Abstract
OBJECTIVES
To examine the association of dietary sodium intake with cognitive function in community-dwelling older adults.
DESIGN
Cross-sectional study
SETTING
Southern California community
PARTICIPANTS
White men (n=373) and women (n=552), aged 50–96 years from the Rancho Bernardo Study, a longitudinal study of cardiovascular disease risk factors and healthy aging.
MEASUREMENTS
During the 1992–1996 research clinic visit, a food frequency questionnaire was used to determine daily sodium intake; cognitive function was assessed with Trails Making Test, part B (Trails B), Mini-Mental State Exam (MMSE), and Verbal Fluency Test (VFT); and medical, clinical and demographic information was obtained. Linear regression was used to assess the association between calorie-adjusted sodium intake and cognitive test scores with adjustment for demographic, behavioral and health measures. Logistic regression examined the odds of having cognitive impairment by sodium intake.
RESULTS
Lower sodium intake was associated with poorer performance on Trails B (p=0.008) and MMSE (p=0.003) after controlling for age, sex, and education. Associations did not differ by sex, but there was a significant interaction by age for the Trails B: older (≥80 years), but not younger, adults showed worse performance with lower sodium intake (p=0.03). Associations remained significant after additional adjustment for smoking, alcohol intake, exercise, body weight, cardiovascular risk factors, kidney function, diuretic medication use, and diet quality. Lower daily sodium intake was associated with increased odds of cognitive impairment on the MMSE (score < 26; OR per SD decrease = 1.12, 95% CI 1.08, 1.16).
CONCLUSION
Lower sodium intake was associated with worse cognitive function in older community-dwelling adults. For the maintenance of cognitive health, older adults may be advised to avoid very low sodium diets.
Keywords: sodium, diet, cognitive function, aging
Introduction
By 2060, 24% of the total U.S. population is projected to be aged 65 years and older, up from 15% in 2014 (1). As the population ages, identifying modifiable lifestyle factors that promote healthy cognitive aging is an increasingly pressing public health concern (2). Dietary sodium intake is one lifestyle factor that may affect cognitive function (3, 4), but its role in cognitive aging has not been thoroughly studied (5, 6).
Sodium is an essential nutrient. The average intake in the U.S. is estimated to be 3,400 mg/day, higher than the recommended amount (2,300 mg/day) (7). High dietary sodium intake has been linked to hypertension and cardiovascular disease (CVD) (8), which has led to dietary guidelines recommending reduction of daily sodium intake (9). Hypertension and CVD have also been associated with reduced cognitive function (10, 11). Dietary sodium intake may also impair cognitive function independently of its effect on blood pressure. For example, studies in rodents (3, 4) suggest that high sodium diets impair spatial memory through an increase in oxidative stress in the hippocampus.
Few studies have examined the association of dietary sodium with cognitive function. The Dietary Approaches to Stop Hypertension (DASH) diet, which emphasizes low sodium intake, has been associated with less cognitive decline in older adults (12, 13). However, sodium intake is only one of ten components that factor into the DASH dietary score. Other putative healthful components of this diet, such as increased omega-3 consumption, reduced saturated fats and trans-saturated fats, increased dietary fiber, fruits and vegetables, have biological plausibility to explain the positive association with cognitive performance and DASH diet adherence (14–17).
Similar interpretation difficulties apply to findings from a recent study of the association of nutrient patterns with cognitive function and biomarkers of Alzheimer’s disease (AD) (18). That study reported that although there were no significant associations between dietary patterns and cognitive performance, a nutrient pattern of higher intake of fats, cholesterol and sodium was associated with neuroimaging biomarkers of AD. It is not clear how much of that association was driven by sodium versus fat intake (18).
Three studies have specifically examined the relation between sodium intake and cognitive function. In a small cross-sectional study (n=119) of middle-aged to early older-aged adults (average age 54.2 years, maximum 70 years) newly diagnosed with hypertension, higher sodium intake, measured by 24-hour urinary sodium excretion, was associated with poorer cognitive function (19). A large, prospective study of 6,426 women aged 65–79 from the Women’s Health Initiative Memory Study (WHIMS) did not find a significant association between sodium intake, as determined with the Block FFQ, and development of mild cognitive impairment (MCI) or AD over a 9-year follow-up period (6). However, when stratified by hypertension status, hypertensive women with higher sodium intake had a higher risk of developing MCI or AD over the follow-up period, although the formal interaction was not significant(6).
A 3-year follow up study of 1,262 men and women aged 67–84 years from the Québec Longitudinal Study on Nutrition and Successful Aging (NuAge Study), found that high dietary sodium intake, assessed with a 78-item FFQ, was associated with greater 3-year decline on the Modified Mini Mental State Exam, but only among those with low physical activity (5). Although this study adjusted for many potential confounders including age, sex, education, smoking, and several health indicators, it did not adjust for several critical variables related to sodium levels including kidney function, blood pressure medication use, and alcohol consumption. Additionally, it only assessed performance on a single cognitive function test.
These mixed findings point to the need for more study of the association of dietary sodium and cognitive function. We sought to extend prior findings by examining the relation of dietary sodium intake to multiple domains of cognitive function in a large, community-dwelling cohort of older men and women with a wide age range (50 to 96 years). Because this cohort is very well-characterized, we were able to control for multiple lifestyle and health variables that may mask or exacerbate associations of sodium intake with cognitive function. Based on the literature reviewed above, we hypothesized that higher sodium intake would be associated with worse cognitive function.
Materials and Methods
Participants
Participants were community-dwelling, middle- to upper-middle class men and women aged 50–96 years from the Rancho Bernardo Study. In 1972–1974, 6,629 adults, representing 82% of the population of adults aged 30 years or older residing in a southern California community, were enrolled in a study to investigate heart disease risk factors as part of the Lipid Research Clinics Prevalence Program. These individuals have been followed ever since with periodic research clinic visits. In 1992–1996, 1,782 participants completed a follow-up research clinic visit when cognitive function was assessed and nutritional information was obtained. After excluding 49 individuals younger than 50 years of age, 320 missing dietary data, 285 missing cognitive function scores, 188 missing education information, and 15 individuals with implausible daily caloric intake data (see below), there remained 552 women and 373 men who form the basis of this report.
This study was approved by the Human Research Protections Program of the University of California San Diego; all participants provided written informed consent prior to participation.
Dietary Assessment
During the 1992–1996 research clinic visit, the 153-item Willet FFQ (20) was administered. This FFQ asks participants to indicate, for a specified standard portion size for each food item, frequency of consumption in the past month on a 9-point scale. Response choices ranged from ‘never or less than once per month’ to ‘six or more times per day’. Daily nutrient intakes were estimated using the Willett nutrient and database program with an algorithm that multiplies the frequency responses by the nutrient compositions of the corresponding portion sizes of each food. (HarvardSSFQ.5/93; Harvard TC Chan School of Public Health, Boston, Massachusetts). FFQ scores were deemed implausible if 70 or more food items were left blank (21), or daily caloric intake was estimated to be lower than 700 or greater than 4200 kcal. The current study examined daily intake of sodium, potassium, calcium and alcohol as well as total caloric intake. A measure of global dietary quality was obtained by determining degree of adherence to a Mediterranean-style diet as previously described (21).
Cognitive Assessment
Trained interviewers administered a battery of cognitive function tests including the Verbal Fluency Test (VFT) (22), Mini-Mental State Examination (MMSE) (23) and Trail-making Test, part B (Trails B) (24). During the VFT, participants are asked to name as many animals as possible within one minute; scores represent the number of animals correctly named. The MMSE assesses global cognition including orientation, registration, attention, calculation, language and recall. MMSE scores range from 0–30 with higher scores indicating better cognitive function. The Trails B test assesses executive function, visuomotor tracking and attention. Participants are asked to draw lines to connect numbers and letters in alternating order as quickly as possible. Scores reflect the time needed to complete the task with 300 seconds as the maximum. Higher scores thus indicate poorer performance. Pre-specified scores of >132 for Trails B, <26 for MMSE, and <12 for VFT were used to indicate categorically-defined poor performance.
Covariate Assessment
A standardized self-administered survey was used to assess age, education (high school diploma or less/some college or more), smoking habits (never/past/current), and exercise (≥ 3 times/week; no/yes). Mood and depressive symptoms were assessed using the self-administered, 21-item Beck Depression Inventory (BDI) (25). On this scale, participants are asked to choose statements that best describe their feelings. Higher scores indicate greater depressed mood; scores ≥ 12 suggest clinical depression (25).
Clinical measurements were obtained by specially trained nurses. Height, weight, and waist-hip ratio (WHR) were measured with the participants wearing light clothing and no shoes. Body mass index (BMI) was calculated as weight in kilograms divided by height in meters squared. BMI was used as an estimate of obesity and WHR was used as an estimate of central adiposity. A nursed trained in the Hypertension Detection and Follow-up Program protocol measured systolic and diastolic blood pressures twice in participants who had been seated quietly for 5 minutes (26). Medical history including self-reported physician diagnosis of morbidity, including angina, heart attack, hypertension, pulmonary disease, cancer, arthritis, osteoporosis, and diabetes, was queried by a trained interviewer. Medication use was verified by examination of prescriptions or pill containers brought to the clinic visit for that purpose. Individuals were categorized based on their number of comorbidities (3 or more vs fewer or none) and medications (3 or more vs fewer or none).
Blood samples were obtained by venipuncture after a requested overnight 12–16 hour fast. Fasting plasma glucose was measured by the glucose oxidase method and hemoglobin A1C (HbA1C) by high performance liquid chromatography. Fasting plasma cholesterol, triglyceride, and high density lipoprotein and low density lipoprotein cholesterol levels were measured in a Centers for Disease Control and Prevention certified Lipid Research Clinic Laboratory. Total cholesterol and triglyceride levels were measured by enzymatic techniques using an ABA-200 biochromatic analyzer (Abbott Laboratories, Abbott Park, Illinois). High density lipoprotein cholesterol was measured according to the standardized procedures of the Lipid Research Clinic’s manual; low density lipoprotein cholesterol was calculated using the Friedewald formula (27). Serum creatinine was measured by SmithKline Beecham clinical Laboratories (King of Prussia, Pennsylvania).
Diabetes was defined according to the 1999 World Health Organization criteria: measured fasting plasma glucose ≥ 126 mg/dL, a physician diagnosis of diabetes, or use of diabetes-specific medication (oral or insulin). Kidney function was assessed by estimating the glomerular filtration rate (eGFR) which was calculated by using the abbreviated Modification of Diet in Renal Disease study equation: eGFR (ml/minute/1.73 m2) = 186 × (serum creatinine (mg/dL))−1.154 × (age)−0.203 × (0.742 if female) × (1.210 if black). Hypertension was defined as systolic blood pressure ≥ 140, diastolic blood pressure ≥ 90 mmHg or use of antihypertensive medication.
Statistical Analysis
For all analyses, sodium was adjusted for total caloric intake using the nutrient residual method as described by Willett & Stampfer (28). We regressed sodium intake on total caloric intake and then added a constant (1900 mg) to the residuals for ease of interpretation. To examine the relation of calorie-adjusted sodium intake to demographic and clinical variables, sodium intake quartiles were created based on frequency distributions. Chi-square tests were used to compare sodium intake quartiles on categorical variables and ANOVAs were used to compare quartiles on continuous variables. Multiple linear regression models were used to examine the association of calorie-adjusted daily sodium intake with each cognitive function test. Calorie-adjusted daily sodium intake was treated as a continuous variable and base models adjusted for age, sex, and education. Base models were also adjusted for total caloric intake as recommended by Willett and Stampfer (28). Additional models adjusted for other potential confounders including cigarette smoking, alcohol, exercise 3 or more times/week, diuretic medication, eGFR, diabetes, BMI, WHR, hypertension, HDL, LDL, and triglycerides, potassium intake, calcium intake and global dietary quality. Potential multicollinearity between covariates was assessed and models included only those variables that were not statistically correlated at variance inflation factor > 4 or a tolerance level < 0.1. Effect modification for the association between dietary sodium intake and cognitive function was assessed for age, sex, hypertensive status and diabetes status. Multiple logistic regression was used to analyze the odds of having categorical cognitive impairment Data were analyzed using SAS, version 9.3 for Windows (SAS Institute Inc., Cary, NC).
Results
The average age of participants was 74.5 years ± 8.7. Table 1 shows unadjusted participant characteristics by quartile of calorie-adjusted daily sodium intake. Those in the highest quartiles of sodium intake had the highest rates of diabetes (p=0.024) and lowest alcohol intake (p<0.001). Participants in both the highest and lowest quartile of sodium intake had higher rates of hypertension (p=0.01) and were more likely to use diuretic medications compared to those in the middle quartiles (p=0.043).
Table 1.
Participant Characteristics by Quartiles of Calorie-Adjusted Daily Sodium Intake; Rancho Bernardo Study, 1992–1996 (n=925)
| Quartile 1 (n=231) |
Quartile 2 (n=231) |
Quartile 3 (n=231) |
Quartile 4 (n=232) |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Characteristic | n (%) | n (%) | n (%) | n (%) | p-value | ||||
| Sex | 0.532 | ||||||||
| Female | 133 | (57.6) | 134 | (58.0) | 147 | (63.6) | 138 | (59.5) | |
| Education | 0.793 | ||||||||
| High School or less |
59 | (25.5) | 63 | (27.3) | 64 | (27.7) | 69 | (29.7) | |
| Some college or more |
172 | (74.5) | 168 | (72.7) | 167 | (72.3) | 163 | (70.3) | |
| Smoking Status | 0.977 | ||||||||
| Never | 98 | (42.8) | 95 | (41.1) | 106 | (45.9) | 100 | (43.1) | |
| Past | 114 | (49.8) | 117 | (50.7) | 108 | (47.8) | 115 | (49.6) | |
| Current | 17 | (7.4) | 19 | (8.2) | 17 | (7.4) | 17 | (7.4) | |
|
Exercisea, 3+ times/wk |
167 | (72.3) | 175 | (75.8) | 163 | (70.6) | 167 | (72.0) | 0.638 |
|
> 2 Chronic Diseasesb |
19 | (8.2) | 21 | (9.1) | 22 | (9.5) | 28 | (12.1) | 0.540 |
| Diabetes*a | 27 | (11.7) | 30 | (13.0) | 35 | (15.2) | 49 | (21.1) | 0.024 |
| Hypertension*a | 90 | (40.0) | 61 | (26.4) | 79 | (34.2) | 92 | (39.7) | 0.010 |
| > 2 Medicationsc | 88 | (38.1) | 77 | (33.3) | 86 | (37.2) | 101 | (43.5) | 0.157 |
|
Any BP Medicationa |
79 | (34.2) | 64 | (27.7) | 74 | (32.0) | 90 | (38.8) | 0.083 |
| Diuretic*a | 45 | (19.5) | 28 | (12.1) | 46 | (19.9) | 50 | (21.6) | 0.043 |
| Betablockera | 21 | (9.1) | 30 | (13.0) | 27 | (11.7) | 28 | (12.1) | 0.592 |
|
Calcium Channel Blockera |
33 | (14.3) | 36 | (15.6) | 26 | (11.3) | 39 | (16.8) | 0.363 |
| Characteristic | Mean (sd) | Mean (sd) | Mean (sd) | Mean (sd) | p-value | ||||
| Age (years) | 74.6 | (9.7) | 72.9 | (8.5) | 73.6 | (9.1) | 75.2 | (9.4) | 0.314 |
|
Daily Sodium Intake (mg/d)** |
1647.5 | (540.4) | 1814.4 | (602.3) | 1950.5 | (522.3) | 2628.5 | (824.9) | <0.001 |
| Calories (kcal) | 1913.7 | (562.3) | 1747.1 | (544.2) | 1680.1 | (470.7) | 1913.4 | (558.2) | 0.679 |
| Alcohol (gm/d)** | 16.9 | (18.8) | 11.4 | (13.9) | 8.1 | (11.9) | 7.7 | (10.7) | <0.001 |
|
Mediterranean Diet Score** |
3.9 | (1.7) | 4.0 | (1.8) | 4.0 | (1.8) | 4.4 | (1.8) | 0.003 |
|
Daily Potassium Intake (mg/d)** |
3185.0 | (1057.2) | 2949.7 | (865.8) | 2973.3 | (947.2) | 3493.6 | (1170.2) | 0.002 |
|
Daily Calcium Intake (mg/d) |
9.2 | (0.4) | 9.2 | (0.5) | 9.2 | (0.5) | 9.2 | (0.4) | 0.399 |
| eGFR | 66.4 | (17.2) | 69.7 | (19.2) | 66.2 | (18.4) | 64.4 | (14.8) | 0.065 |
| BMI (kg/m2) | 25.2 | (3.6) | 25.4 | (4.3) | 25.7 | (3.8) | 25.4 | (4.4) | 0.434 |
| WHR | 0.897 | (0.08) | 0.903 | (0.08) | 0.899 | (0.085) | 0.895 | (0.077) | 0.684 |
| Systolic BP | 139.8 | (21.6) | 136.8 | (21.2) | 139.1 | (20.4) | 140.9 | (22.5) | 0.379 |
| Diastolic BP | 75.8 | (9.7) | 75.5 | (8.3) | 76.1 | (9.5) | 75.5 | (9.9) | 0.923 |
| HDL (mg/dl) | 56.6 | (17.0) | 57.5 | (16.3) | 57.3 | (16.8) | 57.8 | (17.6) | 0.487 |
| LDL (mg/dl) | 123.8 | (31.0) | 128.2 | (35.2) | 128.4 | (31.7) | 122.6 | (32.6) | 0.727 |
|
Triglycerides (mg/dl) |
117.2 | (67.1) | 114.8 | (78.1) | 117.9 | (60.1) | 127.2 | (84.3) | 0.127 |
| BDI | 5.2 | (4.4) | 5.1 | (4.2) | 5.5 | (4.3) | 5.3 | (4.0) | 0.337 |
| Trails B | 137.3 | (66.1) | 126.1 | (55.6) | 131.2 | (60.5) | 130.6 | (53.3) | 0.395 |
| MMSE** | 27.5 | (2.8) | 27.9 | (2.1) | 28.1 | (2.2) | 28.0 | (2.2) | 0.025 |
| VFT | 17.3 | (5.1) | 17.5 | (4.4) | 17.9 | (4.9) | 16.9 | (5.5) | 0.684 |
Abbreviations: BMI, body mass index; WHR, waist to hip ratio; BP, blood pressure; eGFR, estimated glomerular filtration rate; HDL, high density lipoprotein; LDL, low density lipoprotein; BDI, Beck Depression Inventory; MMSE, Mini-mental status exam; VFT, verbal fluency test
Variable significant using chi-square testing
Variable significant using ANOVA testing
Percent
Chronic diseases included: angina, heart attack, hypertension, pulmonary disease, cancer, arthritis, osteoporosis, and diabetes
Medications included: blood pressure medication, diuretic, beta blocker, calcium channel blocker, steroid, blood thinner, thyroid medication, acetaminophen, non-steroidal anti-inflammatory drug, aspirin, calcium supplement, antihistamine, laxative, antianxiety
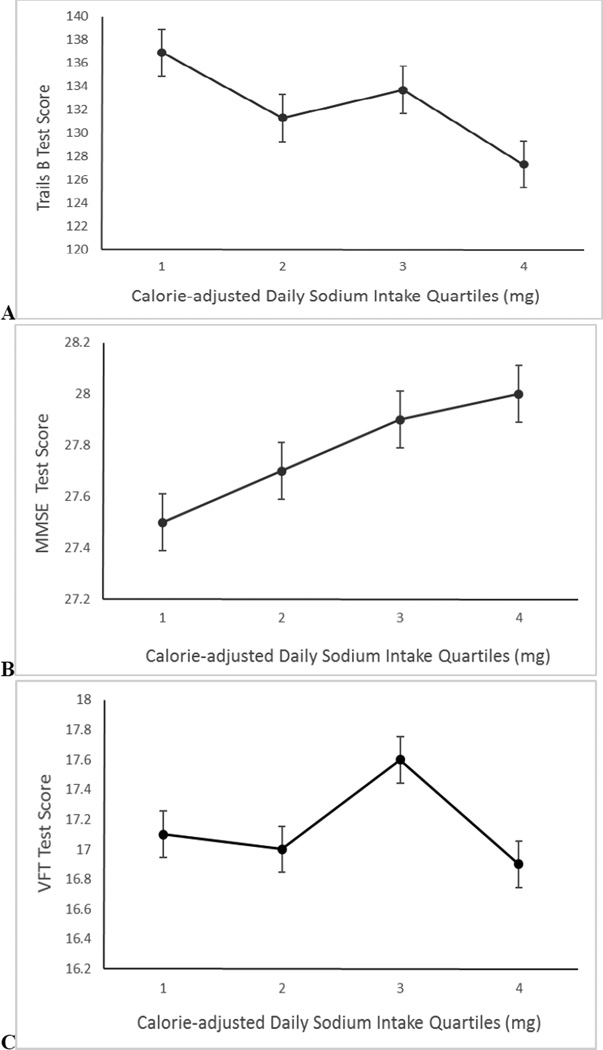
Figure 1 shows mean scores on each cognitive function test by quartile of sodium intake after adjustment for age, sex, education and total caloric intake. As sodium intake quartile increased, scores on Trails B (p=0.073, linear trend) (Fig 1A) and MMSE (p=0.007, linear trend) (Fig 1B) improved. No systematic differences were observed for VFT by quartile of sodium intake (p=0.897, linear trend) (Fig 1C).
Figure 1.
Mean (SEM) performance on (A) Trail-making Test, part B (Trails B) (p=0.073), (B) Mini-Mental State Examination (MMSE) (p=0.007), and (C) Verbal Fluency Test (VFT) (p=0.897) by quartile of calorie-adjusted daily sodium intake. Mean test scores are adjusted for age, sex, education level and total caloric intake. Note: p-values represent linear test for trend; y-axes show test score; note range does not begin at zero.
Table 2 shows the association of sodium intake with performance on each cognitive function test in the base model (adjusting for sex, age, education level, and total caloric intake), and after adjusting for additional covariates. In the base model, there was a significant negative association between daily sodium intake and time to complete Trails B: those with low sodium intake levels took longer to complete the Trails B test (standardized β = −0.07, p = 0.008). This is equivalent to approximately a 2-year increase in age for every standard deviation (SD) increase in sodium intake. Similarly, the base model for MMSE showed that those with low sodium intake had lower scores than those with higher sodium intake (standardized β = 0.091, p = 0.003). This is equivalent to approximately a 3-year increase in age for every SD increase in sodium intake. The association of low sodium intake with poorer performance on Trails B and MMSE remained significant after sequential adjustment for additional covariates including smoking, alcohol, exercise, potassium intake, calcium intake and global dietary quality, diuretic medication use, kidney function, diabetes, BMI, WHR, hypertension, HDL, LDL, triglycerides. There was no significant association between daily sodium intake and VFT score before or after adjustment for covariates.
Table 2.
Association of calorie-adjusted sodium intake with cognitive function; Rancho Bernardo Study, 1992–1996 (n=925)
| Cognitive Test | ||||||
|---|---|---|---|---|---|---|
| Trails B | MMSE | VFT | ||||
| Model | β | p-value | β | p-value | β | p-value |
| Base Modela | −0.07 | 0.008 | 0.091 | 0.003 | −0.0001 | 0.999 |
| Base Model + smoking, alcohol, exercise, BDI | −0.080 | 0.006 | 0.078 | 0.02 | −0.009 | 0.783 |
| Base Model + diuretic, eGFR | −0.080 | 0.014 | 0.088 | 0.004 | 0.002 | 0.940 |
| Base Model + diabetes, BMI, WHR | −0.070 | 0.032 | 0.088 | 0.004 | 0.005 | 0.867 |
| Base Model + hypertension | −0.075 | 0.008 | 0.090 | 0.003 | −0.0003 | 0.993 |
| Base Model + hypertension, HDL, LDL, triglycerides | −0.067 | 0.042 | 0.089 | 0.004 | −0.003 | 0.912 |
| Base Model + daily potassium intake, calcium intake and Mediterranean Diet score |
−0.079 | 0.005 | 0.09 | 0.007 | −0.006 | 0.823 |
Standardized β-coefficients are shown.
Abbreviations: BMI, body mass index; WHR, waist to hip ratio; eGFR, estimated glomerular filtration rate; HDL, high density lipoprotein; LDL, low density lipoprotein; MMSE, Mini-mental status exam; VFT, verbal fluency test.
Base model: adjusted for age, sex, education, total daily caloric intake.
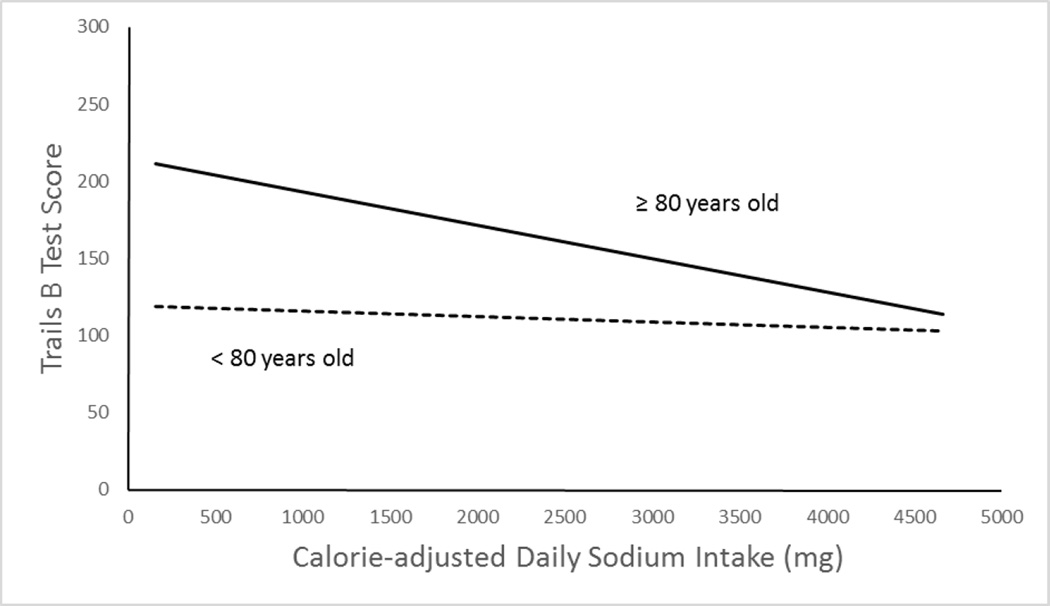
There was significant effect modification by age for the relation between Trails B and sodium intake (p = 0.04) (Figure 2). In individuals 80 years old or younger (n = 631), there was no association between daily sodium intake and Trails B performance (standardized β = −0.02, p = 0.564). However, in individuals older than 80 years of age (n = 294), lower sodium intake was associated with worse Trails B performance after adjusting for sex and education (standardized β = −0.13, p = 0.024). Further adjustment for the behavioral, dietary and health variables shown in Table 2 did not change the results. There was no effect modification by age for the MMSE or VFT scores. There was no effect modification by sex, hypertension status or diabetes status for any test (data not shown).
Figure 2.
Age-stratified Trail-making Test, part B (Trails B) scores by calorie-adjusted daily sodium intake. The lines are based on linear regression of Trails B scores by calorie-adjusted daily sodium intake adjusting for sex, education and total caloric intake. The association is significant for individuals aged 80 years or older (standardized β = −0.15, p = 0.014), solid lines but not significant for those less than 80 years of age (standardized β = −0.04, p = 0.365), dashed line.
Lower sodium intake was associated with increased odds of impairment on the MMSE after adjusting for age, sex, and education (OR per SD decrease = 1.15; 95% CI 1.02, 1.28); and remained significant after further adjustment for all other covariates (data not shown). Sodium intake was not associated with categorical cognitive impairment on Trails B or VFT (data not shown).
Discussion
This study assessed the association between dietary sodium intake and cognitive function among a cohort of community dwelling older adults. Contrary to our hypothesis, and to results of some prior studies (5, 6, 19), we did not find that higher sodium intake was associated with poorer cognitive performance. Instead, we found that lower dietary sodium was associated with poorer performance on tests of global (MMSE) and executive (Trails B) cognitive function independent of lifestyle, comorbidities medication use and kidney function. The association between dietary sodium and executive function and was more pronounced in the oldest adults (those over 80 years of age).
The magnitude of the observed effects are clinically significant. Lower sodium intake was associated with increased odds of clinically significant impairment on the MMSE. Scores below the cut-off used here are indicative of mild cognitive impairment or dementia (29). Results on Trails B showed that those in the lowest quartile of sodium intake took an average of 7 seconds longer to complete the task than those in the highest quartile, equivalent to a 2-year increase in age within this sample. Impairments in global and executive cognitive function can adversely affect instrumental activities of daily life, such as managing finances. With the growing number of older adults at risk for cognitive decline, even small changes in cognitive ability associated with dietary sodium intake may have important public health consequences.
Our findings differ from those of the three prior studies that have examined associations of sodium intake with cognitive function. Direct comparison of the studies is hampered by methodological differences, and differences in study samples. The study by Afsar (19) examined urine sodium excretion levels in a younger sample of adults in Turkey, recently diagnosed with essential hypertension. Results showed that lower urine sodium excretion levels were associated with higher MMSE scores (19). Their sample differed from ours in a number of characteristics that could affect vulnerability of cognitive function to stressors, including higher levels of depression, lower levels of education and lower socioeconomic status. Indeed, Afsar speculated that the observed associations of cognitive function with sodium may have been related to differences in education level or stress levels between individuals with lower and higher sodium intake rather than to sodium itself (19).
Our results also differ from those of the Canadian NuAge study (5). In that study, higher sodium intake was associated with greater 3-year decline on a test of global cognitive function (the Modified MMSE), but only among individuals with low physical activity (5). No significant associations of cognitive function with sodium intake was observed in the cross-sectional analysis at baseline. However, the NuAge study did not adjust for total caloric intake prior to dividing the sample into sodium intake tertiles, so confounding by total caloric intake may have occurred. Additionally, the NuAge study used a shorter (78-item) FFQ, and a different nutrient database for determining sodium levels. Thus estimates of sodium intake may have systematically differed between the two studies. Estimated sodium intake levels in the NuAge study were much higher than those in our study, despite similar caloric intake. The highest tertile of daily sodium intake in the NuAge study was 3919 mg/day (1.66 mg sodium per kcal), higher than the mean of our highest sodium intake quartile (2498 mg/day, 1.39 mg sodium per kcal). This may reflect methodological differences in sodium estimation or real differences in sodium content in diets of individuals in Quebec and California. If the latter, the NuAge study may not have contained sufficient individuals with very low sodium intake to observe the association between low sodium intake and lower cognitive performance found here.
Our results are also difficult to directly compare to those of the WHIMS, which found a tendency for higher sodium intake to be associated with greater odds of clinical decline over a 9-year follow-up in women with hypertension (6). Similar results were obtained when sodium intake was estimated based on a FFQ, and after FFQ estimates were corrected by 24-hour sodium excretion. This correction resulted in higher estimated daily sodium intakes. Because we looked at cross-sectional associations rather than prospective associations with future decline, results are not directly comparable.
Because our study is correlational, we cannot infer causality. Individuals could have restricted their sodium intake due to adverse health conditions that could have also impacted their cognitive performance. The finding that those in the lowest sodium quartile had higher rates of hypertension than those in the middle two quartiles may support this view. However, controlling for a variety of health-related variables, including hypertension and other cardiovascular risk factors, diuretic medication use, diabetes status, kidney function, and number of chronic diseases did not change the results.
In their most recent report, the Institute of Medicine (30) stated that there was no supporting evidence for reducing the daily sodium recommendation to 1500 mg/day in the general population. They advised that further research is needed to examine the associations of low to moderate levels of sodium intake with multiple health outcomes, including cognitive function. Although it has been established that lowering dietary sodium can reduce hypertension (31, 32) it is possible that low levels of dietary sodium may adversely impact insulin regulation as well as the renin-angiotensin and sympathetic systems and this may adversely affect cognitive function (33–35). A recent study reported a J-shaped association of sodium intake with cardiovascular disease and mortality (36). Individuals consuming more than 6 g sodium per day and those consuming less than 3 g sodium per day had a higher risk of death and cardiovascular events (36). Although more research is needed, our findings, along with those of the prior studies (5, 6, 19), may suggest a similar J-shaped associated between sodium intake and cognitive function in older adults.
Hyponatremia, a condition marked by low sodium levels in the blood, is a medical concern in the elderly because it has been associated with medical conditions as well as cognitive function (37). Prevalence of hyponatremia in the elderly has been reported to vary between 7% in healthy individuals to 30% in nursing home residents who need acute hospitalization (38, 39). Disturbances in water intake and output as well as sodium intake can make elderly individuals more susceptible to alterations in serum sodium homeostasis, which can increase the rate of hyponatremia (40, 41). A recent study of older adults (70 ± 12 years) showed that adding a low-dose diuretic to prescribed angiotensin II receptor blockers for controlling high blood pressure significantly decreased serum sodium levels in those categorized with low dietary salt intake (42). Therefore, decreasing dietary sodium intake to low levels could affect an individual’s ability to maintain homeostasis which in turn could lead to alterations in cognitive function. This may be of particular concern among the very old and among those on medications that alter sodium levels.
There are several limitations to our study. Our sample represents a relatively homogenous population of middle-class, White, well-educated older individuals, which may limit generalizability. However, this also minimizes potential confounding due to educational attainment, socio-economic status, access to healthcare, and racial/ethnic differences. Dietary sodium intake was based on the Willett FFQ. Although this instrument has been validated and shown to be a reliable source of dietary data (43), there are important limitations to the use of FFQs for estimating sodium intake (44). For example, the FFQ does not assess the addition of salt during cooking or at the table, and assumptions about salt content of foods in the nutrient database may not be accurate. Additionally, respondents often underestimate amount of food consumed. This is likely to lead to a systematic underestimation of actual sodium intake. As noted in the WHIMS study, correction of sodium estimates based on FFQ by use of 24-hour sodium excretion resulted in higher estimates of daily sodium intake (6). The average sodium intake in our sample, 2010 mg daily, is low relative to national average intake (7) suggesting that such an underestimation occurred. Thus, this study cannot provide guidance on absolute of levels of sodium intake that may be detrimental to cognitive function.
This study also has numerous strengths including the use of multiple measures of cognitive function and examination of a wide age range of community-dwelling men and women. We were also able to incorporate numerous potential confounding factors in the analysis including lifestyle variables such as smoking and exercise, global dietary quality, daily potassium and calcium intake, cardiovascular risk factors and other comorbidities. More importantly, we were also able to adjust for kidney function, which decreases with age, and use of diuretic medication. Both these factors can affect serum sodium concentration, and thus, confound associations between dietary sodium intake and cognitive function and other health outcomes.
Together with prior findings, results of our study suggest that the association of sodium intake with cognitive function may vary by age and cognitive domain. With increasing age, maintaining a sufficient level of sodium in the diet may be important for maintaining cognitive health. More research is needed to confirm these findings and to determine the mechanisms whereby low sodium may adversely affect cognitive function among the very old.
Acknowledgments
The authors would like to thank A. Z. LaCroix, PhD for helpful comments on the study and J. Bergstrom for database assistance.
The current study was funded by grant NIAAA R01AA021187. Data acquisition for the Rancho Bernardo Study was funded by the National Institute of Diabetes and Digestive and Kidney Disease grant number DK31801, and the National Institute on Aging (NIA) grants number AG07181 and AG028507.
The funding organizations had no role in the design and conduct of the study; collection, analysis, or preparation of data; or preparation, review, or approval of the manuscript.
The authors affirm that they have listed everyone who contributed significantly to the work in the Acknowledgements.
Footnotes
Author contributions: All authors had full access to all of the data in the study. Rush: Study design, analysis and interpretation of data, drafting manuscript. Kritz-Silverstein: study conception and design, acquisition of the data, interpretation of data analysis, critical revision of manuscript for important intellectual content. Laughlin: study conception and design, acquisition of the data, interpretation of data analysis, critical revision of manuscript for important intellectual content. Fung: analysis of data, critical revision of manuscript for important intellectual content. Barrett-Connor: study funding, acquisition of the data, interpretation of data, and critical revision of manuscript for important intellectual content. McEvoy: study funding, study conception and design, analysis and interpretation of data, critical revision of manuscript for important intellectual content. All authors approved the final version of the submitted manuscript.
References
- 1.Colby SL, Ortman JM. Projections of the size and composition of the U.S. population: 2014 to 2060. Washington, D.C: U.S. Census Bureau; 2014. [Google Scholar]
- 2.Smith PJ, Blumenthal JA. Dietary factors and cognitive decline. J Prev Alz Dis. 2016;3(1):53–64. doi: 10.14283/jpad.2015.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu YZ, Chen JK, Li ZP, Zhao T, Ni M, Li DJ, Jiang CL, Shen FM. High-salt diet enhances hippocampal oxidative stress and cognitive impairment in mice. Neurobiol Learn Mem. 2014;114:10–15. doi: 10.1016/j.nlm.2014.04.010. [DOI] [PubMed] [Google Scholar]
- 4.Chugh G, Asghar M, Patki G, Bohat R, Jafri F, Allam F, Dao AT, Mowrey C, Alkadhi K, Salim S. A high-salt diet further impairs age-associated declines in cognitive, behavioral, and cardiovascular functions in male Fischer brown Norway rats. J Nutr. 2013;143(9):1406–1413. doi: 10.3945/jn.113.177980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fiocco AJ, Shatenstein B, Ferland G, Payette H, Belleville S, Kergoat MJ, Morais JA, Greenwood CE. Sodium intake and physical activity impact cognitive maintenance in older adults: the NuAge Study. Neurobiol Aging. 2012;33(4):829. doi: 10.1016/j.neurobiolaging.2011.07.004. e21-8. [DOI] [PubMed] [Google Scholar]
- 6.Haring B, Wu C, Coker LH, Seth A, Snetselaar L, Manson JE, Rossouw JE, Wassertheil-Smoller S. Hypertension, Dietary Sodium, and Cognitive Decline: Results from the Women’s Health Initiative Memory Study. Am Journal Hypertension. 2016;29(2):202–216. doi: 10.1093/ajh/hpv081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cohen HW, Hailpern SM, Alderman MH. Sodium intake and mortality follow-up in the Third National Health and Nutrition Examination Survey (NHANES III) J Gen Intern Med. 2008;23(9):1297–1302. doi: 10.1007/s11606-008-0645-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Strazzullo P, D’Elia L, Kandala NB, Cappuccio FP. Salt intake, stroke, and cardiovascular disease: meta-analysis of prospective studies. BMJ. 2009;339:b4567. doi: 10.1136/bmj.b4567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Henney JE, Taylor CL, Boon CS, editors. Institute of Medicine (U.S.). Committee on Strategies to Reduce Sodium Intake. Strategies to reduce sodium intake in the United States. Washington, D.C: National Academies Press; 2010. [PubMed] [Google Scholar]
- 10.Veglio F, Paglieri C, Rabbia F, Bisbocci D, Bergui M, Cerrato P. Hypertension and cerebrovascular damage. Atherosclerosis. 2009;205(2):331–341. doi: 10.1016/j.atherosclerosis.2008.10.028. [DOI] [PubMed] [Google Scholar]
- 11.Manolio TA, Olson J, Longstreth WT. Hypertension and cognitive function: pathophysiologic effects of hypertension on the brain. Curr Hypertens Rep. 2003;5(3):255–261. doi: 10.1007/s11906-003-0029-6. [DOI] [PubMed] [Google Scholar]
- 12.Tangney CC, Li H, Wang Y, Barnes L, Schneider JA, Bennett DA, Morris MC. Relation of DASH- and Mediterranean-like dietary patterns to cognitive decline in older persons. Neurology. 2014;83(16):1410–1416. doi: 10.1212/WNL.0000000000000884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wengreen H, Munger RG, Cutler A, Quach A, Bowles A, Corcoran C, Tschanz JT, Norton MC, Welsh-Bohmer KA. Prospective study of Dietary Approaches to Stop Hypertension- and Mediterranean-style dietary patterns and age-related cognitive change: the Cache County Study on Memory, Health and Aging. Am J Clin Nutr. 2013;98(5):1263–1271. doi: 10.3945/ajcn.112.051276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Morris MC, Evans DA, Tangney CC, Bienias JL, Wilson RS. Fish consumption and cognitive decline with age in a large community study. Arch Neurol. 2005;62(12):1849–1853. doi: 10.1001/archneur.62.12.noc50161. [DOI] [PubMed] [Google Scholar]
- 15.Morris MC, Evans DA, Tangney CC, Bienias JL, Wilson RS. Associations of vegetable and fruit consumption with age-related cognitive change. Neurology. 2006;67(8):1370–1376. doi: 10.1212/01.wnl.0000240224.38978.d8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kalmijn S, Launer LJ, Ott A, Witteman JC, Hofman A, Breteler MM. Dietary fat intake and the risk of incident dementia in the Rotterdam Study. Ann Neurol. 1997;42(5):776–782. doi: 10.1002/ana.410420514. [DOI] [PubMed] [Google Scholar]
- 17.Kalmijn S, van Boxtel MP, Ocke M, Verschuren WM, Kromhout D, Launer LJ. Dietary intake of fatty acids and fish in relation to cognitive performance at middle age. Neurology. 2004;62(2):275–280. doi: 10.1212/01.wnl.0000103860.75218.a5. [DOI] [PubMed] [Google Scholar]
- 18.Berti V, Murray J, Davies M, Spector N, Tsui WH, Li Y, Williams S, Pirraglia E, Vallabhajosula S, McHugh P, Pupi A, de Leon MJ, Mosconi L. Nutrient patterns and brain biomarkers of Alzheimer’s disease in cognitively normal individuals. J Nutr Health Aging. 2015;19(4):413–423. doi: 10.1007/s12603-014-0534-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Afsar B. The relationship between cognitive function, depressive behaviour and sleep quality with 24-h urinary sodium excretion in patients with essential hypertension. High Blood Press Cardiovasc Prev. 2013;20(1):19–24. doi: 10.1007/s40292-013-0002-7. [DOI] [PubMed] [Google Scholar]
- 20.Rimm EB, Giovannucci EL, Stampfer MJ, Colditz GA, Litin LB, Willett WC. Reproducibility and validity of an expanded self-administered semiquantitative food frequency questionnaire among male health professionals. Am J Epidemiol. 1992;135(10):1114–1126. doi: 10.1093/oxfordjournals.aje.a116211. [DOI] [PubMed] [Google Scholar]
- 21.Fung TT, Rexrode KM, Mantzoros CS, Manson JE, Willett WC, Hu FB. Mediterranean diet and incidence of and mortality from coronary heart disease and stroke in women. Circulation. 2009;119(8):1093–1100. doi: 10.1161/CIRCULATIONAHA.108.816736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Borkowski J, Benton AL, Spreen O. Word fluency and brain damage. Neuropsychologia. 1967;5:135–140. [Google Scholar]
- 23.Tombaugh TN, McIntyre NJ. The mini-mental state examination: a comprehensive review. J Am Geriatr Soc. 1992;40(9):922–935. doi: 10.1111/j.1532-5415.1992.tb01992.x. [DOI] [PubMed] [Google Scholar]
- 24.Reitan R. Validity of the Trail Making Test as an indicator of organic brain damage. Percept Mot Skills. 1958;8:271–276. [Google Scholar]
- 25.Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Arch Gen Psychiatry. 1961;4:561–571. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- 26.The hypertension detection and follow-up program: Hypertension detection and follow-up program cooperative group. Prev Med. 1976;5(2):207–215. doi: 10.1016/0091-7435(76)90039-6. [DOI] [PubMed] [Google Scholar]
- 27.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18(6):499–502. PubMed PMID: 4337382. [PubMed] [Google Scholar]
- 28.Willett W, Stampfer MJ. Total energy intake: implications for epidemiologic analyses. Am J Epidemiol. 1986;124(1):17–27. doi: 10.1093/oxfordjournals.aje.a114366. [DOI] [PubMed] [Google Scholar]
- 29.O’Bryant SE, Humphreys JD, Smith GE, Ivnik RJ, Graff-Radford NR, Petersen RC, Lucas JA. Detecting dementia with the mini-mental state examination in highly educated individuals. Arch Neurol. 2008;65(7):963–967. doi: 10.1001/archneur.65.7.963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Institute of Medicine (U.S.). Committee on the Consequences of Sodium Reduction in Populations. Institute of Medicine (U.S.). Food and Nutrition Board, Institute of Medicine (U.S.). Board on Population Health and Public Health Practice. In: Strom BL, Yaktine AL, Oria M, editors. Sodium intake in populations : assessment of evidence. Washington, D.C: The National Academies Press; 2013. [PubMed] [Google Scholar]
- 31.Cook NR. Salt intake, blood pressure and clinical outcomes. Curr Opin Nephrol Hypertens. 2008;17(3):310–314. doi: 10.1097/MNH.0b013e3282f4b720. [DOI] [PubMed] [Google Scholar]
- 32.Stamler J. The INTERSALT Study: background, methods, findings, and implications. Am J Clin Nutr. 1997;65(2 Suppl):626S–642S. doi: 10.1093/ajcn/65.2.626S. [DOI] [PubMed] [Google Scholar]
- 33.Patel SM, Cobb P, Saydah S, Zhang X, de Jesus JM, Cogswell ME. Dietary sodium reduction does not affect circulating glucose concentrations in fasting children or adults: findings from a systematic review and meta-analysis. J Nutr. 2015;145(3):505–513. doi: 10.3945/jn.114.195982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Alderman MH, Madhavan S, Ooi WL, Cohen H, Sealey JE, Laragh JH. Association of the renin-sodium profile with the risk of myocardial infarction in patients with hypertension. N Engl J Med. 1991;324(16):1098–1104. doi: 10.1056/NEJM199104183241605. [DOI] [PubMed] [Google Scholar]
- 35.Grassi G, Dell’Oro R, Seravalle G, Foglia G, Trevano FQ, Mancia G. Short- and long-term neuroadrenergic effects of moderate dietary sodium restriction in essential hypertension. Circulation. 2002;106(15):1957–1961. doi: 10.1161/01.cir.0000033519.45615.c7. [DOI] [PubMed] [Google Scholar]
- 36.O’Donnell M, Mente A, Rangarajan S, McQueen MJ, Wang X, Liu L, Yan H, Lee SF, Mony P, Devanath A, Rosengren A, Lopez-Jaramillo P, Diaz R, Avezum A, Lanas F, Yusoff K, Iqbal R, Ilow R, Mohammadifard N, Gulec S, Yusufali AH, Kruger L, Yusuf R, Chifamba J, Kabali C, Dagenais G, Lear SA, Teo K, Yusuf S, Investigators P. Urinary sodium and potassium excretion, mortality, and cardiovascular events. N Engl J Med. 2014;371(7):612–623. doi: 10.1056/NEJMoa1311889. [DOI] [PubMed] [Google Scholar]
- 37.Renneboog B, Musch W, Vandemergel X, Manto MU, Decaux G. Mild chronic hyponatremia is associated with falls, unsteadiness, and attention deficits. Am J Med. 2006;119(1):71. doi: 10.1016/j.amjmed.2005.09.026. e1–8. [DOI] [PubMed] [Google Scholar]
- 38.Caird FI, Andrews GR, Kennedy RD. Effect of posture on blood pressure in the elderly. Br Heart J. 1973;35(5):527–530. doi: 10.1136/hrt.35.5.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lavizzo-Mourey R, Johnson J, Stolley P. Risk factors for dehydration among elderly nursing home residents. J Am Geriatr Soc. 1988;36(3):213–218. doi: 10.1111/j.1532-5415.1988.tb01803.x. [DOI] [PubMed] [Google Scholar]
- 40.Fulop T, Jr, Worum I, Csongor J, Foris G, Leovey A. Body composition in elderly people I. Determination of body composition by multiisotope method and the elimination kinetics of these isotopes in healthy elderly subjects. Gerontology. 1985;31(1):6–14. doi: 10.1159/000212676. [DOI] [PubMed] [Google Scholar]
- 41.Rolls BJ, Phillips PA. Aging and disturbances of thirst and fluid balance. Nutr Rev. 1990;48(3):137–144. doi: 10.1111/j.1753-4887.1990.tb02915.x. [DOI] [PubMed] [Google Scholar]
- 42.Nakayama M, Tomiyama H, Kuwajima I, Saito T, Hokama Y, Fujii Y, Shimizu T, Nakayama T, Yamashina A, Aizawa Y. Low salt intake and changes in serum sodium levels in the combination therapy of low-dose hydrochlorothiazide and angiotensin II receptor blocker. Circ J. 2013;77(10):2567–2572. doi: 10.1253/circj.cj-13-0287. [DOI] [PubMed] [Google Scholar]
- 43.Feskanich D, Rimm EB, Giovannucci EL, Colditz GA, Stampfer MJ, Litin LB, Willett WC. Reproducibility and validity of food intake measurements from a semiquantitative food frequency questionnaire. J Am Diet Assoc. 1993;93(7):790–766. doi: 10.1016/0002-8223(93)91754-e. [DOI] [PubMed] [Google Scholar]
- 44.Cobb LK, Anderson CA, Elliott P, Hu FB, Liu K, Neaton JD, Whelton PK, Woodward M, Appel LJ American Heart Association Council on L, Metabolic H. Methodological issues in cohort studies that relate sodium intake to cardiovascular disease outcomes: a science advisory from the American Heart Association. Circulation. 2014;129(10):1173–1186. doi: 10.1161/CIR.0000000000000015. [DOI] [PubMed] [Google Scholar]