Abstract
Programmed death-1 (PD-1) is an immunosuppressive receptor functionally bound with programmed death-ligand 1 (PD-L1), which has been reported in various malignancies. However, only a few studies are available for the clinical significance of PD-1/PD-L1 in nasopharyngeal carcinoma (NPC). In this study, we aim to investigate alterations in PD-1/PD-L1 by using immunohistochemistry analysis in a cohort of consecutively enrolled NPC patients (n = 99). To further analyse the correlation between PD-1/PD-L1 and factors involved in clinico-pathology, haematologic biomarkers, EBV-DNA load and outcomes, we collected clinical data for statistical analysis. We observed that lower haemoglobin (HB) and Body Mass Index (BMI) levels were associated with high levels of PD-L1 staining in NPC patients. Importantly, our results suggested that PD-L1 might be a negative indicator for NPC patients. In contrast, a correlation between the PD-1/PD-L1 level and EBV load was not identified. Moreover, PD-1 positivity was suggested to not be significantly correlated with clinical outcomes. Taken together, our results revealed that PD-L1 might be a potential prognostic biomarker for NPC patients. However, further studies are needed to clarify the underlying mechanism of EBV status in the immunosuppression process induced by the PD-1/PD-L1 axis.
Nasopharyngeal carcinoma (NPC) is an Epstein-Barr virus (EBV)-related malignancy, with an annual incidence of 30–80 per 10,000 in endemic regions, such as South China1. NPC is highly sensitive to radiotherapy and chemotherapy. Despite the development of precise radiotherapy technologies and combined chemotherapy, treatment failure for NPC remains quite frequent, with rates of approximately 10% for recurrence and 20% for distant metastasis2,3. Better and novel treatments are urgently needed to improve survival.
NPC is characterized by substantial lymphocytic infiltration (mainly of T cells) in the primary tumour4,5. Programmed death-1 (PD-1 or CD279) is an immunosuppressive receptor that is expressed in T cells6. PD-1 engagement by programmed death-ligand 1 (PD-L1 or CD274) in cancer cells decreases T cell activation and induce tumour immune escape6,7. It has been generally recognized that PD-1/PD-L1 protein abundance is associated with aggressive histology and worse prognosis in multiple tumour types, such as PD-1 in breast cancer8 and soft tissue sarcomas9, as well as PD-L1 in melanoma10 and renal cell carcinoma11. However, contradictory prognostic values have also been reported in several tumours. For example, PD-1-stained T cells were correlated with improved survival in follicular lymphoma12 and HPV-associated head and neck cancer13, and PD-L1 expression was associated with a better prognosis of pulmonary squamous cell carcinomas14 and breast cancer15. However, until recently, only a few studies on the PD-1/PD-L1 axis in NPC are available, and the reported prognostic role of PD-L1 in NPC patients remains inconsistent. For instance, Zhang et al.16 revealed that PD-L1 protein abundance was correlated with a worse outcome. In contrast, a positive correlation of PD-L1 expression and survival of non-metastatic NPC was delineated by Lee et al.17. In addition, Hsu et al.18 showed that PD-1 on CD8 T cells predicted a poor prognosis in a small size of NPC patients, whereas PD-1 expression might not be a prognostic factor in NPC patients when using a larger sample16. Therefore, the prognostic role of PD-1 and PD-L1 in NPC needs to be clarified.
The blockade of the PD-1 and PD-L1 pathway is one of the most promising strategies to activate anti-tumour immunity19. There are also ongoing clinical trials evaluating the safety and efficiency of anti-PD1 antibodies in NPC20. Clinical data have shown that the treatment response to PD-1/PD-L1 blockade was correlated with PD-1 or PD-L1 detected by immunohistochemistry (IHC)21,22. In addition to PD-1/PD-L1 expression, many host-related influences might account for the heterogeneous responses and failures during immunotherapies, such as nutritional status23, smoking24, and inflammation status25. The tumour microenvironment, including hypoxia and immunosuppressive cytokine production, also plays a role in the therapeutic response26. Baseline peripheral blood parameters have been reported as predictive biomarkers for the response to PD-1 antibodies27. Thus, additional information about the clinico-pathological characteristics and haematologic biomarkers related to PD-1/PD-L1 expression could promote a better understanding of their regulatory role in NPC and might be helpful for improving the response to anti PD-1/PD-L1 treatment.
In addition, PD-1 and PD-L1 could induce T cell deactivation during chronic viral infections28,29. The Epstein-Barr virus (EBV) has been reported to be strongly linked with NPC in epidemic areas30. EBV-DNA load significantly correlates not only with NPC development but also with treatment failure31. Experimental studies32 have suggested that there is an upregulation of PD-L1 in EBV-positive NPC cell lines. Despite improvement in preclinical research, only a little information is currently available for the correlation between EBV-DNA load and PD-1/PD-L1 expression in NPC.
Taken together, the prognostic value of PD-1/PD-L1 expression in NPC remains largely unknown. Detailed data on potential related biomarkers and EBV infection status with PD-1/PD-L1 are needed to be confirmed by clinical data. Therefore, considering the tremendous therapeutic potential of targeting the PD-1/PD-L1 interaction in NPC, we analysed the prognostic value of PD-1 and PD-L1 in a cohort of NPC patients (n = 99), which were consecutively recruited form a single medical care group over the course of two years. We also evaluated the correlation between PD-1/PD-L1 expression and clinico-pathological parameters as well as potential related haematologic biomarkers, such as the haemoglobin (HB) level. Our study might provide a new strategy for immune checkpoint-blocking therapy.
Results
General information
All of the nasopharynx biopsy samples (n = 99) were collected and histologically diagnosed as NPC by experienced pathologists. The samples were fixed with formalin and embedded into paraffin using a tissue processor. Among the 99 patients enrolled, including 68 males and 31 females, the median age was 47.5 years old (range from 20 to 78 years). All patients were treated with intensity-modulated radiation therapy (IMRT) with or without combined chemotherapy. The average follow-up time was 49.4 months (range from 6.7 to 64.9 months). In this cohort, 18 patients encountered disease progression (recurrence and/or metastasis), and 9 patients died during the follow-up period. All tumours were classified as the undifferentiated non-keratinizing phenotype. The characteristics of the enrolled patients are summarized in Supplementary Table S1.
Clinico-pathologic correlations
PD-1-positive immune cells were present in 44 of the total 99 tumours (44.4%), and PD-L1 staining was detectable in 96 patients (97.0%) and was mainly located at the membrane or in the cytoplasm region (or both) in the tumour cells. PD-L1 was also expressed in tumour-infiltrating lymphocytes (TILs) in a scattered manner. According to the ROC curve analysis for OS, the optimal cut-off value for the H-score was 155 (AUC: 0.780, sensitivity: 1.000, specificity: 0.419). Thus, when the H-score ≥155, PD-L1 staining was classified as being at a high level (61 cases). PD-1 positivity was defined as cases with PD-1 staining intensity ≥2 in more than 5% of TILs. Representative stainings of PD-1 and PD-L1 in NPC are shown in Figs 1 and 2. The optimal cut-off value for the nasopharynx gross tumour volume (GTVnx) was 29.6 mm3 (AUC: 0.740, sensitivity: 0.917, specificity: 0.505). In this study, neither PD-1 positivity nor a high level of PD-L1 staining was significantly correlated with the clinico-pathological parameters of age, gender, smoking status, family history of cancer, GTVnx or clinical stage at diagnose. Detailed data are summarized in Table 1
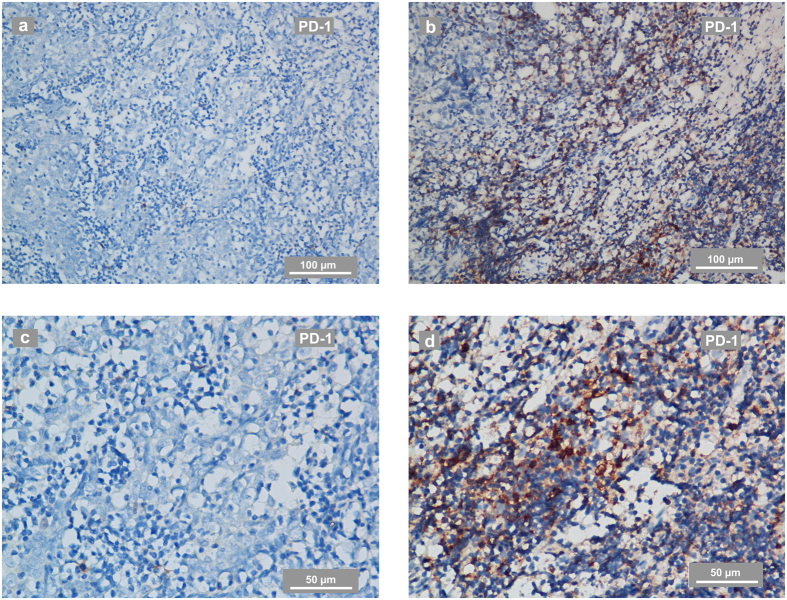
Figure 1. Representative IHC staining of PD-1 in NPC-biopsies.
(a,c) PD-1 staining in a biopsy from an NPC patient evaluated as PD-1 negative. The IHC photos were taken using phase-contrast microscopy and are shown at low (a) and high magnification (c). (b,d) PD-1 staining in a biopsy from an NPC patient evaluated as PD-1-positive. The IHC photos are shown at low (b) and high magnification (d). The scale bars for a-b are 100 μm and for c-d are 50 μm.
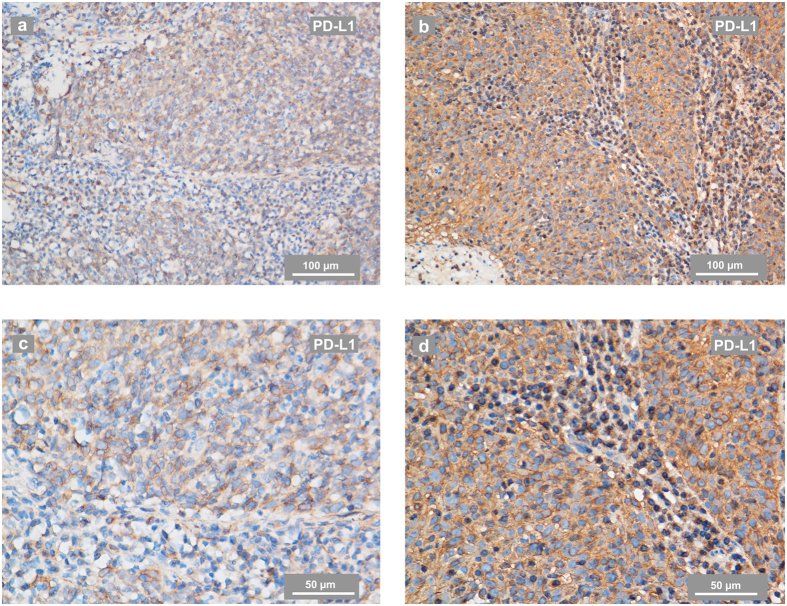
Figure 2. Representative IHC staining of PD-L1 in NPC-biopsies.
(a,c) PD-L1 staining in a biopsy from an NPC patient evaluated as having a low level of PD-L1. The IHC photos were taken using phase-contrast microscopy and are shown at low (a) and high magnification (c). (b,d) PD-L1 staining in a biopsy from an NPC patient evaluated as having a high level of PD-L1. The IHC photos are shown at low (b) and high magnification (d). PD-L1 expressed in tumour-infiltrating lymphocytes in a scattered manner. The scale bars for a-b are 100 μm and for c-d are 50 μm.
Table 1. Clinico-pathologic variables and immunoreactivity status of PD-1 and PD-L1 in 99 NPC patients.
| Variables | n | PD-1 Positive | P value1 | PD-L1 High2 | P value1 | |
|---|---|---|---|---|---|---|
| Gender | Male | 68 | 30 (44. 1%) | 0.923 | 44 (64.7%) | 0.349 |
| Female | 31 | 14 (45.2%) | 17 (54.8%) | |||
| Age (years) | <50 | 60 | 27 (45.0%) | 0.890 | 35 (58.3%) | 0.405 |
| ≥50 | 39 | 17 (43.6%) | 26 (66.7%) | |||
| Smoking status | Smoker or ex-smoker | 34 | 15 (44.1%) | 0.962 | 20 (58.8%) | 0.679 |
| Non-smoker | 65 | 29 (44.6%) | 41 (63.1%) | |||
| Family history | Yes | 25 | 14 (56.0%) | 0.179 | 13 (52.0%) | 0.253 |
| No | 74 | 30 (40.5%) | 48 (64.9%) | |||
| Tumour size | rT1-3 | 71 | 33 (46.5%) | 0.517 | 43 (60.6%) | 0.732 |
| rT4 | 28 | 11 (39.3%) | 18 (64.3%) | |||
| Nodal status | N0-1 | 54 | 22 (40.7%) | 0.417 | 33 (61.1%) | 0.910 |
| N2-3 | 45 | 22 (48.9%) | 28 (62.2%) | |||
| Clinical stage3 | I-III | 65 | 29 (44.6%) | 0.962 | 38 (58.5%) | 0.372 |
| IV | 34 | 15 (44.1%) | 23 (67.6%) | |||
| GTVnx (mm3)2 | <29.6 | 45 | 22 (48.9%) | 0.417 | 28 (62.2%) | 0.910 |
| ≥29.6 | 54 | 22 (40.7%) | 33 (61.1%) | |||
| EBV-DNA load | Undetectable | 22 | 10 (45.5%) | 0.914 | 12 (54.5%) | 0.439 |
| Detectable | 77 | 34 (44.2%) | 49 (63.6%) | |||
| PD-1 positivity | Negative | 55 | 31 (56.4%) | 0.230 | ||
| Positive | 44 | 30 (68.2%) | ||||
1Chi-square test.
2The optimal cut-off points for PD-L1 and GTVnx were determined by the receiver operating characteristic (ROC) curve for overall survival (OS).
3According to the 7th Edition of the AJCC/UICC Staging System for Nasopharyngeal Cancer.
BMI and HB in 99 NPC patients with heterogeneous immunoreactivity of PD-1/PD-L1
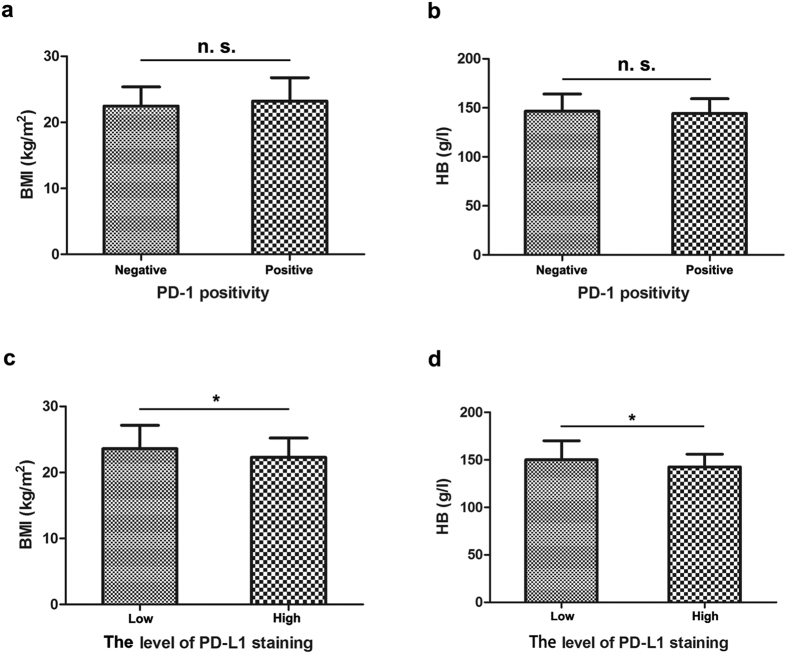
Weight and height were collected to calculate the body mass index (BMI), calculated by dividing the weight in kilograms by the square of the height in metres (kg/m2). The BMI of the 99 NPC patients ranged from 16.26 to 33.83 kg/m2. We compared the difference in the BMI level of patients with low or high levels of PD-L1 staining, and our results suggested that patients with a high level of PD-L1 staining had significantly lower BMI levels (P = 0.048, Fig. 3c). Our results also revealed that the HB level was significantly reduced in the patients with a high level of PD-L1 staining (P = 0.029, Fig. 3d). In contrast, we observed no significant difference in the BMI (a) or HB level (b) in patients with positive or negative PD-1 staining. (Fig. 3a,b).
Figure 3. BMI and HB in 99 NPC patients with different PD-1/PD-L1 levels.
(a,b) Statistical analysis suggested no significant (n. s.) differences in the BMI (a) or HB (b) levels between patients with positive or negative PD-1. (c,d) Statistical analysis revealed a significantly lower BMI (c) and HB (d) level in patients with high levels of PD-L1. A t-test was used to evaluate the association of PD1/PD-L1 with BMI and HB level after normality tests and homogeneity of variance test. *P < 0.05.
No significant association between PD-1/PD-L1 expression and EBV load
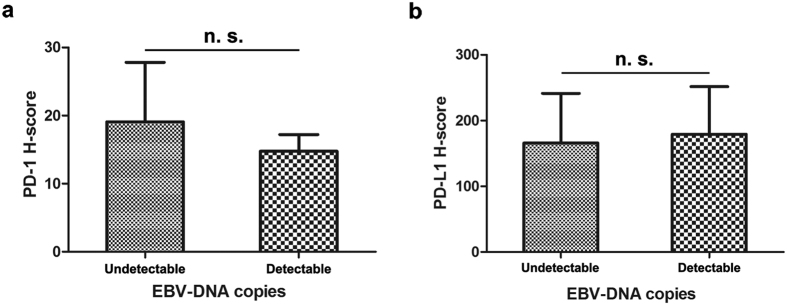
To evaluate the correlation between EBV viral load and immunoreactivity of PD-1 and PD-L1 in the NPC patients, we collected the qRT-PCR data of EBV-DNA copies from our database. In this cohort of 99 patients, EBV-DNA load was detectable in 77 patients. Non-parametric tests showed no significant difference in the PD-1 (P = 0.853, Fig. 4a) or PD-L1 H-scores (P = 0.389, Fig. 4b) between patients with a detectable or undetectable EBV-DNA load. Chi-square tests indicated that there was no significant association between the PD-1 (P = 0.914, Table 1) or PD-L1 level (P = 0.439, Table 1) and the EBV level.
Figure 4.
No significant differences in the PD-1 or PD-L1 H-score between NPC patients with undetectable or detectable baseline EBV-DNA copies Statistical analysis shows no significant (n. s.) difference in the PD-1 H-score (a) or the PD-L1 H-score (b) between patients with undetectable or detectable EBV loads. The differences were evaluated by non-parametric Mann-Whitney U test.
Prognostic values related with PD-1 and PD-L1
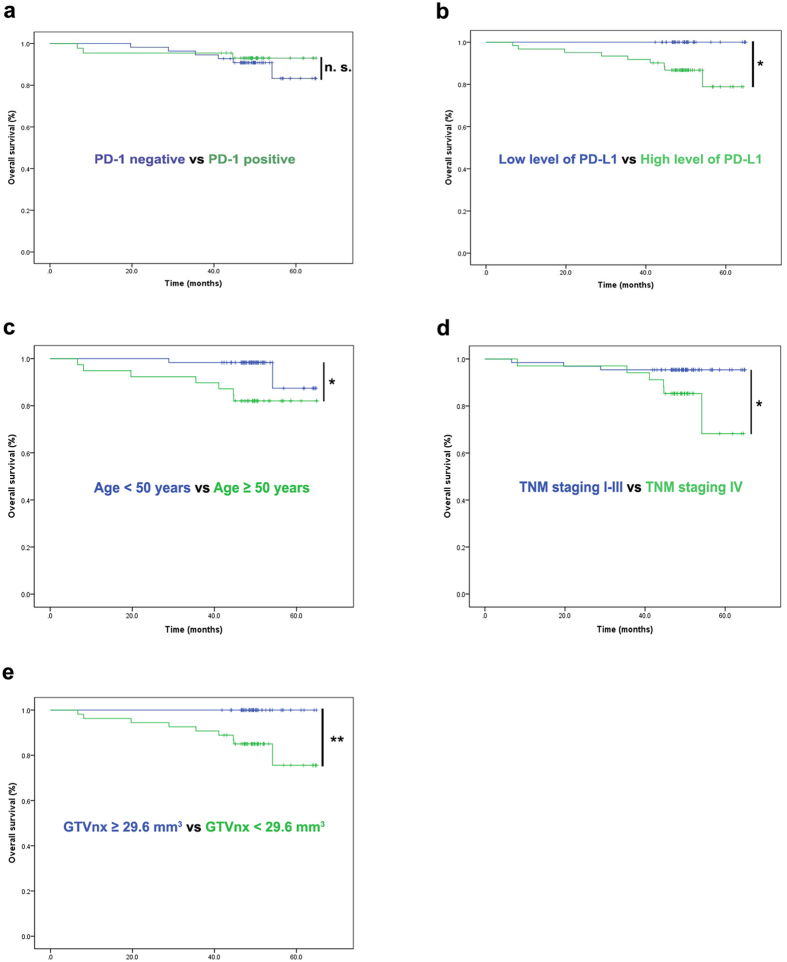
To evaluate the prognostic values in PD-1-positive tumour-infiltrating lymphocytes (TILs) and tumour cells with PD-L1 expression, we used the Kaplan-Meier survival analysis and log-rank tests. In this consecutively enrolled cohort of 99 NPC patients, our results revealed that a high expression of PD-L1 was correlated with shorter OS (P = 0.015, Fig. 5b) and showed trend of a reduced progression-free survival rate (PFS rate) (P = 0.127, Table 2). The factors significantly correlated with OS by univariate analyses were age (Fig. 5c), the level of PD-L1 staining (Fig. 5b), clinical stage (Fig. 5d), and GTVnx (Fig. 5e) before treatment. In contrast, we observed that the PD-1 positivity in TILs was not significantly correlated with OS (P = 0.563, Fig. 5a) or PFS (P = 0.616, Table 2). Moreover, in the PD-L1 highly expressed cases, the positivity of PD-1 could not further predict the OS (P = 0.399) and PFS of patients (P = 0.956). Multivariate analyses of prognostic factors were performed using a Cox regression model. T classification was not put in the multivariate analysis for it was found to be significantly correlated with clinical stage by a chi-square test (φ correlation coefficient = 0.868, P = 0.000) in this group of NPC patients. Thus, the variables included in the multivariate analysis for OS were age, clinical stage, GTVnx and PD-L1 H-score (Table 3). In the multivariate analysis, only the PD-L1 H-score was suggested to be an independent prognostic factor for OS. Patients with a higher PD-L1 H-score could have a greater risk of death (95% CI, 1.002–1.031, P = 0.028, Table 3).
Figure 5. Prognostic factors of the 99 NPC patients by Kaplan-Meier survival analysis.
(a) Statistical analysis suggested that PD-1 positivity on TILs was not significantly correlated with overall survival (OS). The results revealed significant associations between OS and high levels of PD-L1 (b), age (c), TNM staging (d) and GTVnx (e). The cut-off points for PD-L1 and GTVnx were generated from the ROC. Survival analysis used the Kaplan-Meier method. Univariate analysis was performed with a log-rank test. *P < 0.05, **P < 0.01.
Table 2. Univariate analysis of prognostic factors involved in survival.
| Cases (n = 99) | PFS |
OS |
||||
|---|---|---|---|---|---|---|
| Variables | X2 | P value1 | X2 | P value1 | ||
| Gender | Male | 68 | 0.725 | 0.394 | 0.344 | 0.558 |
| Female | 31 | |||||
| Age (years) | <50 | 60 | 2.327 | 0.127 | 5.551 | 0.018* |
| ≥50 | 39 | |||||
| Tumour size | rT1-3 | 71 | 5.030 | 0.025* | 6.959 | 0.008** |
| rT4 | 28 | |||||
| Nodal status | N0-1 | 54 | 2.227 | 0.136 | 0.005 | 0.941 |
| N2-3 | 45 | |||||
| Clinical stage3 | I-III | 65 | 2.306 | 0.129 | 4.372 | 0.037* |
| IV | 34 | |||||
| GTVnx (mm3)2 | <29.6 | 45 | 5.049 | 0.025* | 8.090 | 0.004** |
| ≥29.6 | 54 | |||||
| EBV-DNA load | undetectable | 22 | 0.324 | 0.569 | 0.005 | 0.942 |
| detectable | 77 | |||||
| Treatment | IMRT | 10 | 2.273 | 0.132 | 1.020 | 0.312 |
| IMRT+CT | 89 | |||||
| PD-1 positivity | negative | 55 | 0.251 | 0.616 | 0.334 | 0.563 |
| positive | 44 | |||||
| PD-L1 H-score2 | <155 | 38 | 2.327 | 0.127 | 5.870 | 0.015* |
| ≥155 | 61 | |||||
1Log-rank test.
2The optimal cut-off points for GTVnx and the PD-L1 H-score were determined by the receiver operating characteristic (ROC) curve for overall survival (OS).
3According to the 7th Edition of the AJCC/UICC Staging System for Nasopharyngeal Cancer. *P < 0.05, **P < 0.01, ***P < 0.001.
Table 3. Multivariate analysis of prognostic factors involved in survival.
| Variables | β | P value1 | Hazard ratio | 95% confidence interval |
|---|---|---|---|---|
| Age | 0.051 | 0.130 | 1.053 | 0.985–1.125 |
| Clinical stage (I-III vs IV)2 | −0.967 | 0.307 | 0.380 | 0.060–2.430 |
| GTVnx | 0.017 | 0.156 | 1.017 | 0.994–1.041 |
| PD-L1 H-score | 0.016 | 0.028* | 1.016 | 1.002–1.031 |
1Cox regression analysis. 2According to the 7th Edition of the AJCC/UICC Staging System for Nasopharyngeal Cancer. * P < 0.05.
Discussion
The association of the immunological checkpoint PD-1/PD-L1 and its prognosis of various cancers are currently a research hotspot19. PD-L1 has been reported to be overexpressed in most tumours including NPC16,33 to inhibit T cell-mediated antitumour immunity via PD-1 on TILs34. Recent studies have also revealed that PD-L1 was associated with poor prognosis in most epithelial-originated cancers35, suggesting an effect of PD-L1 in the induction of tumour progression by interrupting anti-tumour immunity36. However, the prognostic significance of PD-L1 as well as PD-1 in NPC has not been clarified yet. We collected biopsies and clinical data from 99 consecutively enrolled NPC patients to analyse the association of PD-L1/PD-1 and related factors. Our results suggested that NPC patients with a high level of PD-L1 had a significantly reduced survival outcome (Fig. 5b), which is consist with the results from 132 NPC patients16. However, another publication noted that there was a longer survival rate in non-metastatic NPC patients with a high PD-L1 level15, which might be explained by heterogeneous applications of different experimental procedures, scoring criteria, patient samples and survival endpoints. These differences could also illustrate why the PD-L1 expression rate was found to be much lower (25%) in the NPC patients reported by Lee et al.17 than that reported in our results (97.0%) and Zhang et al.16 (95.0%).
Furthermore, we also analysed the correlation between PD-1 positivity and the survival outcomes of NPC patients and found that there was no significant association (Fig. 5). Interestingly, previous publications have suggested a worse clinical outcome in NPC patients with PD-1-positive CD8 T cells (total n = 46)18. However, in a larger cohort (total n = 132), the impact of PD-1 was not obvious16.
As of now, standardized testing procedures and consistent scoring criteria for PD-1/PD-L1 in solid malignancies by IHC still need to be optimized35,37,38,39. The H-score has been applied in many IHC studies for its semi-quantitative characteristic39,40. Since no standard scoring criteria for PD-1 and PD-L1 are available35,38,41, we have used an ROC analysis to determine the cut-off value for PD-1 and PD-L1. However, our results suggested no significant cut-off value for PD-1 based on survival endpoints. The cut-off point was 155 for the PD-L1 H-score by ROC analysis in this study, while an intensity ≥217 or an H score ≥3516 was chosen to be the cut-off value in previous publications. As reported by Zhang et al.16, an H-score > 0 was applied as the cut-off value for PD-1. Consistently, we also found no significant correlation with the pathological features or prognostic role of PD-1 when the cut-off was defined as an H-score > 0. We raised the criteria of PD-1 positivity to a staining intensity ≥ 2 in more than 5% of the TILs (H-score ≥ 10), similar to the methods reported by D’Incecco et al.24, to minimize the potential interference of non-specific staining for PD-1.
Our study revealed a non-significant difference between the PD-L1 level and PD-1 positivity (Table 1), suggesting an indirect interaction of PD-L1 and PD-1 in NPC cells. PD-1 belongs to the CD28 family and is characterized as an inhibitory receptor expressed in T cells, dendritic cells (DC), natural killer (NK) cells, macrophages and B cells42. The PD-1 ligands, PD-L1 and PD-L2, belong to the B7 superfamily43. However, binding targets of PD-L1 and PD-L2 are not restricted to PD-1, since PD-L1 and PD-L2 can also bind to CD8044 and Repulsive Guidance Molecule b (RGMb)45, respectively. In addition, PD-L1 may be expressed in T cells, B cells, myeloid dendritic cells (DC) and in tissue macrophages in the tumour microenvironment42. It has also been reported that the predictive significance of PD-L1 can vary depending on if the positivity is defined on tumour cells or TILs46,47. We have found an increased level of PD-L1 in TILs (Fig. 2), and further studies are needed to clarify the underlying mechanism.
The identification of potential factors that can stratify a response to anti-PD-1 therapies will aid in the selection of NPC therapy. In NPC, experimental research has demonstrated an upregulation of PD-L1 by EBV-induced latent membrane protein 1 (LMP1) in EBV-positive NPC cell lines32. Recently, PD-L1 and PD-1 expression were found to be significantly associated with EBV-associated malignancies47,48,49, where EBV-encoded RNA was used to test for EBV infection in most studies. However, our results revealed no significant correlations between baseline EBV load and PD-1/PD-L1 expression (Table 1 and Fig. 4). One possible explanation could be that the circulating cell-free plasma EBV-DNA load might be an inactive remnant of a previously active EBV infection50. Moreover, our study also showed reduced BMI and HB levels in patients with high levels of PD-L1 staining (Fig. 3). Because of a large overlap in confidence intervals of our statistical results, the clinical significance between these factors still needs to be confirmed in larger samples. Notably, previous studies have confirmed that a low baseline HB is an adverse prognostic factor in patients with locally advanced head and neck squamous cell carcinoma51 and NPC52, by inducing treatment resistance via enhancing tumour hypoxia. Thus, we showed these findings to arouse attention for the possible biologic relationship between HB and PD-L1, which might be triggered by hypoxia, inducing immune deficiency through the PD-1/PD-L1 axis53. In addition, we have also compared PD-1/PD-L1 expression with a few baseline haematological parameters, such as neutrophil counts and the lactate dehydrogenase level from peripheral blood in this exploratory analysis, and there was no significant association observed for any of the relevant factors (Supplementary Fig. S1 and S2).
Our study revealed the prognostic role of age, PD-L1, GTVnx, T classification and clinical stage in the 99 NPC patients by log-rank test. Age, smoking history, EBV-DNA load, HB, GTVnx, and T and N classification as well as clinical stage have been reported to have a prognostic role in primary NPC patients in previous studies52,54,55,56,57. In our study, age 50 was chosen to be the cut-off value according to previous studies on NPC patients as reported by Chen et al.54. The baseline EBV-DNA loads were dichotomized to undetectable and detectable levels as reported by Liu et al.56. The optimal cut-off point of GTVnx (29.6 mm3) was determined by an ROC analysis in this study, which was consistent with previous studies in different NPC samples, where 19 mm3 (Guo et al.57) and 25 mm3 (Wu et al.58) were determined to be the optimal cut-off value by ROC analysis. Patients with T1-3 classification showed a similar survival rate in this study, which might be due to the satisfactory local control rates of NPC patients raised by IMRT59 and might be the reason that our multivariate analysis failed to identify the clinical stage as a significant prognostic factor.
In conclusion, PD-L1 and PD-1 are widely expressed in NPC tissue. However, PD-1 positivity could not predict prognosis in our study. Importantly, PD-L1 has been suggested to be a negative prognostic factor for NPC patients. Further studies with a larger sample size are needed to confirm these observations and to evaluate the predictive value of PD-1 and PD-L1 in NPC in the context of PD-1 inhibition as well. Moreover, more studies are warranted to identify the molecular mechanism of the interaction between HB, EBV and PD-1/PD-L1 in NPC.
Materials and Methods
Ethics Statement
The study was approved by the Institutional Review Board of Sun Yat-sen University Cancer Center. As this was a retrospective analysis of routine data, we requested and were granted a waiver of individual informed consent from the ethics committee. Patient records/information was anonymized and de-identified prior to analysis.
Patients and samples
For this study, 115 NPC patients were consecutively sampled by one medical care group at Sun Yat-sen University Cancer Center (Guangzhou, China) from December 2010 to December 2012. The cases were selected based on the following criteria: (1) histologically proven NPC with available biopsy specimens; (2) complete clinical data; (3) a new diagnosis; (4) non-distant metastatic NPC; (5) no treatment history or history of other malignant disease; (6) Karnofsky score ≥ 70; (7) received IMRT at Sun Yat-sen University; (8) regular follow-up. Patients with active hepatitis, diabetes, or who failed the follow-up requirements were excluded. Therefore, there were 99 cases that qualified for this study. All medical records were reviewed retrospectively, and all patients were restaged according to the 7th edition of the UICC/AJCC system. FFPE blocks of NPC were retrieved by fibrescope biopsy from the Department of Pathology at Sun Yat-sen University Cancer Center. The related clinical data of these patients were retrospectively collected from our database or hospital records, including gender, age, body weight, height, clinical stage, GTVnx (gross tumour volume of primary tumour), pre-therapy laboratory counts of neutrophils, lymphocytes, HB, platelets, albumin, lactate dehydrogenase (LDH), and EBV-DNA copies by quantitative PCR test, as well as follow-up records. All of the patients were followed-up for at least 40 months.
Radiotherapy
All patients received a computed tomography simulation scan at the radiotherapy position. The images included plain and enhanced computed tomography scans. The scope of each scan was from the top of the head to 2 cm below sub-clavicle head (thickness: 3 mm per slice). According to the institutional treatment protocol from Sun Yat-sen University Cancer Center, contouring of targets and organs at risk was performed under the IMRT planning system. The prescribed doses for the NPC patients were applied as follows: (1) planning target volume (PTV) of the primary gross tumour volume (GTVnx): 66–72 Gy at 2.12–2.43 Gy/fraction; (2) PTV of the GTV of the involved lymph nodes (GTVnd): 64–70 Gy; (3) PTV of the high-risk clinical target volume (CTV1): 60–63 Gy; (4) PTV of the low-risk clinical target volume (CTV2): 54–56 Gy. All targets were treated simultaneously using a simultaneous integrated boost technique.
Chemotherapy (CT)
According to the institutional treatment protocol from Sun Yat-sen University Cancer Centre, IMRT was applied for stage I disease as a sole treatment, concurrent chemoradiotherapy for stage II disease, and concurrent chemoradiotherapy (CCRT) +/− neoadjuvant/adjuvant chemotherapy for stage III to IVA-B disease. Among the 99 patients involved in this study, 59 patients (59.6%) received both neoadjuvant chemotherapy (NCT) and concurrent chemotherapy (CCRT); 2 patients (2.0%) received sole NCT; 28 patients (28.3%) received CCRT; and 10 (10.1%) patients received IMRT as a sole treatment. Neoadjuvant chemotherapy consisted of cisplatin (100 mg/m2) with 5-fluorouracil (4.0 g/m2), cisplatin (75 mg/m2) with toxoids (75 mg/m2) or triple agent treatment with cisplatin (70 mg/m2), 5-fluorouracil (3.0 g/m2) and toxoids (70 mg/m2) every three weeks for two or three cycles. Concurrent chemotherapy consisted of cisplatin (100 mg/m2) given in week 1, 4, and 7 of radiotherapy.
Immunohistochemistry staining
The samples were fixed with 4% paraformaldehyde (PFA) containing 2% sucrose in PBS at 4 °C overnight and embedded into paraffin using a tissue processor (EG1150, Leica, Germany). FFPE sections (3 μm thick) were cut with a rotation microtome (RM2255, Leica, Germany). Dewaxed paraffin sections were rehydrated by alcohol series, treated with 3% H2O2 for 10 min at room temperature and steam-heated for 2.5 min to retrieve the antigen using ethylene diamine tetra acetic acid buffer (PH = 8.0). Subsequent immunostaining was performed with a 50-min incubation period in 37 °C with the monoclonal antibodies for PD-1 and PD-L1 (Supplementary Table S2). The IHC staining of PD-1 and PD-L1 proteins was performed on two different slides. Tonsil tissue was taken as a positive control. Immunoreaction was visualized using a Peroxidase/DAB kit (Cat. K5007, Dako, Denmark). Images were taken with a phase contrast microscope (Eclipse 80i, Nikon, Japan). Details of all reagents with reference to the immunohistochemical staining procedure are listed in Supplementary Table S3.
Evaluation standard of IHC
The percentages of PD-L1-positive tumour cells and PD-1-positive lymphocytes and staining intensity were evaluated by two pathologists who counted 20 sequential high-power fields (0.54 mm field diameter) judged to be representative of the sample, while remaining blinded to clinical information. Staining intensity was scored as follows: 0 for negative or trace; 1 for weak; 2 for moderate; 3 for high. The percentage of stained cells (0–100%) was multiplied by the dominant intensity pattern of staining ranging from 0 to 3. Therefore, the overall semi-quantitative score (H-score) ranged from 0 to 300 (maximum value of 300 corresponding to 100% of tumour cells positive for PD-L1 or TILs positive for PD-1 with an overall staining intensity score of 3).
Statistical analysis
The optimal cut-off point for the expression level of each marker including the PD-L1 H-score and GTVnx were determined by the area under the curve (AUC) of the receiver operating characteristic (ROC) curve at the highest positive likelihood ratio point for overall survival (OS). PD-L1 expression was dichotomized into two groups (High and Low), using a cut-off score of ≥155. PD-1 positivity was defined as cases with PD-1 staining intensity ≥2 in more than 5% of TILs.
OS was measured from the date of diagnosis to the date of death or the date of the last follow-up through April 2016. PFS was measured from the date of diagnosis to the date of death or the date of tumour progression (recurrence and/or metastasis) through April 2016. Patients who were still alive at last contact or died from other causes were treated as censored for OS analysis.
A chi-square test was used to assess the correlation of the expression of PD-1 and PD-L1 with various clinical parameters such as age, clinical stage and EBV-DNA status. A t-test was used to compare the differences between the BMI, HB level, and neutrophil and lymphocyte counts in patients with different PD-1/PD-L1 immunoreactivity after a normality test (Kolmogorov-Smirnov test). A non-parametric Mann-Whitney U test was applied to compare the differences in albumin, LDH and the neutrophil/lymphocyte ratio in patients with different PD-1/PD-L1 immunoreactivities. A non-parametric Mann-Whitney U test was also applied to compare the differences in the PD-1/PD-L1 H-score in patients with different baseline EBV-DNA status. The number that follows the ± sign is the standard error (s.e.m.) in this article. Survival analysis was depicted by the Kaplan-Meier method defined by cut-off points generated from the ROC. Univariate analyses and multivariate analyses were performed with a log-rank test and Cox regression analysis, respectively. A P value < 0.05 was used to denote statistical significance, and all reported P values are two sided. P values were marked as *P < 0.05, **P < 0.01, ***P < 0.001, indicating different level of significance. These statistical analyses were performed with SPSS 16.0 48,49,50,51,52,53,54,55,56,57,58,59.
Additional Information
How to cite this article: Zhou, Y. et al. PD-L1 predicts poor prognosis for nasopharyngeal carcinoma irrespective of PD-1 and EBV-DNA load. Sci. Rep. 7, 43627; doi: 10.1038/srep43627 (2017).
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Material
Acknowledgments
We thank the Department of Nasopharyngeal Carcinoma from Sun Yat-sen University Cancer Center for providing the tissue samples. We thank Prof. Dr. Chuanhua Yu from the School of Public Health, Wuhan University for suggestions to improve our statistical analysis. This study was supported by the Natural Sciences Foundation of Hubei Province (grant number 2013CFA006). The funder had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
The authors declare no competing financial interests.
Author Contributions C.X., C.Z., W.D. and Y.Z. conceived and designed the experiments; Y.Z., J.M., H.W., J.C. and D.S performed the experiments; C.X., J.C., D.H., X.Z. and Y.Z. analysed the results; C.Z., J.M., W.D., Y.Z. and C.X. contributed reagents/materials/analysis tools; C.Z., Y.Z. and C.X. contributed to the writing of the manuscript. All authors reviewed the manuscript.
References
- Strazzulla A. et al. Nasopharyngeal carcinoma: review of the literature with a focus on therapeutical implications. Infez Med. 23, 224–229 (2015). [PubMed] [Google Scholar]
- Sun X. et al. Long-term outcomes of intensity-modulated radiotherapy for 868 patients with nasopharyngeal carcinoma: an analysis of survival and treatment toxicities. Radiother Oncol. 110, 398–403 (2014). [DOI] [PubMed] [Google Scholar]
- Li A. C. et al. Distant metastasis risk and patterns of nasopharyngeal carcinoma in the era of IMRT: long-term results and benefits of chemotherapy. Oncotarget. 6, 24511–24521 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khanna R. et al. Molecular characterization of antigen-processing function in nasopharyngeal carcinoma (NPC): evidence for efficient presentation of Epstein-Barr virus cytotoxic T-cell epitopes by NPC cells. Cancer Res. 58, 310–314 (1998). [PubMed] [Google Scholar]
- Pai S. et al. Nasopharyngeal carcinoma-associated Epstein-Barr virus-encoded oncogene latent membrane protein 1 potentiates regulatory T-cell function. Immunol Cell Biol. 85, 370–377 (2007). [DOI] [PubMed] [Google Scholar]
- Fife B. T. et al. Interactions between PD-1 and PD-L1 promote tolerance by blocking the TCR-induced stop signal. Nat Immunol. 10, 1185–1192 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carreno B. M. & Collins M. The B7 family of ligands and its receptors: new pathways for costimulation and inhibition of immune responses. Annu Rev Immunol. 20, 29–53 (2002). [DOI] [PubMed] [Google Scholar]
- Muenst S. et al. The presence of programmed death 1 (PD-1)-positive tumor-infiltrating lymphocytes is associated with poor prognosis in human breast cancer. Breast Cancer Res Treat. 139, 667–676 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J. R. et al. Tumor infiltrating PD1-positive lymphocytes and the expression of PD-L1 predict poor prognosis of soft tissue sarcomas. Plos One 8, e82870 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleffel S. et al. Melanoma Cell-Intrinsic PD-1 Receptor Functions Promote Tumor Growth. Cell. 162, 242–1256 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu F. et al. Clinicopathological and prognostic value of programmed death ligand-1 (PD-L1) in renal cell carcinoma: a metaanalysis. Int J Clin Exp Med. 8, 14595–14603 (2015). [PMC free article] [PubMed] [Google Scholar]
- Yang Z. Z. et al. PD-1 expression defines two distinct T-cell sub-populations in follicular lymphoma that differentially impact patient survival. Blood Cancer J. 5, e281 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badoual C. et al. PD-1-expressing tumor-infiltrating T cells are a favorable prognostic biomarker in HPV-associated head and neck cancer. Cancer Res. 73, 128–138 (2013). [DOI] [PubMed] [Google Scholar]
- Schmidt L. H. et al. PD-1 and PD-L1 Expression in NSCLC Indicate a Favorable Prognosis in Defined Subgroups. Plos One 10, e136023 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baptista M. Z., Sarian L. O., Derchain S. F., Pinto G. A. & Vassallo J. Prognostic significance of PD-L1 and PD-L2 in breast cancer. Hum Pathol. 47, 78–84 (2016). [DOI] [PubMed] [Google Scholar]
- Zhang J. et al. Co-expression of PD-1 and PD-L1 predicts poor outcome in nasopharyngeal carcinoma. Med Oncol. 32, 86 (2015). [DOI] [PubMed] [Google Scholar]
- Lee V. H. et al. Correlation of PD-L1 Expression of Tumor Cells with Survival Outcomes after Radical Intensity-Modulated Radiation Therapy for Non-Metastatic Nasopharyngeal Carcinoma. Plos One. 11, e157969 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu M. C. et al. Increase of programmed death-1-expressing intratumoral CD8 T cells predicts a poor prognosis for nasopharyngeal carcinoma. Mod Pathol. 23, 1393–1403 (2010). [DOI] [PubMed] [Google Scholar]
- Flemming A. Cancer: PD1 makes waves in anticancer immunotherapy. Nat Rev Drug Discov. 11, 601 (2012). [DOI] [PubMed] [Google Scholar]
- Jain A., Chia W. K. & Toh H. C. Immunotherapy for nasopharyngeal cancer-a review. Chin Clin Oncol. 5, 22 (2016). [DOI] [PubMed] [Google Scholar]
- Fehrenbacher L. et al. Atezolizumab versus docetaxel for patients with previously treated non-small-cell lung cancer (POPLAR): a multicentre, open-label, phase 2 randomised controlled trial. Lancet. 387, 1837–1846 (2016). [DOI] [PubMed] [Google Scholar]
- Spain L. & Larkin J. Combination immune checkpoint blockade with ipilimumab and nivolumab in the management of advanced melanoma. Expert Opin Biol Ther. 16, 389–396 (2016). [DOI] [PubMed] [Google Scholar]
- Bobbio A. & Alifano M. Immune therapy of non-small cell lung cancer. The future. Pharmacol Res. 99, 17–222 (2015). [DOI] [PubMed] [Google Scholar]
- D’Incecco A. et al. PD-1 and PD-L1 expression in molecularly selected non-small-cell lung cancer patients. Br J Cancer. 112, 95–102 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Concha-Benavente F. et al. Identification of the Cell-Intrinsic and -Extrinsic Pathways Downstream of EGFR and IFNgamma That Induce PD-L1 Expression in Head and Neck Cancer. Cancer Res. 76, 1031–1043 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitt J. M. et al. Resistance Mechanisms to Immune-Checkpoint Blockade in Cancer: Tumor-Intrinsic and -Extrinsic Factors. Immunity. 44, 1255–1269 (2016). [DOI] [PubMed] [Google Scholar]
- Martens A. et al. Baseline Peripheral Blood Biomarkers Associated with Clinical Outcome of Advanced melanoma Patients Treated with Ipilimumab. Clin Cancer Res. 22, 2908–2918 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto T. et al. Surface expression patterns of negative regulatory molecules identify determinants of virus-specific CD8+ T-cell exhaustion in HIV infection. Blood. 117, 4805–4815 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massanella M. et al. Methamphetamine Use in HIV-infected Individuals Affects T-cell Function and Viral Outcome during Suppressive Antiretroviral Therapy. Sci Rep. 5, 13179 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burrows J. M. et al. Selection pressure-driven evolution of the Epstein-Barr virus-encoded oncogene LMP1 in virus isolates from Southeast Asia. J Virol. 78, 7131–7137 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yip T. T., Ngan R. K., Fong A. H. & Law S. C. Application of circulating plasma/serum EBV DNA in the clinical management of nasopharyngeal carcinoma. Oral Oncol. 50, 527–538 (2014). [DOI] [PubMed] [Google Scholar]
- Fang W. et al. EBV-driven LMP1 and IFN-gamma up-regulate PD-L1 in nasopharyngeal carcinoma: Implications for oncotargeted therapy. Oncotarget. 5, 12189–121202 (2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L., Co-inhibitory molecules of the B7-CD28 family in the control of T-cell immunity. Nat Rev Immunol. 4, 336–347 (2004). [DOI] [PubMed] [Google Scholar]
- Lu J., Lee-Gabel L., Nadeau M. C., Ferencz T. M. & Soefje S. A. Clinical evaluation of compounds targeting PD-1/PD-L1 pathway for cancer immunotherapy. J. Oncol Pharm Pract. 21, 451–467 (2015). [DOI] [PubMed] [Google Scholar]
- Zhang Y. et al. Prognostic significance of programmed cell death 1 (PD-1) or PD-1 ligand 1 (PD-L1) Expression in epithelialoriginated cancer: a meta-analysis. Medicine (Baltimore). 94, e515 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardoll D. M., The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 12, 252–264 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr K. M. et al. Programmed Death-Ligand 1 Immunohistochemistry in Lung Cancer: In what state is this art? J Thorac Oncol. 10(7), 985–989 (2015). [DOI] [PubMed] [Google Scholar]
- Scognamiglio G. et al. Variability in Immunohistochemical Detection of Programmed Death Ligand 1 (PD-L1) in Cancer Tissue Types. Int J Mol Sci. 17(5), 790 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebelatto M. C. et al. Development of a programmed cell death ligand-1 immunohistochemical assay validated for analysis of nonsmall cell lung cancer and head and neck squamous cell carcinoma. Diagn Pathol. 811(1), 95 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budwit-Novotny D. A. et al. Immunohistochemical analyses of estrogen receptor in endometrial adenocarcinoma using a monoclonal antibody. Cancer Res. 46(10), 5419–5425 (1986). [PubMed] [Google Scholar]
- Chakravarti N. & Prieto V. G., Predictive factors of activity of anti-programmed death-1/programmed death ligand-1 drugs: immunohistochemistry analysis. Transl Lung Cancer Res. 4(6), 743–751 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boussiotis V. A., Chatterjee P. & Li L. Biochemical signaling of PD-1 on T cells and its functional implications. Cancer J. 20, 265–271 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L., Co-inhibitory molecules of the B7-CD28 family in the control of T-cell immunity. Nat Rev Immunol. 4, 336–347 (2004). [DOI] [PubMed] [Google Scholar]
- Ostrand-Rosenberg S., Horn L. A. & Alvarez J. A. Novel strategies for inhibiting PD-1 pathway-mediated immune suppression while simultaneously delivering activating signals to tumor-reactive T cells. Cancer Immunol Immunother. 64, 1287–1293 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao Y., et al. RGMb is a novel binding partner for PD-L2 and its engagement with PD-L2 promotes respiratory tolerance. J Exp Med. 211, 943–959 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellmunt J. et al. Association of PD-L1 expression on tumor-infiltrating mononuclear cells and overall survival in patients with urothelial carcinoma. Ann Oncol. 26, 812–817 (2015). [DOI] [PubMed] [Google Scholar]
- Li Z. et al. PD-L1 expression is associated with massive lymphocyte infiltration and histology in gastric cancer. Hum Pathol. 55, 182–189 (2016). [DOI] [PubMed] [Google Scholar]
- Fang W. et al. PD-L1 is remarkably over-expressed in EBV-associated pulmonary lymphoepithelioma-like carcinoma and related to poor disease-free survival. Oncotarget. 6, 33019–33032 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paydas S., Bagir E., Seydaoglu G., Ercolak V. & Ergin M. Programmed death-1 (PD-1), programmed death-ligand 1 (PD-L1), and EBV-encoded RNA (EBER) expression in Hodgkin lymphoma. Ann Hematol. 94, 1545–1552 (2015). [DOI] [PubMed] [Google Scholar]
- Hu H. et al. Epstein-Barr Virus Infection of Mammary Epithelial Cells Promotes Malignant Transformation. EBioMedicine. 9, 148–160 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghadjar P. et al. Haemoglobin and creatinine values as prognostic factors for outcome of concurrent radiochemotherapy in locally advanced head and neck cancers: Secondary results of two European randomized phase III trials (ARO 95-06, SAKK 10/94). Strahlenther Onkol. 192, 552–560 (2016). [DOI] [PubMed] [Google Scholar]
- Liang X. X. et al. Significant prognostic impact of chemoradiotherapy-induced hemoglobin decrease on treatment outcomes of nasopharyngeal carcinoma. J. Cancer. 6, 502–510 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barsoum I. B., Smallwood C. A., Siemens D. R. & Graham C. H. A mechanism of hypoxia-mediated escape from adaptive immunity in cancer cells. Cancer Res. 74, 665–674 (2014). [DOI] [PubMed] [Google Scholar]
- Chen C., Shen L. J., Li B. F. Gao J. & Xia Y. F. Smoking is a poor prognostic factor for male nasopharyngeal carcinoma treated with radiotherapy. Radiother Oncol. 110, 409–415 (2014). [DOI] [PubMed] [Google Scholar]
- Peng H. et al. Prognostic Impact of Plasma Epstein-Barr Virus DNA in Patients with Nasopharyngeal Carcinoma Treated using Intensity-Modulated Radiation Therapy. Sci Rep. 6, 22000 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L. T. et al. The Prognostic Value of Plasma Epstein-Barr Viral DNA and Tumor Response to Neoadjuvant Chemotherapy in Advanced-Stage Nasopharyngeal Carcinoma. Int J Radiat Oncol Biol Phys 93, 862 (2015). [DOI] [PubMed] [Google Scholar]
- Guo R. et al. Is primary tumor volume still a prognostic factor in intensity modulated radiation therapy for nasopharyngeal carcinoma? Radiother Oncol. 104(3), 294–299 (2012). [DOI] [PubMed] [Google Scholar]
- Wu Z., Zeng R. F., Su Y., Gu M. F. & Huang S. M. Prognostic significance of tumor volume in patients with nasopharyngeal carcinoma undergoing intensity-modulated radiation therapy. Head Neck. 35(5), 689–694 (2013). [DOI] [PubMed] [Google Scholar]
- Kang M. et al. A new staging system for nasopharyngeal carcinoma based on intensity-modulated radiation therapy: results of a prospective multicentric clinical study. Oncotarget. 7(12), 15252–15261 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.