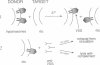Abstract
The variant surface glycoprotein (VSG) of trypanosomes is attached to the cell surface by means of a phosphatidylinositol-containing glycolipid membrane anchor. The studies presented in this paper support the hypothesis that the transfer of VSG from trypanosomes to erythrocytes could lead to one of the pathological features associated with trypanosome infection--i.e., anemia. Migration of trypanosome VSG from live trypanosomes to target cells (sheep erythrocytes) could be shown by preincubating erythrocytes with trypanosomes and subsequently testing the washed erythrocytes for insertion of VSG by their susceptibility to lysis by complement in the presence of an anti-VSG antibody. Complement-mediated lysis was found to depend on the strain-specific anti-VSG antibody used. Extent of erythrocyte lysis increased with time of cell exposure to trypanosomes and with trypanosome concentration. No erythrocyte lysis was observed when trypanosomes were preincubated with anti-VSG antibody before adding erythrocytes. Purified membrane-form VSG (which retains the glycolipid anchor), but not soluble VSG (which no longer has the terminal diacylglycerol moiety), could sensitize erythrocytes to anti-VSG antibody-mediated complement lysis. The intermembrane transfer of VSG from trypanosomes to cells of the infected host could provide a molecular mechanism for the pathogenesis of trypanosomiasis.
Full text
PDF
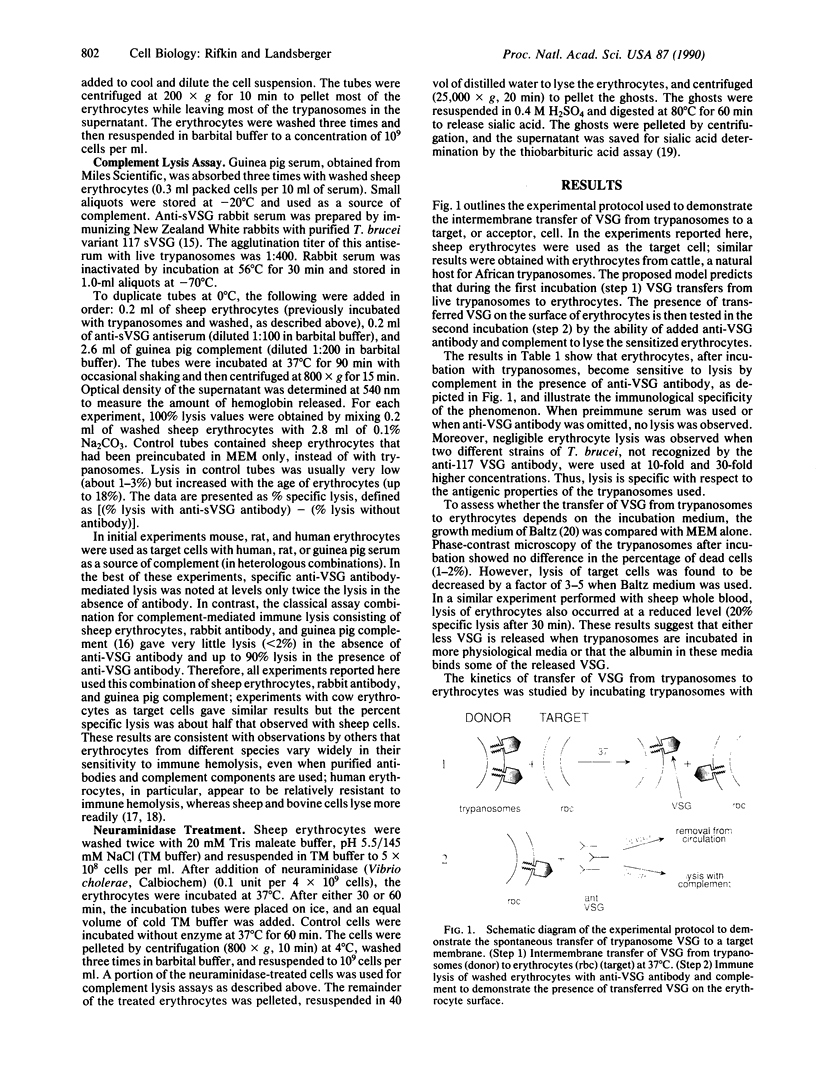
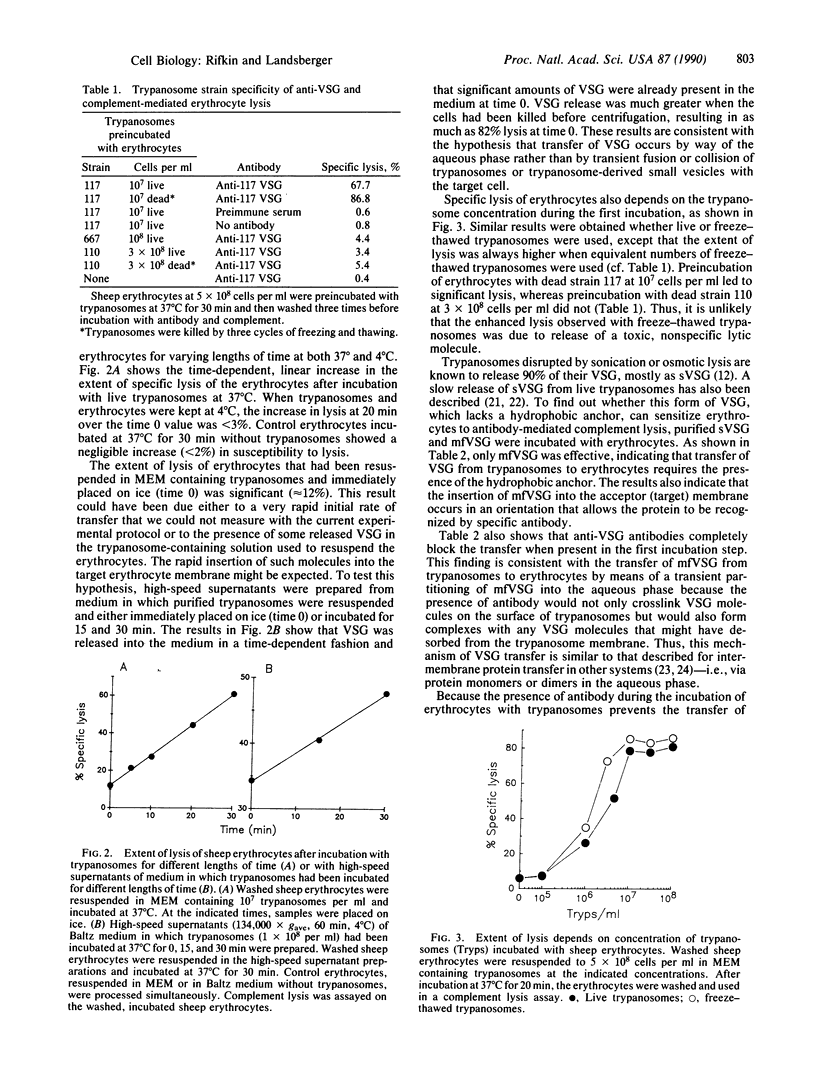


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amole B. O., Clarkson A. B., Jr, Shear H. L. Pathogenesis of anemia in Trypanosoma brucei-infected mice. Infect Immun. 1982 Jun;36(3):1060–1068. doi: 10.1128/iai.36.3.1060-1068.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baltz T., Baltz D., Giroud C., Crockett J. Cultivation in a semi-defined medium of animal infective forms of Trypanosoma brucei, T. equiperdum, T. evansi, T. rhodesiense and T. gambiense. EMBO J. 1985 May;4(5):1273–1277. doi: 10.1002/j.1460-2075.1985.tb03772.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouma S. R., Drislane F. W., Huestis W. H. Selective extraction of membrane-bound proteins by phospholipid vesicles. J Biol Chem. 1977 Oct 10;252(19):6759–6763. [PubMed] [Google Scholar]
- Bülow R., Nonnengässer C., Overath P. Release of the variant surface glycoprotein during differentiation of bloodstream to procyclic forms of Trypanosoma brucei. Mol Biochem Parasitol. 1989 Jan 1;32(1):85–92. doi: 10.1016/0166-6851(89)90132-1. [DOI] [PubMed] [Google Scholar]
- Capron A., Dessaint J. P., Capron M., Ouma J. H., Butterworth A. E. Immunity to schistosomes: progress toward vaccine. Science. 1987 Nov 20;238(4830):1065–1072. doi: 10.1126/science.3317823. [DOI] [PubMed] [Google Scholar]
- Cardoso de Almeida M. L., Turner M. J. The membrane form of variant surface glycoproteins of Trypanosoma brucei. Nature. 1983 Mar 24;302(5906):349–352. doi: 10.1038/302349a0. [DOI] [PubMed] [Google Scholar]
- Clarkson A. B., Jr, Bacchi C. J., Mellow G. H., Nathan H. C., McCann P. P., Sjoerdsma A. Efficacy of combinations of difluoromethylornithine and bleomycin in a mouse model of central nervous system African trypanosomiasis. Proc Natl Acad Sci U S A. 1983 Sep;80(18):5729–5733. doi: 10.1073/pnas.80.18.5729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook S. L., Bouma S. R., Huestis W. H. Cell to vesicle transfer of intrinsic membrane proteins: effect of membrane fluidity. Biochemistry. 1980 Sep 30;19(20):4601–4607. doi: 10.1021/bi00561a010. [DOI] [PubMed] [Google Scholar]
- Cross G. A. Eukaryotic protein modification and membrane attachment via phosphatidylinositol. Cell. 1987 Jan 30;48(2):179–181. doi: 10.1016/0092-8674(87)90419-3. [DOI] [PubMed] [Google Scholar]
- Cross G. A. Identification, purification and properties of clone-specific glycoprotein antigens constituting the surface coat of Trypanosoma brucei. Parasitology. 1975 Dec;71(3):393–417. doi: 10.1017/s003118200004717x. [DOI] [PubMed] [Google Scholar]
- Cross G. A. Release and purification of Trypanosoma brucei variant surface glycoprotein. J Cell Biochem. 1984;24(1):79–90. doi: 10.1002/jcb.240240107. [DOI] [PubMed] [Google Scholar]
- Davis C. E. Thrombocytopenia: a uniform complication of African trypanosomiasis. Acta Trop. 1982 Jun;39(2):123–133. [PubMed] [Google Scholar]
- Enoch H. G., Fleming P. J., Strittmatter P. Cytochrome b5 and cytochrome b5 reductase-phospholipid vesicles. Intervesicle protein transfer and oreintation factors in protein-protein interactions. J Biol Chem. 1977 Aug 25;252(16):5656–5660. [PubMed] [Google Scholar]
- Greenwood B. M. Asymptomatic malaria infections--do they matter? Parasitol Today. 1987 Jul;3(7):206–214. doi: 10.1016/0169-4758(87)90061-5. [DOI] [PubMed] [Google Scholar]
- Haas R., Brandt P. T., Knight J., Rosenberry T. L. Identification of amine components in a glycolipid membrane-binding domain at the C-terminus of human erythrocyte acetylcholinesterase. Biochemistry. 1986 Jun 3;25(11):3098–3105. doi: 10.1021/bi00359a005. [DOI] [PubMed] [Google Scholar]
- Haldar K., Ferguson M. A., Cross G. A. Acylation of a Plasmodium falciparum merozoite surface antigen via sn-1,2-diacyl glycerol. J Biol Chem. 1985 Apr 25;260(8):4969–4974. [PubMed] [Google Scholar]
- Herbert W. J., Inglis M. D. Immunization of mice, against T. brucei infection, by the administration of released antigen adsorbed to erythrocytes. Trans R Soc Trop Med Hyg. 1973;67(2):268–268. doi: 10.1016/0035-9203(73)90174-0. [DOI] [PubMed] [Google Scholar]
- Hereld D., Krakow J. L., Bangs J. D., Hart G. W., Englund P. T. A phospholipase C from Trypanosoma brucei which selectively cleaves the glycolipid on the variant surface glycoprotein. J Biol Chem. 1986 Oct 15;261(29):13813–13819. [PubMed] [Google Scholar]
- Hotez P. J., Le Trang N., Fairlamb A. H., Cerami A. Lipoprotein lipase suppression in 3T3-L1 cells by a haematoprotozoan-induced mediator from peritoneal exudate cells. Parasite Immunol. 1984 May;6(3):203–209. doi: 10.1111/j.1365-3024.1984.tb00793.x. [DOI] [PubMed] [Google Scholar]
- Huestis W. H., Newton A. C. Intermembrane protein transfer. Band 3, the erythrocyte anion transporter, transfers in native orientation from human red blood cells into the bilayer of phospholipid vesicles. J Biol Chem. 1986 Dec 5;261(34):16274–16278. [PubMed] [Google Scholar]
- Jarvinen J. A., Dalmasso A. P. Trypanosoma musculi: immunologic features of the anemia in infected mice. Exp Parasitol. 1977 Oct;43(1):203–210. doi: 10.1016/0014-4894(77)90024-8. [DOI] [PubMed] [Google Scholar]
- Lamont G. S., Tucker R. S., Cross G. A. Analysis of antigen switching rates in Trypanosoma brucei. Parasitology. 1986 Apr;92(Pt 2):355–367. doi: 10.1017/s003118200006412x. [DOI] [PubMed] [Google Scholar]
- Lanham S. M., Godfrey D. G. Isolation of salivarian trypanosomes from man and other mammals using DEAE-cellulose. Exp Parasitol. 1970 Dec;28(3):521–534. doi: 10.1016/0014-4894(70)90120-7. [DOI] [PubMed] [Google Scholar]
- Leto T. L., Roseman M. A., Holloway P. W. Mechanism of exchange of cytochrome b5 between phosphatidylcholine vesicles. Biochemistry. 1980 Apr 29;19(9):1911–1916. doi: 10.1021/bi00550a028. [DOI] [PubMed] [Google Scholar]
- Linscott W. D. Immune hemolysis: an inter-species study. J Immunol. 1967 May;98(5):991–1003. [PubMed] [Google Scholar]
- McKeone B. J., Massey J. B., Knapp R. D., Pownall H. J. Apolipoproteins C-I, C-II, and C-III: kinetics of association with model membranes and intermembrane transfer. Biochemistry. 1988 Jun 14;27(12):4500–4505. doi: 10.1021/bi00412a042. [DOI] [PubMed] [Google Scholar]
- Medof M. E., Kinoshita T., Nussenzweig V. Inhibition of complement activation on the surface of cells after incorporation of decay-accelerating factor (DAF) into their membranes. J Exp Med. 1984 Nov 1;160(5):1558–1578. doi: 10.1084/jem.160.5.1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medof M. E., Walter E. I., Roberts W. L., Haas R., Rosenberry T. L. Decay accelerating factor of complement is anchored to cells by a C-terminal glycolipid. Biochemistry. 1986 Nov 4;25(22):6740–6747. doi: 10.1021/bi00370a003. [DOI] [PubMed] [Google Scholar]
- Phillips R. E., Warrell D. A. The pathophysiology of severe falciparum malaria. Parasitol Today. 1986 Oct;2(10):271–282. doi: 10.1016/0169-4758(86)90136-5. [DOI] [PubMed] [Google Scholar]
- Rifkin M. R. Trypanosoma brucei: new radioisotope assay for quantitating cell lysis. Exp Parasitol. 1978 Dec;46(2):207–212. doi: 10.1016/0014-4894(78)90132-7. [DOI] [PubMed] [Google Scholar]
- Sadun E. H., Johnson A. J., Nagle R. B., Duxbury R. E. Experimental infections with African trypanosomes. V. Preliminary parasitological, clinical, hematological, serological, and pathological observations in rhesus monkeys infected with Trypanosoma rhodesiense. Am J Trop Med Hyg. 1973 May;22(3):323–330. doi: 10.4269/ajtmh.1973.22.323. [DOI] [PubMed] [Google Scholar]
- Shak S., Davitz M. A., Wolinsky M. L., Nussenzweig V., Turner M. J., Gurnett A. Partial characterization of the cross-reacting determinant, a carbohydrate epitope shared by decay accelerating factor and the variant surface glycoprotein of the African Trypanosoma brucei. J Immunol. 1988 Mar 15;140(6):2046–2050. [PubMed] [Google Scholar]
- Shapiro S. Z. Trypanosoma brucei: release of variant surface glycoprotein during the parasite life cycle. Exp Parasitol. 1986 Jun;61(3):432–437. doi: 10.1016/0014-4894(86)90199-2. [DOI] [PubMed] [Google Scholar]
- WARREN L. The thiobarbituric acid assay of sialic acids. J Biol Chem. 1959 Aug;234(8):1971–1975. [PubMed] [Google Scholar]
- Yamamoto K. I. Lytic activity of C5-9 complexes for erythrocytes from the species other than sheep: C9 rather than C8-dependent variation in lytic activity. J Immunol. 1977 Oct;119(4):1482–1485. [PubMed] [Google Scholar]