ABSTRACT
Cwc24 is an essential splicing factor but only transiently associates with the spliceosome, with an unknown function. The protein contains a RING finger and a zinc finger domain in the carboxyl terminus. The human ortholog of Cwc24, RNF113A, has been associated with the disorder trichothiodystrophy. Here, we show that the zinc finger domain is essential for Cwc24 function, while the RING finger domain is dispensable. Cwc24 binds to the spliceosome after the Prp19-associated complex and is released upon Prp2 action. Cwc24 is not required for Prp2-mediated remodeling of the spliceosome, but the spliceosome becomes inactive if remodeling occurs before the addition of Cwc24. Cwc24 binds directly to pre-mRNA at the 5′ splice site, spanning the splice junction. In the absence of Cwc24, U5 and U6 modes of interaction with the 5′ splice site are altered, and splicing is very inefficient, with aberrant cleavage at the 5′ splice site. Our data suggest roles for Cwc24 in orchestrating organization of the spliceosome into an active configuration prior to Prp2-mediated spliceosome remodeling and in promoting specific interaction of U5 and U6 with the 5′ splice site for fidelity of 5′ splice site selection.
KEYWORDS: Cwc24, Prp2, spliceosome, first catalytic step, splicing fidelity
INTRODUCTION
The spliceosome is a large ribonucleoprotein complex that catalyzes the removal of intervening sequences (introns) from precursor mRNAs by two-step transesterification reactions. Consisting of five small nuclear RNAs (snRNAs) and a range of protein factors, the spliceosome recognizes short conserved sequence stretches within the introns through base pairing between the snRNAs and splice sites, while the protein factors play roles in stabilizing base-paired interactions and mediating structural changes of the spliceosome (for reviews, see references 1 and 2).
The spliceosome is assembled by sequential binding of the snRNPs in the order U1, U2, and then the U4/U6.U5 tri-snRNP. Subsequent activation of the spliceosome involves a major structural rearrangement in the spliceosome, leading to the release of U1 and U4 and formation of new base pairs between U2 and U6 and between U6 and the 5′ splice site (5′SS) (1–3). The Prp19-associated complex (NTC, for nineteen complex) is required to stabilize the interactions of U6 and U5 with the pre-mRNA after the release of U1 and U4 during formation of the active spliceosome (4). At least eight proteins have been identified as associating with the NTC, and several others have been suggested as putative NTC components (5–10).
DEXD/H-box proteins are a family of RNA-dependent ATPases that utilize the energy from ATP hydrolysis to unwind RNA duplexes or to displace proteins from binding to RNA. They function in a wide range of biological processes that involve RNA molecules. Eight DEXD/H-box proteins are required for the splicing process. Each of the catalytic steps requires a DEXD/H-box protein, Prp2 for the first step and Prp16 for the second step, to remodel the spliceosome prior to the catalytic reaction (11–14). After the spliceosome has been activated, Prp2 mediates destabilization of the U2 snRNP components SF3a and SF3b, presumably to expose the branch point to allow the transesterification reaction (15–19), and the NTC-related proteins Cwc24 and Cwc27 are concomitantly removed from the spliceosome (15, 16). The G-patch protein Spp2 is required for spliceosome association and functioning of Prp2 (14, 20–22). Another NTC-related protein, Cwc22, has also been shown to be necessary for the Prp2 functioning step (23). After Prp2 action, Yju2 and Cwc25 are required to promote the first catalytic reaction, and they become stably associated with the postcatalytic spliceosome (24, 25). Their removal, mediated by Prp16, is required for the spliceosome to proceed to the second catalytic step (26). Several structures of the spliceosome revealed by high-resolution cryo-electron microscopy (cryo-EM), representing the activated spliceosome, the catalytic step 1 spliceosome, and the lariat intron-containing spliceosome, have recently been determined, providing molecular insights into not only the architecture of the spliceosomes but also how the conformation of the spliceosome might change between steps during the splicing reaction (27–31).
Cwc24 is a RING finger protein and also contains a Zn finger (ZF) motif. Cwc24 was initially identified in a proteomic study as a component, associated with Cef1/Ntc85 (32), involved in spliceosome activation (4, 6), but in later studies, it was shown to associate only transiently with the spliceosome (18, 33). Cwc24 interacts with the U5 component Brr2 and is essential for splicing both in vivo and in vitro (34, 35). The Drosophila ortholog of Cwc24, midlife crisis, is required to maintain neuronal differentiation (36), while the human ortholog, RNF113A, is associated with the disorder trichothiodystrophy (37). We investigated the functional roles of Cwc24 in the splicing reaction and dissected the structural requirements of the protein for its functionality. We found that the Zn finger motif is required for the function of Cwc24 in splicing, whereas the RING finger domain is dispensable. In the absence of Cwc24, Prp2 binds less tightly to the spliceosome, but its function in destabilizing SF3a/b is not greatly affected. Nevertheless, splicing is hindered if Cwc24 is added after Prp2-mediated SF3a/b destabilization. Cwc24 binds directly to pre-mRNA at the 5′ splice site, spanning the 5′ splice junction. In the absence of Cwc24, the modes of U6-5′SS and U5-5′SS interactions are altered. Splicing could proceed at very low efficiency in the absence of Cwc24, but a fraction of the pre-mRNA was cleaved at the −5 position of the 5′ exon. Our results reveal a crucial role of Cwc24 in orchestrating an active RNA catalytic center prior to the Prp2 step and a role of Cwc24 in fidelity of 5′ splice site selection.
RESULTS
Cwc24 is required for the Prp2 functioning step.
To study the functional role of Cwc24 in the splicing reaction, we raised antibodies against recombinant full-length Cwc24 protein. The antibody could be used for depletion of Cwc24 from splicing extracts (Fig. 1A, lane 2). Addition of recombinant Cwc24 to the depleted extracts restored full splicing activity (lanes 3 to 7), suggesting that Cwc24 is not tightly associated with other essential splicing factors in the extracts. Using mass spectrometry and dual-color fluorescence cross-correlation spectroscopy, Cwc24 has been shown to be displaced from the spliceosome during Prp2-mediated remodeling of the spliceosome (18, 33), but when and how it is recruited to the spliceosome are unknown. We first examined the functional relationship between Cwc24 and NTC, since Cwc24 was initially identified because of its association with the NTC component Cef1/Ntc85 (32).
FIG 1.
Cwc24 is not required for NTC-associated spliceosome activation. (A) Splicing was carried out in mock-treated (lane 1) or Cwc24-depleted (lanes 2 to 7) extracts, with the addition of 1 ng (lane 3), 2 ng (lane 4), 5 ng (lane 5), 10 ng (lane 6), and 25 ng (lane 7) recombinant Cwc24. (B) The spliceosome formed with nonbiotinylated (lanes 1, 3, 5, and 7) or biotinylated (lanes 2, 4, 6, and 8) ACAC pre-mRNA in mock-treated (lanes 1 and 2), NTC-depleted (lanes 3 and 4), Cwc24-depleted (lanes 5 and 6), or Spp2-depleted (lanes 7 and 8) extracts was isolated by precipitation with streptavidin-Sepharose, and the components were analyzed by Western blotting. (C) Spliceosomes were formed with biotinylated ACAC pre-mRNA in mock-treated (lanes 2 to 6), NTC-depleted (lanes 7 to 11), or Cwc24-depleted (lanes 12 to 16) extracts and precipitated with streptavidin-Sepharose. The purified spliceosomes were incubated in splicing buffer in the presence or absence of ATP, and RNA was analyzed by Northern blotting after separation of supernatant and pellet fractions. T, total precipitate; P, pellet; S, supernatant. (D) Splicing was carried out in Cwc24-depleted extracts. To the reaction mixture, 2 mM glucose was added (lanes 4 to 7) or not added (lanes 2 and 3), followed by incubation for 5 min, and then Cwc24 was added (lanes 5 and 7) or not added (lanes 4 and 6), together with 2 mM glucose (lanes 4 and 5) or 2 mM ATP (lanes 6 and 7), for further incubation. M, mock; ΔATP, ATP depletion; +24, Cwc24 added; +Glu, glucose added; Inc, incubation.
Spliceosomes formed with biotinylated actin pre-mRNA in NTC-depleted or Cwc24-depleted extracts were pulled down, and the components were subjected to Western blotting (Fig. 1B). To accumulate spliceosomes in large amounts, the 3′ splice site mutant ACAC was used to avoid recycling of the spliceosome. Since Cwc24 is displaced from the spliceosome during Prp2 action, we also depleted Spp2 from the extract to block the Prp2 functional step so that Cwc24 could be arrested on the spliceosome. In mock-treated extracts, only a small amount of Cwc24 was detected (lane 2), but large amounts of Cwc24 accumulated in Spp2-depleted extracts (lane 8), consistent with previous reports (18, 33). While depletion of Cwc24 did not affect the binding of NTC (as revealed by Prp19 and Ntc85 signals), Yju2, or Cwc22 (lane 6), depletion of NTC prevented binding of Cwc24 (lane 4), suggesting that the presence of NTC is required for the recruitment of Cwc24 to the spliceosome.
We then examined whether Cwc24 is required for spliceosome activation, which requires NTC. We have previously shown that after spliceosome activation, U5 and U6 become stably associated with the spliceosome. In NTC-depleted extracts, U5 and U6 dissociate more easily from the spliceosome upon incubation of the purified spliceosome in splicing buffer (38). Figure 1C shows that, similar to mock-treated extracts (lanes 2 to 6) but unlike NTC-depleted extracts (lanes 7 to 11), U5 and U6 remained stably associated with the spliceosome formed in Cwc24-depleted extracts (lanes 12 to 16) when incubated in the presence or absence of ATP. This suggests that Cwc24 is not required for stabilizing U5 and U6 after the release of U1 and U4 upon activation of the spliceosome but may be required for the first catalytic step.
The first catalytic step involves an ATP-dependent reaction that requires Prp2, followed by an ATP-independent step. We first tested whether the functioning step of Cwc24 requires ATP (Fig. 1D). As shown in the flow chart, splicing was carried out in Cwc24-depleted extracts (lane 2), followed by addition of 2 mM glucose to exhaust ATP (lanes 4 to 7). Cwc24 was then added back to the reaction mixture, together with ATP or glucose for further incubation. As a control, Cwc24 was also added prior to the splicing reaction (lane 3). The results show that Cwc24 restored splicing activity only when ATP was also added, albeit not to mock or control levels (lane 7) (see below), indicating a requirement for ATP during or after the Cwc24 functional step.
Cwc24 stabilizes the association of Prp2 with the spliceosome but is not required for Prp2-mediated spliceosome remodeling.
Prp2 has been shown to destabilize the binding of the U2 components SF3a/b and RES (retention and splicing) complex components to the spliceosome before the first catalytic reaction (17, 18). After Prp2 action, Yju2 and Cwc25 are required to promote the first transesterification in an ATP-independent manner (24, 25). Considering the ATP requirement for Cwc24 functioning, it is unlikely that Cwc24 is involved in the post-Prp2 step. We investigated whether Cwc24 is required for the functioning of Prp2 by first examining the effect of Cwc24 on the binding of Prp2 to the spliceosome. Splicing was performed in Cwc24-depleted extracts in the presence of a dominant-negative Prp2-V5 mutant, S378L, which carries a mutation in the SAT motif and remains stably associated with the spliceosome even in the presence of ATP (39). The reaction mixtures were precipitated with anti-V5 antibody to investigate the association of Prp2 with the spliceosome. Figure 2A shows that fewer spliceosomes coprecipitated with Prp2 (lane 8) than with Ntc20 (lane 7) without readding Cwc24, whereas in mock-treated extracts (lanes 1 to 4) or when Cwc24 was readded to depleted extracts (lanes 9 to 12), comparable amounts of precatalytic spliceosomes coprecipitated with Prp2 and Ntc20 (lanes 3, 4, 11, and 12). These findings indicate that Cwc24 is required for stable association of Prp2 with the spliceosome.
FIG 2.
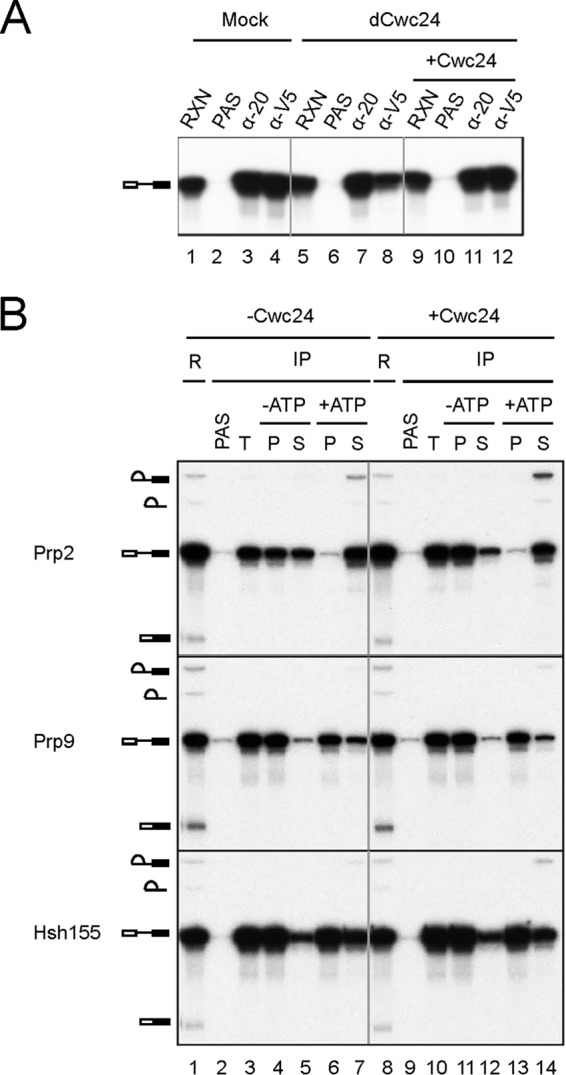
Cwc24 stabilizes the association of Prp2 with the spliceosome but is not required for Prp2-mediated spliceosome remodeling. (A) Splicing was carried out in mock-treated (lanes 1 to 4) or Cwc24-depleted (lanes 5 to 12) extracts without (lanes 5 to 8) or with (lanes 9 to 12) prior addition of Cwc24 in the presence of 60 nM V5-prp2-S378L, and the reaction mixtures were precipitated with anti-Ntc20 (lanes 3, 7, and 11) or anti-V5 (lanes 4, 8, and 12) antibody. RXN, 1/10 of the reaction mixture used for immunoprecipitation; PAS, protein A-Sepharose; α-20, anti-Ntc20. (B) Splicing was carried out in Cwc24-depleted Prp9-V5 (middle) or Hsh155-HA (bottom) extracts, and ATP was then depleted. Following addition (lanes 8 to 14) or no addition (lanes 1 to 7) of 10 ng of recombinant Cwc24 and incubation for 5 min, the reaction mixtures were precipitated with anti-Prp2 (top), anti-V5 (middle), or anti-HA (bottom) antibody under standard conditions but using 75 mM instead of 150 mM NaCl in the wash buffer. The precipitated spliceosome was then incubated in splicing buffer with or without ATP for 20 min. Supernatant and pellet fractions were separated and analyzed. R, 1/5 of the reaction mixture used for immunoprecipitation; IP, immunoprecipitation; T, total precipitate; P, pellet; S, supernatant.
We then addressed whether the function of Prp2 in destabilizing SF3a/b requires Cwc24. The SF3a component Prp9 and the SF3b component Hsh155 were tagged with V5 and hemagglutinin (HA), respectively. Spliceosomes were assembled in Cwc24-depleted Prp9-V5 or Hsh155-HA extracts with or without prior addition of recombinant Cwc24 and precipitated with anti-Prp2, anti-V5, or anti-HA antibody to isolate Prp2, Prp9, and Hsh155-associated spliceosomes, respectively (Fig. 2B, lanes 3 and 10). The spliceosomes were then incubated in splicing buffer with or without ATP to see whether pre-mRNA would be released from Sepharose beads. Upon incubation without ATP, nearly half of the spliceosomes dissociated from Prp2 in the absence of Cwc24 (lanes 4 and 5), whereas only a small fraction was dissociated in the presence of Cwc24 (lanes 11 and 12). This further corroborates the notion that, in the absence of Cwc24, Prp2 is associated less tightly with the spliceosome. Prp2 was nearly completely dissociated from the spliceosome upon incubation with ATP regardless of the presence or absence of Cwc24 (lanes 6, 7, 13, and 14). Incubation of the Prp9- or Hsh155-associated spliceosome with ATP resulted in approximately 30% dissociation of Prp9 and more than 40% of Hsh155 from the spliceosome in the absence of Cwc24 (lanes 6 and 7). Dissociation of both Prp9 and Hsh155 was only slightly less efficient in the presence of Cwc24 (lanes 13 and 14). These results suggest that although Cwc24 is required for stabilization of Prp2 on the spliceosome, it is not required for productive action of Prp2 in destabilizing SF3a/b.
Cwc24 has an essential function prior to Prp2 action.
CWC24 is essential for cellular growth, and the protein is essential for the splicing reaction. Although Cwc24 can stabilize the association of Prp2 with the spliceosome, it is not required for the function of Prp2 in mediating destabilization of SF3a/b prior to the catalytic reaction. Its role in stabilizing Prp2 cannot account for the necessity for Cwc24 in splicing, since depletion of Cwc24 nearly completely abolished the splicing activity. We noted that Cwc24 added to the reaction mixture after performing splicing in Cwc24-depleted extracts restored much lower splicing activity than if it was added before the reaction (Fig. 1D). We speculated that Cwc24 might have to act before Prp2 to orchestrate a functional conformation of the spliceosome prior to destabilization of SF3a/b. If Cwc24 is added after Prp2-mediated remodeling, the spliceosome may not take a functional configuration, thereby preventing further progression of the reaction. Such a structure may not be changed even when Cwc24 is added back. To see if prior action of Prp2 prevents normal functioning of Cwc24, Spp2 was depleted from metabolically Cwc24-depleted extracts (see Fig. S1 in the supplemental material) to allow splicing without progression into Prp2-mediated remodeling (Fig. 3A), followed by ordered addition of Cwc24 and Spp2 to the reaction mixtures, as shown in the flow chart in Fig. 3B. Either Cwc24 or Spp2 was added and allowed to react for 5 min before addition of the other reciprocal protein. The reaction mixtures were then incubated for 5 or 10 min (lanes 10 to 13). As a control, the two proteins were combined before incubation (lane 8). Figure 3B shows that splicing activity was indeed restored to higher levels when Cwc24 was added before Spp2 (compare lanes 12 and 13 with 10 and 11), confirming that the ordered action of Cwc24 before Prp2 is important for efficient splicing.
FIG 3.
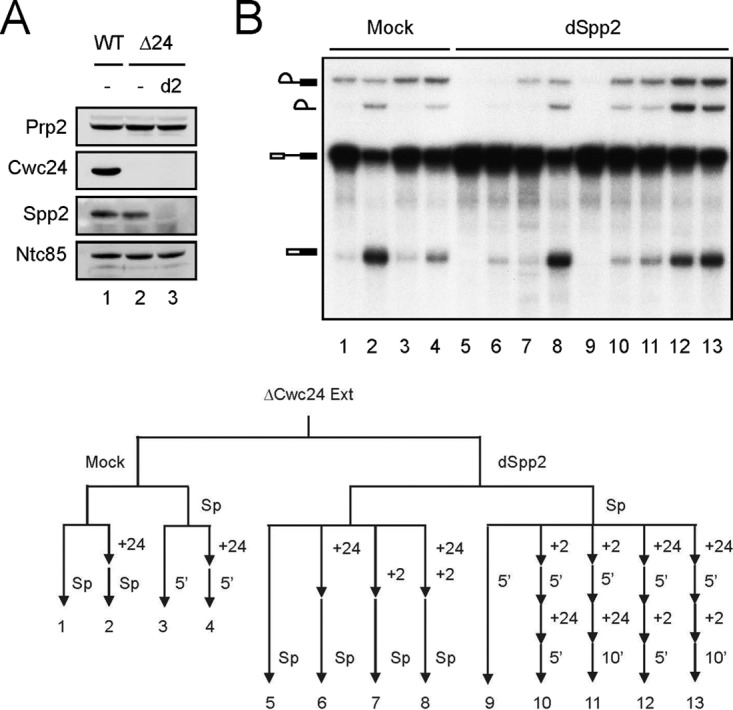
Cwc24 has an essential function prior to Prp2 action. (A) Western blotting of wild-type (lane 1), metabolically Cwc24-depleted (lanes 2 and 3), and Spp2-depleted (lane 3) extracts. (B) Splicing was carried out in mock-treated (lanes 1 to 4) or Spp2-depleted (lanes 5 to 13) metabolically Cwc24-depleted extracts without (lanes 3, 4, and 9 to 13) or with (lanes 2 and 6 to 8) the addition of Cwc24 and/or Spp2 for 30 min. To the extracts without prior addition of Cwc24 or Spp2, either Cwc24 (lanes 12 and 13) or Spp2 (lanes 10 and 11) was then added and incubated for 5 min, followed by addition of the other reciprocal protein and incubation for 5 min (lanes 10 and 12) or 10 min (lanes 11 and 13). For the mock-treated reaction mixture, Cwc24 was added and incubated for 5 min (lane 4). Δ24, Cwc24 metabolically depleted extracts; d2, Spp2 depletion; Sp, splicing; +24, Cwc24 added; +2, Spp2 added.
The ZF domain is required for Cwc24 function.
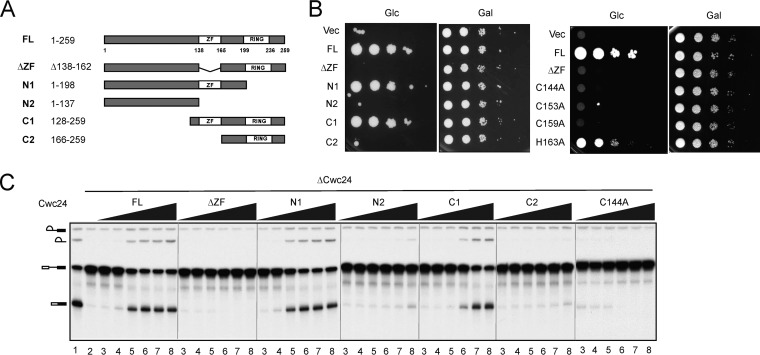
Cwc24 contains a zinc finger and a RING finger domain. To address the functional roles of these domains, Cwc24 was deleted of the ZF domain (ΔZF), a C-terminal fragment including the RING finger domain (N1), a C-terminal fragment including both the RING finger and ZF domains (N2), the N-terminal region while retaining both the ZF and RING finger domains (C1), and the N-terminal region while retaining only the RING finger domain (C2) for functional analyses (Fig. 4A). Yeast Saccharomyces cerevisiae strains were constructed in which the chromosomal copy of CWC24 was controlled by the GAL1 promoter and various CWC24 mutants were controlled by its own promoter on a single-copy plasmid. Yeast cells were grown in medium supplemented with raffinose and then spotted on glucose- or galactose-containing plates in series dilutions (Fig. 4B). The result showed that only the N1 and C1 mutants were viable, indicating that the ZF domain, but not the RING finger domain, is essential for cellular viability. To further address the importance of the ZF motif, we individually mutated conserved cysteine and histidine residues in the ZF domain for growth analyses. Except for H163, which showed a poor-growth phenotype when changed to alanine, changes of any of the cysteine residues to alanine resulted in cellular lethality, indicating that the ZF domain is important for cellular functions.
FIG 4.
Domain analysis for the function of Cwc24 in splicing. (A) Map of Cwc24 and deletion mutants. The positions of the ZF and RING finger motifs are indicated. (B) Spot assays for growth of deletion and ZF point mutants on glucose- or galactose-supplemented synthetic medium. (C) Splicing assays of Cwc24 deletion mutants. Splicing reactions were performed in in vivo Cwc24-depleted extracts with the addition of 0 nM (lane 2), 0.035 nM (lanes 3), 0.35 nM (lanes 4), 3.5 nM (lanes 5), 35 nM (lanes 6), 350 nM (lanes 7), or 3500 nM (lanes 8) full-length (FL) Cwc24 or deletion mutants. Vec, vector.
To analyze the functions of the domains in splicing, we expressed and purified from Escherichia coli the mutant proteins with V5 tagged at the N terminus of each protein (see Fig. S2 in the supplemental material) for complementation of the Cwc24-depleted extracts. Extracts prepared from metabolically Cwc24-depleted cells were used for the experiment (see Fig. S1 in the supplemental material). Titration of purified proteins for complementation analysis revealed that recombinant N1 protein restored the splicing activity of Cwc24-depleted extracts to nearly the level of full-length protein, whereas nearly 100-fold C1 protein was required to restore splicing activity to the same level. None of the mutants with the ZF domain deleted were functional for complementation to any significant level (Fig. 4C). Mutation in C144 also completely abolished the splicing activity. These results suggest that the ZF motif is essential for the function of Cwc24 in splicing, and the N-terminal region also plays an auxiliary role.
Cwc24 directly interacts with the 5′ splice site, spanning the splice junction.
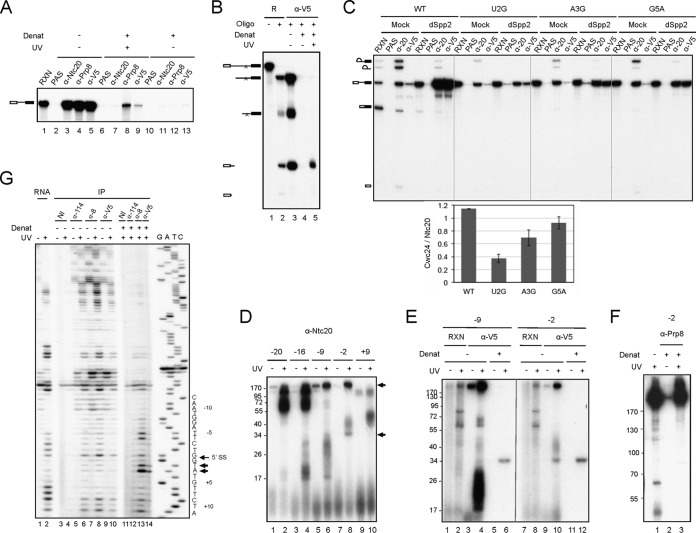
Since the ZF domain is essential for splicing, we examined whether Cwc24 directly interacts with pre-mRNA by UV cross-linking. Splicing was carried out in Spp2-depleted Cwc24-V5 extracts to arrest Cwc24 on the spliceosome, and the reaction mixtures were irradiated with UV at 254 nm. After denaturation, the mixtures were precipitated with anti-V5 antibody to see whether pre-mRNA was covalently linked to Cwc24. Prp8 is known to directly interact with pre-mRNA (40), so it was used as a control. Figure 5A shows that under nondenaturation conditions, spliceosomes were efficiently precipitated by anti-Ntc20, anti-Prp8, and anti-V5 antibodies (lanes 3 to 5). When the reaction mixtures were denatured, only anti-Prp8 and anti-V5 antibodies could precipitate pre-mRNA following UV irradiation (lanes 8 and 9), indicating cross-linking of Cwc24 and Prp8 but not Ntc20 to pre-mRNA. Cross-linking of Cwc24 to snRNAs was not detected (see Fig. S3 in the supplemental material).
FIG 5.
Cwc24 directly binds to pre-mRNA at the 5′ splice site across the splice junction. Splicing was carried out in Spp2-depleted Cwc24-V5 extracts. (A) The reaction mixtures (lane 1) were irradiated (lanes 6 to 9) or not irradiated (lanes 2 to 5 and 10 to 13) with UV at 254 nm and then subjected to immunoprecipitation without (lanes 2 to 5) or with (lanes 6 to 13) prior denaturation. The relative amounts of samples loaded on each lane were 1:10:1,000 for RXN, no-denaturation IP, and denaturation (Denat) IP. (B) A DNA primer, Pre-III, complementary to positions 51 to 71 of IVS, was added to the reaction mixture (lanes 2 to 5), followed by UV irradiation (lane 5), denaturation (lanes 4 and 5), and immunoprecipitation with anti-V5 antibody (lanes 3 to 5). (C) Splicing was carried out with wild-type (WT), U2G, A3G, and G5A actin pre-mRNA in mock-treated or Spp2-depleted Cwc24-V5 extracts. The reaction mixtures were precipitated with anti-Ntc20 or anti-V5 antibody. RXN, equivalent to 1 μl of the reaction mixture, and 1/10 (wild type), 1/200 (G2G and A3G), or 1/30 (G5A) of the reaction mixture used for immunoprecipitation. The relative RNA intensity on autoradiography was quantified using Vision Works LS image acquisition and analysis software (UVP) and normalized by arbitrarily setting the densitometry of the control to 1. (D to F) Splicing was carried out under standard conditions in Spp2-depleted Cwc24-V5 extracts using actin pre-mRNA 4sU labeled at the indicated positions. The reaction mixture was irradiated with UV at 365 nm, treated with nuclease P1, and then analyzed on 12.5% SDS-PAGE (D), precipitated with anti-V5 antibody (lanes 3 to 6 and 9 to 12) with (lanes 5, 6, 11, and 12) or without (lanes 1 to 4 and 7 to 10) prior denaturation before being analyzed on SDS-10% PAGE (E), or precipitated with anti-Prp8 antibody following denaturation (lanes 2 and 3) and analyzed on SDS-7% PAGE (F). (G) UV-cross-linked products isolated from the gel in panel A were analyzed by primer extension using primer Pre-II. The relative amounts of samples loaded on each lane were 1:10:1,000 for RNA (lanes 1 and 2), no-denaturation IP (lanes 3 to 10), and denaturation IP (lanes 11 to 14). RNA, deproteinized RNA; NI, nonimmune serum; α-114, anti-Snu114 antibody; α-8, anti-Prp8 antibody.
Cwc24 cross-linking sites were first mapped by oligonucleotide-directed RNase H cleavage (Fig. 5B). An oligonucleotide complementary to positions 41 to 61 of the pre-mRNA intron was added to the reaction mixture after the splicing reaction, but prior to UV irradiation, to direct cleavage of pre-mRNA by RNase H (lane 2). Upon UV irradiation and denaturation, the reaction mixture was precipitated with anti-V5 antibody. While both 5′ and 3′ RNA fragments were precipitated by the antibody without denaturation of the reaction mixture (lane 3), only the 5′ fragment was precipitated after UV irradiation and denaturation (lane 5), indicating cross-linking of Cwc24 to the 5′ fragment, which contains the 5′ exon and 40 to 60 bases of the intron sequence. This suggests that Cwc24 may bind to pre-mRNA at the 5′ splice site.
We reasoned that if Cwc24 binds to pre-mRNA at the 5′ splice site, mutations in the 5′ splice site might interfere with its binding. To examine whether stable association of Cwc24 with the spliceosome is affected by mutations at the 5′ splice site, spliceosomes were assembled in Spp2-depleted Cwc24-V5 extracts using pre-mRNA with mutations at the 5′ splice site and precipitated with anti-Ntc20 or anti-V5 antibody. The amount of pre-mRNA coprecipitated with Cwc24 was normalized to that with Ntc20. Spliceosome formation with various 5′ splice site-mutated pre-mRNAs was inefficient, so much larger amounts of the reaction mixtures than for wild-type pre-mRNA were used for immunoprecipitation. The result shows that all the 5′ splice site mutants, G1A, U2G, A3G, and G5A, exhibited reduced Cwc24 association, but branch point mutants had no effect (Fig. 5C; see Fig. S4 in the supplemental material). Figure 5C also shows that mutations at sites proximal to the splice junction had a stronger effect than those at distal sites, suggesting possible binding of Cwc24 close to the splice junction on the 5′ exon or within the intron.
Site-specific cross-linking was performed to identify the Cwc24-interacting site. A single 4-thiouridine (4sU) residue was introduced at the +9, −2, −9, −16, and −20 positions of pre-mRNA (the first nucleotide of the intron is +1) and with 32P-labeled 5′ ends. Splicing was carried out with 4sU-labeled pre-mRNA in Spp2-depleted Cwc24-V5 extracts, and total activated spliceosomes were isolated by precipitation with anti-Ntc20 antibody. The spliceosome was irradiated with UV at 365 nm and then digested with nuclease P1 to remove RNA residues prior to electrophoresis on SDS-PAGE (Fig. 5D). The 34-kDa protein cross-linked to the pre-mRNA at the −2 and −9 positions (lanes 6 and 8) was speculated to be Cwc24, which has a calculated molecular weight of 29,700, and was confirmed by precipitation with anti-V5 antibody after denaturation of the reaction mixture (Fig. 5E, lanes 6 and 12). Prp8 was also seen to cross-link to the −2 and −9 positions (Fig. 5F). These results show that Cwc24 binds to the 5′ splice site and directly contacts the exon near the splice junction.
Since introducing 4sU within the conserved splice site sequence might interfere with RNA base pairing, we performed primer extension of UV cross-linked products, using a primer downstream of the 5′ splice site, to see whether Cwc24 also cross-linked to the pre-mRNA intron sequence. As shown in Fig. 5G, Prp8 cross-linked strongly to the intron sequence at the +1 and +2 positions and to the exon sequence at the −5 and −6 positions, but less strongly at the −7 to −9 positions (lane 13), indicating that Prp8-5′SS interactions span the splice junction. Cross-linking of Cwc24 to the +1 and +2 positions was also detected, but the signals were weaker than those of Prp8 (lane 14). Although Cwc24 was seen to cross-link to the −2 position when using 4sU-labeled pre-mRNA, no clear signal was detected in the exon by UV cross-linking/primer extension due to high background in this region with naked RNA (lane 2). Nevertheless, a combination of UV cross-linking and site-specific cross-linking analyses revealed that, like Prp8, Cwc24 also binds to the 5′ splice site across the splice junction.
A role of Cwc24 in fidelity of the 5′ splice site selection.
The fact that both Cwc24 and Prp8 bind at the 5′ splice site across the splice junction suggests their roles in positioning of the 5′ splice site. We noted that although Cwc24 is essential for splicing, a small amount of splicing activity was retained in extracts depleted of Cwc24 either in vivo (Fig. 6A) or in vitro (see Fig. S5 in the supplemental material). Interestingly, a band below free exon 1 was detected in the reaction (Fig. 6A, lane 1) and was more evident when the spliceosome was enriched by precipitation with anti-Ntc20 antibody (lane 5), indicating aberrant cleavage at the 5′ splice site. We had noted previously that depletion of several other first-step factors failed to completely abolish the splicing activity, despite the proteins not being detected by Western blotting. However, except for Cwc24, no aberrant cleavage was detected in any of the depleted extracts (see Fig. S5 in the supplemental material). Primer extension of purified lariat intron-exon 2 RNA resulted in stops at the authentic 5′ splice site and the −5 position of the 5′ exon, each as doublets, possibly due to addition of an extra nontemplated nucleotide by the reverse transcriptase (Fig. 6B, lane 1). More than half of the splicing intermediates were generated from cleavage at the −5 position. It has previously been shown (see Fig. S6 in the supplemental material) that pre-mRNA with a G5A mutation at the 5′ splice site induces aberrant cleavage at the −5 position (41). We reasoned that aberrant cleavage of the 5′ splice site might be attributable to altered RNA structure at the catalytic center, which might also be the reason for the spliceosome being nonfunctional after destabilization of SF3a/b. We therefore examined U6-5′SS and U5-5′SS interactions by UV cross-linking using Ac/Cla actin pre-mRNA (38).
FIG 6.
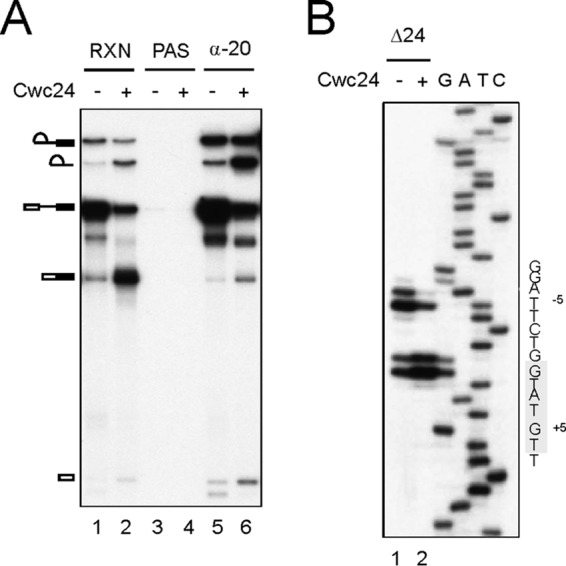
Aberrant 5′ splice site cleavage. (A) Splicing was carried out in metabolically Cwc24-depleted extracts with or without prior addition of 35 nM recombinant Cwc24. The reaction mixtures were precipitated with anti-Ntc20 antibody. RXN, 1/10 of the reaction mixture; α-20, anti-Ntc20 antibody. (B) Primer extension to map the cleavage site at the 5′ splice site using purified lariat intron-exon 2 RNA and primer Pre-II. The conserved 5′ splice site sequence is highlighted. Δ24, metabolically Cwc24-depleted extracts.
A role for Cwc24 in formation of the RNA catalytic center.
The Ac/Cla substrate, containing only 4 nucleotides downstream of the branch point, is functional for spliceosome activation but does not allow Prp2 to function (19). We previously identified three U6 pre-mRNA cross-linked products, X1, X2a, and X2b, after selection with a 5′-biotinylated U6 oligonucleotide (38) (Fig. 7A and B, lane 1). X1 is generated from cross-links of U6 in the Lsm-binding region with the intron sequence around 30 nucleotides downstream of the 5′ splice site, and it is generated only in the presence of NTC. X2a arises from cross-links of the intron at the +9 position with nucleotides of U6 at positions 36 to 38, and it is generated from spliceosomes primarily at the stage prior to NTC binding. X2b is generated from cross-links of U6 at positions 43 to 46 with the intron at the +7 and +9 positions (Fig. 7A). From mock-treated extracts, we indeed observed stronger X2b and weaker X2a and X1 signals (Fig. 7B, lane 1). In Cwc24-depleted extracts, a strong signal below X2b that we named X2c was also seen (lane 2). For the G5A mutant, X2c was not obvious, but the strong signal seemed to migrate between X2a and X2b (lane 3). Cross-links at the position of X1 were detected in all cases.
FIG 7.
UV cross-linking analysis of U5- and U6-5′ splice site interactions. (A) Proposed modes of U6-5′SS interactions. Residues involved in X2a cross-linking are shaded in green, X2b in blue, X2c in red, and X2G5A in brown. Residues circled in gray are cross-linking from dCwc24 extracts, and those circled in brown are from G5A pre-mRNA. The pink arrow indicates an authentic 5′ splice site, and the open arrow indicates an aberrant 5′ splice site. (B to F) Splicing was performed in mock-treated or Cwc24-depleted extracts using wild-type Ac/Cla pre-mRNA or in untreated extracts using G5A Ac/Cla pre-mRNA, and the reaction mixtures were precipitated with anti-Smd1 or anti-Ntc20 antibody. Following UV irradiation and deproteinization, U6 and U5 cross-linked products and total pre-mRNA were affinity selected with U6-Abio (B and C), U5-Cbio (E and F), and Pre-IVbio (D) oligonucleotides, respectively, for primer extension analysis using Pre-V (C), U6-B (D), and Pre-III (F). The conserved 5′ splice site sequence is highlighted in yellow, and the ACAGA box is highlighted in pink. M, mock treated; d24, Cwc24 depleted; 2, mixtures of RNA in X2 region; a+b, mixture of RNA in X2a and X2b regions; c, X2c; 1, 3, 4, Y1, Y3, and Y4.
RNAs from X2c, X2a plus X2b, or the area comprising X2a, X2b, and X2c were isolated for primer extension analysis to determine the cross-linking sites on pre-mRNA (Fig. 7C) and U6 (Fig. 7D). As expected, cross-links at U7 and U9 of the intron were seen in mock-treated extracts (lane 1). In Cwc24-depleted extracts, extra cross-links at G−1, U2, and U6 of the pre-mRNA were observed (lane 2). Cross-links at G−1 and U2 were specific for X2c (lane 4) and were not present in X2a/b (lane 3) or the G5A mutant (lane 5). Cross-linking at U6 was particularly strong in the G5A mutant (lane 5). Primer extension for U6 cross-linked sites revealed a strong cross-link at A51 only from X2c and, in X2, two additional cross-links at U46 and G39 (Fig. 7D, lane 2). Cross-links at both U46 and G39 were also observed in the G5A mutant (lane 5). We speculated that the cross-link at G39 involves cross-linking of U6 to Cwc2, which has also been shown to cross-link to pre-mRNA near the 5′ splice site (42). These results suggest that X2c might be the product of cross-links of U6-A51 to G−1 and U2 of pre-mRNA and that U6-U46 might cross-link to U6 of pre-mRNA in both Cwc24-depleted extracts and the G5A mutant (Fig. 7A, X2G5A). X2c was not detected in the presence of Cwc24 either in mock-treated extracts or for the G5A mutant, probably due to protection of the 5′ splice site by Cwc24 during normal splicing reactions.
U6 is known to interact with the 5′ splice site in a dynamic manner during the splicing reaction (38). Two different types of U6-5′SS base pairings have been proposed based on data from UV cross-linking and mutational analyses, which suggest that the conserved 5′ splice site UGU at positions 4 to 6 of the intron forms base pairs with U6 at positions 42 to 44 (type I) or 47 to 49 (type II) (Fig. 7A) at different stages of the pathway (38, 43–46). Our cross-linking data suggested a novel mode of U6-5′SS base pairings when splicing was performed with G5A mutant pre-mRNA or in the absence of Cwc24. In this mode of interaction, the UGU of the 5′ splice site forms base pairs with the AUA at positions 45 to 47 of U6 (Fig. 7A, type III). Notably, the G5A mutation that changes UGU to UAU does not affect these base pairings.
U5 is known to interact with the last two bases of the 5′ exon during the first catalytic reaction, and such U5-5′SS contact persists through both catalytic steps (47–50). U5-5′SS interactions are more distributive at initial binding of the tri-snRNP to the spliceosome and are confined to residues at the −1 and −2 positions after the binding of NTC (50). We examined whether U5-5′SS interactions are affected in the absence of Cwc24 by UV cross-linking. Four U5 pre-mRNA cross-linked products, Y1, Y2, Y3, and Y4, have previously been identified (50) (Fig. 7E). Y1 and Y3 are the major products detected under normal splicing conditions, probably representing pre-mRNA cross-linked to the short and long forms of U5, respectively, whereas Y2 and Y4 are more prominent when splicing is carried out in the absence of NTC (50). A previously unseen fast-migrating band was noticed in the experiment (Fig. 7E, lane 1, question mark). In Cwc24-depleted extracts (lane 2), Y1 and Y3 appeared to migrate slightly faster than those from mock-treated extracts, and a strong band migrating a little faster than Y4 emerged (lane 2). The G5A mutant also has Y1-, Y3-, and Y4-like cross-linked products, but the Y3- and Y4-like signals are relatively weak (lane 3).
Primer extension analysis revealed that Y1, Y3, and Y4 had identical cross-linking sites (Fig. 7F). Although U5 was previously observed to cross-link to the −1 and −2 positions of pre-mRNA (50), cross-linking was seen predominantly at G−1 in this experiment (lanes 1 to 3). In Cwc24-depleted extracts, cross-linking of U5 shifted to positions −4 and −5 (lanes 4 to 6), and in the G5A mutant, cross-linking at both G−1 and G−7 was detected (lanes 7 to 9). Together, these results show that without Cwc24, both U5 and U6 interact with the 5′ splice site in different ways, which may result in a spatial alteration of the RNA catalytic core, leading to inefficient and aberrant cleavage of the 5′ splice site.
DISCUSSION
Cwc24 was initially found to associate with the NTC component Cef1 in a proteomic study (32). Here, we show that Cwc24 has a function distinct from that of NTC and is not required for NTC-associated spliceosome activation in stabilizing U5 and U6 on the spliceosome. Cwc24 requires the presence of NTC for its recruitment to the spliceosome and is released from the spliceosome upon Prp2 action. When the function of Prp2 is inhibited, Cwc24 accumulates on the spliceosome in large amounts. Previous proteomic and spectroscopic studies have also revealed transient association of Cwc24 with the spliceosome (17, 18, 33). Thus, Cwc24 represents a unique spliceosomal factor that is present on the spliceosome only at a very specific stage of the spliceosome pathway, i.e., after spliceosome activation and prior to Prp2-mediated spliceosome remodeling. Cwc24 can therefore serve in structural studies as a signature protein for purification of this specific precatalytic complex immediately before the first reaction.
We also showed that Cwc24 is not required for the recruitment of Prp2 to the spliceosome or for its function in destabilizing SF3a/b, but it does stabilize the association of Prp2 with the spliceosome. Cwc24 is required not only for stabilizing Prp2 but also for orchestrating organization of the activated spliceosome into a functional configuration prior to destabilization of SF3a/b. Although Cwc24 is not required for NTC-mediated stabilization of U5 and U6, U5 and U6 interact atypically with the 5′ splice site in the absence of Cwc24. Under such conditions, Prp2 can still mediate remodeling of the spliceosome, but this results in an inactive form of the spliceosome that allows Cwc24 to bind (see Fig. S7 in the supplemental material) but not to function, as evidenced by the recovery of low splicing activity when Cwc24 was added after the action of Prp2. In this context, proper configuration of the RNA catalytic core requires the coordination of Cwc24 with SF3a/b. This notion is supported by the recent report of the cryo-EM structure, which revealed that both Cwc24 and Prp11 interact with the first residue of the 5′ splice site and also interact with each other on the activated spliceosome (28).
By UV cross-linking analysis, U5 and U6 were found to interact atypically with the 5′ splice site in the absence of Cwc24. Two cross-linked products derived from cross-linking of U6 to residues near the 5′ splice junction were observed in the absence of Cwc24 but not in the G5A mutant, likely due to lack of protection of these residues by Cwc24. A novel cross-linking at U6 of the intron sequence was detected under Cwc24 depletion and was particularly prominent for the G5A mutant. A corresponding cross-linked residue on U6 was not as obvious but was speculated to be U46, which is also involved in the formation of X2b. The cross-linking results suggest a different mode of U6-5′SS base pairing in which the 5′ splice site UGU is at positions 4 to 6, with AUA at positions 45 to 47, of U6 in the absence of Cwc24 instead of with ACA at positions 47 to 49 of U6, as in the activated spliceosome under normal conditions. The G5A mutation that changes UGU to UAU does not affect these base pairings.
U5 cross-linking also shifted to positions −4 and −5 in the absence of Cwc24 but remained at position −1 in G5A, with an additional cross-link observed at position −7. U5 is proposed to play a role in positioning the 5′ splice site for cleavage at the first catalytic step. Cross-linking at G−7 may explain the aberrant cleavage at the −5 position in the G5A mutant. Notably, the amount of U5 cross-linked product in G5A was much smaller than in mock or Cwc24-depleted extracts. This suggests that the G5A mutation may hinder the interaction of U5 with the 5′ splice site, resulting in low splicing activity. In contrast, cross-linking of U5 to pre-mRNA in Cwc24-depleted extracts was only slightly less efficient but was at atypical sites, which, in combination with the aberrant U6-pre-mRNA interactions, may cause inactivation of the spliceosome.
Despite the aberrant interactions of U5 and U6 with the 5′ splice site, a low level of splicing activity was detected in the absence of Cwc24 or with G5A pre-mRNA (see Fig. S6 in the supplemental material). U5 and U6 may interact with the 5′ splice site in a dynamic manner so that the spliceosome switches between different conformations, infrequently assuming a configuration suitable for the reaction to take place. However, the occasional activity is not precise, since cleavage at the −5 position was detected for both Cwc24-lacking and G5A spliceosomes, with more cleavage at the aberrant site on G5A than on Cwc24-lacking spliceosomes. Aberrant cleavage may occur on the spliceosomes that form an incorrect structure. It is not clear how the spatial arrangement of the RNA elements may result in cleavage at the −5 position of the 5′ splice site. Cross-linking of U5 to the 5′ splice site was mapped to the −4 and −5 positions in Cwc24-depleted extracts instead of at the −1 and −2 positions under normal splicing conditions. This indicates that upon cleavage, U5 would not interact with the excised exon. Since U5 plays a role in the alignment of the two exons for exon ligation by interacting with residues of both exons at the splice junction (44, 51), loss of U5 interactions with the 5′ exon may block the second reaction. Indeed, sequence analysis of clones of reverse transcription (RT)-PCR products of mature mRNA revealed high accuracy of the ligated products. Out of 484 clones, only one was generated from cleavage at the −5 position and another one at the +12 position.
Through a combination of UV cross-linking and site-specific photo-cross-linking analyses, we showed that Cwc24 binds directly to the 5′ splice site of pre-mRNA. Cross-linking was detected at the −2 and −9 positions in the 5′ exon using 4sU-labeled pre-mRNA, with stronger signals at the −2 than at the −9 position. Primer extension of UV cross-linked products revealed cross-linking in the intron region at the +1 and +2 positions. Furthermore, mutations in the intron sequence of the 5′ splice site destabilized the association of Cwc24 with the spliceosome, with mutations at proximal sites exhibiting a more severe effect than those at distal sites. Together, these data suggest that Cwc24 binds directly to the 5′ splice site, spanning the splice junction. Consistent with these results, the cryo-EM structure of the activated yeast spliceosome also revealed binding of Cwc24 to the 5′ splice site, with several amino acid residues in the ZF domain contacting the RNA residues at the 5′ splice site (30).
Prp8 has previously been shown to interact with the 5′ splice site before the first catalytic step (40, 47, 52, 53). In agreement with this, we also observed Prp8 cross-linking to the −2, −9, and −16 positions of the exon, as well as to the +1 and +2 positions of the intronic sequence. Residues that cross-linked to Prp8 largely overlapped with those cross-linked to Cwc24, but in a broader region and with much stronger signals. The fact that the same residues could cross-link to both proteins suggests that Prp8 and Cwc24 interact with this region of the RNA sequence in a dynamic manner so that either protein can contact the RNA in different time frames. In the cryo-EM structure of the yeast-activated spliceosome investigated by Yan et al., the disordered 1585 loop was shown to be located close to the 5′ splice site, but no detailed interaction of Prp8 in the region was revealed (28). It is possible that the interaction of the 1585 loop with the 5′ splice site is rather dynamic, so that the side chains could not be clearly assigned. The fact that the 1585 loop falls within one of the three domains found to cross-link to the −1 position of the 5′ splice site suggests this disordered loop is the region that cross-linked to the 5′ splice site in the spliceosomal state (54). The SF3a component Prp11 was also seen to bind to the 5′ splice site and, together with Cwc24, shields the 5′ splice junction (28). We have also detected Prp11 cross-linked to the −2 position of the 5′ splice site by 4sU cross-linking. Cwc24 and Prp11 may act together to modulate specific interaction of Prp8 with the 5′ splice site prior to their release from the spliceosome upon catalytic activation. They play roles similar to that of Hsh155 for the branch site in positioning and shielding the 5′ splice site prior to the catalytic reaction and need to be removed to expose the catalytic residues before the reaction can take place. Although the RNA structure of the catalytic core of the spliceosome is largely established and stabilized by Prp8 and NTC after the release of U1 and U4, further conformational change mediated by Prp2 is necessary to position the splice sites to allow the catalytic reaction (28, 29).
Prp2 is proposed to be recruited to the spliceosome via interaction with Brr2 and is then translocated to the pre-mRNA, docking downstream of the branch site (19), and has also been shown to interact with Hsh155 (29). Prp2 then catalyzes ATP hydrolysis to destabilize SF3a/b from the spliceosome. SF3a/b is known to bind to the branch site, as several of its components could cross-link to pre-mRNA upstream and downstream of the branch site (55–57). Among them, Prp11 has been seen to cross-link upstream of the branch site (57). The release of Cwc24 and Prp11 from the spliceosome suggests that Prp2 action also elicits remodeling of the spliceosome at the 5′ splice site, and Cwc24 may be dissociated from the spliceosome via its interaction with Prp11.
Deletion analysis revealed that the ZF domain of Cwc24 is essential for cellular viability and for splicing in vitro, whereas the RING finger domain is dispensable. Mutation at each cysteine residue of the ZF motif also resulted in cellular lethality, suggesting that the ZF motif is important for cellular function. Furthermore, changing C144 to adenine completely abolished the function of Cwc24 in splicing. Consistent with this result, the cryo-EM structure of the yeast-activated spliceosome investigated by Yan et al. revealed several amino acid residues of the ZF motif interacting with the first two bases of the intron (28). Thus, the ZF domain plays a central role in the function of Cwc24. The N-terminal region, amino acid residues 1 to 127, although not necessary to support cellular growth, may play an auxiliary role in splicing, since its deletion reduced splicing activity more than 10-fold. Several residues in this region have been shown to cross-link to the N-terminal, RNase H, and Jab1 domains of Prp8 (29). Cwc24-Prp8 interaction may play a role in stabilizing the association of Cwc24 with the spliceosome in support of the splicing reaction. Deletion of the C-terminal 60 amino acid residues of Cwc24, including the RING finger domain, had no effect on cellular viability or splicing. Altogether, the essential region for Cwc24 function is within the region of amino acids 138 to 199, comprising the ZF domain.
MATERIALS AND METHODS
Splicing extracts, substrates, and reactions.
Splicing extracts were prepared according to the method of Cheng et al. (58). For metabolically Cwc24-depleted extracts, YSCC38 cells grown in synthetic complete medium supplemented with raffinose were inoculated into yeast extract-peptone-dextrose (YPD) and grown for 23 h before harvesting for the preparation of extracts. Splicing substrates were synthesized in vitro with SP6 RNA polymerase using the EcoRI-linearized plasmid pSPAct6-88 as the template. Splicing assays were carried out according to the methods described by Cheng and Abelson (59). For UV cross-linking of Cwc24 and Prp8 to pre-mRNA, pre-mRNA was labeled with 32P at approximately 108 dpm/pmol transcript. For site-specific cross-linking, DNA templates for in vitro transcription were generated by PCR using the pSP6Act6-88 plasmid as a template. Two primer pairs were used for generation of templates by PCR for each of the 5′ and 3′ RNA fragments for 4sU labeling at each position (see Table S1 in the supplemental material). RNA was synthesized with SP6 RNA polymerase. Transcription reactions for the 5′ fragment contained 40 mM Tris-HCl (pH 7.9), 6 mM MgCl2, 2 mM spermidine, 10 mM NaCl, 10 mM dithiothreitol (DTT), 2 U/μl RNasin, 0.5 mM (each) the four nucleoside triphosphates (NTPs), 6.6 nM [α-32P]UTP (3,000 Ci/mmol), 30 nM DNA template, and 1.9 U/μl SP6 RNA polymerase. The 3′ fragment was transcribed under the same conditions, with the addition of 2.5 mM 4-thioUpG or UpG dinucleotide to the reaction mixture, and the transcript was further labeled with 32P at the 5′ end using T4 polynucleotide kinase. The 5′- and 3′-half RNA fragments were mixed with a bridging oligonucleotide in the ratio 3:1:2 for ligation in a buffer containing 50 mM Tris-HCl (pH 7.5), 10 mM MgCl2, 1 mM DTT, 1 mM ATP, 2 U/μl RNasin, and 1 U/μl T4 Rnl2. The ligated products were purified by electrophoresis on 5% denaturing polyacrylamide gels.
Assay for spliceosome activation.
To assay for stable association of U5 and U6 with the spliceosome, 120 μl of splicing reaction mixture was precipitated with 60 μl of streptavidin-Sepharose. The precipitates were separated into three fractions, one for total precipitate and two for reincubation in the absence or presence of ATP. For reincubation, 60 μl each of the splicing buffer with or without 2 mM ATP was added to the precipitated spliceosome, followed by incubation at room temperature for 20 min. After removing the supernatant, the pellet was further washed, and RNAs in all the fractions were analyzed by electrophoresis on 5% polyacrylamide (29:1), 8 M urea gels and electroblotted onto GeneScreen membranes for Northern blotting and probing with five snRNAs.
Immunodepletion, immunoprecipitation, and precipitation of the spliceosome by streptavidin-agarose.
Depletion of the NTC was performed as described previously by Chan et al. (38). For depletion of Cwc24, 150 μl of anti-Cwc24 antiserum coupled to 50 μl of protein A-Sepharose was used for each 100 μl of splicing extract. For depletion of Spp2, 200 μl of anti-Spp2 antibody was used for each 100 μl of extract. Immunoprecipitation was performed as described previously by Tarn et al. (60) unless otherwise indicated. The amounts of antibody used for precipitation of the spliceosome from 20 μl of splicing reaction mixture were 1 μl for anti-Ntc20, 1.5 μl for anti-Prp8, and 8 μl for anti-Cwc24 antibody. For splicing in Hsh155-HA extracts, 10 μl of monoclonal 8G5F antibody was used. For Cwc24-V5 and Prp9-V5 extracts, 1 μl of monoclonal anti-V5 antibody was used. Precipitation of the spliceosome with streptavidin-Sepharose was conducted according to the method of Chan et al. (38).
Purification of recombinant Cwc24 proteins.
Full-length Cwc24 and fragments of it were expressed as HIS-SUMO fusion proteins in E. coli. Recombinant proteins were purified on a Ni-nitrilotriacetic acid (Ni-NTA) affinity column (Novagen) according to the manufacturer's instructions. The purified Cwc24 proteins were cleaved with SUMO protease and then passed through a Ni-NTA column to remove the SUMO domain and SUMO protease.
Cross-linking analysis.
UV cross-linking of Cwc24 and Prp8 to pre-mRNA and primer extension analysis were performed according to the method of Chiang and Cheng (61), except that splicing was carried out in Cwc24-V5 extracts for precipitation of cross-linked products with anti-V5 antibody. Primer Pre-II (see the supplemental material) was used for primer extension analysis. UV cross-linking of U5 and U6 to pre-mRNA was performed according to the method of Chan et al. (38). The 5′-biotinylated oligonucleotides Pre-IVbio, U5-Cbio, and U6-Abio were used for selection of cross-linked products for primer extension analysis. Primers Pre-III, Pre-V, and U6-B were used for primer extension to map cross-linking sites on pre-mRNA and U6. For site-specific cross-linking of Cwc24 to the 5′ splice site, splicing reactions were carried out under standard conditions with 0.4 nM 4sU-labeled actin pre-mRNA in the presence of 0.4 U/μl RNasin in Spp2-depleted extracts. The reaction mixtures were chilled in an ice water bath and then spread onto a piece of parafilm covering an ice-cold aluminum block. Droplets were irradiated for 10 min at a distance of ∼2 cm with a 365-nm UV lamp (model UVGL-25; UVP Inc.). Following addition of a 0.12 volume of a mixture containing 0.25 U/μl nuclease P1 and 12.5× Complete EDTA-free protease inhibitor cocktail (Roche), the reaction mixtures were incubated in an Eppendorf tube at 37°C for 30 min and centrifuged at 13,200 rpm for 2 min to remove insoluble materials. The soluble fractions were precipitated with anti-V5 antibody with or without denaturation. The reaction mixtures were denatured by the addition of 1% (wt/vol) SDS, 1% (vol/vol) Triton X-100, and 100 mM DTT, followed by heat treatment at 100°C for 1.5 min and then centrifugation at 13,200 rpm for 2 min to remove insoluble materials. The soluble fraction was diluted 10-fold with a buffer containing 50 mM Tris-HCl (pH 7.5), 300 mM NaCl, and 0.05% NP-40 prior to immunoprecipitation.
Supplementary Material
ACKNOWLEDGMENTS
We thank J. O'Brien for English editing and members of the Cheng laboratory for helpful discussions.
This work was supported by a grant from Academia Sinica and the Minister of Science and Technology (Taiwan) (MoST104-2321-B-001-068).
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/MCB.00580-16.
REFERENCES
- 1.Brow DA. 2002. Allosteric cascade of spliceosome activation. Annu Rev Genet 36:333–360. doi: 10.1146/annurev.genet.36.043002.091635. [DOI] [PubMed] [Google Scholar]
- 2.Wahl MC, Will CL, Lührmann R. 2009. The spliceosome: design principles of a dynamic RNP machine. Cell 136:701–718. doi: 10.1016/j.cell.2009.02.009. [DOI] [PubMed] [Google Scholar]
- 3.Will CL, Lührmann R. 2011. Spliceosome structure and function. Cold Spring Harb Perspect Biol 3:a003707. doi: 10.1101/cshperspect.a003707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tarn W-Y, Hsu C-H, Huang K-T, Chen H-R, Kao H-Y, Lee K-R, Cheng S-C. 1994. Functional association of essential splicing factor(s) with PRP19 in a protein complex. EMBO J 13:2421–2431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen H-R, Jan S-P, Tsao TY, Sheu Y-J, Banroques J, Cheng S-C. 1998. Snt309p, a component of the Prp19p-associated complex that interacts with Prp19p and associates with the spliceosome simultaneously with or immediately after dissociation of U4 in the same manner as Prp19p. Mol Cell Biol 18:2196–2204. doi: 10.1128/MCB.18.4.2196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tsai W-Y, Chow Y-T, Chen H-R, Huang K-T, Hong R-I, Jan S-P, Kuo N-Y, Tsao TY, Chen C-H, Cheng S-C. 1999. Cef1p is a component of the Prp19p-asociated complex and essential for pre-mRNA splicing. J Biol Chem 274:9455–9462. doi: 10.1074/jbc.274.14.9455. [DOI] [PubMed] [Google Scholar]
- 7.Chen C-H, Tsai W-Y, Chen H-R, Wang C-H, Cheng S-C. 2001. Identification and characterization of two novel components of the Prp19p-associated complex, Ntc30p and Ntc20p. J Biol Chem 276:488–494. doi: 10.1074/jbc.M006958200. [DOI] [PubMed] [Google Scholar]
- 8.Chen C-H, Yu W-C, Tsao TY, Wang L-Y, Chen H-R, Lin J-Y, Tsai W-Y, Cheng S-C. 2002. Functional and physical interactions between components of the Prp19p-associated complex. Nucleic Acids Res 30:1029–1037. doi: 10.1093/nar/30.4.1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Albers M, Diment A, Muraru M, Russell CS, Beggs JD. 2003. Identification and characterization of Prp45p and Prp46p, essential pre-mRNA splicing factors. RNA 9:138–150. doi: 10.1261/rna.2119903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ohi MD, Vander Kooi CW, Rosenberg JA, Ren L, Hirsch JP, Chazin WJ, Walz T, Gould K. 2005. Structural and functional analysis of essential pre-mRNA splicing factor Prp19p. Mol Cell Biol 25:451–460. doi: 10.1128/MCB.25.1.451-460.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lin R-J, Lustig AJ, Abelson J. 1987. Splicing of yeast nuclear pre-mRNA in vitro requires a functional 40S spliceosome and several extrinsic factors. Genes Dev 1:7–18. doi: 10.1101/gad.1.1.7. [DOI] [PubMed] [Google Scholar]
- 12.Schwer B, Guthrie C. 1991. PRP16 is an RNA-dependent ATPase that interacts transiently with the spliceosome. Nature 349:494–499. doi: 10.1038/349494a0. [DOI] [PubMed] [Google Scholar]
- 13.Schwer B, Guthrie C. 1992. A conformational rearrangement in the spliceosome is dependent on PRP16 and ATP hydrolysis. EMBO J 11:5033–5040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim S-H, Lin R-J. 1996. Spliceosome activation by PRP2 ATPase prior to the first transesterification reaction of pre-mRNA splicing. Mol Cell Biol 16:6810–6819. doi: 10.1128/MCB.16.12.6810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bessonov S, Anokhina M, Will CL, Urlaub H, Lührmann R. 2008. Isolation of an active step I spliceosome and composition of its RNP core. Nature 452:846–850. doi: 10.1038/nature06842. [DOI] [PubMed] [Google Scholar]
- 16.Fabrizio P, Dannenberg J, Dube P, Kastner B, Stark H, Urlaub H, Lührmann R. 2009. The evolutionarily conserved core design of the catalytic activation step of the yeast spliceosome. Mol Cell 36:593–608. doi: 10.1016/j.molcel.2009.09.040. [DOI] [PubMed] [Google Scholar]
- 17.Warkocki Z, Odenwälder P, Schmitzová J, Platzmann F, Stark H, Urlaub H, Ficner R, Fabrizio P, Lührmann R. 2009. Reconstitution of both steps of Saccharomyces cerevisiae splicing with purified spliceosomal components. Nat Struct Mol Biol 16:1237–1243. doi: 10.1038/nsmb.1729. [DOI] [PubMed] [Google Scholar]
- 18.Lardelli RM, Thompson JX, Yates JR III, Stevens SW. 2010. Release of SF3 from the intron branchpoint activates the first step of pre-mRNA splicing. RNA 16:516–528. doi: 10.1261/rna.2030510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu H-L, Cheng S-C. 2012. The interaction of Prp2 with a defined region of the intron is required for the first splicing reaction. Mol Cell Biol 32:5056–5066. doi: 10.1128/MCB.01109-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Roy J, Kim K, Maddock JR, Anthony JG, Woolford JJL. 1995. The final stages of spliceosome maturation require Spp2p that can interact with the DEAH box protein Prp2p and promote step 1 of splicing. RNA 1:375–390. [PMC free article] [PubMed] [Google Scholar]
- 21.Silverman EJ, Maeda A, Wei J, Smith P, Beggs JD, Lin R-J. 2004. Interaction between a G-patch protein and a spliceosome DEXD/H-box ATPase that is critical for splicing. Mol Cell Biol 24:10101–10110. doi: 10.1128/MCB.24.23.10101-10110.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Warkocki Z, Schneider C, Mozaffari-Jovin S, Schmitzova J, Hobartner C, Fabrizio P, Lührmann R. 2015. The G-patch protein Spp2 couples the spliceosome-stimulated ATPase activity of the DEAH-box protein Prp2 to catalytic activation of the spliceosome. Genes Dev 29:94–107. doi: 10.1101/gad.253070.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yeh T-C, Liu H-L, Chung C-S, Wu N-Y, Liu Y-C, Cheng S-C. 2011. Cwc22 is a novel splicing factor required for the function of Prp2 and for the spliceosome to escape from a futile pathway. Mol Cell Biol 31:43–53. doi: 10.1128/MCB.00801-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu Y-C, Chen H-C, Wu N-Y, Cheng S-C. 2007. A novel splicing factor Yju2 is associated with NTC and acts after Prp2 in promoting the first catalytic reaction of pre-mRNA splicing. Mol Cell Biol 27:5403–5413. doi: 10.1128/MCB.00346-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chiu Y-F, Liu Y-C, Chiang T-W, Yeh T-C, Tseng C-K, Wu NY, Cheng S-C. 2009. Cwc25 is a novel splicing factor required after Prp2 and Yju2 to facilitate the first catalytic reaction. Mol Cell Biol 29:5671–5678. doi: 10.1128/MCB.00773-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tseng C-K, Liu H-L, Cheng S-C. 2011. DEAH-box ATPase Prp16 has dual roles in remodeling of the spliceosome in catalytic steps. RNA 17:145–154. doi: 10.1261/rna.2459611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yan C, Hang J, Wan R, Huang M, Wong CCL, Shi Y. 2015. Structure of a yeast spliceosome at 3.6-angstrom resolution. Science 349:1182–1191. doi: 10.1126/science.aac7629. [DOI] [PubMed] [Google Scholar]
- 28.Yan C, Wan R, Bai R, Huang G, Shi Y. 2016. Structure of a yeast activated spliceosome at 3.5-Å resolution. Science 353:904–911. [DOI] [PubMed] [Google Scholar]
- 29.Rauhut R, Fabrizio P, Dybkov O, Hartmuth K, Pena V, Chari A, Kumar V, Lee C-T, Urlaub H, Kastner B, Stark H, Lührmann R. 2016. Molecular architecture of the Saccharomyces cerevisiae activated spliceosome. Science 353:1399–1405. doi: 10.1126/science.aag1906. [DOI] [PubMed] [Google Scholar]
- 30.Wan R, Yan C, Bai R, Huang G, Shi Y. 2016. Structure of a yeast catalytic step I spliceosome at 3.4 Å resolution. Science 353:895–904. doi: 10.1126/science.aag2235. [DOI] [PubMed] [Google Scholar]
- 31.Galej WP, Wilkinson ME, Fica SM, Oubridge C, Newman AJ, Nagai K. 2016. cryo-EM structure of the spliceosome immediately after branching. Nature 537:197–201. doi: 10.1038/nature19316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ohi MD, Link AJ, Ren L, Jennings JL, McDonald WH, Gould KL. 2002. Proteomics analysis reveals stable multiprotein complexes in both fission and budding yeasts containing Myb-related Cdc5p/Cef1p, novel pre-mRNA splicing factors, and snRNAs. Mol Cell Biol 22:2011–2024. doi: 10.1128/MCB.22.7.2011-2024.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ohrt T, Prior M, Dannenberg J, Odenwalder P, Dybkov O, Rasche N, Schmitzova J, Gregor I, Fabrizio P, Enderiein J, Lührmann R. 2012. Prp2-mediated protein rearrangements at the catalytic core of the spliceosome as revealed by dcFCCS. RNA 18:1244–1256. doi: 10.1261/rna.033316.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Goldfeder M, Oliveira CC. 2008. Cwc24p, a novel Saccharomyces cerevisiae nuclear ring finger protein, affects pre-snoRNA U3 splicing. J Biol Chem 283:2644–2653. doi: 10.1074/jbc.M707885200. [DOI] [PubMed] [Google Scholar]
- 35.Coltri PP, Oliveira CC. 2012. Cwc24p is a general Saccharomyces cerevisiae splicing factor required for the stable U2 snRNP binding to primary transcripts. PLoS One 7:e45678. doi: 10.1371/journal.pone.0045678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Carney TD, Struck AJ, Doe CQ. 2013. midlife crisis encodes a conserved zinc-finger protein required to maintain neuronal differentiation in Drosophila. Development 140:4155–4164. doi: 10.1242/dev.093781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Corbett MA, Dudding-Byth T, Crock PA, Botta E, Christie LM, Nardo T, Aligiuri G, Hobson L, Boyle J, Mansour A, Friend KL, Crawford J, Jackson G, Vandeleur L, Hackett A, Tarpey P, Stratton MR, Turner G, Gécz J, Field M. 2015. A novel X-linked trichothiodystrophy associated with a nonsense mutation in RNF113A. J Med Genet 52:269–274. doi: 10.1136/jmedgenet-2014-102418. [DOI] [PubMed] [Google Scholar]
- 38.Chan S-P, Kao D-I, Tsai W-Y, Cheng S-C. 2003. The Prp19p-associated complex in spliceosome activation. Science 302:279–282. doi: 10.1126/science.1086602. [DOI] [PubMed] [Google Scholar]
- 39.Plumpton M, McGarvey M, Beggs JD. 1994. A dominant negative mutation in the conserved RNA helicase motif ‘SAT’ causes splicing factor PRP2 to stall in spliceosomes. EMBO J 13:879–887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Teigelkamp S, Newman AJ, Beggs JD. 1995. Extensive interactions of PRP8 protein with the 5′ and 3′ splice sites during splicing suggest a role in stabilization of exon alignment by U5 snRNA. EMBO J 14:2602–2612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Parker R, Guthrie C. 1985. A point mutation in the conserved hexanucleotide at a yeast 5′ splice junction uncouples recognition, cleavage, and ligation. Cell 41:107–118. doi: 10.1016/0092-8674(85)90065-0. [DOI] [PubMed] [Google Scholar]
- 42.Rasche N, Dybkov O, Schmitzová J, Akyildiz B, Fabrizio P, Lühurmann L. 2012. Cwc2 and its human homologue RBM22 promote an active conformation of the spliceosome catalytic centre. EMBO J 31:1591–1604. doi: 10.1038/emboj.2011.502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sawa H, Abelson J. 1992. Evidence for a base-pairing interaction between U6 small nuclear RNA and the 5′ splice site during the splicing reaction in yeast. Proc Natl Acad Sci U S A 89:11269–11273. doi: 10.1073/pnas.89.23.11269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sontheimer EJ, Steitz JA. 1993. The U5 and U6 small nuclear RNAs as active site components of the spliceosome. Science 262:1989–1996. doi: 10.1126/science.8266094. [DOI] [PubMed] [Google Scholar]
- 45.Lesser CF, Guthrie C. 1993. Mutational analysis of pre-mRNA splicing in Saccharomyces cerevisiae using a sensitive new reporter gene, CUP1. Genetics 133:851–863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kim CH, Abelson J. 1996. Site-specific crosslinks of yeast U6 snRNA to the pre-mRNA near the 5′ splice site. RNA 2:995–1010. [PMC free article] [PubMed] [Google Scholar]
- 47.Wyatt JR, Sontheimer EJ, Steitz JA. 1992. Site-specific cross-linking of mammalian U5 snRNP to the 5′ splice site before the first step of pre-mRNA splicing. Genes Dev 6:2542–2553. doi: 10.1101/gad.6.12b.2542. [DOI] [PubMed] [Google Scholar]
- 48.Newman AJ, Norman C. 1992. U5 snRNA interacts with exon sequences at 5′ and 3′ splice sites. Cell 68:743–754. doi: 10.1016/0092-8674(92)90149-7. [DOI] [PubMed] [Google Scholar]
- 49.Newman AJ, Teigelkamp S, Beggs JD. 1995. snRNA interactions at 5′ and 3′ splice sites monitored by photoactivated crosslinking in the yeast spliceosome. RNA 1:968–980. [PMC free article] [PubMed] [Google Scholar]
- 50.Chan S-P, Cheng S-C. 2005. The Prp19-associated complex is required for specifying interactions of U5 and U6 with pre-mRNA during spliceosome activation. J Biol Chem 280:31190–31199. doi: 10.1074/jbc.M505060200. [DOI] [PubMed] [Google Scholar]
- 51.O'Keefe RT, Newman AJ. 1998. Functional analysis of the U5 snRNA loop 1 in the second catalytic step of yeast pre-mRNA splicing. EMBO J 17:565–574. doi: 10.1093/emboj/17.2.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Teigelkamp S, Whittaker E, Beggs JD. 1995. Interaction of the yeast splicing factor PRP8 with substrate RNA during both steps of splicing. Nucleic Acids Res 23:320–326. doi: 10.1093/nar/23.3.320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chiara MD, Gozani O, Bennett M, Champion-Arnaud P, Palandjian L, Reed R. 1996. Identification of proteins that interact with exon sequences, splice sites, and the branchpoint sequence during each stage of spliceosome assembly. Mol Cell Biol 16:3317–3326. doi: 10.1128/MCB.16.7.3317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Turner IA, Norman CM, Churcher MF, Newman AJ. 2006. Dissection of Prp8 protein defines multiple interactions with crucial RNA sequences in the catalytic core of the spliceosome. RNA 12:375–386. doi: 10.1261/rna.2229706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gozani O, Potashkin J, Reed R. 1998. A potential role for U2AF SAP155 interactions in recruiting U2 snRNP to the branch site. Mol Cell Biol 18:4752–4760. doi: 10.1128/MCB.18.8.4752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.McPheeters DS, Muhlenkamp P. 2003. Spatial organization of protein-RNA interactions in the branch site-3′ splice site region during pre-mRNA splicing in yeast. Mol Cell Biol 23:4174–4186. doi: 10.1128/MCB.23.12.4174-4186.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schneider C, Agafonov DE, Schmitzová J, Hartmuth K, Fabrizio P, Lührmann R. 2015. Dynamic contacts of U2, RES, Cwc25, Prp8 and prp45 proteins with the pre-mRNA branch-site and 3′ splice site during catalytic activation and step 1 catalysis in yeast spliceosomes. PLoS Genet 11:e1005539. doi: 10.1371/journal.pgen.1005539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cheng S-C, Newman A, Lin R-J, McFarland GD, Abelson JN. 1990. Preparation and fractionation of yeast splicing extract. Methods Enzymol 181:89–96. doi: 10.1016/0076-6879(90)81114-A. [DOI] [PubMed] [Google Scholar]
- 59.Cheng S-C, Abelson J. 1986. Fractionation and characterization of a yeast mRNA splicing extract. Proc Natl Acad Sci U S A 83:2387–2391. doi: 10.1073/pnas.83.8.2387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tarn W-Y, Lee K-R, Cheng S-C. 1993. The yeast PRP19 protein is not tightly associated with small nuclear RNAs, but appears to associate with the spliceosome after binding of U2 to the pre-mRNA and prior to formation of the functional spliceosome. Mol Cell Biol 13:1883–1891. doi: 10.1128/MCB.13.3.1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chiang T-W, Cheng S-C. 2013. A weak spliceosome-binding domain of Yju2 functions in first step and bypasses Prp16 in second step of splicing. Mol Cell Biol 33:1746–1755. doi: 10.1128/MCB.00035-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.