Abstract
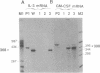
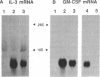
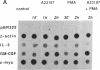
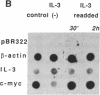
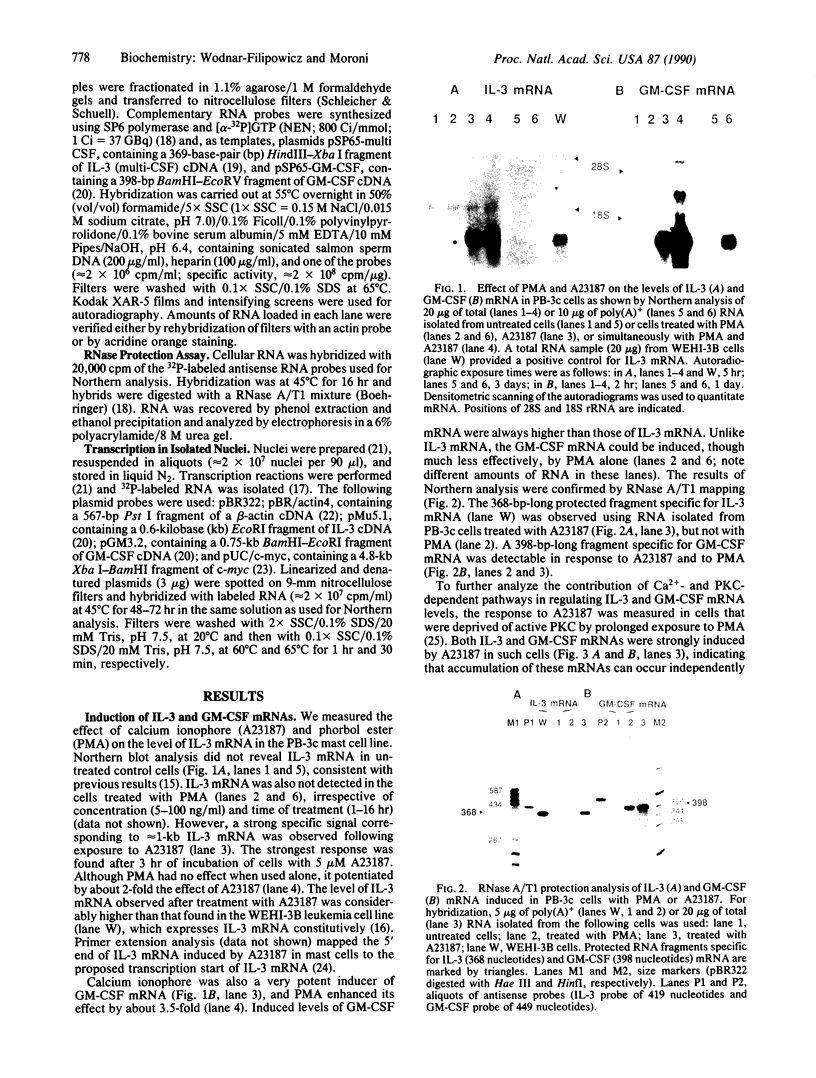
Interleukin 3 (IL-3) is transiently produced by murine bone marrow-derived mast cells in response to antigen stimulation of the high-affinity immunoglobulin E receptors. We have studied the postreceptor signaling pathways involved in regulating expression of the IL-3 gene in the murine mast cell line PB-3c. Large amounts of IL-3 mRNA accumulated after exposure of cells to calcium ionophore A23187, a reagent that increases intracellular Ca2+. Phorbol 12-myristate 13-acetate, which stimulates protein kinase C, did not induce IL-3 mRNA accumulation, although it did potentiate the effect of A23187. Nuclear run-on analysis showed that the IL-3 gene is constitutively transcribed in unstimulated cells and that treatment with A23187 and/or phorbol ester has no influence on its transcription rate. The effect of A23187 was found to be due to stabilization of the IL-3 mRNA. In cells maintained in the presence of A23187 the IL-3 mRNA was stable during 3 hr of incubation with actinomycin D, whereas removal of A23187 under the same conditions resulted in rapid degradation of the mRNA. These results indicate that control of expression of the IL-3 gene in mast cells is primarily at the posttranscriptional level and that the Ca2(+)-dependent signal-transduction pathway plays an important role in this process. Synthesis of granulocyte/macrophage colony-stimulating factor mRNA in response to A23187 and phorbol ester was found to be subject to both transcriptional and posttranscriptional regulation.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrejauskas E., Moroni C. Reversible abrogation of IL-3 dependence by an inducible H-ras oncogene. EMBO J. 1989 Sep;8(9):2575–2581. doi: 10.1002/j.1460-2075.1989.tb08396.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ball P. E., Conroy M. C., Heusser C. H., Davis J. M., Conscience J. F. Spontaneous, in vitro, malignant transformation of a basophil/mast cell line. Differentiation. 1983;24(1):74–78. doi: 10.1111/j.1432-0436.1983.tb01305.x. [DOI] [PubMed] [Google Scholar]
- Barlow D. P., Bućan M., Lehrach H., Hogan B. L., Gough N. M. Close genetic and physical linkage between the murine haemopoietic growth factor genes GM-CSF and Multi-CSF (IL3). EMBO J. 1987 Mar;6(3):617–623. doi: 10.1002/j.1460-2075.1987.tb04799.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaven M. A., Cunha-Melo J. R. Membrane phosphoinositide-activated signals in mast cells and basophils. Prog Allergy. 1988;42:123–184. [PubMed] [Google Scholar]
- Berridge M. J. Inositol trisphosphate and diacylglycerol: two interacting second messengers. Annu Rev Biochem. 1987;56:159–193. doi: 10.1146/annurev.bi.56.070187.001111. [DOI] [PubMed] [Google Scholar]
- Caput D., Beutler B., Hartog K., Thayer R., Brown-Shimer S., Cerami A. Identification of a common nucleotide sequence in the 3'-untranslated region of mRNA molecules specifying inflammatory mediators. Proc Natl Acad Sci U S A. 1986 Mar;83(6):1670–1674. doi: 10.1073/pnas.83.6.1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Conscience J. F., Verrier B., Martin G. Interleukin-3-dependent expression of the c-myc and c-fos proto-oncogenes in hemopoietic cell lines. EMBO J. 1986 Feb;5(2):317–323. doi: 10.1002/j.1460-2075.1986.tb04215.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dexter T. M., Spooncer E. Growth and differentiation in the hemopoietic system. Annu Rev Cell Biol. 1987;3:423–441. doi: 10.1146/annurev.cb.03.110187.002231. [DOI] [PubMed] [Google Scholar]
- Gough N. M., Metcalf D., Gough J., Grail D., Dunn A. R. Structure and expression of the mRNA for murine granulocyte-macrophage colony stimulating factor. EMBO J. 1985 Mar;4(3):645–653. doi: 10.1002/j.1460-2075.1985.tb03678.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg M. E., Ziff E. B. Stimulation of 3T3 cells induces transcription of the c-fos proto-oncogene. Nature. 1984 Oct 4;311(5985):433–438. doi: 10.1038/311433a0. [DOI] [PubMed] [Google Scholar]
- Hofstetter P., Kikinis Z., Altus M. S., Pearson D., Nagamine Y. A new genetic approach for studying hormonal regulation of urokinase-type plasminogen activator gene expression in LLC-PK1 cells. Mol Cell Biol. 1987 Dec;7(12):4535–4541. doi: 10.1128/mcb.7.12.4535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurley J. B., Simon M. I., Teplow D. B., Robishaw J. D., Gilman A. G. Homologies between signal transducing G proteins and ras gene products. Science. 1984 Nov 16;226(4676):860–862. doi: 10.1126/science.6436980. [DOI] [PubMed] [Google Scholar]
- Ihle J. N., Pepersack L., Rebar L. Regulation of T cell differentiation: in vitro induction of 20 alpha-hydroxysteroid dehydrogenase in splenic lymphocytes from athymic mice by a unique lymphokine. J Immunol. 1981 Jun;126(6):2184–2189. [PubMed] [Google Scholar]
- Ihle J. N., Weinstein Y. Immunological regulation of hematopoietic/lymphoid stem cell differentiation by interleukin 3. Adv Immunol. 1986;39:1–50. doi: 10.1016/s0065-2776(08)60347-8. [DOI] [PubMed] [Google Scholar]
- Kelso A., Gough N. M. Coexpression of granulocyte-macrophage colony-stimulating factor, gamma interferon, and interleukins 3 and 4 is random in murine alloreactive T-lymphocyte clones. Proc Natl Acad Sci U S A. 1988 Dec;85(23):9189–9193. doi: 10.1073/pnas.85.23.9189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelso A., Gough N. M. Differential inhibition by cyclosporin A reveals two pathways for activation of lymphokine synthesis in T cells. Growth Factors. 1989;1(2):165–177. doi: 10.3109/08977198909029126. [DOI] [PubMed] [Google Scholar]
- Kindler V., Thorens B., de Kossodo S., Allet B., Eliason J. F., Thatcher D., Farber N., Vassalli P. Stimulation of hematopoiesis in vivo by recombinant bacterial murine interleukin 3. Proc Natl Acad Sci U S A. 1986 Feb;83(4):1001–1005. doi: 10.1073/pnas.83.4.1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koeffler H. P., Gasson J., Tobler A. Transcriptional and posttranscriptional modulation of myeloid colony-stimulating factor expression by tumor necrosis factor and other agents. Mol Cell Biol. 1988 Aug;8(8):3432–3438. doi: 10.1128/mcb.8.8.3432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Land H., Parada L. F., Weinberg R. A. Tumorigenic conversion of primary embryo fibroblasts requires at least two cooperating oncogenes. Nature. 1983 Aug 18;304(5927):596–602. doi: 10.1038/304596a0. [DOI] [PubMed] [Google Scholar]
- Le Beau M. M., Epstein N. D., O'Brien S. J., Nienhuis A. W., Yang Y. C., Clark S. C., Rowley J. D. The interleukin 3 gene is located on human chromosome 5 and is deleted in myeloid leukemias with a deletion of 5q. Proc Natl Acad Sci U S A. 1987 Aug;84(16):5913–5917. doi: 10.1073/pnas.84.16.5913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melton D. A., Krieg P. A., Rebagliati M. R., Maniatis T., Zinn K., Green M. R. Efficient in vitro synthesis of biologically active RNA and RNA hybridization probes from plasmids containing a bacteriophage SP6 promoter. Nucleic Acids Res. 1984 Sep 25;12(18):7035–7056. doi: 10.1093/nar/12.18.7035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metcalf D., Begley C. G., Johnson G. R., Nicola N. A., Lopez A. F., Williamson D. J. Effects of purified bacterially synthesized murine multi-CSF (IL-3) on hematopoiesis in normal adult mice. Blood. 1986 Jul;68(1):46–57. [PubMed] [Google Scholar]
- Metzger H. Molecular aspects of receptors and binding factors for IgE. Adv Immunol. 1988;43:277–312. doi: 10.1016/s0065-2776(08)60368-5. [DOI] [PubMed] [Google Scholar]
- Miyatake S., Yokota T., Lee F., Arai K. Structure of the chromosomal gene for murine interleukin 3. Proc Natl Acad Sci U S A. 1985 Jan;82(2):316–320. doi: 10.1073/pnas.82.2.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair A. P., Diamantis I. D., Conscience J. F., Kindler V., Hofer P., Moroni C. A v-H-ras-dependent hemopoietic tumor model involving progression from a clonal stage of transformation competence to autocrine interleukin 3 production. Mol Cell Biol. 1989 Mar;9(3):1183–1190. doi: 10.1128/mcb.9.3.1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishizuka Y. Studies and perspectives of protein kinase C. Science. 1986 Jul 18;233(4761):305–312. doi: 10.1126/science.3014651. [DOI] [PubMed] [Google Scholar]
- Plaut M., Pierce J. H., Watson C. J., Hanley-Hyde J., Nordan R. P., Paul W. E. Mast cell lines produce lymphokines in response to cross-linkage of Fc epsilon RI or to calcium ionophores. Nature. 1989 May 4;339(6219):64–67. doi: 10.1038/339064a0. [DOI] [PubMed] [Google Scholar]
- Schuler G. D., Cole M. D. GM-CSF and oncogene mRNA stabilities are independently regulated in trans in a mouse monocytic tumor. Cell. 1988 Dec 23;55(6):1115–1122. doi: 10.1016/0092-8674(88)90256-5. [DOI] [PubMed] [Google Scholar]
- Shaw G., Kamen R. A conserved AU sequence from the 3' untranslated region of GM-CSF mRNA mediates selective mRNA degradation. Cell. 1986 Aug 29;46(5):659–667. doi: 10.1016/0092-8674(86)90341-7. [DOI] [PubMed] [Google Scholar]
- Taniguchi T. Regulation of cytokine gene expression. Annu Rev Immunol. 1988;6:439–464. doi: 10.1146/annurev.iy.06.040188.002255. [DOI] [PubMed] [Google Scholar]
- Thorens B., Mermod J. J., Vassalli P. Phagocytosis and inflammatory stimuli induce GM-CSF mRNA in macrophages through posttranscriptional regulation. Cell. 1987 Feb 27;48(4):671–679. doi: 10.1016/0092-8674(87)90245-5. [DOI] [PubMed] [Google Scholar]
- Wodnar-Filipowicz A., Heusser C. H., Moroni C. Production of the haemopoietic growth factors GM-CSF and interleukin-3 by mast cells in response to IgE receptor-mediated activation. Nature. 1989 May 11;339(6220):150–152. doi: 10.1038/339150a0. [DOI] [PubMed] [Google Scholar]
- Ymer S., Tucker W. Q., Sanderson C. J., Hapel A. J., Campbell H. D., Young I. G. Constitutive synthesis of interleukin-3 by leukaemia cell line WEHI-3B is due to retroviral insertion near the gene. Nature. 1985 Sep 19;317(6034):255–258. doi: 10.1038/317255a0. [DOI] [PubMed] [Google Scholar]