Abstract
Background
Adult porcine islets (APIs) constitute a promising alternative to human islets in treating Type 1 diabetes. The intrahepatic site has been used in pre-clinical primate studies of API xenografts; however, an estimated two-thirds of donor islets are destroyed after intraportal infusion due to a number of factors, including the instant blood mediated inflammatory reaction (IBMIR), immunosuppressant toxicity, and poor reestablishment of extracellular matrix connections. Intraperitoneal (i.p.) transplantation of non-vascularized encapsulated islets offers several advantages over intrahepatic transplantation of free islets, including avoidance of IBMIR, immunoprotection, accommodation of a larger graft volume, and reduced risk of hemorrhage. However, there exists evidence that the peritoneal site is hypoxic, which likely impedes islet function.
Methods
We tested the effect of hypoxia (2-5% oxygen or pO2: 15.2-38.0 mmHg) on free and encapsulated APIs over a period of 6 days in culture. Free and encapsulated APIs under normoxia served as controls. Islet viability was evaluated with a viability/cytotoxicity assay using calcein AM and ethidium bromide on days 1, 3 and 6 of culture. Alamar blue assay was used to measure the metabolic activity on days 1 and 6. Insulin in spent medium was assayed by ELISA on days 1 and 6.
Results
Viability staining indicated that free islet clusters lost their integrity and underwent severe necrosis under hypoxia; encapsulated islets remained intact, even when they began to undergo necrosis. Under hypoxia, thae metabolic activity and insulin secretion (normalized to metabolic activity) of both free and encapsulated islets decreased relative to islets cultured under normoxic conditions.
Conclusions
Hypoxia (2-5% oxygen or pO2: 15.2-38.0 mmHg) affects the viability, metabolic activity and insulin secretion of both free and encapsulated APIs over a 6-day culture period. Encapsulation augments islet integrity under hypoxia, but it does not prevent loss of viability, metabolic activity, or insulin secretion.
Keywords: porcine islets, microencapsulation, hypoxia, insulin secretion, islet viability
Introduction
Islet transplantation has proven to be a viable option for the treatment of people with Type 1 diabetes (1, 2) but the shortage of human cadaveric pancreata and deleterious consequences of long-term immunosuppression limit the of number of patients that can be treated to a small fraction of the diabetic population (3, 4). Xenotransplantation of porcine islets is a promising alternative for human islets, as pigs are an abundant source of pancreata (5), pigs and humans have similar blood glucose levels, and porcine insulin differs from human insulin by only one amino acid. Hence, pigs are considered one of the most suitable xenogeneic sources of islets for potential clinical transplantation under immunosuppression (6, 7). To improve immune acceptance and to reduce or eliminate the need for immunosuppression, porcine islets can be immunoprotected by micro- or macroencapsulation using biocompatible polymeric materials (8). Recently, the field of cellular microencapsulation has been invigorated by new advancements, such as the development of alginate derivatives that inhibit the foreign body response and the demonstration that encapsulated human stem cell-derived beta cells can correct hyperglycemia in immunocompetent mice long-term, without immunosuppression (9, 10).
The intraperitoneal cavity (i.p.) has been used as the transplantation site for encapsulated islet allografts (11, 12) and xenografts (13), but long term survival of these grafts has been difficult to achieve in this site. One likely reason is the hypoxic environment experienced by i.p.-transplanted encapsulated islets. In native islets, whether human or porcine, insulin-secreting β cells are highly oxygenated by an extensive vascular glomerular-like network of capillaries that delivers arterial blood throughout the islets in vivo (14). This vasculature is disrupted during islet isolation and cannot be re-established when islets are encapsulated. Therefore, transplanted islets must receive oxygen and other nutrients from the surrounding tissue by diffusion alone, and as a result, cells at the center of the islet are less oxygenated than cells at the periphery. In addition, the i.p. graft site is hypoxic, further reducing the oxygenation of cells in islets. Goh et al., reported that dissolved oxygen (DO) in the peritoneal cavity of mice (both normal and diabetic) is in the range of 6.5%-7.3% O2 (pO2: 49.21-55.27 mmHg) and that DO decreases in a dose-dependent manner upon transplantation of encapsulated βTC-tet cells (a conditionally-transformed mouse β-cell line) or encapsulated porcine islets (15, 16). Other factors that can contribute to islet loss after transplantation include islet size, the percentage of acinar tissue in the graft, and the islet tissue oxygen consumption rate (17).
Several studies reported in the literature have shown that hypoxia indeed compromises the viability and function of islets. For example, Schrezenmeir et al reported that hypoxia causes central necrosis in encapsulated rat islets and decreases glucose-stimulated insulin secretion (18). Dionne et al demonstrated that hypoxia (1.31% O2 or pO2: 10.0 mmHg) affects the second phase of insulin secretion from perfused rat islets over a 25 min period (19). Tomotsune et al reported that 1% O2 (pO2: 7.6 mmHg) could severely affect insulin secretion by rat islets within 24 h and was associated with cell death by necrosis (20). Neonatal and adult rat islets were found to be more resistant to hypoxia (5% O2 or pO2: 38.0 mmHg for 24 hr) than APIs in terms of insulin secretion (21).
Encapsulated APIs are indeed promising for xenotransplantation, and oxygen levels of 2-5% have been measured in mice that received encapsulated mouse insulinoma or encapsulated API grafts (15, 16). However, to our knowledge, there have been no reports on the effects of sustained hypoxia, at levels found in vivo, on a transplantation-relevant islet system. Therefore, in this study, we evaluated the effect of hypoxia at 2-5% O2 (pO2: 15.2-38.0 mmHg) on the viability, metabolic activity and insulin secretion of free and encapsulated APIs during culture over a period of 6 days. The implications of our findings regarding i.p. therapeutic transplantation of APIs are discussed.
Methods
Materials
For API isolation from the pancreata of adult pigs, the following reagents were used: Collagenase P/neutral protease solution (VitaCyte, Indianapolis, IN); Cold Storage/Purification Stock Solution (Mediatech, Manassas, VA), Hanks Balanced Salt Solution with calcium and magnesium (HBSS) (Mediatech), PentaStarch (Mediatech), porcine serum (Biologos, Inc., Montgomery, IL), heparin and insulin (McKesson Medical-Surgical Inc, Atlanta, GA). Islets were cultured in API medium composed of Medium E199 (Mediatech), 100 U/mL of penicillin and100 μg/mL streptomycin (Thermo Scientific, Logan, UT), 20 μg/mL ciprofloxacin (Sigma Aldrich, Saint Louis, USA), and 10% heat inactivated porcine serum (Biologos, Inc.,). Reagents used for encapsulation included sodium alginate (FMC Biopolymer, Oslo, Norway); and BaCl2 (Sigma Aldrich, St. Louis, MO). Assays used in this study included a viability/cytotoxicity assay (calcein AM/ethidium bromide) for animal live & dead cells (Biotium, Inc, CA), alamar blue assay (Life Technologies, Carlsbad, CA), and the porcine insulin ELISA kit (Mercodia, Uppsala, Sweden).
Animals
Pancreata from adult (>2 years of age) retired breeder sows (White Landrace) were provided by Drs. Lawrence Gazda and Robert Holdcraft of the Rogosin Institute, Xenia, OH. All pancreas collection procedures were performed at Bob Evans Farms, Inc., in Xenia OH, under USDA regulation, as the pancreata were technically a by-product of the food production process.
Islet isolation
API isolation was performed as described (22). Briefly, the entire adult porcine pancreas was dissected ex situ and perfused with collagenase and protease in Xenia, OH, then transported commercially from Ohio to Emory University in Atlanta within about 6 h of cold ischemia time. In our laboratory at Emory, APIs were prepared by digesting the pancreas during static incubation with the enzyme. Briefly, the whole pancreas and collagenase/protease enzyme solution present during transport were placed in a sterile wire basket (500 μm mesh screen) containing 7 sterile marbles in a canister in a 32°C water bath. Cold Storage/Purification Solution was added to the basket, so the pancreas was covered by 4-5 inches of solution. When the pancreas appeared to be dissociating (by visual inspection), the digestion was stopped by adding HBSS with 10% heat inactivated porcine serum (3L) followed by dilution with HBSS and 2% porcine serum (18 L). The digest was collected into 500 mL conical centrifuge tubes; each tube was centrifuged (233 G-force, 3 min at 4°C), and the pellets were pooled into one flask containing Cold Storage/Wash Solution, up to a total of less than 500 mL of digest. The digest was divided into two 250 mL conical tubes, the volume brought up to 250 mL with Cold Storage/Purification solution, and centrifuged (233 G-force, 3 min at 4°C). The supernatant was aspirated from each of the two tubes, 20 mL of heat-inactivated porcine serum was added to each tube, HBSS was added to bring the volume to up 250 mL per tube, and the islets were purified by continuous density gradient purification on a Cobe 2991 at room temperature. The purified islets were cultured in suspension flasks in API medium.
Islet quantitation
After overnight culture, all of the islets were pooled into one T-75 flask in a 50 mL volume, and islets in three aliquots of 50 μl each were quantitated by staining with dithizone and counting using an inverted phase contrast microscope with an ocular micrometer for sizing the islets. The total number of islets was determined by counting the number of islets of the following sizes in each sample (50-100 μm, 101-150 μm, 151-200 μm, 201-250 μm, 251-300 μm, 301-350 μm, 351-400 μm, and >400 μm) and converting to islet equivalents (IEQs) by multiplying the number of islets in each size category by the appropriate conversion factor (0.140, 0.62, 1.625, 3.42, 6.22, 10.24, 15.71, and 30.00 respectively). The sum of the average IEQ (n=3) in each category was multiplied by the dilution factor (1000) to determine the total IEQ in the digested tissue. In our hands, the average yield was 799 ±133 IEQ per gram (n=13 isolations), which amounts to 240,000 IEQ for a 300 gram adult porcine pancreas.
Islet encapsulation
Islets were encapsulated in highly purified, low endotoxin (49 EU/gram), low viscosity (52 mPa.s), high mannuronic acid (57%) (LVM) sodium alginate gelled with barium chloride (50 mM) using a protocol modified from the methods of Duvivier-Kali, et al.(23). For encapsulation, islets were centrifuged (149 G-force for 3 min) supernatant aspirated and islets were resuspended in 3.3% LVM sodium alginate (20,000 islets/1mL alginate). Briefly, for Ba-gelled in-homogeneous alginate capsules, APIs in 3.3% sodium alginate were aspirated through a 16-gauge air jet encapsulation needle (pump speed 2 mL/min, air flow 6 L/min) and dropped into a bath containing 50 mM BaCl2 in 0.9% saline having 10 mM 3-(N-Morpholino) propane-sulfonic acid hemisodium salt, 140 mM mannitol, and 0.025% Tween 20, on a magnetic stirrer. Gelling in mannitol rather than sodium chloride was used to form in-homogeneous alginate microcapsules (24). The gelled capsules were incubated at room temperature in 50 mM BaCl2 for 5 min on a rotating platform and successively washed in 25 mM BaCl2, 12.5 mM BaCl2, followed by six washes with sterile saline. The diameter of the resulting Ba-gelled alginate capsules was 854 ± 11 μm (range 830–880 μm, n = 50). The encapsulated APIs were cultured for 24 h at 37°C in a humidified atmosphere of 5% CO2 in air before starting the experiment.
Hypoxic chamber
A Lexan box (21.5 cm X 12.5 cm X 15.5 cm (custom designed at Georgia Institute of Technology, Atlanta, USA) was kept on an orbital shaker and housed in a CO2 incubator at 37°C. Hypoxia was established by supplying continuously a gas mixture of 5% CO2 balanced with N2 through the chamber, lowering the oxygen to hypoxic levels in the range of 2-5% O2 (or 0.02-0.05 mM). The oxygen concentration within the Lexan chamber was measured by an Orion 083005MD O2 probe (Thermo Fisher Scientific, Middletown, NJ). Prior to the study, the hypoxic chamber was equilibrated for at least 36 h. After the initiation of the study, in order to avoid temporary reoxygenation during medium changes, the medium was pre-equilibrated in the chamber for 24 h prior to use.
Experimental design
For each experiment, islets were isolated from one adult pig pancreas donor. Our experiments were repeated four times, each with islets from different islet isolation. Non-encapsulated APIs and Ba alginate-encapsulated APIs (both 60 IEQs / well of 48 well plate, 6 wells per condition) were cultured in API medium for 24 hours under normoxia (5% CO2 and 21% O2 at 37°C) before beginning the experiment, to allow cells to recover from isolation and encapsulation. After this overnight culture (on day 0) alamar blue (AB) assays were performed to measure the metabolic activity of the islets at time zero, and these AB values were used subsequently for normalization. On day 0, all spent medium was removed and fresh medium was added to the appropriate plates (normoxic medium for plates to be cultured under normoxia or medium that had been pre-incubated under hypoxia, 2-5% O2, for plates to be cultured under hypoxia). Next, the culture plates were incubated for 1, 3 and 6 days under normoxic or hypoxic conditions. For assays performed on days 3 and 6, culture media was changed on days 2 and 5. On days 1, 3, and 6, live/dead viability experiments were performed. On days 1 and 6, metabolic activity by alamar blue assays and quantification of secreted insulin in spent medium (500 μL, conditioned over 24 h) was determined by porcine insulin ELISA.
Islet viability assessment
Islet viability was evaluated with a viability/cytotoxicity assay using calcein AM (Ex 488 nm / Em 515 nm) /ethidium bromide (Ex 488 nm / Em 610 nm) to detect live (green) and dead (red) cells on days 1, 3 and 6 of culture. Fluorescent images were captured under 10 x magnification using a Zeiss LSM 510 UV Confocal Microscope (Carl Zeiss, Germany).
Islet viability quantitation
To quantify the number of live (green) and dead (red) cells from confocal micrographs, spots-based analysis was performed using Imaris (Bitplane AG, MA, USA). This software calculates cell size and point spread function (PSF) elongation values empirically using clearly distinguishable single cells. PSF elongation provides more accurate results with low magnification lenses (10X), as were used in our study. Threshold values were kept as similar as possible between confocal z-stack images. If required, manually determined surfaces were used as a mask to include only cells within a single capsule. The percent viability was calculated by dividing the number of live cells by the total number of cells (both live and dead) multiplied by 100. For simplicity, the percentage of viability is henceforth referred to as viability.
Alamar blue (AB) assay
The alamar blue (AB) assay has been used to assess the effects of hypoxia on cellular metabolic activity (25). In this study, we used the AB assay to measure the metabolic activity of APIs, whether free or encapsulated, under hypoxic and normoxic conditions. AB, a water-soluble dye, was chosen because it is non-toxic to cells and can be added as a sterile solution to viable cells in culture for continuous monitoring of the metabolic activity of cell cultures over time (26). When added to cell cultures, the oxidized form of AB (resazurin) is converted within the cells to the reduced form (resorufin) by mitochondrial enzymes. As a result of this redox reaction, the culture medium changes from indigo blue to fluorescent pink, for analysis by fluorescence spectroscopy using a plate reader (23). The metabolic activity under various conditions is reported as the AB ratio.
A sterile 10% non-fluorescent alamar blue (resazurin) solution in medium was added to APIs (5 wells per condition) in a 96-well plate, and the plates were incubated in a standard 5% CO2 incubator at 37°C for 3 h. Due to the metabolic activity of the islets, the non-fluorescent resazurin was reduced to fluorescent resorufin; fluorescence was measured on a SpectraMax M2 Multi-Mode Micro Plate Reader (Molecular Devices, CA, USA) by exciting at 540 nm, and measuring the emission at 580 nm. Fluorescence intensity was reported in fluorescent arbitrary units (FAU).
The alamar blue assay was performed on Days 0, 1 and 6. The results on Days 1 and 6 were normalized to the fluorescence of Day 0, which was measured immediately before the plates were placed under hypoxia or normoxia. These ratios henceforth will be referred to as AB ratios.
Porcine insulin ELISA assay
To assess the effect(s) of hypoxia on islets, we chose to use insulin secretion as a measure of islet function. The insulin secreted over 24 h in spent medium was measured on Days 1 and 6 using a porcine insulin ELISA kit. Insulin concentrations (ng/500 μL) were normalized to the corresponding Day 0 alamar blue values and expressed as ng/(500 μL*FAU).
Statistics
Results were expressed as mean ± SD. The student’s t test for unpaired data and ANOVA were used as appropriate to compare results in each experimental condition. Differences were considered significant if p ≤0.05.
Results
Effect of hypoxia on the viability of free and encapsulated APIs in vitro
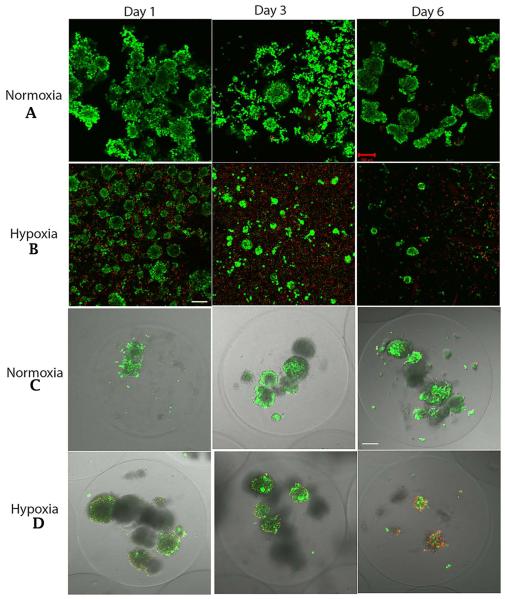
To elucidate the effects of hypoxia on islet viability, and to determine whether encapsulation protects islets from the effects of hypoxia, we exposed free vs microencapsulated APIs to hypoxic conditions for 1, 3, and 6 days, and we assessed islet viability at each time point by staining with calcein AM and ethidium bromide. In parallel, free and encapsulated APIs were cultured under normoxia and stained, as controls.
Under hypoxia, the free islets began to dissociate and to lose viability after one day of culture, and these changes became more apparent by day 3. By day 6, very few viable, intact free islets remained under hypoxic conditions, but under normoxic conditions a substantial number of free islets remained viable and intact (Figure 1A and 1B). By comparison, when islets were encapsulated they remained intact under both hypoxia and normoxia until day 6. Under normoxic culture conditions, encapsulated islets maintained their integrity and viability for the 6 day culture period (Figure 1C). However, in spite of maintaining their integrity, under hypoxic conditions encapsulated islets began to die by day 3, and the loss of viability was more pronounced by day 6 (Figure 1D).
Figure 1.
Confocal micrographs of live / dead assay. A&B: free APIs on Days 1, 3, and 6 under normoxia and hypoxia. C&D: Encapsulated APIs on Days 1, 3, and 6 under normoxia and hypoxia. Green=Live; Red=Dead; Scale bar -100 μm.
The confocal qualitative data are corroborated by the viability data described below. In the text below and in the figures, the following abbreviations will be used to identify the islet samples and culture conditions: NF1 and NF6 = Free APIs under normoxia on days 1 and 6 of culture; HF1 and HF6 = Free APIs under hypoxia on days 1 and 6 of culture; NE1 and NE6 = Encapsulated APIs under normoxia on days 1 and 6 of culture; HE1 and HE6 = Encapsulated APIs under hypoxia on days 1 and 6 of culture.
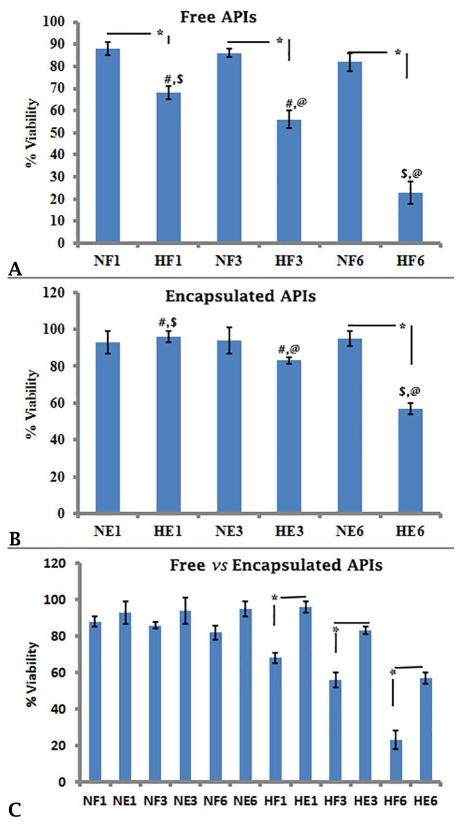
Under normoxic conditions, there was no change in the viability of free and encapsulated islets over time (Figure 2A; NF1 vs NF3 88 ± 3 vs 86 ± 2; NF1 vs NF6, 88 ± 3 vs 82 ± 4; NF3 vs NF6, 86 ± 2 vs 82 ± 4; Figure 2B NE1 vs NE3, 93 ± 6 vs 94 ± 7; NE1 vs NE6, 93 ± 6 vs 95 ± 4; NE3 vs NE6, 94 ± 7 vs 95 ± 4; p≥0.05 for all). However, the viability of free islets under hypoxia was significantly lower than the viability under normoxia at each time point (Figure 2A, NF1 vs HF1, 88 ± 3 vs 68 ± 3; NF3 vs HF3, 86 ± 2 vs 56 ± 4; NF6 vs HF6, 82 ± 4 vs 23 ± 5; p≤0.05). Furthermore, hypoxia caused the viability of free islets to decrease with time over the 6 day period of our study (Figure 2A, HF1 vs HF3, 68 ± 3 vs 56 ± 4, HF1 vs HF6, 68 ± 3 vs 23 ± 5, HF3 vs HF6, 56 ± 4 vs 23 ± 5; p≤0.05). With encapsulation, on days 1 and 3, there was no statistical difference in islet viability between normoxic and hypoxic conditions (Figure 2B, NE1 vs HE1, 93 ± 6 vs 96 ± 3 and NE3 vs HE3, 94 ± 7 vs 83 ± 2; p≥0.05), but on day 6, the viability was lower under hypoxia relative to normoxia (Figure 2B, NE6 vs HE6, 95 ± 4 vs 57 ± 3; p≤0.05). Hypoxia caused the viability of encapsulated islets to decrease with time over the 6 day period (Figure 2B, HE1 vs HE3, 96 ± 3 vs 83 ± 2, HE1 vs HE6, 96 ± 3 vs 57 ± 3; HE3 vs HE6, 83 ± 2 vs 57 ± 3; p≤0.05).
Figure2.
The viability of free and encapsulated APIs under normoxia and hypoxia. Representative results are shown in Figures 2A, 2B and 2C. The viability was calculated by counting number of live (green) and dead (red) cells from confocal micrographs and the results were expressed as average ± standard deviation (SD). Statistically significant differences (*, #, $, @) between groups (n=3 confocal micrographs from each experimental condition) were determined using the unpaired students t test (p≤0.05). NF1, NF3, NF6 = Free APIs under normoxia on Days 1, 3 and 6 of culture; HF1, HF3 and HF6 = Free APIs under hypoxia on Days 1, 3 and 6 of culture; NE1, NE3 and NE6 = Encapsulated APIs under normoxia on Days 1, 3 and 6 of culture; HE1, HE3 and HE6 = Encapsulated APIs under hypoxia on Days 1, 3 and 6 of culture.
There was no significant difference in the viability of free vs encapsulated islets under normoxic conditions at each time point (Figure 2C, NF1 vs NE1, 88±3 vs 93 ± 6; NF3 vs NE3, 86 ± 2 vs 94 ± 7; NF6 vs NE6, 82 ± 4 vs 95 ± 4; p≥0.05). However, under hypoxia, encapsulated APIs exhibited higher viability relative to free APIs at all three time points (Figure 2C, HF1 vs HE1, 68±3 vs 96 ± 3; HF3 vs HE3, 56 ± 4 vs 83 ± 2; HF6 vs HE6 23 ± 5 vs 57 ± 3; p≤0.05).
Effect of hypoxia on metabolic activity of free and encapsulated APIs in vitro
We evaluated the metabolic activity of APIs that were either free or encapsulated and cultured for either 1 day or 6 days under normoxic vs hypoxic conditions.
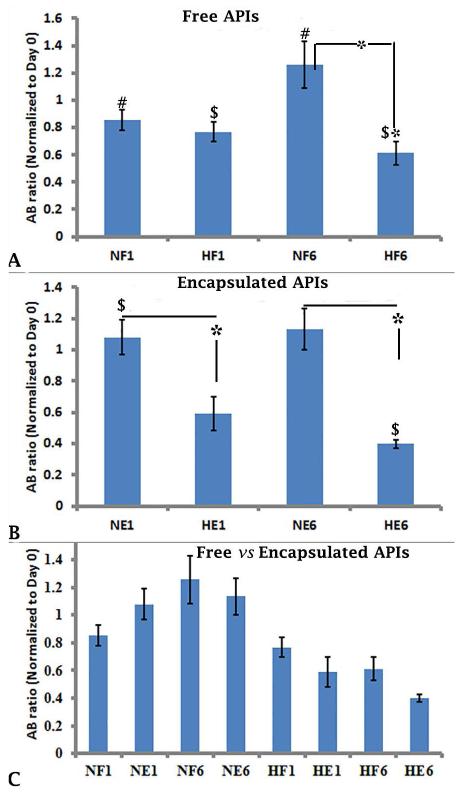
On day 1 of culture, there was no difference between the metabolic activity (AB ratio) of free APIs under nomoxic and hypoxic conditions (Figure 3A, NF1 vs HF1, 0.85 ± 0.07 vs 0.76 ± 0.07; p≥0.05). By day 6 of culture, free APIs exhibited significantly lower metabolic activity under hypoxia compared to normoxia (Figure 3A, NF6 vs HF6, 1.25 ± 0.17 vs 0.61 ± 0.08; p≤0.05), suggesting that free islets require an oxygenated environment in order to survive. Under normoxia, there was a significant increase in metabolic activity over time (Figure 3A, NF1 vs NF6, 0.85 ± 0.07 vs 1.25 ± 0.17; p≤0.05). However, under hypoxic conditions, there was a significant decrease in the metabolic activity of free islets between days 1 and 6 (Figure 3A, HF1vs HF6, 0.76 ± 0.07 vs 0.61 ± 0.08; p≤0.05). These results correlate with our findings shown in Figures 1A and 2A, in which very few viable, intact free islets remained under hypoxic conditions by day 6 of culture, but under normoxic conditions a substantial number of free islets were viable and intact.
Figure 3.
Metabolic activity as determined by the alamar blue assay of free and encapsulated APIs under normoxia and hypoxia. The experiments were performed 4 times, each with islets from separate islet isolation, and representative experiments are shown in Figures 3A, 3B, and 3C. The metabolic activity (fluorescence arbitrary unit FAU, n=5) was determined at each time point after being normalized to Day 0, and is shown as the average ± standard deviation (SD). Statistically significant differences (#, $ and *) among all groups (n=5 each) was performed using non-parametric ANOVA post hoc analysis (p≤0.05). AB ratio is the ratio of fluorescence at each time point to the fluorescence of the same well at day 0. NF1 and NF6 = Free APIs under normoxia on Days 1 and 6 of culture; HF1 and HF6 = Free APIs under hypoxia on Days 1 and 6 of culture; NE1 and NE6 = Encapsulated APIs under normoxia on Days 1 and 6 of culture; HE1 and HE6 = Encapsulated APIs under hypoxia on Days 1 and 6 of culture.
With encapsulation, there was no change in the metabolic activity of islets between days 1 and 6 of culture under normoxic or hypoxic conditions (Figure 3B, NE1 vs NE6, 1.07 ± 0.11 vs 1.13 ± 0.13; HE1 vs HE6, 0.59 ± 0.1 vs 0.39 ± 0.02; p≥0.05). However, the metabolic activity of encapsulated islets was decreased significantly under hypoxia compared to normoxia at each time point (Figure 3B NE1 vs HE1, 1.07 ± 0.11 vs 0.59 ± 0.1 and NE6 vs HE6, 1.13 ± 0.13 vs 0.39 ± 0.02; p≤0.05). These results correlate with our findings shown in Figure 1C & D and 2B, in which the viability of encapsulated islets was maintained on days 1, 3, and 6 in the presence of sufficient oxygen; However, under hypoxic conditions, the viability did not change on day 1 but there was a pronounced decrease by day 6 of culture.
Encapsulation did not change the metabolic activity of islets under either normoxia or hypoxia on days 1 and 6 (Figure 3C, NF1 vs NE1, 0.85 ± 0.07 vs 1.08 ± 0.11; NF6 vs NE6, 1.25 ± 0.17 vs 1.13 ± 0.10; HF1 vs HE1, 0.76 ± 0.07 vs 0.59 ± 0.10; HF6 vs HE6, 0.61 ± 0.08 vs 0.39 ± 0.02, p≥0.05).
Effect of hypoxia on insulin secretion by free and encapsulated APIs in vitro
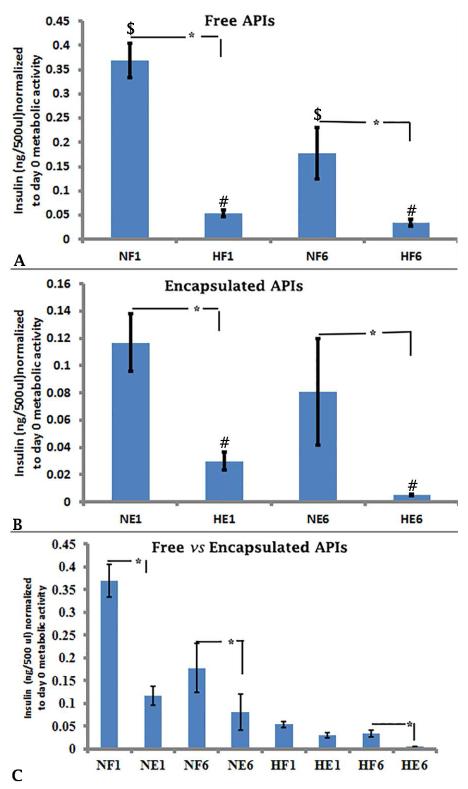
To assess the effect(s) of hypoxia on the insulin secretory function of APIs over time, spent medium was assayed on days 1 and 6 of culture using a porcine insulin ELISA. On days 1 and 6 of culture, insulin secretion by both free and encapsulated islets was significantly lower under hypoxic conditions compared to normoxia (Figure 4A, NF1 vs HF1, 0.37 ± 0.03 vs 0.05 ± 0.01; NF6 vs HF6, 0.18 ± 0.05 vs 0.03 ± 0.01, Figure 4B, NE1 vs HE1, 0.12 ± 0.02 vs 0.02 ± 0.01; NE6 vs HE6, 0.08 ± 0.03 vs 0.005 ± 0.001, p≤0.05). These results show that hypoxia suppresses insulin secretory function in vitro, regardless of encapsulation state.
Figure 4.
Insulin secretion determined by ELISA of free and encapsulated under normoxia and hypoxia. The experiments were performed 4 times, each with islets from separate islet isolation, and representative experiments are shown in Figures 4A, 4B, and 4C.Insulin concentrations (ng/500 μl, n=3 at each time point were normalized to metabolic activity of the same well on Day 0. Statistically significant differences ($, #, *) between two groups (n=3 each) using the unpaired students t test (p≤0.05). The treatments indicated on the x-axis are the same as in Figure 3.
Under normoxia, free APIs secreted more insulin than encapsulated APIs on both days 1 and 6 (Figure 4C, NF1 vs NE1, 0.37 ± 0.03 vs 0.17 ± 0.02; NF6 vs NE6, 0.18 ± 0.05 vs 0.08 ± 0.003, p≤0.05). Under hypoxia, insulin secretion by encapsulated APIs was lower than secretion by free APIs only on day 6 (Figure 4C, HF1 vs HE1 0.05 ± 0.006 vs 0.02± 0.006, p≥0.05; HF6 vs HE6 0.03± 0.007 vs 0.005 ± 0.001, p≤0.05).
Under normoxia as well as under hypoxia, there was a significant decrease in insulin secretion of free APIs over time (Figure 4A, NF1 vs NF6, 0.36 ± 0.03 vs 0.17 ± 0.05; HF1 vs HF6, 0.054 ± 0.006 vs 0.033 ± 0.007, p≤0.05). However, with encapsulation, under normoxia, there was no change in insulin secretion (Figure 4B, NE1 vs NE6, 0.12 ± 0.02 vs 0.08 ± 0.34, p≥0.05). However, under hypoxia, insulin secretion from encapsulated islets declined over time as well (Figure 4B, HE1 vs HE6, 0.03 ± 0.06 vs 0.005 ± 0.00, p≤0.05).
Discussion
In this study, we evaluated the effects of hypoxic levels typically found in the peritoneal cavity of mice (2-5% O2 or pO2: 15.4- 37.81 mmHg) on free and encapsulated APIs in vitro. Though these values are lower than what is found i.p in non-transplanted mice, they are comparable to i.p DO in mice that have received grafts of encapsulated βTC-tet cells or APIs (12, 13). Confocal images demonstrated that under hypoxia, free APIs disintegrate and undergo necrosis over a period of 6 days in culture. Under identical hypoxic culture conditions, encapsulated APIs maintain their shape and integrity, but by day 6, they also show signs of necrosis. Under hypoxic conditions, the viability of free islets was drastically decreased by day 6 compared to normoxia. When encapsulated, there was little loss of viability on days 1 and 3, under both normoxic and hypoxic conditions. However, there was a significant decrease in viability by day 6 under hypoxic conditions. Compared to free islets, encapsulated islets maintained better viability under hypoxic conditions. Similar to our findings, Moritz et al., have reported that under 1% O2 (pO2: 7.57 mmHg), human pancreatic islets begin disintegrating after 2 days in culture. During the first 6 h of hypoxia, human pancreatic islets undergo apoptosis, but by 48 h, many cells undergo necrosis (27). Giuliani et al., have reported that when human islets are cultured under 1% O2 (pO2: 7.57 mmHg) for 24 h, islets undergo central necrosis with nuclear pyknosis and DNA fragmentation (the features of apoptosis), and MIN6 cells (a murine insulinoma cell line) cultured under the same conditions show signs of apoptosis, such as two-fold increase in 3-caspase activity, but not necrosis, as determined by release of lactate dehydrogenase (28).
Metabolic activity is considered to be a marker for cell viability, and it can be determined by the alamar blue assay (29). In our study, on day 1, there was no difference between the metabolic activity of free APIs under normoxia and hypoxia, but on day 6, the metabolic activity of free APIs under hypoxia was significantly lower relative to normoxia. However, encapsulated APIs exhibited a trend toward lower metabolic activity under hypoxia relative to normoxia this difference is significant on both days 1 and 6. Others have made similar findings. De Groot et al., found that the viability of encapsulated rat islets decreased significantly over a period of 5 days in hypoxic culture conditions (1% O2 or pO2: 7.57 mmHg). The percentage of viable cells was 71±7% after two days of hypoxia relative to 86±5% under normoxia. Indeed, the De Groot study showed that after 5 days of hypoxia, the percentage of viable cells decreased to 62±9% relative to normoxia where viability was 96±0%. The loss of viability was associated with a substantial increase in necrosis and a slight increase in apoptosis (30). Emamaullee et al., (31) tested the effect of 1% O2 (pO2:10 mm Hg) on neonatal porcine islets (NPIs) and APIs. Following exposure to hypoxia for 24 h, >84% of NPIs remained viable by the MTT (3-(4,5-Dimethylthiazol-2-yl)-2,5-Diphenyltetrazolium Bromide) assay relative to 93% cell viability under normoxia. However, under hypoxia, 95% of APIs were apoptotic by the TUNEL (Terminal deoxynucleotidyl transferase dUTP nick end labeling) assay relative to 50% apoptotic under normoxia.
Interestingly, in our study there was a significant increase in metabolic activity of free islets on day 6 under normoxia, and there was a trend toward increased metabolic activity in encapsulated islets by day 6, as well. This increase could be due to proliferation of endothelial and beta cells (32) within APIs.
It has been reported that hypoxia reduces glucose-stimulated insulin secretion by both free (21) and encapsulated islets (32). Indeed, we have found similar reductions in insulin release by APIs cultured under hypoxia vs normoxia, irrespective of encapsulation. Though both encapsulated and free islets had reduced insulin secretion under hypoxic conditions, this effect was more pronounced when the islets were encapsulated. De Groot et al., reported that when encapsulated rat islets were cultured under 1% O2 (pO2: 7.57 mmHg) for 2 and 5 days, on the fifth day of hypoxia, there was 50% decrease in accumulated insulin in culture medium when compared to encapsulated islets which were cultured under normoxia. Furthermore, rat islets secreted insulin under basal conditions in both normoxic and hypoxic conditions, but they did not respond to high glucose under hypoxia (32). These results were also consistent with the results of Dionne et al (15), who found that though there was a slight decrease in basal secretion, the second-phase insulin secretion was almost eliminated during perfusion under a PO2 of 1.31% (pO2:10 mmHg).
Studies suggest that insulin secretion from transformed β cells is more resistant to hypoxia than secretion from pancreatic islets. For example, Papas et al., found that when βTC3 cell monolayers were cultured for 4 h under 20.4-1.04% O2 (pO2:137-7 mmHg), insulin secretion remained constant; but it was inhibited at 0% O2 (pO2:0 mmHg) (33). In another study, Papas et al., also reported that when encapsulated βTC3 cells were cultured for 4 h and 24 h under different oxygen levels (approximately 4.1-5.21% O2 or 28-35 mmHg) there was not a significant change in the insulin secretion rate (34).
We found that under normoxic conditions the insulin levels in spent medium were significantly lower when the islets were encapsulated compared to free. Our data is in agreement with the findings of de Haan et al (35) who showed that the presence of a capsule may interfere with the exchange of glucose and insulin during a glucose-stimulated insulin release assay.
In our study, under hypoxic conditions, the viability of APIs was significantly improved by encapsulation, compared to free islets which exhibited severe cell death by day 6. There was a trend toward decreased metabolic activity in free as well as in encapsulated APIs. Furthermore, insulin secretory function was significantly reduced by encapsulation under hypoxia. One possible explanation for our finding of reduced insulin secretion in spite of improved viability with encapsulation could be that the encapsulated islets were not lysed, while the free islets had undergone necrosis and were lysed. These lysed islets likely released their stored insulin into the medium in contrast to the encapsulated islets which retained stored insulin.
The hypoxia-induced compromise of islet viability and function suggests that strategies protecting API grafts from in vivo hypoxic effects should be implemented. This is especially true for encapsulated islets because the literature suggests that gradients within the encapsulation matrix may further reduce oxygen availability for the islets (36). Tactics that may be employed include the incorporation of CXCL12 in the capsules housing the islets (37), and using hypoxia-resistant teleost piscine islets (38) or NPIs (35) instead of APIs. Other strategies that have been implemented with various degrees of success include vascularizing the transplantation site by using islets which expresses vascular endothelial growth factors (39) or creating neovessels in a pouch (40), employing oxygen releasing biomaterials (41), or incorporating photosynthetic microorganisms and a light source along with the islets (42).
In conclusion, our study shows that hypoxia representative of the murine peritoneal environment severely decreases API viability and metabolic activity as well as insulin secretion compared to normoxia, We also conclude that under hypoxic conditions encapsulation helps maintain islet integrity and viability, but our study shows that encapsulation does not improve metabolic activity or insulin secretion relative to free islets over a period of 6 days in culture.
Acknowledgements
We gratefully acknowledge Dr. Lawrence Gazda and Dr. Robert Holdcraft of the Rogosin Institute, Xenia Division, OH for providing adult pig pancreata from Bob Evans Farms for our API isolations. We also thank Neil Anthony (The Emory Integrated Cellular Imaging core) for assistance with Imaris (3D Image analysis software). This work was funded by Juvenile Diabetes Research Foundation and National Institute of Health under award R90DK098981.
Abbreviations
- APIs
Adult Porcine Islets
- IBMIR
Immediate Blood Mediated Inflammatory Reaction
- DO
Dissolved Oxygen
- HBSS
Hanks Balanced Salt Solution
- IEQs
Islet equivalents (IEQs)
- AB
Alamar blue
- FAU
Fluorescent Arbitrary Units
Footnotes
Author Contributions
Sudhakar Muthyla performed all experiments, cell culture, insulin ELISA assays, alamar blue metabolic assays, confocal microscopy, data analyses and graphing, and writing of the manuscript; Susan Safley assisted with writing the manuscript and reviewed the manuscript; Kereen Gordon performed API isolation and islet encapsulation; Graham Barer cultured islets and assisted with API isolation and islet encapsulation. Collin Weber was PI of the laboratory at Emory University in which much of the work was performed; Athanassios Sambanis was PI of the laboratory at Georgia Institute of Technology, in which much of the work was performed, and he assisted with interpretation of the data and reviewing the manuscript.
References
- 1.SHAPIRO AM, LAKEY JR, RYAN EA, et al. Islet transplantation in seven patients with type1 diabetes mellitus using a glucocorticoid-free immunosuppressive regimen. The New England journal of medicine. 2000;343:230–238. doi: 10.1056/NEJM200007273430401. [DOI] [PubMed] [Google Scholar]
- 2.MARKMANN JF, DENG S, HUANG X, et al. Insulin independence following isolated islet transplantation and single islet infusions. Annals of surgery. 2003;237:741–749. doi: 10.1097/01.SLA.0000072110.93780.52. discussion 9-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.KAUFMAN DB, LOWE WL., JR Clinical islet transplantation. Current diabetes reports. 2003;3:344–350. doi: 10.1007/s11892-003-0028-7. [DOI] [PubMed] [Google Scholar]
- 4.SMITH RM, MANDEL TE. Pancreatic islet xenotransplantation: the potential for tolerance induction. Immunology today. 2000;21:42–48. doi: 10.1016/s0167-5699(99)01554-6. [DOI] [PubMed] [Google Scholar]
- 5.KIRCHHOF N, SHIBATA S, WIJKSTROM M, et al. Reversal of diabetes in non-immunosuppressed rhesus macaques by intraportal porcine islet xenografts precedes acute cellular rejection. Xenotransplantation. 2004;11:396–407. doi: 10.1111/j.1399-3089.2004.00157.x. [DOI] [PubMed] [Google Scholar]
- 6.GROTH CG, KORSGREN O, TIBELL A, et al. Transplantation of porcine fetal pancreas to diabetic patients. Lancet. 1994;344:1402–1404. doi: 10.1016/s0140-6736(94)90570-3. [DOI] [PubMed] [Google Scholar]
- 7.HERING BJ, WIJKSTROM M, GRAHAM ML, et al. Prolonged diabetes reversal after intraportal xenotransplantation of wild-type porcine islets in immunosuppressed nonhuman primates. Nature medicine. 2006;12:301–303. doi: 10.1038/nm1369. [DOI] [PubMed] [Google Scholar]
- 8.NARANG AS, MAHATO RI. Biological and biomaterial approaches for improved islet transplantation. Pharmacological reviews. 2006;58:194–243. doi: 10.1124/pr.58.2.6. [DOI] [PubMed] [Google Scholar]
- 9.VEGAS AJ, VEISEH O, DOLOFF JC, MA M, Tam HH, Bratlie K, et al. Corrigendum: Combinatorial hydrogel library enables identification of materials that mitigate the foreign body response in primates. Nature biotechnology. 2016;34:345–352. doi: 10.1038/nbt.3462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.VEGAS AJ, VEISEH O, GURTLER M, et al. Long-term glycemic control using polymer-encapsulated human stem cell-derived beta cells in immune-competent mice. 2016;22:306–311. doi: 10.1038/nm.4030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.SOON-SHIONG P, FELDMAN E, NELSON R, et al. Long-term reversal of diabetes by the injection of immunoprotected islets. Proceedings of the National Academy of Sciences of the United States of America. 1993;90:5843–5847. doi: 10.1073/pnas.90.12.5843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.SOON-SHIONG P, HEINTZ RE, MERIDETH N, et al. Insulin independence in a type 1 diabetic patient after encapsulated islet transplantation. Lancet. 1994;343:950–951. doi: 10.1016/s0140-6736(94)90067-1. [DOI] [PubMed] [Google Scholar]
- 13.ELLIOTT RB, ESCOBAR L, CALAFIORE R, et al. Transplantation of micro- and macroencapsulated piglet islets into mice and monkeys. Transplantation proceedings. 2005;37:466–469. doi: 10.1016/j.transproceed.2004.12.198. [DOI] [PubMed] [Google Scholar]
- 14.MENGER MD, VAJKOCZY P, BEGER C, Messmer K. Orientation of microvascular blood flow in pancreatic islet isografts. The Journal of clinical investigation. 1994;93:2280–2285. doi: 10.1172/JCI117228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.GOH F, SAMBANIS A. In vivo noninvasive monitoring of dissolved oxygen concentration within an implanted tissue-engineered pancreatic construct. Tissue engineering Part C, Methods. 2011;17:887–894. doi: 10.1089/ten.tec.2011.0098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.GOH F. The effect on cell viability and function and their use in noninvasively monitoring the cellular microenvironment.Thesis. Georgia Institute of Technology; May, 2011. The use of perflurocarbons in encapsulated cell systems. [Google Scholar]
- 17.SUSZYNSKI TM, AVGOUSTINIATOS ES, PAPAS KK. Intraportal islet oxygenation. Journal of diabetes science and technology. 2014;8:575–580. doi: 10.1177/1932296814525827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.SCHREZENMEIR J, GERO L, LAUE C, et al. The role of oxygen supply in islet transplantation. Transplantation proceedings. 1992;24:2925–2929. [PubMed] [Google Scholar]
- 19.DIONNE KE, COLTON CK, YARMUSH ML. Effect of hypoxia on insulin secretion by isolated rat and canine islets of Langerhans. Diabetes. 1993;42:12–21. doi: 10.2337/diab.42.1.12. [DOI] [PubMed] [Google Scholar]
- 20.TOMOTSUNE KO, HASEGAWA N, KUDO T, et al. Hypoxia induced dysfunction and cell death of the rat pancreatic islets. Hirosaki Med J. 2009;60:54–62. [Google Scholar]
- 21.HYDER A, LAUE C, SCHREZENMEIR J. Variable responses of islet cells of different ages and species to hypoxia. Transplantation proceedings. 1998;30:578–580. doi: 10.1016/s0041-1345(97)01411-5. [DOI] [PubMed] [Google Scholar]
- 22.HOLDCRAFT RW, GAZDA LS, CIRCLE L, et al. Enhancement of in vitro and in vivo function of agarose-encapsulated porcine islets by changes in the islet microenvironment. Cell transplantation. 2014;23:929–944. doi: 10.3727/096368913X667033. [DOI] [PubMed] [Google Scholar]
- 23.DUVIVIER-KALI VF, OMER A, PARENT RJ, O'Neil JJ, Weir GC. Complete protection of islets against allorejection and autoimmunity by a simple barium-alginate membrane. Diabetes. 2001;50:1698–1705. doi: 10.2337/diabetes.50.8.1698. [DOI] [PubMed] [Google Scholar]
- 24.MORCH YA, DONATI I, STRAND BL, SKJAK-BRAEK G. Effect of Ca2+, Ba2+, and Sr2+ on alginate microbeads. Biomacromolecules. 2006;7:1471–1480. doi: 10.1021/bm060010d. [DOI] [PubMed] [Google Scholar]
- 25.LI W, HU ZF, CHEN B, Ni GX. Response of C2C12 myoblasts to hypoxia: the relative roles of glucose and oxygen in adaptive cellular metabolism. BioMed research international. 2013;2013:326346–326356. doi: 10.1155/2013/326346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.RAMPERSAD SN. Multiple applications of Alamar Blue as an indicator of metabolic function and cellular health in cell viability bioassays. Sensors (Basel, Switzerland) 2012;12(9):12347–60. doi: 10.3390/s120912347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.MORITZ W, MEIER F, STROKA DM, et al. Apoptosis in hypoxic human pancreatic islets correlates with HIF-1alpha expression. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2002;16:745–747. doi: 10.1096/fj.01-0403fje. [DOI] [PubMed] [Google Scholar]
- 28.GIULIANI M, MORITZ W, BODMER E, et al. Central necrosis in isolated hypoxic human pancreatic islets: evidence for postisolation ischemia. Cell transplantation. 2005;14:67–76. doi: 10.3727/000000005783983287. [DOI] [PubMed] [Google Scholar]
- 29.AL-NASIRY S, GEUSENS N, HANSSENS M, LUYTEN C, PIJNENBORG R. The use of Alamar Blue assay for quantitative analysis of viability, migration and invasion of choriocarcinoma cells. Human reproduction. 2007;22:1304–1309. doi: 10.1093/humrep/dem011. [DOI] [PubMed] [Google Scholar]
- 30.DE GROOT M, SCHUURS TA, KEIZER PP, et al. Response of encapsulated rat pancreatic islets to hypoxia. Cell transplantation. 2003;12:867–875. [PubMed] [Google Scholar]
- 31.EMAMAULLEE JA, SHAPIRO AM, RAJOTTE RV, KORBUTT G, ELLIOTT JF. Neonatal porcine islets exhibit natural resistance to hypoxia-induced apoptosis. Transplantation. 2006;82:945–952. doi: 10.1097/01.tp.0000238677.00750.32. [DOI] [PubMed] [Google Scholar]
- 32.JOHANSSON M, MATTSSON G, ANDERSSON A, JANSSON L, CARLSSON PO. Islet endothelial cells and pancreatic beta-cell proliferation: studies in vitro and during pregnancy in adult rats. Endocrinology. 2006;147:2315–2324. doi: 10.1210/en.2005-0997. [DOI] [PubMed] [Google Scholar]
- 33.PAPAS KK, LONG RC, JR., CONSTANTINIDIS I, SAMBANIS A. Effects of oxygen on metabolic and secretory activities of beta TC3 cells. Biochimica et biophysica acta. 1996;1291:163–166. doi: 10.1016/0304-4165(96)00062-1. [DOI] [PubMed] [Google Scholar]
- 34.PAPAS KK, LONG RC, JR., CONSTANTINIDIS I, SAMBANIS A. Effects of short-term hypoxia on a transformed cell-based bioartificial pancreatic construct. Cell transplantation. 2000;9:415–422. doi: 10.1177/096368970000900312. [DOI] [PubMed] [Google Scholar]
- 35.DE HAAN BJ, FAAS MM, DE VOS P. Factors influencing insulin secretion from encapsulated islets. Cell transplantation. 2003;12:617–625. doi: 10.3727/000000003108747226. [DOI] [PubMed] [Google Scholar]
- 36.SCHREZENMEIR J, KIRCHGESSNER J, GERO L, et al. Effect of microencapsulation on oxygen distribution in islets organs. Transplantation. 1994;57:1308–1314. doi: 10.1097/00007890-199405150-00003. [DOI] [PubMed] [Google Scholar]
- 37.DUNCANSON S, SAMBANIS A. Dual factor delivery of CXCL12 and Exendin-4 for improved survival and function of encapsulated beta cells under hypoxic conditions. Biotechnology and bioengineering. 2013;110:2292–2300. doi: 10.1002/bit.24872. [DOI] [PubMed] [Google Scholar]
- 38.SAFLEY SA, CUI H, CAUFFIEL SM, et al. Encapsulated piscine (tilapia) islets for diabetes therapy: studies in diabetic NOD and NOD-SCID mice. Xenotransplantation. 2014;21:127–139. doi: 10.1111/xen.12086. [DOI] [PubMed] [Google Scholar]
- 39.ZHANG N, RICHTER A, SURIAWINATA J, et al. Elevated vascular endothelial growth factor production in islets improves islet graft vascularization. Diabetes. 2004;53:963–970. doi: 10.2337/diabetes.53.4.963. [DOI] [PubMed] [Google Scholar]
- 40.PEPPER AR, GALA-LOPEZ B, PAWLICK R, MERANI S, KIN T. A prevascularized subcutaneous device-less site for islet and cellular transplantation. 2015;33:518–523. doi: 10.1038/nbt.3211. [DOI] [PubMed] [Google Scholar]
- 41.PEDRAZA E, CORONEL MM, FRAKER CA, RICORDI C, STABLER CL. Preventing hypoxia-induced cell death in beta cells and islets via hydrolytically activated, oxygen-generating biomaterials. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:4245–4250. doi: 10.1073/pnas.1113560109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.EVRON Y, ZIMERMANN B, LUDWIG B, et al. Oxygen supply by photosynthesis to an implantable islet cell device. Hormone and metabolic research. 2015;47:24–30. doi: 10.1055/s-0034-1394375. [DOI] [PubMed] [Google Scholar]