Abstract
Worldwide, osteoarthritis (OA) is one of the leading causes of chronic pain, for which adequate relief is not available. Ongoing peripheral input from the affected joint is a major factor in OA-associated pain. Therefore, this review focuses predominantly on peripheral targets emerging in the preclinical and clinical arena. Nerve growth factor is the most advanced of these targets, and its blockade has shown tremendous promise in clinical trials in knee OA. A number of different types of ion channels, including voltage-gated sodium channels and calcium channels, transient receptor potential channels and acid-sensing ion channels, are important for neuronal excitability and play a role in pain genesis. Few channel blockers have been tested in preclinical models of OA, with varying results. Finally, we discuss some examples of G-protein coupled receptors, which may offer attractive therapeutic strategies for OA pain, including receptors for bradykinin, calcitonin gene-related peptide, and chemokines. Since many of the pathways described above can be selectively and potently targeted, they offer an exciting opportunity for pain management in OA, either systemically or locally.
Keywords: Osteoarthritis, Pain, Targets, Ion channels, Nerve Growth Factor, GPCRs
Introduction
Osteoarthritis (OA) is a painful disease of synovial joints that results in failure of the joint as an organ [1]. It is by far the most common form of arthritis and pain associated with OA is quickly becoming one of the leading causes of chronic pain in the world [2]. This is because the prevalence of this chronic joint disease is increasing as the population ages and adequate treatments are not available [3, 4].
Chronic pain associated with OA can be generated, modified, and maintained at different levels along the neuraxis, all of which may provide opportunity for pharmacological intervention [5, 6]. There is ample clinical evidence to indicate that ongoing peripheral input from the affected joint drives OA pain. Firstly, intra-articularly administered local anesthetics alleviate knee pain [7]. Further, recent clinical trials with antibodies that neutralize the peripherally acting pain mediator, nerve growth factor (NGF), have shown promising analgesic efficacy [8]. Finally, in the majority of cases, total joint replacement results in pain relief [4]. Therefore, the focus of this narrative review is on peripheral mechanisms of pain, in particular nociceptor activation and sensitization. We will discuss emerging targets that show promise in preclinical models of OA and/or in clinical trials.
Peripheral mechanisms of pain
Acute pain is a normal physiological response to a potentially harmful event resulting in the avoidance of tissue damage. The neuronal substrates for pain include a variety of pathways such as the dorsal root ganglion and trigeminal sensory neurons in the periphery as well as central pathways linking the dorsal horn of the spinal cord to higher centers of consciousness [9]. Under some circumstances, including tissue damage and infection, plastic changes in pain pathways produce abnormal excitability and pain in the absence of normal stimulation. Small fiber neuropathies associated with numerous systemic disorders including diabetes mellitus, hyperlipidemia, amyloidosis, Fabry syndrome, celiac disease, sarcoidosis, and human immunodeficiency virus infection affect small non-myelinated C-fibers and lightly myelinated A□-fibers and present with pain and autonomic symptoms.
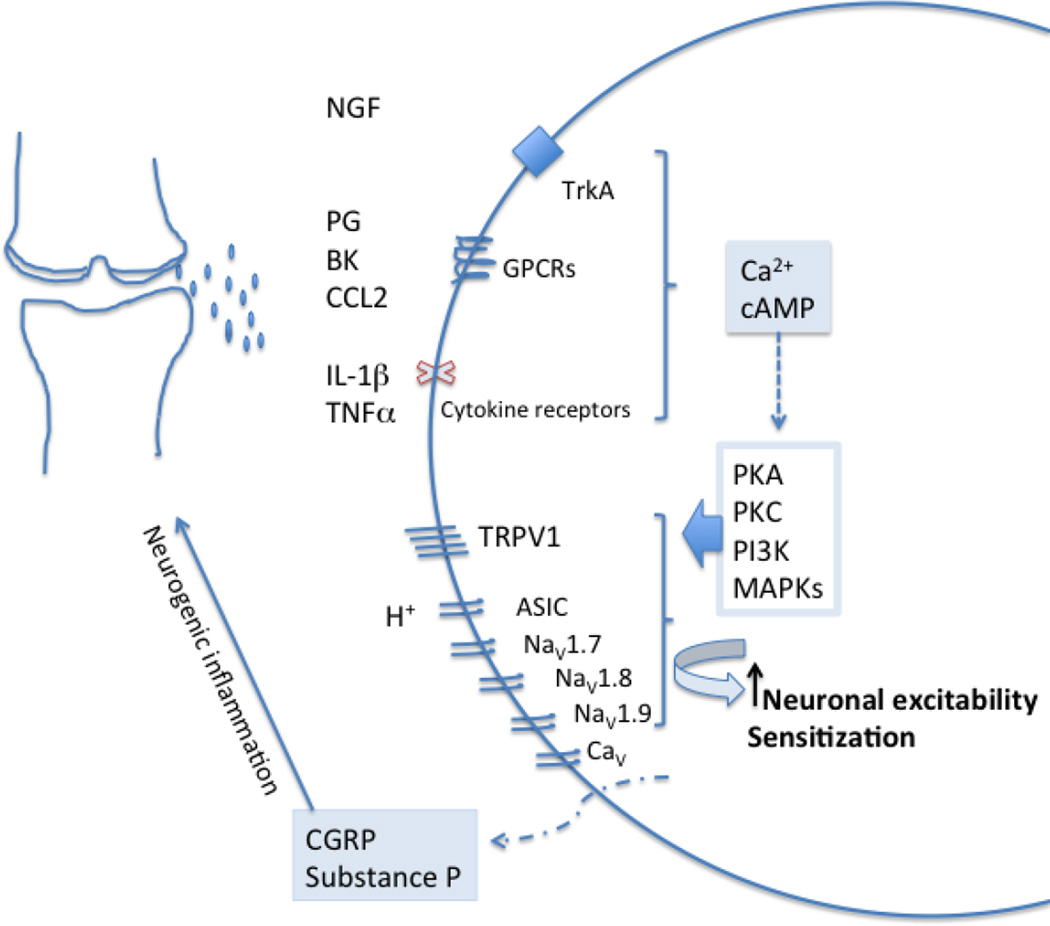
Tissue damage and inflammation result in release of a wide array of mediators that can bind specific receptors on nociceptors that innervate the affected tissues, resulting in different biological effects, including: neuronal excitation, eliciting pain; peripheral sensitization; and release of neuropeptides such as substance P and calcitonin-gene related peptide (CGRP), which contribute to neurogenic inflammation (Fig. 1). Excessive neuronal activity of primary sensory neurons can also trigger neuroinflammation, which is characterized by activation of satellite glial cells and infiltration by immune cells such as macrophages in the dorsal root ganglia (DRG), where the cell bodies of the sensory neurons reside [10, 11].
Fig. 1.
Inflammatory mediators present in the OA joint include prostaglandins, bradykinin, H+, nerve growth factor (NGF), pro-inflammatory cytokines (TNF-α, IL-1β), and pro-inflammatory chemokines such as CCL2. Receptors for all these mediators, including GPCRs, tyrosine kinase receptors, and cytokine receptors, are present on nociceptors. Their activation results in the generation of second messengers such as Ca2+ and cAMP, which in turn activates several kinases, such as the PKA, PKC, CaMK, PI3K, and MAPKs (ERK, p38, and JNK). This modulates key ion channels, such as transient receptor potential ion channel V1 (TRPV1) and voltage-gated sodium-channels, NaV1.7, NaV1.8 and NaV1.9, causing hypersensitivity and hyperexcitability of nociceptors. This process is known as peripheral sensitization. Nociceptor activation also results in the release of the neuropeptides, substance P and CGRP, which cause vasodilation and neurogenic inflammation (adapted from [11]).
The pathways outlined above (Fig. 1) indicate that a broad array of peripheral targets is potentially “druggable” with the aim of achieving relief of joint pain. However, few of these have been studied specifically in the context of experimental models of OA-related pain, and very few have been assessed in clinical trials in OA patients. Below, we will discuss a selection of novel peripheral targets that are emerging in the preclinical and clinical arena.
1. BLOCKADE OF NERVE GROWTH FACTOR ACTIVITY
Inhibition of NGF activity has been explored as a powerful strategy for pain management in OA. NGF is a member of the neurotrophin family, proteins that are critical to the development and maintenance of the nervous system. In addition, NGF is a pro-algesic molecule that contributes to peripheral nociceptor sensitization [12]. Experimental evidence for a pivotal role for NGF in OA joint pain is steadily increasing. Expression of NGF in the inflamed synovium of knee OA patients is associated with the presence of pain [13]. Other joint tissues can also produce NGF: many years ago, it was shown that human chondrocytes produce NGF and express its high affinity receptor, TrkA, and that these molecules are upregulated in OA [14]. Renewed interest in the contribution of NGF to joint pain led to several recent reports that stimuli present in the OA joint may promote production of NGF by chondrocytes, including interleukin (IL)-1β, the adipokine visfatin [15], transforming growth factor (TGF)β [16], as well as mechanical injury in murine cartilage [17]. Therefore, OA cartilage - though aneural -may provide a rich source of NGF and thus locally contribute to pain generation. In two rat OA models, meniscal transection and mono-iodoacetate (MIA)-induced OA, it was shown that pain responses to NGF locally administered into the knee cavity are increased in OA compared to non-OA joints [18], indicating that local production of NGF may be crucial for the generation of pain. In the rat MIA model, a single dose of anti-NGF antibody exerted a long-lasting beneficial effect on pain during motion, as assessed by monitoring gait [19]. A soluble NGF receptor fragment containing the NGF binding domain, TrkAd5, had a beneficial effect on weightbearing deficits 16 weeks after destabilization of the medial meniscus (DMM) in the mouse [20]. A recent clinical trial in dogs with degenerative joint disease suffering from chronic pain demonstrated a clear analgesic effect of an anti-NGF antibody [21].
There are different strategies for reducing NGF activity [22], including monoclonal antibodies that bind and sequester free NGF as well as small molecules that block its high-affinity receptor, TrkA. Initial clinical trials with monoclonal anti-NGF antibodies in knee OA patients were very encouraging but abruptly halted in 2010 after reports of unexpected accelerated joint destruction leading to total joint replacement, especially in subjects where anti-NGF antibodies were combined with non steroidal anti-inflammatory drugs (NSAIDs). Trials have now resumed (reviewed in [8]). Selective small molecule inhibitors of TrkA are in development and their analgesic efficacy has been demonstrated in preclinical models of OA [23, 24]. A first-in-human study with ascending single intra-articular doses of the TrkA inhibitor, GZ389988, in patients with painful osteoarthritis of the knee has recently commenced (https://clinicaltrials.gov/ct2/show/NCT02424942?term=trka+and+osteoarthritis&rank=1).
2. ION CHANNELS
DRG neurons that mediate pain signaling under physiological conditions express a number of different types of ion channels that can enhance neuronal excitability (Fig. 1). Electrogenesis in DRG and trigeminal neurons is dependent on the activity of a variety of voltage-dependent ion channels, including voltage-gated sodium and calcium channels (VGSC and VGCC). Changes in the properties of these channels may drive hyperexcitability in a variety of chronic pain states and so they are widely believed to be suitable drug targets for the treatment of chronic pain conditions.
2.1. Voltage-gated sodium channels (VGSC)
VGSCs are responsible for the upstroke of the action potential as well as other features that have important effects on neuronal excitability. The molecules that form the pore of these channels are large multidomain proteins with molecular weights of around 270 kDa. VGSCs in mammalian cells consist of a gene family encoding 9 different proteins which exhibit an approximately 50% sequence homology. The action potential in DRG neurons is particularly dependent on NaV1.3, NaV1.7, NaV1.8 and NaV1.9. These channels are all found in non-myelinated and thinly myelinated DRG neurons, populations that have been traditionally associated with pain. NaV1.7 channels are also found in sympathetic neurons. NaV1.3 is normally expressed in embryos but can also be upregulated in DRG neurons in response to tissue damage. Both NaV1.3 and NaV1.7 are blocked by the classic sodium channel blocker, tetrodotoxin, whereas NaV1.8 and NaV1.9 are resistant to this toxin (reviewed in [25, 26]. The role of these channels in the genesis of pain has been particularly indicated through several mutations recently detected in the human population. For example, loss of function mutations in SCN9A, the gene encoding NaV 1.7, results in dramatic reductions in pain sensation in the few families that are known to have survived with these types of mutations [27]. Dominant gain-of-function mutations in SCN9A were first described in two severe pain syndromes, inherited erythromelalgia (IEM) and paroxysmal extreme pain disorder (PEPD) [28, 29] both of which are autosomal dominant. These observations have encouraged the idea that small molecule NaV1.7 blockers may be effective treatments for pain. The mechanism of action of these substances, however, may not be completely clear as it has also been recently demonstrated that strong antagonism of NaV1.7 activity leads to strongly increased expression of opioid peptide levels, which may contribute to the analgesic effects of loss of NaV1.7 function. Whatever their precise mechanism, several of these substances have entered the drug development pipeline (for recent review, see [30]).
In the context of OA, one study reported a single nucleotide polymorphism (SNP) in SCN9A that was correlated with increased pain sensitivity in OA patients [31], but this could not be replicated in larger cohorts [32]. However, because several different types of sodium channels contribute to nociceptor electrogenesis, blockers of several of these might be useful therapeutically in OA pain. In preclinical models, the effect of selective blockade of either NaV1.7 or NaV1.8 has been explored. In rat MIA, a selective blocker of NaV1.8 significantly reduced the firing rate of joint afferents during noxious rotation of the joint but had no effect during non-noxious rotation, while injecting the compound into the knee cavity attenuated hindlimb incapacitance and secondary allodynia [33]. ProTxII, a tarantula toxin that potently and selective inhibits NaV1.7 channels, or the selective NaV1.8 blocker, A-803467, inhibited neuronal responses evoked by both low-threshold and suprathreshold stimuli in MIA but not control rats, indicating that these channels contribute to arthritic pain [34]. The role of sodium channels in OA pain is also supported by the observation that lacosamide, an anticonvulsant drug that acts as a state-dependent sodium channel blocker, reduced hypersensitivity to mechanical stimuli in the same model, although it failed to correct weightbearing deficits [35]. Information on the effect of selective blockade of VGSC in more translational models of OA [36] is not yet available, but can be expected to provide very useful information with respect to the specific relative contribution of these channels to OA-related pain.
2.2. Voltage-gated calcium channels (VGCC)
In addition to VGSCs, voltage-sensitive calcium channels (VGCCs) may also be targets for OA pain. N-type calcium channels (CaV2.2) are known to be the major route for Ca2+ entry into the nerve terminals of nociceptors. Therefore, blockers of these channels would be expected to produce antinociceptive effects by reducing transmitter release. For example, ziconitide, a peptide CaV2.2 blocker obtained from a Cone snail, has been shown to be effective in several chronic pain conditions [37]. It has been suggested that VGCCs are important for mechanical excitability of slowly conducting afferents innervating the rat knee [38]. Spinal or systemic administration of the CaV2 channel blocker, N-triazole oxindole (TROX-1), significantly reduced neuronal measures of nociception in the rat MIA model [39].
There has also been wide interest in the use of gabapentinoids such as gabapentin and pregabalin in the treatment of chronic pain conditions, including OA. It is most likely that the beneficial effects of these molecules are due to their binding to the α2δ non-pore forming subunit of VGCCs. Hence, both VGCCs in the DRG and the central and peripheral terminals of nociceptors may be targeted by gabapentinoids. Both gabapentin and pregabalin have also been shown to have beneficial effects in the rat MIA model [40] and in surgical models in rats and mice [41, 42].
2.3. Transient Receptor Potential Channels (TRP)
Transient Receptor Potential (TRP) channels are a superfamily of non-selective cation channels widely distributed throughout the body. These channels can be activated by a variety of animal and plant substances that produce pain and other sensory effects. These include the well-known effects of the hot pepper-derived molecule, capsaicin (TRPV1), the cooling substances menthol and eucalyptus (TRPM8) and several irritants such as wasabi and mustard oil (TRPA1) (for review, see [43]). Members of the TRP vanniloid (TRPV) family, TRPV1 in particular, are highly expressed in nociceptive neurons and can be activated by noxious stimuli either directly (temperature, protons) or indirectly through transactivation by activation of receptors such as TrkA (NGF) or diverse G-protein coupled receptors (GPCRs) (bradykinin, prostanoids, chemokines) (Fig. 1). Not only are these channels involved in physiological pain responses but their phosphorylation and other modifications results in sensitization in diverse chronic pain conditions. The role of TRPVI has been investigated in particular and, following its discovery [44], numerous small molecule antagonists or desensitizing agonists were produced with the expectation that they would be therapeutically effective. Unfortunately, their clinical development has been restricted due to a variety of side effects such as hyperthermia that were rapidly apparent [45]. Nevertheless, the important roles that these channels play in pain physiology has encouraged the view that they should still be considered potential targets for pain conditions including OA [46]. A second generation of TRP channel modulators is now under development with the aim of trying to divest these molecules of their side effects.
TRPV1 has been implicated in genetic susceptibility to symptomatic knee OA [47]. Human OA synovium displays increased TRPV1 immunoreactivity and both local and systemic administration of the selective TRPV1 receptor antagonist, JNJ-17203212, reversed sensitization of joint afferents and inhibited weight-bearing asymmetry in the rat MIA model of OA pain [48].
TRPV1 can also be targeted by channel agonists, such as capsaicin and the potent vanilloid analogue, resiniferatoxin, which cause an initial burning pain followed by a desensitization of the primary afferents. Topical capsaicin derivatives (creams) are used in OA (reviewed in [49]), and compounds for intra-articular administration are in development (https://clinicaltrials.gov/ct2/show/NCT02558439).
Interestingly, it was recently reported that TRPV1 channels are molecular targets of hyaluronan (HA) [50], which is used intra-articularly in knee OA and reportedly provides analgesia. In this study, HA reduced excitability of TRPV1 channels, which may be exploited to optimize its analgesic properties.
2.4 Acid Sensing Ion Channels (ASIC)
ASIC channels are a group of amiloride sensitive non-specific voltage insensitive cation channels that are activated by extracellular protons. Each channel represents a homo or heterotrimer formed from a family of six ASIC protein subunits. ASIC channels are found in both neuronal and non-neuronal tissues and are highly expressed in small nociceptive DRG neurons. Activation of ASIC channels in these neurons produces an excitatory depolarizing current and hypersensitive pain behavior (reviewed in [51]). The potential role of ASIC channels in pain physiology is illustrated by the effects of a number of toxins. For example, toxins from the venom of the Texas coral snake strongly activated ASIC channels in DRG neurons accompanied by intense pain [52]. On the other hand, toxins isolated from the venom of the black mamba (mambalgins) blocked pain behavior in a variety of experimental paradigms (formalin test, carrageenan etc). In some tests the effects of mambalgins approached those produced by morphine [53].
In rats, repeated injections of acidic saline into the knee joint cavity induced weight-bearing asymmetry and decreased knee compression and paw withdrawal threshold. This appeared to be mediated through ASIC3, since a selective ASIC3 antagonist (but not ASIC1 or TRPV blockers) reduced the observed hyperalgesia [54]. ASIC channels are targeted by a number of other toxins from a wide variety of species. In rat MIA, daily intra-articular administration of APETx2, a 42-amino acid toxin component from the venom of the sea anemone Anthopleura elegantissima [55], reduced pain-related behavior and secondary hyperalgesia [56]. Similar effects were produced by the non-specific ASIC blocker amiloride.
3. G-PROTEIN COUPLED RECEPTORS (GPCRs)
The human genome codes for some 800 GPCRs, representing about 4% of the genome. GPCRs are well represented in all of the cells that constitute the peripheral and central components of the pain pathway. GPCRs have proved to be highly “druggable” targets in the past [57]. Depending on the G-protein involved in signaling, GPCRs may have excitatory or inhibitory effects on pain neurons. Examples of the influence of GPCRs on pain are the pro-algesic effects of bradykinin and the analgesic effects of morphine. In some instances, important GPCRs are expressed directly by nociceptive neurons and in other instances by cells in direct communication with these neurons. In either case, the pro- or analgesic effects of activating different GPCRs may offer attractive therapeutic strategies depending on their role in OA. Major examples are discussed below.
3.1 Bradykinin Receptors
Kinins such as bradykinin (BK) and Lys-BK are peptides involved in both acute and chronic inflammation. They induce prostaglandin synthesis and contribute to symptoms that include pain, vasodilation, and edema, indicating a putative role for kinins at several levels of OA pathology [58]. There are at least two kinin receptors, B1 and B2, which belong to the Class I of GPCRs. B1 receptors mediate the effects of C-terminal des-Arg kinin metabolites, while B2 receptors mediate the effect of BK and Lys-BK. It appears that B2 receptors are expressed not only on knee nociceptors but also on synoviocytes and chondrocytes [58]. BK has been shown to evoke release of prostaglandin E2 and maybe CGRP in knee joint preparations of STR/N1 mice, which spontaneously develop OA [59]. Observations such as these have encouraged the view that B2 antagonists might be therapeutically useful in OA. The synthetic decapeptide, Icatibant, is a potent, stable, specific, and long acting antagonist of the B2 receptor while Fasitibant is a small-molecule B2 receptor antagonist. Results of clinical trials with intra-articular injection of these inhibitors in subjects with painful knee OA have been encouraging [60, 61] and a large randomized placebo-controlled trial was recently completed (https://clinicaltrials.gov/ct2/show/study/NCT02205814)
3.2 CGRP and Substance P Receptors
Nerves containing the peptide neurotransmitter CGRP have been widely implicated in a number of pain scenarios. In particular the peripheral terminals of CGRP-containing DRG nociceptors innervate several structures in the knee and show alterations in their pattern of innervation in OA [62, 63]. Release of CGRP from the peripheral terminals of nociceptors is thought to play an important role in the genesis of neurogenic inflammation and other pain-associated phenomena. For example, the vasodilation of cerebral blood vessels observed in association with migraine is thought to be a consequence of CGRP release and antagonism of the action of CGRP by small molecule receptor antagonists or antibodies to CGRP itself is currently a promising therapy for migraine [64]. CGRP release has been observed in the joints of OA affected rodents and CGRP receptors are expressed by different joint tissues. In rat models of OA (MIA and MMT), a role for CGRP in peripheral sensitization has been demonstrated [65]. Such observations have indicated that drugs or antibodies that antagonize the effects of CGRP may be useful in counteracting the effects of this peptide in OA. Indeed, a newly developed antibody against CGRP had a potent and prolonged analgesic effect in preclinical models, as measured by weight-bearing asymmetry [66].
In addition to CGRP, it is well established that neuropeptides of the tachykinin family, particularly substance P and neurokinin A, are also localized in a large number of nociceptors and it is thought that release of these peptides in the dorsal horn and from peripheral terminals can produce pain and neurogenic inflammation, respectively. The tachykinin receptor, NK1R, is expressed by neurons in the dorsal horn and tachykinins can be released from central terminals of nociceptors. Tachykinin receptors are also expressed peripherally on blood vessels and a variety of immune cells. These data have encouraged the idea that NK1R antagonists might be effective interventions in pain. However, although several small molecule NK1R antagonists have shown promise in preclinical studies they are yet to show clinical effectiveness in humans, including patients with osteoarthritis pain [67, 68].
Finally, there are numerous other neuropeptides associated with DRG neurons (e.g., VIP, NPY, galanin). Here again, in preclinical studies these substances can be shown to modulate pain related behaviors but the therapeutic potential of drugs that block receptors for these neuropeptides in OA pain remains to be established.
3.3 Chemokine Receptors
Chemokines are a family of small, secreted proteins that have been shown to play a fundamental role in leukocyte chemotaxis and tissue/stem cell development. Several investigations noted the expression of chemokine receptors in the nervous system including DRG nociceptors [69] and the CXCR4 receptor was shown to be responsible for guiding the migration of DRG progenitors from the neural crest during development [70]. In the adult, the expression of several chemokines and their receptors was shown to be upregulated in the DRG in association with the development of chronic pain. Electrophysiological studies demonstrated that activation of chemokine receptors expressed in the DRG had a potent excitatory effect, indicating that chemokine signaling may play an important role in the genesis of hyperexcitability in chronic pain [69].
Although the expression of several chemokine receptors by DRG neurons has been demonstrated, particular attention has been centered on the role of the chemokine, monocyte chemoattractant protein (MCP)-1 (CCL2), and its receptor, CCR2. CCR2 antagonists and knock out of the Ccr2 gene protected mice from pain hypersensitivity in a number of mouse models [71, 72]. In a surgical mouse model of OA, CCL2/CCR2 were found to be highly expressed in DRG neurons and Ccr2 null mice showed an abbreviated pain phenotype, while a CCR2 antagonist was also able to ameliorate pain behaviors [73]. Hence, it has been suggested that chemokine receptor antagonists, and CCR2 antagonists in particular, may be a novel therapeutic intervention in OA. CCR2 antagonism has been viewed as a promising drug mechanism in OA and several specific CCR2 antagonists are undergoing clinical trials. However, no positive data have been forthcoming to date.
3.4 Proteinase-activated receptors (PARs)
PARs constitute a unique family of GPCRs that are widely expressed, including on sensory neurons. Signaling through PARs requires serine-protease-mediated proteolytic cleavage of the extracellular domain, which reveals a new N-terminus that then acts as the ligand for the receptor. Evidence is increasing that PARs play a role in inflammatory pain, including arthritis pain (reviewed in [74]). PAR2 is expressed in DRG neurons that innervate the rat knee, and its activation resulted in joint nociceptor firing rate during non-noxious and noxious rotation of the knee [75]. Different strategies to target these receptors for pain relief are being explored, with many examples in preclinical models [74]. For instance, selective blockade of PAR4 inhibited PAR4-ligand-induced firing of joint nociceptors in rats [76]. However, data in experimental or clinical OA pain are not yet in the public domain.
4. OTHER TARGETS
Naturally, these are not the only potential targets for novel therapeutic interventions in the periphery. For example, botulinum neurotoxins A1 and B1 can alter nociceptive processing when administered locally into joints, and there is great interest in their potential application for OA pain (reviewed in [77]). Manipulation of the cannabinoid system is also heavily studied as an approach for OA pain management [78]. Efforts have been made to produce agents that only activate CB1 cannabinoid receptors peripherally and so are free of any psychotropic effects. Finally, several inflammatory cytokines are elevated in the OA joint, and these cytokines may well contribute to sensitization of sensory nerves innervating the joint, and thus to pain (for a detailed review, see [79]). Clinical trials up to date have had mixed results. Monoclonal antibodies against TNF-α had an analgesic effect in a small open-label trial in symptomatic knee OA with joint effusion [80], but were not efficacious in a randomized, placebo-controlled trial in patients with hand OA who were unresponsive to analgesics and NSAIDs [81]. Intra-articular administration of the IL-1 receptor antagonist, anakinra, was not efficacious in a multicenter, randomized, double-blind, placebo-controlled study in patients with symptomatic radiographic knee [82]. A phase2 double-blind placebo controlled trial in symptomatic knee OA with effusion is currently in progress, testing the effect of a dual variable domain immunoglobulin molecule that specifically and potently neutralizes both IL-1α and IL-1β [83].
Conclusions
Clearly, the abundance of peripheral targets that are potentially expressed on nociceptors in the joint (Fig. 1) begs the question as to how they each contribute to sensitization and pain in the course of OA. It seems that future research should be directed toward carefully documenting the localization of these targets in the OA joint and their contribution to pain, as well as elucidating how these different pathways integrate into networks that drive OA joint pain. Since many of the pathways described above can be selectively and potently targeted, they offer an exciting opportunity for analgesic drug development. However, it should be realized that OA is a multifactorial chronic disease, and human brain imaging studies are increasingly uncovering the complex networks of sensory and emotional experiences that underlie chronic pain [84]. Therefore, a final description of OA pain will presumably involve integration of peripheral and central components at all levels of the neuraxis.
Acknowledgments
A.M. Malfait (R01AR060364 and R01AR064251) and R.J. Miller (R01AR064251) acknowledge the support by the National Institute of Arthritis and Musculoskeletal and Skin Diseases.
Footnotes
Compliance with Ethics Guidelines
Conflict of Interest
Anne-Marie Malfait and Richard J. Miller declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human subjects performed by any of the authors. Animal studies performed by the authors were approved by the Institutional Animal Care and Use Committee at Rush University Medical Center and at Northwestern University.
References
Papers of particular interest, published recently, have been highlighted as:
* Of importance
** Of major importance
- 1.Loeser RF, Goldring SR, Scanzello CR, Goldring MB. Osteoarthritis: a disease of the joint as an organ. Arthritis Rheum. 2012;64(6):1697–1707. doi: 10.1002/art.34453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Global, regional, and national incidence, prevalence, and years lived with disability for 301 acute and chronic diseases and injuries in 188 countries, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2015;386(9995):743–800. doi: 10.1016/S0140-6736(15)60692-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Neogi T, Zhang Y. Epidemiology of osteoarthritis. Rheum Dis Clin North Am. 2013;39(1):1–19. doi: 10.1016/j.rdc.2012.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Neogi T. The epidemiology and impact of pain in osteoarthritis. Osteoarthritis Cartilage. 2013;21(9):1145–1153. doi: 10.1016/j.joca.2013.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Malfait AM, Schnitzer T. Toward a mechanism-based approach of pain management in osteoarthritis. Nature Reviews Rheumatology. 2013;9:654–664. doi: 10.1038/nrrheum.2013.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Miller RE, Tran PB, Obeidat AM, Raghu P, Ishihara S, Miller RJ, Malfait AM. The Role of Peripheral Nociceptive Neurons in the Pathophysiology of Osteoarthritis Pain. Curr Osteoporos Rep. 2015;13(5):318–326. doi: 10.1007/s11914-015-0280-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Creamer P, Hunt M, Dieppe P. Pain mechanisms in osteoarthritis of the knee: effect of intraarticular anesthetic. J Rheumatol. 1996;23(6):1031–1036. [PubMed] [Google Scholar]
- 8.Schnitzer TJ, Marks JA. A systematic review of the efficacy and general safety of antibodies to NGF in the treatment of OA of the hip or knee. Osteoarthritis Cartilage. 2015;(23 Suppl 1):S8–S17. doi: 10.1016/j.joca.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 9.Basbaum AI, Bautista DM, Scherrer G, Julius D. Cellular and molecular mechanisms of pain. Cell. 2009;139(2):267–284. doi: 10.1016/j.cell.2009.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ren K, Dubner R. Interactions between the immune and nervous systems in pain. Nat Med. 2010;16(11):1267–1276. doi: 10.1038/nm.2234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ji RR, Xu ZZ, Gao YJ. Emerging targets in neuroinflammation-driven chronic pain. Nat Rev Drug Discov. 2014;13(7):533–548. doi: 10.1038/nrd4334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lewin GR, Lechner SG, Smith ES. Nerve growth factor and nociception: from experimental embryology to new analgesic therapy. Handb Exp Pharmacol. 2014;220:251–282. doi: 10.1007/978-3-642-45106-5_10. [DOI] [PubMed] [Google Scholar]
- 13.Stoppiello LA, Mapp PI, Wilson D, Hill R, Scammell BE, Walsh DA. Structural associations of symptomatic knee osteoarthritis. Arthritis Rheumatol. 2014;66(11):3018–3027. doi: 10.1002/art.38778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Iannone F, De Bari C, Dell’Accio F, Covelli M, Patella V, Lo Bianco G, Lapadula G. Increased expression of nerve growth factor (NGF) and high affinity NGF receptor (p140 TrkA) in human osteoarthritic chondrocytes. Rheumatology (Oxford) 2002;41(12):1413–1418. doi: 10.1093/rheumatology/41.12.1413. [DOI] [PubMed] [Google Scholar]
- 15.Pecchi E, Priam S, Gosset M, Pigenet A, Sudre L, Laiguillon MC, Berenbaum F, Houard X. Induction of nerve growth factor expression and release by mechanical and inflammatory stimuli in chondrocytes: possible involvement in osteoarthritis pain. Arthritis Res Ther. 2014;16(1):R16. doi: 10.1186/ar4443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Blaney Davidson EN, van Caam AP, Vitters EL, Bennink MB, Thijssen E, van den Berg WB, Koenders MI, van Lent PL, van de Loo FA, van der Kraan PM. TGF-beta is a potent inducer of Nerve Growth Factor in articular cartilage via the ALK5-Smad2/3 pathway. Potential role in OA related pain? Osteoarthritis Cartilage. 2015;23(3):478–486. doi: 10.1016/j.joca.2014.12.005. [DOI] [PubMed] [Google Scholar]
- 17.Driscoll C, Chanalaris A, Knights C, Ismail H, Sacitharan PK, Gentry C, Bevan S, Vincent TL. Nociceptive Sensitizers Are Regulated in Damaged Joint Tissues, Including Articular Cartilage, When Osteoarthritic Mice Display Pain Behavior. Arthritis Rheumatol. 2016;68(4):857–867. doi: 10.1002/art.39523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ashraf S, Mapp PI, Burston J, Bennett AJ, Chapman V, Walsh DA. Augmented pain behavioural responses to intra-articular injection of nerve growth factor in two animal models of osteoarthritis. Ann Rheum Dis. 2014;73(9):1710–1718. doi: 10.1136/annrheumdis-2013-203416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ishikawa G, Koya Y, Tanaka H, Nagakura Y. Long-term analgesic effect of a single dose of anti-NGF antibody on pain during motion without notable suppression of joint edema and lesion in a rat model of osteoarthritis. Osteoarthritis Cartilage. 2015;23(6):925–932. doi: 10.1016/j.joca.2015.02.002. [DOI] [PubMed] [Google Scholar]
- 20.McNamee KE, Burleigh A, Gompels LL, Feldmann M, Allen SJ, Williams RO, Dawbarn D, Vincent TL, Inglis JJ. Treatment of murine osteoarthritis with TrkAd5 reveals a pivotal role for nerve growth factor in non-inflammatory joint pain. Pain. 2010;149(2):386–392. doi: 10.1016/j.pain.2010.03.002. [DOI] [PubMed] [Google Scholar]
- 21.Lascelles BD, Knazovicky D, Case B, Freire M, Innes JF, Drew AC, Gearing DP. A canine-specific anti-nerve growth factor antibody alleviates pain and improves mobility and function in dogs with degenerative joint disease-associated pain. BMC Vet Res. 2015;11:101. doi: 10.1186/s12917-015-0413-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Eibl JK, Strasser BC, Ross GM. Structural, biological, and pharmacological strategies for the inhibition of nerve growth factor. Neurochem Int. 2012;61(8):1266–1275. doi: 10.1016/j.neuint.2012.10.008. [DOI] [PubMed] [Google Scholar]
- 23. Nwosu LN, Mapp PI, Chapman V, Walsh DA. Blocking the tropomyosin receptor kinase A (TrkA) receptor inhibits pain behaviour in two rat models of osteoarthritis. Ann Rheum Dis. 2015 doi: 10.1136/annrheumdis-2014-207203. * First evidence that TrkA inhibition blocks pain in experimental OA.
- 24.Flannery C, Moran N, Blasioli D, Donahue K, Kane J, Gladysheva T, Dagher R, Fang R, Vardanyan A, Bangari D, et al. A Novel, Locally Delivered TrkA Inhibitor for the Treatment of Joint Pain: Efficacy in Preclinical Models of Arthritis. ORS abstract. 2015 [Google Scholar]
- 25.Catterall WA, Goldin AL, Waxman SG. International Union of Pharmacology. XLVII. Nomenclature and structure-function relationships of voltage-gated sodium channels. Pharmacol Rev. 2005;57(4):397–409. doi: 10.1124/pr.57.4.4. [DOI] [PubMed] [Google Scholar]
- 26.Liu M, Wood JN. The roles of sodium channels in nociception: implications for mechanisms of neuropathic pain. Pain Med. 2011;(12 Suppl 3):S93–S99. doi: 10.1111/j.1526-4637.2011.01158.x. [DOI] [PubMed] [Google Scholar]
- 27.Cox JJ, Reimann F, Nicholas AK, Thornton G, Roberts E, Springell K, Karbani G, Jafri H, Mannan J, Raashid Y, et al. An SCN9A channelopathy causes congenital inability to experience pain. Nature. 2006;444(7121):894–898. doi: 10.1038/nature05413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Habib AM, Wood JN, Cox JJ. Sodium channels and pain. Handb Exp Pharmacol. 2015;227:39–56. doi: 10.1007/978-3-662-46450-2_3. [DOI] [PubMed] [Google Scholar]
- 29.McDonnell A, Schulman B, Ali Z, Dib-Hajj SD, Brock F, Cobain S, Mainka T, Vollert J, Tarabar S, Waxman SG. Inherited erythromelalgia due to mutations in SCN9A: natural history, clinical phenotype and somatosensory profile. Brain. 2016 doi: 10.1093/brain/aww007. [DOI] [PubMed] [Google Scholar]
- 30.Emery EC, Luiz AP, Wood JN. Na1.7 and other voltage-gated sodium channels as drug targets for pain relief. Expert Opin Ther Targets. 2016 doi: 10.1517/14728222.2016.1162295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Reimann F, Cox JJ, Belfer I, Diatchenko L, Zaykin DV, McHale DP, Drenth JP, Dai F, Wheeler J, Sanders F, et al. Pain perception is altered by a nucleotide polymorphism in SCN9A. Proc Natl Acad Sci U S A. 2010;107(11):5148–5153. doi: 10.1073/pnas.0913181107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Valdes AM, Arden NK, Vaughn FL, Doherty SA, Leaverton PE, Zhang W, Muir KR, Rampersaud E, Dennison EM, Edwards MH, et al. Role of the Nav1.7 R1150W amino acid change in susceptibility to symptomatic knee osteoarthritis and multiple regional pain. Arthritis Care Res (Hoboken) 2011;63(3):440–444. doi: 10.1002/acr.20375. [DOI] [PubMed] [Google Scholar]
- 33.Schuelert N, McDougall JJ. Involvement of Nav 1.8 sodium ion channels in the transduction of mechanical pain in a rodent model of osteoarthritis. Arthritis Res Ther. 2012;14(1):R5. doi: 10.1186/ar3553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Rahman W, Dickenson AH. Osteoarthritis-dependent changes in antinociceptive action of Nav1.7 and Nav1.8 sodium channel blockers: An in vivo electrophysiological study in the rat. Neuroscience. 2015;295:103–116. doi: 10.1016/j.neuroscience.2015.03.042. * Important papers illustrating the role of sodium channels in OA pain.
- 35.Rahman W, Dickenson AH. Antinociceptive effects of lacosamide on spinal neuronal and behavioural measures of pain in a rat model of osteoarthritis. Arthritis Res Ther. 2014;16(6):509. doi: 10.1186/s13075-014-0509-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Malfait AM, Little CB, McDougall JJ. A commentary on modelling osteoarthritis pain in small animals. Osteoarthritis Cartilage. 2013;21(9):1316–1326. doi: 10.1016/j.joca.2013.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Duggan PJ, Tuck KL. Bioactive Mimetics of Conotoxins and other Venom Peptides. Toxins (Basel) 2015;7(10):4175–4198. doi: 10.3390/toxins7104175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Just S, Heppelmann B. Voltage-gated calcium channels may be involved in the regulation of the mechanosensitivity of slowly conducting knee joint afferents in rat. Exp Brain Res. 2003;150(3):379–384. doi: 10.1007/s00221-003-1465-x. [DOI] [PubMed] [Google Scholar]
- 39. Rahman W, Patel R, Dickenson AH. Electrophysiological evidence for voltage-gated calcium channel 2 (Cav2) modulation of mechano- and thermosensitive spinal neuronal responses in a rat model of osteoarthritis. Neuroscience. 2015;305:76–85. doi: 10.1016/j.neuroscience.2015.07.073. * First in vivo evidence for an increased functional role of CaV2, likely CaV2.2, channels in mediating OA pain.
- 40.Vonsy JL, Ghandehari J, Dickenson AH. Differential analgesic effects of morphine and gabapentin on behavioural measures of pain and disability in a model of osteoarthritis pain in rats. Eur J Pain. 2009;13(8):786–793. doi: 10.1016/j.ejpain.2008.09.008. [DOI] [PubMed] [Google Scholar]
- 41.Bove SE, Laemont KD, Brooker RM, Osborn MN, Sanchez BM, Guzman RE, Hook KE, Juneau PL, Connor JR, Kilgore KS. Surgically induced osteoarthritis in the rat results in the development of both osteoarthritis-like joint pain and secondary hyperalgesia. Osteoarthritis Cartilage. 2006;14(10):1041–1048. doi: 10.1016/j.joca.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 42.Knights CB, Gentry C, Bevan S. Partial medial meniscectomy produces osteoarthritis pain-related behaviour in female C57BL/6 mice. Pain. 2012;153(2):281–292. doi: 10.1016/j.pain.2011.09.007. [DOI] [PubMed] [Google Scholar]
- 43.Julius D. TRP channels and pain. Annu Rev Cell Dev Biol. 2013;29:355–384. doi: 10.1146/annurev-cellbio-101011-155833. [DOI] [PubMed] [Google Scholar]
- 44.Caterina MJ, Rosen TA, Tominaga M, Brake AJ, Julius D. A capsaicin-receptor homologue with a high threshold for noxious heat. Nature. 1999;398(6726):436–441. doi: 10.1038/18906. [DOI] [PubMed] [Google Scholar]
- 45.Gavva NR, Treanor JJ, Garami A, Fang L, Surapaneni S, Akrami A, Alvarez F, Bak A, Darling M, Gore A, et al. Pharmacological blockade of the vanilloid receptor TRPV1 elicits marked hyperthermia in humans. Pain. 2008;136(1–2):202–210. doi: 10.1016/j.pain.2008.01.024. [DOI] [PubMed] [Google Scholar]
- 46.Wong GY, Gavva NR. Therapeutic potential of vanilloid receptor TRPV1 agonists and antagonists as analgesics: Recent advances and setbacks. Brain Res Rev. 2009;60(1):267–277. doi: 10.1016/j.brainresrev.2008.12.006. [DOI] [PubMed] [Google Scholar]
- 47.Valdes AM, De Wilde G, Doherty SA, Lories RJ, Vaughn FL, Laslett LL, Maciewicz RA, Soni A, Hart DJ, Zhang W, et al. The Ile585Val TRPV1 variant is involved in risk of painful knee osteoarthritis. Ann Rheum Dis. 2011;70(9):1556–1561. doi: 10.1136/ard.2010.148122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kelly S, Chapman RJ, Woodhams S, Sagar DR, Turner J, Burston JJ, Bullock C, Paton K, Huang J, Wong A, et al. Increased function of pronociceptive TRPV1 at the level of the joint in a rat model of osteoarthritis pain. Ann Rheum Dis. 2015;74(1):252–259. doi: 10.1136/annrheumdis-2013-203413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Laslett LL, Jones G. Capsaicin for osteoarthritis pain. Prog Drug Res. 2014;68:277–291. doi: 10.1007/978-3-0348-0828-6_11. [DOI] [PubMed] [Google Scholar]
- 50. Caires R, Luis E, Taberner FJ, Fernandez-Ballester G, Ferrer-Montiel A, Balazs EA, Gomis A, Belmonte C, de la Pena E. Hyaluronan modulates TRPV1channel opening, reducing peripheral nociceptor activity and pain. Nat Commun. 2015;6:8095. doi: 10.1038/ncomms9095. ** Study provides evidence that extracellular hyaluronan can reduce the excitability of the TRPV1 channel.
- 51.Sluka KA, Gregory NS. The dichotomized role for acid sensing ion channels in musculoskeletal pain and inflammation. Neuropharmacology. 2015;94:58–63. doi: 10.1016/j.neuropharm.2014.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bohlen CJ, Chesler AT, Sharif-Naeini R, Medzihradszky KF, Zhou S, King D, Sanchez EE, Burlingame AL, Basbaum AI, Julius D. A heteromeric Texas coral snake toxin targets acid-sensing ion channels to produce pain. Nature. 2011;479(7373):410–414. doi: 10.1038/nature10607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Diochot S, Baron A, Salinas M, Douguet D, Scarzello S, Dabert-Gay AS, Debayle D, Friend V, Alloui A, Lazdunski M, et al. Black mamba venom peptides target acid-sensing ion channels to abolish pain. Nature. 2012;490(7421):552–555. doi: 10.1038/nature11494. ** Two very interesting papers using toxins to highlight the role of ASIC channels in pain.
- 54.Sugimura N, Ikeuchi M, Izumi M, Kawano T, Aso K, Kato T, Ushida T, Yokoyama M, Tani T. Repeated intra-articular injections of acidic saline produce long-lasting joint pain and widespread hyperalgesia. Eur J Pain. 2015;19(5):629–638. doi: 10.1002/ejp.584. [DOI] [PubMed] [Google Scholar]
- 55.Diochot S, Loret E, Bruhn T, Beress L, Lazdunski M. APETx1, a new toxin from the sea anemone Anthopleura elegantissima, blocks voltage-gated human ether-a-go-go-related gene potassium channels. Mol Pharmacol. 2003;64(1):59–69. doi: 10.1124/mol.64.1.59. [DOI] [PubMed] [Google Scholar]
- 56.Izumi M, Ikeuchi M, Ji Q, Tani T. Local ASIC3 modulates pain and disease progression in a rat model of osteoarthritis. J Biomed Sci. 2012;19:77. doi: 10.1186/1423-0127-19-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pierce KL, Premont RT, Lefkowitz RJ. Seven-transmembrane receptors. Nat Rev Mol Cell Biol. 2002;3(9):639–650. doi: 10.1038/nrm908. [DOI] [PubMed] [Google Scholar]
- 58.De Falco L, Fioravanti A, Galeazzi M, Tenti S. Bradykinin and its role in osteoarthritis. Reumatismo. 2013;65(3):97–104. doi: 10.4081/reumatismo.2013.97. [DOI] [PubMed] [Google Scholar]
- 59.Averbeck B, Rudolphi K, Michaelis M. Osteoarthritic mice exhibit enhanced prostaglandin E2 and unchanged calcitonin gene-related peptide release in a novel isolated knee joint model. J Rheumatol. 2004;31(10):2013–2020. [PubMed] [Google Scholar]
- 60.Flechtenmacher J, Talke M, Veith D. Bardykinin-receptor-inhibition-atherapeutic option in osteoarthritis? Osteoarthritis Cartilage. 2004;12(Suppl 137):S332. (abstract) [Google Scholar]
- 61.Song IH, Althoff CE, Hermann KG, Scheel AK, Knetsch T, Burmester GR, Backhaus M. Contrast-enhanced ultrasound in monitoring the efficacy of a bradykinin receptor 2 antagonist in painful knee osteoarthritis compared with MRI. Ann Rheum Dis. 2009;68(1):75–83. doi: 10.1136/ard.2007.080382. [DOI] [PubMed] [Google Scholar]
- 62.Suri S, Gill SE, Massena de Camin S, Wilson D, McWilliams DF, Walsh DA. Neurovascular invasion at the osteochondral junction and in osteophytes in osteoarthritis. Ann Rheum Dis. 2007;66(11):1423–1428. doi: 10.1136/ard.2006.063354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Suri S, Walsh DA. Osteochondral alterations in osteoarthritis. Bone. 2012;51(2):204–211. doi: 10.1016/j.bone.2011.10.010. [DOI] [PubMed] [Google Scholar]
- 64.Russo AF. Calcitonin gene-related peptide (CGRP): a new target for migraine. Annu Rev Pharmacol Toxicol. 2015;55:533–552. doi: 10.1146/annurev-pharmtox-010814-124701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bullock CM, Wookey P, Bennett A, Mobasheri A, Dickerson I, Kelly S. Peripheral calcitonin gene-related peptide receptor activation and mechanical sensitization of the joint in rat models of osteoarthritis pain. Arthritis Rheumatol. 2014;66(8):2188–2200. doi: 10.1002/art.38656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Benschop RJ, Collins EC, Darling RJ, Allan BW, Leung D, Conner EM, Nelson J, Gaynor B, Xu J, Wang XF, et al. Development of a novel antibody to calcitonin gene-related peptide for the treatment of osteoarthritis-related pain. Osteoarthritis Cartilage. 2014;22(4):578–585. doi: 10.1016/j.joca.2014.01.009. [DOI] [PubMed] [Google Scholar]
- 67.Steinhoff MS, von Mentzer B, Geppetti P, Pothoulakis C, Bunnett NW. Tachykinins and their receptors: contributions to physiological control and the mechanisms of disease. Physiol Rev. 2014;94(1):265–301. doi: 10.1152/physrev.00031.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Goldstein DJ, Wang O, Todd LE, Gitter BD, DeBrota DJ, Iyengar S. Study of the analgesic effect of lanepitant in patients with osteoarthritis pain. Clin Pharmacol Ther. 2000;67(4):419–426. doi: 10.1067/mcp.2000.105243. [DOI] [PubMed] [Google Scholar]
- 69.Miller RJ, Jung H, Bhangoo SK, White FA. Cytokine and chemokine regulation of sensory neuron function. Handb Exp Pharmacol. 2009;(194):417–449. doi: 10.1007/978-3-540-79090-7_12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Belmadani A, Tran PB, Ren D, Assimacopoulos S, Grove EA, Miller RJ. The chemokine stromal cell-derived factor-1 regulates the migration of sensory neuron progenitors. J Neurosci. 2005;25(16):3995–4003. doi: 10.1523/JNEUROSCI.4631-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Abbadie C, Bhangoo S, De Koninck Y, Malcangio M, Melik-Parsadaniantz S, White FA. Chemokines and pain mechanisms. Brain Res Rev. 2009;60(1):125–134. doi: 10.1016/j.brainresrev.2008.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Abbadie C, Lindia JA, Cumiskey AM, Peterson LB, Mudgett JS, Bayne EK, DeMartino JA, MacIntyre DE, Forrest MJ. Impaired neuropathic pain responses in mice lacking the chemokine receptor CCR2. Proc Natl Acad Sci U S A. 2003;100(13):7947–7952. doi: 10.1073/pnas.1331358100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Miller RE, Tran PB, Das R, Ghoreishi-Haack N, Ren D, Miller RJ, Malfait AM. CCR2 chemokine receptor signaling mediates pain in experimental osteoarthritis. Proceedings of the National Academy of Sciences of the United States of America. 2012;109(50):20602–20607. doi: 10.1073/pnas.1209294110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.McDougall JJ, Muley MM. The role of proteases in pain. Handb Exp Pharmacol. 2015;227:239–260. doi: 10.1007/978-3-662-46450-2_12. [DOI] [PubMed] [Google Scholar]
- 75.Russell FA, Schuelert N, Veldhoen VE, Hollenberg MD, McDougall JJ. Activation of PAR(2) receptors sensitizes primary afferents and causes leukocyte rolling and adherence in the rat knee joint. Br J Pharmacol. 2012;167(8):1665–1678. doi: 10.1111/j.1476-5381.2012.02120.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Russell FA, Veldhoen VE, Tchitchkan D, McDougall JJ. Proteinase-activated receptor-4 (PAR4) activation leads to sensitization of rat joint primary afferents via a bradykinin B2 receptor-dependent mechanism. J Neurophysiol. 2010;103(1):155–163. doi: 10.1152/jn.00486.2009. [DOI] [PubMed] [Google Scholar]
- 77.Pellett S, Yaksh TL, Ramachandran R. Current status and future directions of botulinum neurotoxins for targeting pain processing. Toxins (Basel) 2015;7(11):4519–4563. doi: 10.3390/toxins7114519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.La Porta C, Bura SA, Negrete R, Maldonado R. Involvement of the endocannabinoid system in osteoarthritis pain. Eur J Neurosci. 2014;39(3):485–500. doi: 10.1111/ejn.12468. [DOI] [PubMed] [Google Scholar]
- 79.Miller RE, Miller RJ, Malfait AM. Osteoarthritis joint pain: the cytokine connection. Cytokine. 2014;70(2):185–193. doi: 10.1016/j.cyto.2014.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Maksymowych WP, Russell AS, Chiu P, Yan A, Jones N, Clare T, Lambert RG. Targeting tumour necrosis factor alleviates signs and symptoms of inflammatory osteoarthritis of the knee. Arthritis Res Ther. 2012;14(5):R206. doi: 10.1186/ar4044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Chevalier X, Ravaud P, Maheu E, Baron G, Rialland A, Vergnaud P, Roux C, Maugars Y, Mulleman D, Lukas C, et al. Adalimumab in patients with hand osteoarthritis refractory to analgesics and NSAIDs: a randomised, multicentre, double-blind, placebo-controlled trial. Ann Rheum Dis. 2015;74(9):1697–1705. doi: 10.1136/annrheumdis-2014-205348. [DOI] [PubMed] [Google Scholar]
- 82.Chevalier X, Goupille P, Beaulieu AD, Burch FX, Bensen WG, Conrozier T, Loeuille D, Kivitz AJ, Silver D, Appleton BE. Intraarticular injection of anakinra in osteoarthritis of the knee: a multicenter, randomized, double-blind, placebo-controlled study. Arthritis Rheum. 2009;61(3):344–352. doi: 10.1002/art.24096. [DOI] [PubMed] [Google Scholar]
- 83.Lacy SE, Wu C, Ambrosi DJ, Hsieh CM, Bose S, Miller R, Conlon DM, Tarcsa E, Chari R, Ghayur T, et al. Generation and characterization of ABT-981, a dual variable domain immunoglobulin (DVD-Ig(TM)) molecule that specifically and potently neutralizes both IL-1alpha and IL-1beta. MAbs. 2015;7(3):605–619. doi: 10.1080/19420862.2015.1026501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Vachon-Presseau E, Centeno MV, Ren W, Berger SE, Tetreault P, Ghantous M, Baria A, Farmer M, Baliki MN, Schnitzer TJ, et al. The Emotional Brain as a Predictor and Amplifier of Chronic Pain. J Dent Res. 2016 doi: 10.1177/0022034516638027. [DOI] [PMC free article] [PubMed] [Google Scholar]