Abstract
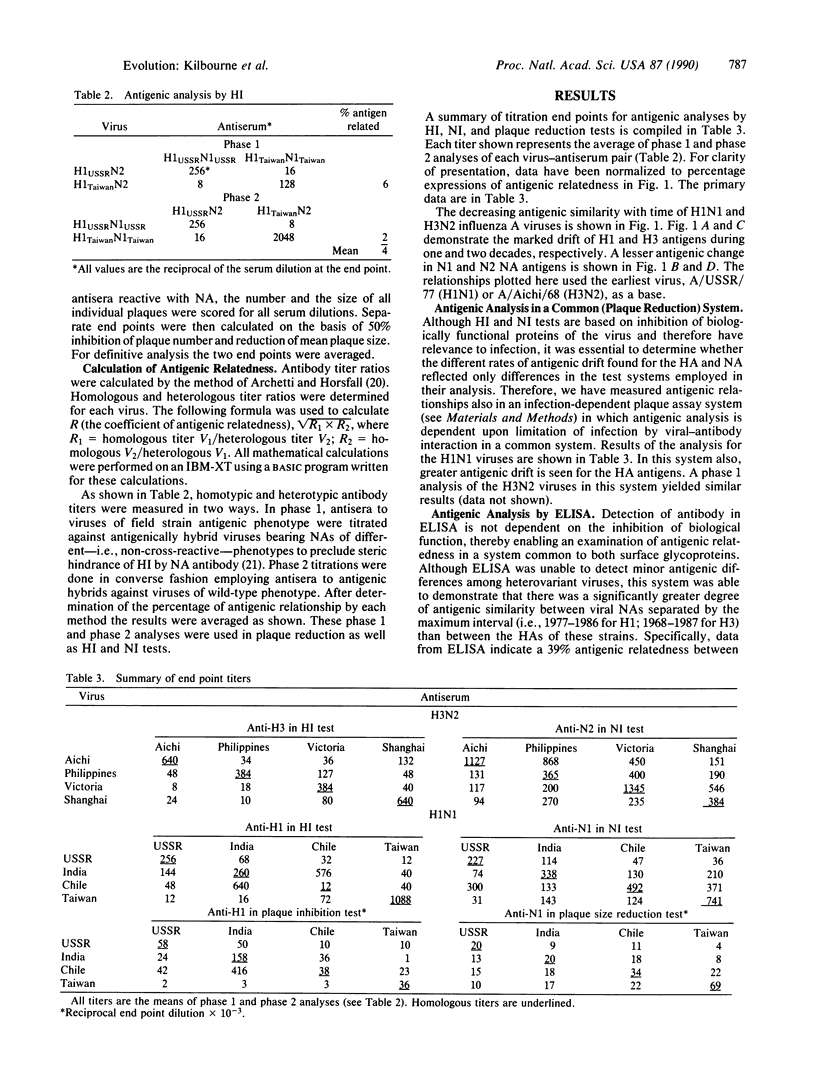
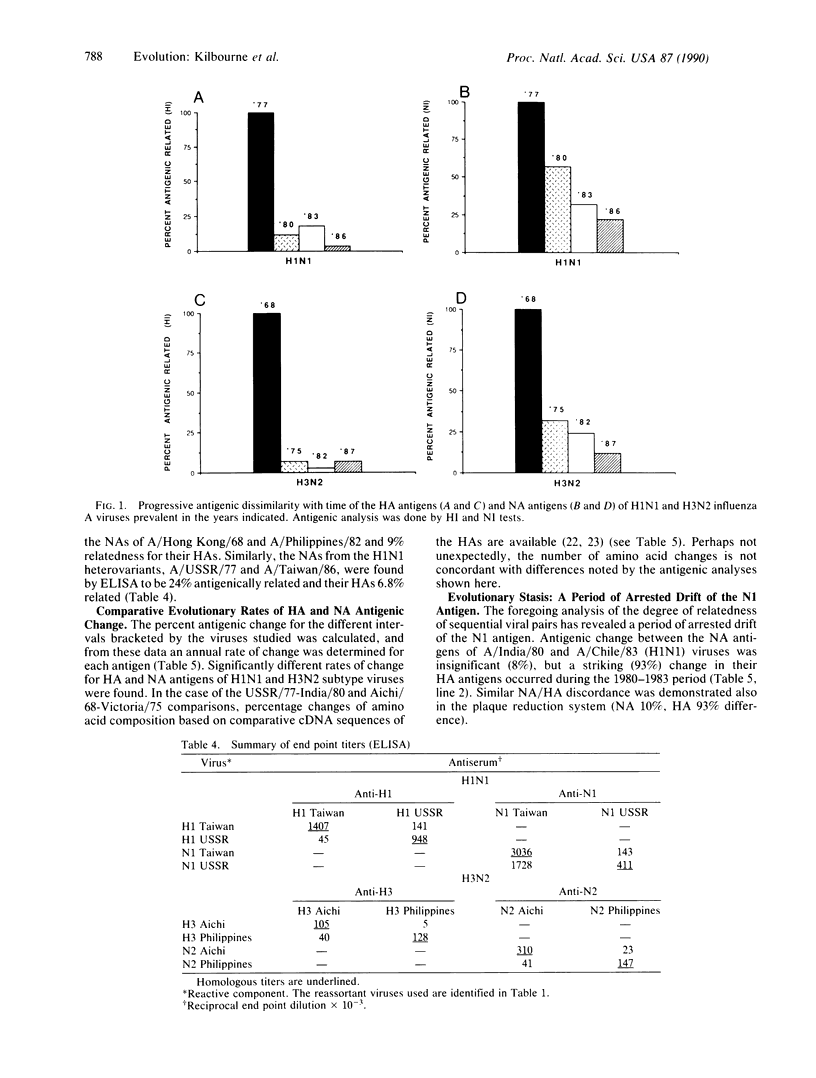
The hemagglutinin (HA) and neuraminidase (NA) external glycoprotein antigens of H1N1 and H3N2 subtypes of epidemiologically important influenza A viruses prevalent during recent decades were subjected to intensive antigenic analysis by four different methods. Prior to serological analysis with polyclonal rabbit antisera, HA and NA antigens of four viruses of each subtype were segregated by genetic reassortment to forestall nonspecific steric hindrance during antigen-antibody combination. This analysis has demonstrated that with respect to antigenic phenotype, HA and NA proteins have evolved at different rates. With H1N1 viruses, an arrest of significant evolution of the NA discordant with the continuing antigenic drift of HA was found in the 1980-1983 period. It is probable that the different and independent rates of evolution of HA and NA reflect the greater selective pressure of HA antibodies, which forces the more rapid emergence of HA escape mutants. The slower antigenic change found for NA further supports the potential for NA-specific infection-permissive immunization as a useful stratagem against influenza.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ARCHETTI I., HORSFALL F. L., Jr Persistent antigenic variation of influenza A viruses after incomplete neutralization in ovo with heterologous immune serum. J Exp Med. 1950 Nov 1;92(5):441–462. doi: 10.1084/jem.92.5.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosch F. X., Von Hoyningen-Huene V., Scholtissek C., Rott R. The overall evolution of the H7 influenza virus haemagglutinins is different from the evolution of the proteolytic cleavage site. J Gen Virol. 1982 Jul;61(Pt 50):101–104. doi: 10.1099/0022-1317-61-1-101. [DOI] [PubMed] [Google Scholar]
- Buonagurio D. A., Nakada S., Parvin J. D., Krystal M., Palese P., Fitch W. M. Evolution of human influenza A viruses over 50 years: rapid, uniform rate of change in NS gene. Science. 1986 May 23;232(4753):980–982. doi: 10.1126/science.2939560. [DOI] [PubMed] [Google Scholar]
- Curry R. L., Brown J. D., Baker F. A., Hobson D. Serological studies with purified neuraminidase antigens of influenza B viruses. J Hyg (Lond) 1974 Apr;72(2):197–204. doi: 10.1017/s0022172400023408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang R., Min Jou W., Huylebroeck D., Devos R., Fiers W. Complete structure of A/duck/Ukraine/63 influenza hemagglutinin gene: animal virus as progenitor of human H3 Hong Kong 1968 influenza hemagglutinin. Cell. 1981 Aug;25(2):315–323. doi: 10.1016/0092-8674(81)90049-0. [DOI] [PubMed] [Google Scholar]
- Hayashida H., Toh H., Kikuno R., Miyata T. Evolution of influenza virus genes. Mol Biol Evol. 1985 Jul;2(4):289–303. doi: 10.1093/oxfordjournals.molbev.a040352. [DOI] [PubMed] [Google Scholar]
- Holland J., Spindler K., Horodyski F., Grabau E., Nichol S., VandePol S. Rapid evolution of RNA genomes. Science. 1982 Mar 26;215(4540):1577–1585. doi: 10.1126/science.7041255. [DOI] [PubMed] [Google Scholar]
- Huddleston J. A., Brownlee G. G. The sequence of the nucleoprotein gene of human influenza A virus, strain A/NT/60/68. Nucleic Acids Res. 1982 Feb 11;10(3):1029–1038. doi: 10.1093/nar/10.3.1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahiel R. I., Kilbourne E. D. Reduction in plaque size and reduction in plaque number as differing indices of influenza virus-antibody reactions. J Bacteriol. 1966 Nov;92(5):1521–1534. doi: 10.1128/jb.92.5.1521-1534.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson B. E., Bucher D. J., Kilbourne E. D. Purified influenza virus hemagglutinin and neuraminidase are equivalent in stimulation of antibody response but induce contrasting types of immunity to infection. J Virol. 1989 Mar;63(3):1239–1246. doi: 10.1128/jvi.63.3.1239-1246.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson B. E., Bucher D. J., Pokorny B. A., Mikhail A., Kilbourne E. D. Identification of PR8 M1 protein in influenza virus high-yield reassortants by M1-specific monoclonal antibodies. Virology. 1989 Aug;171(2):634–636. doi: 10.1016/0042-6822(89)90638-7. [DOI] [PubMed] [Google Scholar]
- Johansson B. E., Moran T. M., Kilbourne E. D. Antigen-presenting B cells and helper T cells cooperatively mediate intravirionic antigenic competition between influenza A virus surface glycoproteins. Proc Natl Acad Sci U S A. 1987 Oct;84(19):6869–6873. doi: 10.1073/pnas.84.19.6869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jukes T. H. Silent nucleotide substitutions and the molecular evolutionary clock. Science. 1980 Nov 28;210(4473):973–978. doi: 10.1126/science.7434017. [DOI] [PubMed] [Google Scholar]
- Khan M. W., Gallagher M., Bucher D., Cerini C. P., Kilbourne E. D. Detection of influenza virus neuraminidase-specific antibodies by an enzyme-linked immunosorbent assay. J Clin Microbiol. 1982 Jul;16(1):115–122. doi: 10.1128/jcm.16.1.115-122.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilbourne E. D. Comparative efficacy of neuraminidase-specific and conventional influenza virus vaccines in induction of antibody to neuraminidase in humans. J Infect Dis. 1976 Oct;134(4):384–394. doi: 10.1093/infdis/134.4.384. [DOI] [PubMed] [Google Scholar]
- Kilbourne E. D. Future influenza vaccines and the use of genetic recombinants. Bull World Health Organ. 1969;41(3):643–645. [PMC free article] [PubMed] [Google Scholar]
- Kilbourne E. D., Laver W. G., Schulman J. L., Webster R. G. Antiviral activity of antiserum specific for an influenza virus neuraminidase. J Virol. 1968 Apr;2(4):281–288. doi: 10.1128/jvi.2.4.281-288.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laver W. G., Air G. M., Webster R. G., Markoff L. J. Amino acid sequence changes in antigenic variants of type A influenza virus N2 neuraminidase. Virology. 1982 Oct 30;122(2):450–460. doi: 10.1016/0042-6822(82)90244-6. [DOI] [PubMed] [Google Scholar]
- Laver W. G., Valentine R. C. Morphology of the isolated hemagglutinin and neuraminidase subunits of influenza virus. Virology. 1969 May;38(1):105–119. doi: 10.1016/0042-6822(69)90132-9. [DOI] [PubMed] [Google Scholar]
- Martínez C., del Rio L., Portela A., Domingo E., Ortín J. Evolution of the influenza virus neuraminidase gene during drift of the N2 subtype. Virology. 1983 Oct 30;130(2):539–545. doi: 10.1016/0042-6822(83)90108-3. [DOI] [PubMed] [Google Scholar]
- Raymond F. L., Caton A. J., Cox N. J., Kendal A. P., Brownlee G. G. Antigenicity and evolution amongst recent influenza viruses of H1N1 subtype. Nucleic Acids Res. 1983 Oct 25;11(20):7191–7203. doi: 10.1093/nar/11.20.7191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholtissek C., Rohde W., Von Hoyningen V., Rott R. On the origin of the human influenza virus subtypes H2N2 and H3N2. Virology. 1978 Jun 1;87(1):13–20. doi: 10.1016/0042-6822(78)90153-8. [DOI] [PubMed] [Google Scholar]
- Schulman J. L., Khakpour M., Kilbourne E. D. Protective effects of specific immunity to viral neuraminidase on influenza virus infection of mice. J Virol. 1968 Aug;2(8):778–786. doi: 10.1128/jvi.2.8.778-786.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulman J. L., Kilbourne E. D. Independent variation in nature of hemagglutinin and neuraminidase antigens of influenza virus: distinctiveness of hemagglutinin antigen of Hong Kong-68 virus. Proc Natl Acad Sci U S A. 1969 Jun;63(2):326–333. doi: 10.1073/pnas.63.2.326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varghese J. N., Laver W. G., Colman P. M. Structure of the influenza virus glycoprotein antigen neuraminidase at 2.9 A resolution. Nature. 1983 May 5;303(5912):35–40. doi: 10.1038/303035a0. [DOI] [PubMed] [Google Scholar]
- Verhoeyen M., Fang R., Jou W. M., Devos R., Huylebroeck D., Saman E., Fiers W. Antigenic drift between the haemagglutinin of the Hong Kong influenza strains A/Aichi/2/68 and A/Victoria/3/75. Nature. 1980 Aug 21;286(5775):771–776. doi: 10.1038/286771a0. [DOI] [PubMed] [Google Scholar]
- Webster R. G., Hinshaw V. S., Laver W. G. Selection and analysis of antigenic variants of the neuraminidase of N2 influenza viruses with monoclonal antibodies. Virology. 1982 Feb;117(1):93–104. doi: 10.1016/0042-6822(82)90510-4. [DOI] [PubMed] [Google Scholar]
- Webster R. G., Laver W. G., Kilbourne E. D. Reactions of antibodies with surface antigens of influenza virus. J Gen Virol. 1968 Dec;3(3):315–326. doi: 10.1099/0022-1317-3-3-315. [DOI] [PubMed] [Google Scholar]
- Webster R. G., Reay P. A., Laver W. G. Protection against lethal influenza with neuraminidase. Virology. 1988 May;164(1):230–237. doi: 10.1016/0042-6822(88)90640-x. [DOI] [PubMed] [Google Scholar]
- Werner J., Schudrowitz C., Köhler H. Antigenic variation of neuraminidase of human type A influenza (H3N2) viruses isolated in Berlin (West). Zentralbl Bakteriol Orig A. 1975 Dec;233(4):440–446. [PubMed] [Google Scholar]
- Wilson A. C. The molecular basis of evolution. Sci Am. 1985 Oct;253(4):164–173. doi: 10.1038/scientificamerican1085-164. [DOI] [PubMed] [Google Scholar]



