. (Blue = cell nuclei, magnification ×200)
Discussion
A retrocorneal membrane can develop as a complication after corneal allotransplantation, trauma, or infection (e.g., herpes simplex virus-1 infection) (17), if these insults affect the endothelium. After corneal allotransplantation, its development has been reported after failed penetrating keratoplasty (18), Descemet stripping endothelial keratoplasty (19,20), and Descemet membrane endothelial keratoplasty (21). The reported incidence varies considerably from 17% to 83% (22–24).
Risk factors include young patient age (<1 year), microcornea (corneal diameter <10mm), preexisting CEC dysfunction, mental retardation in the recipient, and combining the corneal transplant with cataract surgery (25). It has been well documented that corneal transplantation in pediatric patients is more challenging, even for experienced corneal surgeons, due to anatomical differences from adults (e.g., low scleral rigidity, higher vitreous pressure, and a narrow anterior chamber), and from an increased inflammatory response with fibrin reaction (26,27). The present pig-to-monkey corneal transplantation model has similarities to pediatric allotransplantation with regard to the size of eye and the age of the donor.
Briefly, three different origins of retrocorneal membranes have been suggested - (i) epithelial cell ingrowth, (ii) fibroblastic or stromal (keratocytic) downgrowth, and (iii) fibrous metaplasia of the corneal endothelium (14). Although clinical features may help determine the cause of the retrocorneal membrane, immunohistochemical staining can provide further information (14). In the present study, our data suggest that the retrocorneal membranes were derived from stromal keratocytes (fibroblasts).
The mechanism by which retrocorneal membranes originate from fibroblastic cells has not been determined. We suggest the possible causes include: (i) mismatched structures (e.g., comparative thickness of the cornea between graft and host), (ii) mismatched biomechanics (e.g., rigidity), (iii) increased inflammatory reaction to a cornea from a pig of a young age (see below), and (iv) abnormal wound healing. In the present study, we selected 1–3 months-old piglets as sources of grafts, mainly to match graft and host corneal thickness. However, the grafts were extremely flaccid and hardly maintained their concave shape, we believe was a major factor in the development of the retrocorneal membranes. Rigidity of the graft would appear to be a more important factor than its thickness.
Both groups that have reported long-term survival of pig corneal grafts in monkeys used rather older pigs than we did, and, furthermore, both used miniature swine as the sources of the grafts, e.g., 4 months-old Wuzhishan pigs (5), and 2–3 years-old Seoul National University miniature pigs (7), although the thickness of the grafts did not match that of the host cornea very well. The benefit of using miniature pigs is that, although the grafts are fully matured and demonstrate normal (adult) rigidity, their thickness was less (700~850µm) compared to those of regular pigs (e.g., large White) of the same age (900~>1000µm).
In our study, all of the source pigs (both WT and GE) were of Large White background. The average corneal thickness of adult (>1 year) Large White pigs is approximately 1000µm (13). Furthermore, in our experience, corneas from GE pigs are thinner and more flexible/flaccid compared to corneas from age-matched WT pigs, again making surgical manipulation more difficult.
In addition to differences in graft flexibility/rigidity, corneal grafts from younger donors are also associated with an increased inflammatory reaction after corneal allotransplantation (26). More graft rejection episodes have been documented in patients who received allografts from younger (0–5 years old) donors compared to those who received grafts from adults (40–70 years old) (28). Belmonte et al. suggested that high CEC density increases the risk of irreversible graft failure, probably due to exposure to a greater antigenic load (29). Although corneas from GE pigs (e.g., GTKO/CD46) have been proven in vitro to be immunologically beneficial (8), a significant in vivo immune/inflammatory response is still likely.
A retrocorneal membrane may also develop as a result of abnormal wound healing. If there is poor wound apposition (as may occur when there is a great discrepancy in corneal thickness), quiescent keratocytes become activated and migrate to the margins where they proliferate. Hyperplasia of fibrous tissue forms a mass of spindle-shaped fibers and cells, which protrude into the anterior chamber and form a retrocorneal membrane (30). These membranes have been reported to be positive for α-SMA and vimentin expression (14), which is consistent with our results. The presence of irregularly-aligned collagen fibers in the graft also suggests that there may have been abnormal wound healing. Wunderlich et al., reported that connective tissue growth factor may play a crucial role in corneal wound healing and thus in membrane formation (30).
Recently, our collaborators in Beijing (Dr. Zhiqiang Pan’s group) also experienced retrocorneal membrane development after pig-to-monkey corneal xenotransplantation using WT and GTKO/CD46 pigs (both on a Large White background) (Dong X, et al, manuscript submitted). To our knowledge, our own study and that of the Beijing group are the first to report the development of retrocorneal membranes in pig-to-monkey corneal transplantation models.
In our own study, although a retrocorneal membrane developed, in no case was there conclusive evidence of acute rejection. The grafts did not become severely edematous, possibly because the remaining CECs in either the graft or the recipient were functioning well, and there was no inflammatory cell infiltrate in the graft or anterior chamber. This suggests that local/systemic corticosteroid treatment was sufficient to prevent rejection, but not able to prevent retrocorneal membrane development. Additional local and/or systemic anti-inflammatory therapy may be beneficial if there is evidence of an inflammatory response.
In conclusion, we report on the formation of retrocorneal membranes after the transplantation of corneas from young pigs into monkeys. The retrocorneal membrane was derived from donor pig stromal keratocytes. As vision can be fully restored only with a transparent cornea, prevention of retrocorneal membrane development will be crucial to achieve successful corneal xenotranplantation. In summary, the risk of development of a retrocorneal membrane after pig corneal transplantation in monkeys is high, and is probably associated with discrepancies in the thickness and biomechanics of young pig corneas and older monkey corneas. There is evidence from others that it can be avoided if corneas are obtained from adult miniature swine. Further studies of corneal xenotransplantation using adult GE miniature swine as sources of corneas are necessary.
Acknowledgments
Work on xenotransplantation in the Thomas E. Starzl Transplantation Institute at the University of Pittsburgh has been supported in part by NIH grants U01 AI068642, and U19 AI090959, and by Sponsored Research Agreements between the University of Pittsburgh and Revivicor, Inc., Blacksburg, VA.
DA is an employee of Revivicor, Inc.
ABBREVIATIONS
- α-SMA
α-smooth muscle actin
- CECs
corneal endothelial cells
- CK
cytokeratin
- GE
genetically-engineered
- GTKO
α1,3-galactosyltransferase gene-knockout
- WT
wild-type
Footnotes
Conflict of interest
The other authors have no conflict of interest.
References
- 1.Lamm V, Hara H, Mammen A, Dhaliwal D, Cooper DK. Corneal blindness and xenotransplantation. Xenotransplantation. 2014;21:99–114. doi: 10.1111/xen.12082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kim MK, Hara H. Current status of corneal xenotransplantation. Int J Surg. 2015;23:255–260. doi: 10.1016/j.ijsu.2015.07.685. [DOI] [PubMed] [Google Scholar]
- 3.Hara H, Cooper DK. Xenotransplantation-the future of corneal transplantation? Cornea. 2011;30:371–378. doi: 10.1097/ICO.0b013e3181f237ef. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kim MK, Wee WR, Park CG, Kim SJ. Xenocorneal transplantation. Cur Opin Organ Transplant. 2011;16:231–236. doi: 10.1097/MOT.0b013e328344870c. [DOI] [PubMed] [Google Scholar]
- 5.Pan Z, Cun S, Ying J, Ningli W, Li W. WZS-pig is a potential donor alternative in corneal xenotransplantation. Xenotransplantation. 2007;14:603–611. doi: 10.1111/j.1399-3089.2007.00432.x. [DOI] [PubMed] [Google Scholar]
- 6.Choi HJ, Kim MK, Lee HJ, Ko JH, Jeong SH, Lee JI, et al. Efficacy of pig-to-rhesus lamellar corneal xenotransplantation. Invest Ophthalmol Vis Sci. 2011;52:6643–6650. doi: 10.1167/iovs.11-7273. [DOI] [PubMed] [Google Scholar]
- 7.Choi HJ, Lee JJ, Kim DH, Kim MK, Lee HJ, Ko AY, et al. Blockade of CD40–CD154 Costimulatory Pathway Promotes Long-Term Survival of Full-Thickness Porcine Corneal Grafts in Nonhuman Primates: Clinically Applicable Xenocorneal Transplantation. Am J Transplant. 2015;15:628–641. doi: 10.1111/ajt.13057. [DOI] [PubMed] [Google Scholar]
- 8.Hara H, Koike N, Long C, Piluek J, Roh DS, Sundarraj N, et al. Initial in vitro investigation of the human immune response to corneal cells from genetically engineered pigs. Invest Ophthalmol Vis Sci. 2011;52:5278–5286. doi: 10.1167/iovs.10-6947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cohen D, Miyagawa Y, Mehra R, Lee W, Isse K, Long C, et al. Distribution of non-gal antigens in pig cornea: relevance to corneal xenotransplantation. Cornea. 2014;33:390–397. doi: 10.1097/ICO.0000000000000069. [DOI] [PubMed] [Google Scholar]
- 10.Hara H, Cooper DK. The immunology of corneal xenotransplantation: a review of the literature. Xenotransplantation. 2010;17:338–349. doi: 10.1111/j.1399-3089.2010.00608.x. [DOI] [PubMed] [Google Scholar]
- 11.Oh JY, Kim MK, Lee HJ, Ko JH, Kim Y, Park CS, et al. Complement depletion with cobra venom factor delays acute cell-mediated rejection in pig-to-mouse corneal xenotransplantation. Xenotransplantation. 2010;17:140–146. doi: 10.1111/j.1399-3089.2010.00574.x. [DOI] [PubMed] [Google Scholar]
- 12.Hara H, Mammen A, Lee W, Miyagawa Y, Long C, Ayares D, et al. Development of anti-pig antibodies and characterization of B cell phenotype following wild-type pig-to-monkey corneal xenotransplantation. Xenotransplantation. 2013;20:347–348. [Google Scholar]
- 13.Lee SE, Mehra R, Fujita M, Roh DS, Long C, Lee W, et al. Characterization of porcine corneal endothelium for xenotransplantation. Semin Ophthalmol. 2014;29:127–135. doi: 10.3109/08820538.2013.787104. [DOI] [PubMed] [Google Scholar]
- 14.Jakobiec FA, Bhat P. Retrocorneal membranes: a comparative immunohistochemical analysis of keratocytic, endothelial, and epithelial origins. Am J Ophthalmol. 2010;150:230–242. e232. doi: 10.1016/j.ajo.2010.03.011. [DOI] [PubMed] [Google Scholar]
- 15.Okumura N, Hirano H, Numata R, Nakahara M, Ueno M, Hamuro J, et al. Cell surface markers of functional phenotypic corneal endothelial cells. Invest Ophthalmol Vis Sci. 2014;55:7610–7618. doi: 10.1167/iovs.14-14980. [DOI] [PubMed] [Google Scholar]
- 16.Carlson EC, Liu CY, Chikama T, Hayashi Y, Kao CW, Birk DE, et al. Keratocan, a cornea-specific keratan sulfate proteoglycan, is regulated by lumican. J Biol Chem. 2005;280:25541–25547. doi: 10.1074/jbc.M500249200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sherrard ES, Rycroft PV. Retrocorneal membranes. I. Their origin and structure. Br J Ophthalmol. 1967;51:379–386. doi: 10.1136/bjo.51.6.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lifshitz T, Oshry T, Rosenthal G. Retrocorneal membrane after penetrating keratoplasty. Ophthalmic Surg Lasers. 2001;32:159–161. [PubMed] [Google Scholar]
- 19.Young AL, Tam PM, Lau TT, Cheng LL, Rao SK, Lam PT. Case of post Descemet stripping endothelial keratoplasty retrocorneal fibrous membrane. Clin Experiment Ophthalmol. 2009;37:418–419. doi: 10.1111/j.1442-9071.2009.02050.x. [DOI] [PubMed] [Google Scholar]
- 20.Alkatan H, Al-Rajhi A, Al-Shehri A, Khairi A. Histopathological findings of failed grafts following Descemet's stripping automated endothelial keratoplasty (DSAEK) Saudi J Ophthalmol. 2012;26:79–85. doi: 10.1016/j.sjopt.2011.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yum HR, Kim MS, Kim EC. Retrocorneal membrane after Descemet membrane endothelial keratoplasty. Cornea. 2013;32:1288–1290. doi: 10.1097/ICO.0b013e318296e0f7. [DOI] [PubMed] [Google Scholar]
- 22.Kremer I, Rapuano CJ, Cohen EJ, Laibson PR, Eagle RC., Jr Retrocorneal fibrous membranes in failed corneal grafts. Am J Ophthalmol. 1993;115:478–483. doi: 10.1016/s0002-9394(14)74450-2. [DOI] [PubMed] [Google Scholar]
- 23.Calabrese S, Wenkel H, Rummelt C, Kruse F, Cursiefen C. Histopathology of retrocorneal membranes after keratoplasty. Klin Monbl Augenheilkd. 2010;227:815–818. doi: 10.1055/s-0029-1245481. [DOI] [PubMed] [Google Scholar]
- 24.Li Y, Yi Y, Feng G, Chen J, Lin J. Clinical and histological findings of retrocorneal membrane after keratoplasty. Yan Ke Xue Bao. 1997;13:82–84. 81. [PubMed] [Google Scholar]
- 25.Cheng KP, Hiles DA, Biglan AW, Pettapiece MC, Behler SC, Moore MB. Risk factors for complications following pediatric epikeratoplasty. J Cataract Refract Surg. 1992;18:270–279. doi: 10.1016/s0886-3350(13)80904-2. [DOI] [PubMed] [Google Scholar]
- 26.Bachmann B, Avgitidou G, Siebelmann S, Cursiefen C. Pediatric corneal surgery and corneal transplantation. Ophthalmologe. 2015;112:110–117. doi: 10.1007/s00347-014-3053-9. [DOI] [PubMed] [Google Scholar]
- 27.O'hara MA, Mannis MJ. Pediatric penetrating keratoplasty. Int Ophthalmol Clin. 2013;53:59–70. doi: 10.1097/IIO.0b013e3182782a4b. [DOI] [PubMed] [Google Scholar]
- 28.Palay DA, Kangas TA, Stulting RD, Winchester K, Litoff D, Krachmer JH. The effects of donor age on the outcome of penetrating keratoplasty in adults. Ophthalmology. 1997;104:1576–1579. doi: 10.1016/s0161-6420(97)30094-3. [DOI] [PubMed] [Google Scholar]
- 29.Belmonte J, Moral R, Vallcanera S. Suitability of newborn donor corneal graft in penetrating keratoplasty. Arch Soc Esp Oftalmol. 2008;83:219–230. doi: 10.4321/s0365-66912008000400004. [DOI] [PubMed] [Google Scholar]
- 30.Wunderlich K, Senn BC, Reiser P, Pech M, Flammer J, Meyer P. Connective tissue growth factor in retrocorneal membranes and corneal scars. Ophthalmologica. 2000;214:341–346. doi: 10.1159/000027517. [DOI] [PubMed] [Google Scholar]