ABSTRACT
Streptococcus salivarius is an abundant isolate of the oral cavity. The genome of S. salivarius 57.I consists of a 2-Mb chromosome and a 40,758-bp circular molecule, designated YMC-2011. Annotation of YMC-2011 revealed 55 open reading frames, most of them associated with phage production, although plaque formation is not observed in S. salivarius 57.I after lytic induction using mitomycin C. Results from Southern hybridization and quantitative real-time PCR confirmed that YMC-2011 exists extrachromosomally, with an estimated copy number of 3 to 4. Phage particles were isolated from the supernatant of mitomycin C-treated S. salivarius 57.I cultures, and transmission electron microscopic examination indicated that YMC-2011 belongs to the Siphoviridae family. Phylogenetic analysis suggests that phage YMC-2011 and the cos-type phages of Streptococcus thermophilus originated from a common ancestor. An extended −10 element (pL) and a σ70-like promoter (pR) were mapped 5′ to Ssal_phage00013 (encoding a CI-like repressor) and Ssal_phage00014 (encoding a hypothetical protein), respectively, using 5′ rapid amplification of cDNA ends, indicating that YMC-2011 transcribes at least two mRNAs in opposite orientations. Studies using promoter-chloramphenicol acetyltransferase reporter gene fusions revealed that pR, but not pL, was sensitive to mitomycin C induction, suggesting that the switch from lysogenic growth to lytic growth was controlled mainly by the activity of these two promoters. In conclusion, a lysogenic state is maintained in S. salivarius 57.I, presumably by the repression of genes encoding proteins for lytic growth.
IMPORTANCE The movement of mobile genetic elements such as bacteriophages and the establishment of lysogens may have profound effects on the balance of microbial ecology where lysogenic bacteria reside. The discovery of phage YMC-2011 from Streptococcus salivarius 57.I suggests that YMC-2011 and Streptococcus thermophilus-infecting phages share an ancestor. Although S. salivarius and S. thermophilus are close phylogenetically, S. salivarius is a natural inhabitant of the human mouth, whereas S. thermophilus is commonly found in the mammary mucosa of bovine species. Thus, the identification of YMC-2011 suggests that horizontal gene transfer via phage infection could take place between species from different ecological niches.
KEYWORDS: Streptococcus salivarius, temperate phage, lysogeny, plasmid, Siphoviridae family
INTRODUCTION
Phages, prokaryotic viruses, are the most abundant biological entities on the planet (1). Although phages have been isolated from the oral cavity (2, 3), relatively little is known about the phages of oral streptococci, compared to those of other lactic acid bacteria, particularly Lactococcus lactis and Streptococcus thermophilus. To date, the better characterized phages of oral streptococci are the virulent phage M102AD of Streptococcus mutans (4) and the temperate phage SM1 of Streptococcus mitis (5). A Streptococcus salivarius-infecting phage of the family Cystoviridae was described recently (6), but that phage has not been characterized. Although prophages have been found in the genomes of S. salivarius strains JF, NCTC8618, and JIM8777 (7), phage production has not been reported. In contrast, more than 300 virulent and temperate phages have been observed in S. thermophilus (8), a close relative of S. salivarius. Analyses indicate that all S. thermophilus-infecting phages are from a common ancestor, with a hexagonal capsid and a long noncontractile tail (9), belonging to the Siphoviridae family (10). S. thermophilus-infecting phages have been commonly classified as cos-type or pac-type phages based on their DNA-packaging machinery and structural proteins (11). For instance, the virulent phages DT1 (12), 7201 (11), Sfi19 (13), and Abc2 (14), as well as the temperate phage Sfi21 (15), are cos-type phages, whereas the temperate phages O1205 (11) and TP-J34/TP-778L (16) and the virulent phages Sfi11 (17), 2972 (18), 858 (19), and ALQ13.2 (14) are pac-type phages. Additional groups, including phage 5093 (20) and the newly described 987 phage group (21), are classified independently, as both possess mosaic genomes, presumably resulting from a relatively recent horizontal gene transfer and/or recombination event. Similarly, two DNA replication modules, represented by phages Sfi21 and 7201, have been observed with S. thermophilus-infecting phages; however, most such phages possess the conserved Sfi21-like replication module (22).
Temperate phages usually exist in a latent form but can become lytic. In the lysogenic cycle, the phage genome is either integrated into the host chromosome or exists as a low-copy-number plasmid. For example, P1 of Enterobacteriaceae (23) and pZL12 of Streptomyces spp. (24) exist as closed circular plasmids in the cognate host. During lysogenic growth, the bacterial host grows normally, and no phage particle is produced. When the survival of the lysogen is threatened by harmful growth conditions, the prophage is released from the chromosome and exists as a plasmid, and DNA replication begins (25). As seen for phage λ and many other temperate phages, the genetic switch from lysogenic growth to lytic growth is generally controlled by the availability of two phage-encoded transcription factors, CI and Cro. Genes encoding these two proteins are arranged in opposite orientations, and the presence of the transcription factor inhibits transcription from the opposite promoter by binding to specific operators, thereby establishing lysogenic growth. When the SOS response is activated in a λ lysogen, the extensive DNA damage activates RecA, which acts as a corepressor to stimulate specific cleavage of CI, leading to lytic growth (26). Upon entering lytic growth, the phage genome replicates autonomously, head and tail proteins are synthesized, the phage genome is packaged, and the phage particles are released when the host cells lyse.
Advances in genomic analysis have allowed for the identification of previously unknown elements. The complete genome sequence of S. salivarius strain 57.I reveals a chromosome and a 40,758-bp plasmid, designated YMC-2011 (27). Most of the open reading frames (ORFs) annotated from YMC-2011 share significant homology with ORFs related to phage production, suggesting that strain 57.I is lysogenically infected with a temperate phage. In this study, we found that phage particles could be isolated from mitomycin C-treated S. salivarius 57.I cultures, although plaque formation was not detected.
RESULTS
Sequence analysis of YMC-2011.
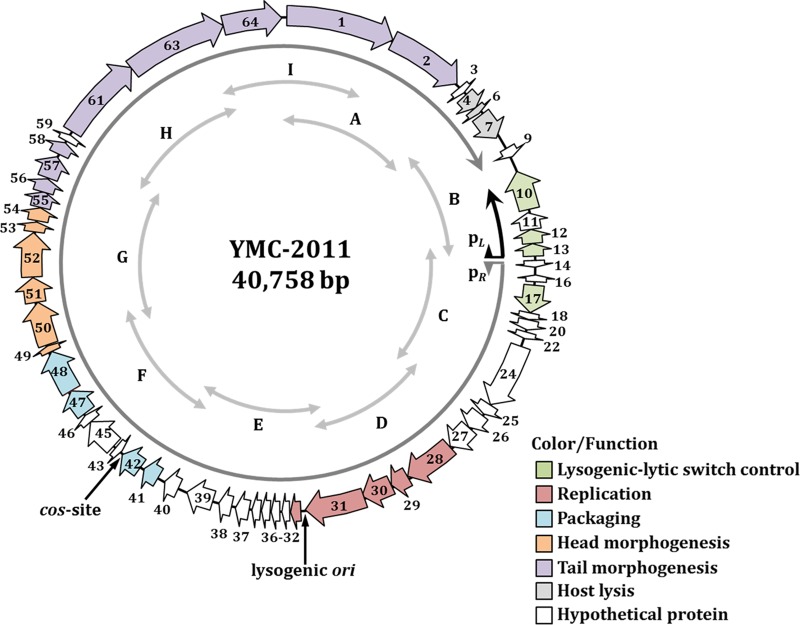
Analysis of the YMC-2011 sequence (GenBank accession number CP002889.1) (27) revealed that YMC-2011 has a GC content of 41% and encodes a total of 55 ORFs (Fig. 1), the basic characteristics of which are summarized in Table S1 in the supplemental material. Of particular note, the original designations Ssal_phage00005, Ssal_phage00008, Ssal_phage00015, Ssal_phage00019, Ssal_phage00021, Ssal_phage00023, Ssal_phage00044, Ssal_phage00060, and Ssal_phage00062 are not identified as ORFs in the database. Twenty-four of the ORFs encode hypothetical proteins, and 10 of them are without homology in the nonredundant GenBank database. The remaining ORFs share significant levels of homology, at the deduced amino acid level, with ORFs found in S. thermophilus phages, including those encoding structural proteins, transcriptional regulators governing the lysogenic-lytic switch, and enzymes for integration, DNA replication, packaging, and host cell lysis, indicating that YMC-2011 likely produces phage particles.
FIG 1.
Schematic representation of the organization of YMC-2011. The relative location and orientation of each Ssal_phage ORF are shown. ORFs that participate in the same pathway for phage production are in the same color. ORFs in white are of unknown function. The locations of the putative ori and cos site are indicated by arrows. The relative orientation and location of the two transcripts generated from pL and pR are indicated. The locations of the amplicons (A to I) presented in Fig. 3B are indicated by double-headed arrows.
Two potential ori regions, with AT contents of >70%, were identified using Ori-Finder. The first region (nucleotides 9928 to 10078), located between Ssal_phage00013 (encoding a CI-like protein) and Ssal_phage00014 (encoding a hypothetical protein), has an AT content of 73% and contains 3 putative DnaA boxes (with no more than 2 mismatches with respect to the consensus sequence 5′-TTWTSCAMA). The second region (nucleotides 19816 to 20057), located between Ssal_phage00031 (encoding a primase) and Ssal_phage00032 (encoding a phage protein), has an AT content of 71% and contains 2 putative DnaA boxes. Most of the characterized ori sequences in S. thermophilus phages are located in noncoding regions close to genes encoding proteins involved in DNA replication (18), suggesting that the region between Ssal_phage00031 and Ssal_phage00032 (region 2) contains the putative ori of YMC-2011. This hypothesis is supported by the sequence similarity between this region and the putative ori sequences of S. thermophilus phages belonging to the Sfi21 replication module. Specifically, significant sequence homology was found between region 2 and the proposed ori sequences of phages ALQ13.2 (14), O1205 (28), DT1 (12), and 2972 (18) (Fig. S1A). Furthermore, ori sequences are known to contain repeat regions (29) and, indeed, two sets of direct repeats (5′-GTTACCKT and 5′-TAAATAAA) and one imperfect inverted repeat (5′-TTATTTATATATT-N5-AATAAATAAATAA) were found in region 2 (Fig. S1B). A putative cos site, 5′-CCGCCACAAGGTG, was also identified 30 bases 3′ to the TTG of Ssal_phage00042 (encoding a terminase small subunit), based on comparisons with other known cos sequences. This cos site is highly homologous to the cos sites of phages 7201, Abc2, DT1, Sfi19, and Sfi21 (Fig. S2).
Based on the results of the homology analysis (Table S1), 15 S. thermophilus-infecting phages, 3 putative S. salivarius prophages, and phage YMC-2011 were selected for phylogenetic analysis (Fig. 2). As expected, the cos-type phages DT1, Abc2, Sfi19, Sfi21, and 7201 are in one cluster, and the pac-type phages 2972, 858, ALQ13.2, Sfi11, and O1205 are in a separate cluster. The newly identified 987 group (phages 9871, 9872, 9873, and 9874) is in a separate cluster. The prophages of S. salivarius (strains JIM8777, JF, and NCTC8618) and phage YMC-2011 are relatively distant from each other, with the exception of the prophage of strain JIM8777 and phage YMC-2011. This prophage and YMC-2011 are more closely related to the cos-type phages than other S. thermophilus-infecting phages, suggesting that they originated from a common ancestor. The prophage from strain NCTC8618 and phage 5093 are in one cluster, suggesting that they share a common ancestor.
FIG 2.
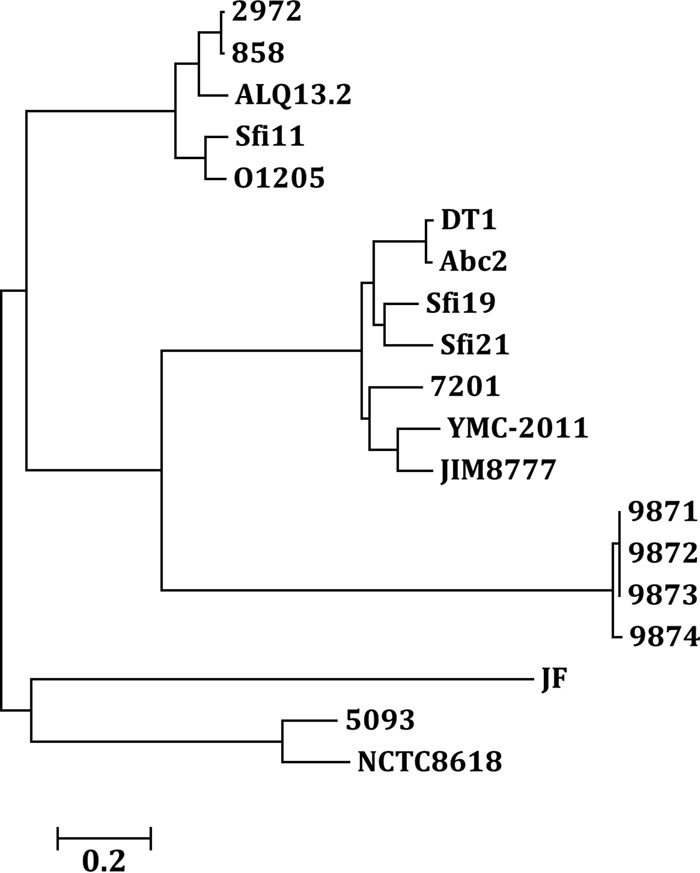
Phylogenetic tree of YMC-2011 and other streptococcal phages. The whole-genome sequences of phages 2972, 858, ALQ13.2, Sfi11, O1205, DT1, Abc2, Sfi19, Sfi21, 7201, 9871, 9872, 9873, 9874, and 5093 of S. thermophilus, the prophages extracted from the genomes of S. salivarius strains JIM8777, JF, and NCTC8618, and phage YMC-2011 of S. salivarius 57.I were used to construct the tree. The tree was built using MEGA 6.0.
Nature of the phage-specific genome.
To confirm that YMC-2011 exists as a circular plasmid, rather than as an integrated or linear prophage, the localization of YMC-2011 in S. salivarius 57.I was first determined by Southern hybridization, using YMC-2011-specific (an internal fragment of Ssal_phage00042) and chromosome-specific (an internal fragment of ureC) probes. A YMC-2011-specific signal was observed in both the plasmid preparation and the total cellular DNA digested with NcoI (a unique cutter for YMC-2011), whereas the ureC-specific signals were detected only in NcoI-digested total cellular DNA, indicating that YMC-2011 exists extrachromosomally (Fig. 3A). To confirm that YMC-2011 is in a circular form, 9 pairs of primers were designed to generate overlapping amplicons around YMC-2011 (Fig. 1, fragments A through I). The amplicons were approximately 5 kbp and overlapped their flanking amplicons by at least 200 bp. All primer pairs were able to generate a PCR product of the expected length, confirming that YMC-2011 is a circular molecule (Fig. 3B). The copy number of YMC-2011 was then determined by quantitative real-time PCR (qPCR) with total DNA isolated from a stationary-phase culture (optical density at 600 nm [OD600] of 0.65) of S. salivarius 57.I. The YMC-2011 copy number in S. salivarius was estimated to be 3 or 4 copies per chromosome (Table 1), confirming that YMC-2011 exists as a circular plasmid.
FIG 3.
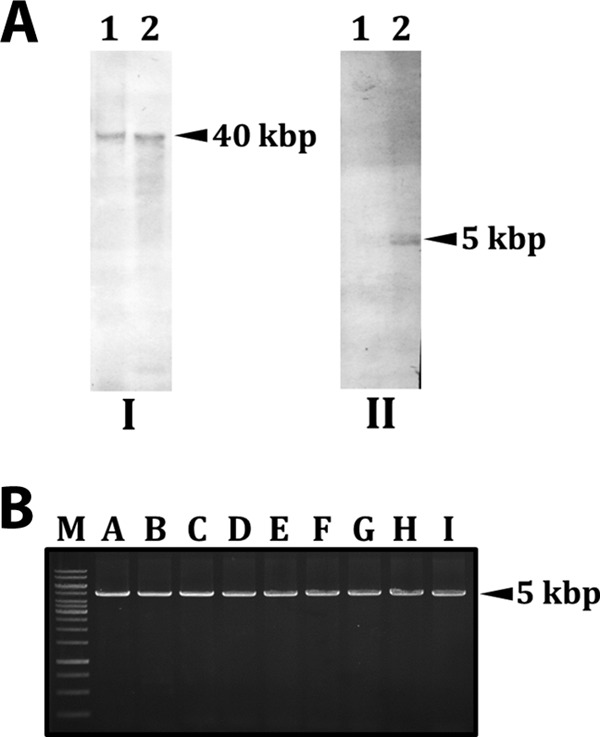
Analysis of the location and conformation of YMC-2011. (A) Southern hybridization with DIG-labeled DNA probes internal to Ssal_phage00042 (I) and ureC (II). The phage- and ureC-specific signals in panels I and II, respectively, are indicated by arrowheads. Lanes 1, uncut plasmid preparation; lanes 2, total cellular DNA digested with NcoI. (B) Overlapping PCR products (fragments A to I) amplified from plasmid YMC-2011. The relative locations of the amplicons are indicated in Fig. 1. M, 1-kb marker.
TABLE 1.
Estimation of the copy number of YMC-2011 by qPCR
| Comparison | ΔCq (mean ± SD)a | Copy numberb |
|---|---|---|
| ureC-Ssal vs phage00002 | 2.12 ± 0.27 | |
| codY-Ssal vs phage00002 | 2.29 ± 0.16 | |
| lacZ-Ssal vs phage00002 | 2.42 ± 0.52 | |
| ureC-Ssal vs phage00031 | 1.63 ± 0.26 | |
| codY-Ssal vs phage00031 | 2.19 ± 0.15 | |
| lacZ-Ssal vs phage00031 | 2.31 ± 0.52 | |
| ureC-Ssal vs phage00063 | 1.13 ± 0.19 | |
| codY-Ssal vs phage00063 | 1.61 ± 0.35 | |
| lacZ-Ssal vs phage00063 | 1.74 ± 1.01 | |
| Mean ± SDc | 1.94 ± 0.43 | 3.84 |
ΔCq is the Cq obtained from reactions specific for the chromosome (ureC, codY, and lacZ) minus the Cq obtained from reactions specific for YMC-2011 (Ssal_phage00002, Ssal_phage00031, and Ssal_phage00063). The values are the means ± standard deviations (SDs) of three independent samples.
The copy number was calculated as 2ΔCq.
The values are the means ± SDs of ΔCq values (mean values) obtained for all comparison pairs.
Induction and observation of phage particles.
We next wanted to examine phage particle production from YMC-2011. If a phage particle were to be produced upon mitomycin C treatment, addition of SDS to destroy the capsid would be essential in order to obtain phage genomic DNA. To test this hypothesis, the assumed phage preparation was subjected to SDS treatment prior to PCR analysis. An Ssal_phage00002-specific product, but not a lacZ-specific product, was obtained from the SDS-treated preparation, suggesting that mitomycin C treatment could induce phage production (Fig. 4A). An Ssal_phage00002-specific PCR product, in lower abundance, was also observed in the sample without the SDS treatment, presumably resulting from phage particles damaged during the denaturation step of the PCR.
FIG 4.
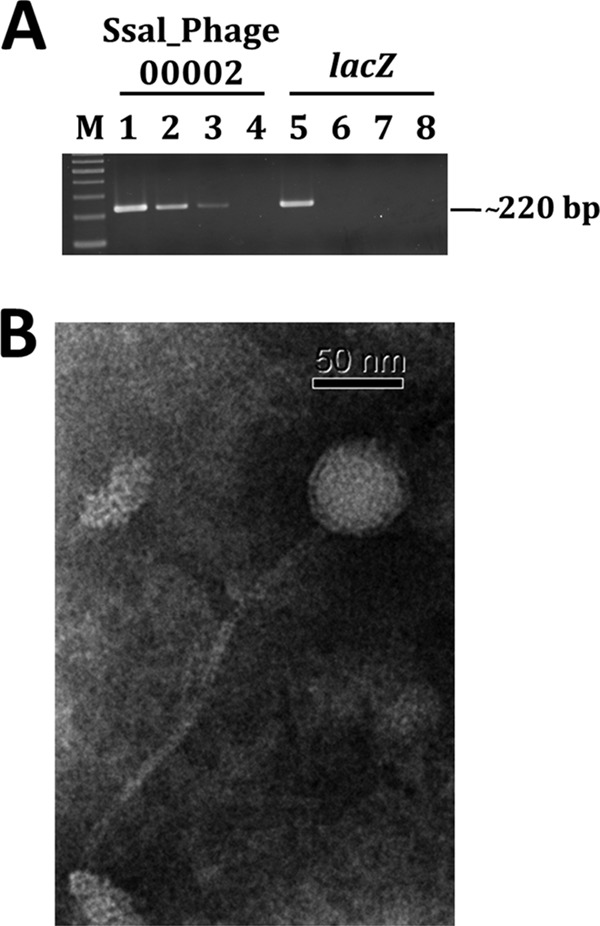
Isolation and examination of S. salivarius 57.I phage YMC-2011. (A) PCR products generated from the phage preparation. Primers specific for Ssal_phage00002 of YMC-2011 (lanes 1 to 4) and the chromosome-borne lacZ (lanes 5 to 8) were used in PCRs with total cellular DNA (lanes 1 and 5), SDS-treated phage preparation (lanes 2 and 6), untreated phage preparation (lanes 3 and 7), or double-distilled water (lanes 4 and 8). M, 100-bp marker. (B) TEM of S. salivarius 57.I phage YMC-2011. The phage was negatively stained with PTA.
The purified phage preparation was negatively stained and examined by transmission electron microscopy (TEM). An icosahedral capsid with a long noncontractile tail, consistent with the Siphoviridae family, was observed (Fig. 4B). From four randomly selected images, the sizes for the icosahedral capsid and the tail were estimated to be 60 ± 1 nm in diameter and 232 ± 1 nm in length, respectively.
Transcriptional organization of YMC-2011.
Based on the annotation, 49 ORFs of YMC-2011 are transcribed from one direction and 6 ORFs (Ssal_phage00010 to Ssal_phage00013, Ssal_phage00016, and Ssal_phage00043) are transcribed from the opposite orientation (Fig. 1). Among those 6 ORFs, Ssal_phage00010 to Ssal_phage00013 are located in a cluster, and the products of the ORFs include an integrase (Ssal_phage00010) and CI-like repressors (Ssal_phage00012 and Ssal_phage00013) that may participate in lysogenic growth. Neither Ssal_phage00016 nor Ssal_phage00043 shares homology with any known sequences. The 49 ORFs that are transcribed in the opposite orientation encode proteins required for phage production. The arrangement of the YMC-2011 genome suggests that the juncture between Ssal_phage00013 and Ssal_phage00014 or that between Ssal_phage00016 and Ssal_phage00017, where two divergent transcripts are initiated, may contain one of the key cis elements controlling the switch between lysogenic growth and lytic growth. To test this hypothesis, reverse transcription (RT)-PCR was performed to analyze whether Ssal_phage00014 to Ssal_phage00064 and Ssal_phage00001 to Ssal_phage00009 were cotranscribed. The results revealed contiguous transcripts between Ssal_phage00014 through Ssal_phage00017 and Ssal_phage00042 through Ssal_phage00045 (Fig. 5). Expression in both regions was enhanced upon mitomycin C treatment, which is in agreement with the mitomycin C-induced phage production described above. In contrast, a contiguous transcript was not detected between Ssal_phage00013 and Ssal_phage00016 or Ssal_phage00017, with or without mitomycin C treatment (Fig. 5), indicating that Ssal_phage00013 and Ssal_phage00016 are not cotranscribed and that a promoter is likely located 5′ to Ssal_phage00013. Using the same approach, we also investigated whether a contiguous transcript could be found between ORFs (in the same orientation), possessing an intergenic region of >150 bp; in all cases, a PCR product was obtained (data not shown). Taken together, the above observations suggest that at least two divergent transcripts, initiated from the 5′-flanking regions of Ssal_phage00013 and Ssal_phage00014, are transcribed from YMC-2011.
FIG 5.
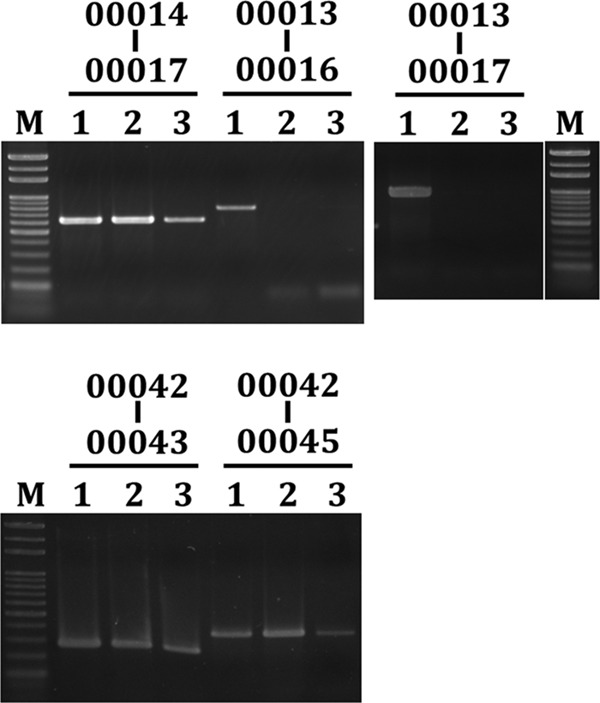
Transcriptional organization of YMC-2011. RT-PCR was used to detect contiguous transcripts between two ORFs. Products for each of the intergenic regions are listed above the gel photograph. Lanes 1, PCR products generated from the total cellular DNA of S. salivarius 57.I; lanes 2 and 3, products generated from the cDNA of wild-type 57.I, with and without mitomycin C treatment, respectively. M, 1-kb marker.
Determination of transcription initiation sites of Ssal_phage00013 and Ssal_phage00014.
Given the results described above, we next determined, using 5′ rapid amplification of cDNA ends (RACE), the transcription initiation sites for Ssal_phage00013 and Ssal_phage00014. Sequence analysis of the final PCR products mapped the transcription initiation site of Ssal_phage00013 to an A located 26 bases 5′ to the ATG (Fig. S3A). An extended −10 sequence (5′-TGTGGTAATAT) was found to be located 6 bases 5′ to the transcription initiation site (Fig. S3B). The transcription initiation site of Ssal_phage00014 was mapped to an A located 22 bases 5′ to the ATG (Fig. S3A), and a typical σ70-like promoter sequence (5′-TTGACA-N17-TATACT) was found 6 bases 5′ to the transcription initiation site (Fig. S3B). Based on the relative locations of the two ORFs, the promoters for Ssal_phage00013 and Ssal_phage00014 were designated pL and pR, respectively.
The operator regulating the expression of lytic and lysogenic genes of a temperate phage often contains direct- or inverted-repeat sequences. Two imperfect inverted repeats were found in the intergenic region between Ssal_phage00013 and Ssal_phage00014. One (5′-ACTCAATTTTCTGT) overlapped the extended −10 sequence of pL, and the other (5′-TACGAAAATTAGTA) was located between the −10 and −35 elements of pR (Fig. S3B). Based on the locations of the inverted repeats, it was suggested that they serve as targets for the antirepressor (encoded by Ssal_phage00017) and the repressor (encoded by Ssal_phage00012 and Ssal_phage00013) of YMC-2011, respectively.
Enhancement of pR transcriptional activity with mitomycin C treatment.
Because the expression of pR could potentially initiate lytic growth, whereas the expression of pL may be required for the maintenance of the lysogenic cycle, it was hypothesized that the expression of pR, but not pL, would be upregulated by mitomycin C treatment. To verify this hypothesis, the effects of mitomycin C treatment on the activity of pL and pR were examined using various chloramphenicol (Cm) acetyltransferase (cat) gene-reporter fusion strains. Mitomycin C treatment enhanced the activity of pR (2.4-fold increase), whereas it had no effect on the activity of pL (Fig. 6), suggesting that the expression of pR and pL is regulated, which determines the switch between lysogenic growth and lytic growth.
FIG 6.
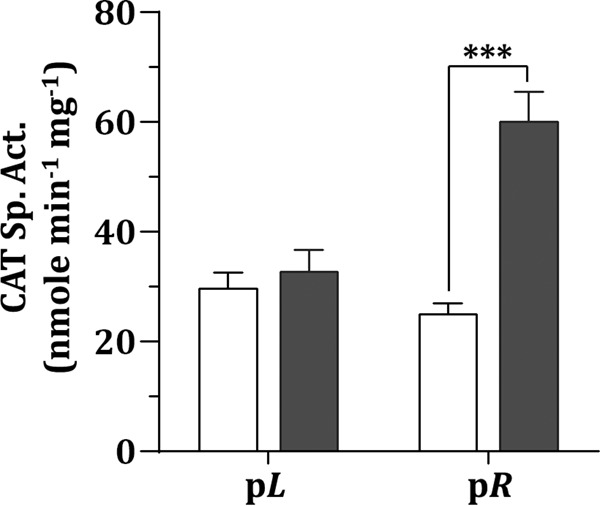
Expression analysis of the promoters of Ssal_phage00013 (pL) and Ssal_phage00014 (pR). The CAT specific activity of the pL-cat and pR-cat fusion strains was assayed after treatment with mitomycin C (■) or no treatment (□). Values are the means and standard deviations from three independent experiments. Significant differences between mitomycin C-treated samples and untreated samples were analyzed using Student's t test. ***, P < 0.001.
DISCUSSION
The significant homology between the ORFs of YMC-2011 and ORFs from S. thermophilus phages suggests that YMC-2011 is associated with phage production. However, several approaches used to induce plaque formation in S. salivarius 57.I, including treatments with UV light and mitomycin C, failed to result in the observation of plaques or complete cell lysis. Nevertheless, phage particles were isolated from mitomycin C-induced S. salivarius 57.I cultures, indicating that a lysogenic stage of YMC-2011 has been established in strain 57.I. Furthermore, YMC-2011-specific PCR products were obtained from DNA isolated from phage particles (Fig. 4), confirming that the purified phages were generated from YMC-2011, rather than an unknown phage genome integrated into the chromosome. We also investigated, by PCR, whether YMC-2011 could exist as a phage particle in S. salivarius, in addition to a plasmid, without mitomycin C treatment. We found that DNase I treatment of total cell lysates of S. salivarius in the exponential growth phase abolished the phage-specific PCR product completely, and an additional SDS treatment did not result in the production of a phage-specific PCR product (data not shown), confirming that YMC-2011 is a “naked” virion without induction.
The expression of the bacteriophage holin-lysin system is essential for the degradation of host cell peptidoglycan and the development of a lytic cycle (30). When genes encoding the putative holin-lysin system of YMC-2011, Ssal_phage00004, Ssal_phage00006, and Ssal_phage00007, were replaced with a nonpolar antibiotic resistance gene, we failed to isolate phage particles from mitomycin C-treated cultures using the standard procedure. However, phage particles were readily detected if the culture was lysed by mechanical disruption in a bead beater prior to phage isolation. Thus, the activity of the holin-lysin system is able to release phage particles but is ineffective in completely lysing host cells. Ssal_phage00004 and Ssal_phage00006 share 68% similarity, at the deduced amino acid level, with the holins (the products of lyt49 and lyt50) of phage O1205 (31). A putative ribosome binding site (RBS) (5′-AGAGG) was found 6 bases 5′ to the ATG translational start site of Ssal_phage00004, but no RBS could be found 5′ to Ssal_phage00006. These two ORFs are 16 bases apart, and thus Ssal_phage00006 may be translated via ribosome hopping, as has been seen for phage T4 (32). The translation of gene 60 of phage T4 utilizes programmed translational bypassing, in which two disparate ORFs, separated by a 50-nucleotide noncoding segment of mRNA, are translated into a single polypeptide. Further, studies have shown that only one-half of the bypassing events resume translation of the second ORF (33). Thus, it is possible that only a small amount of Ssal_phage00006 is translated. However, a putative RBS (5′-GGAGG) is located 9 bases 5′ to the ATG of Ssal_phage00007, encoding a putative phage-associated cell wall hydrolase; therefore, even if the hydrolase is translated, the enzyme may cross the membrane ineffectively to hydrolyze the peptidoglycan of the host cell.
Phages generally infect host cells via specific receptors. For instance, phage DT1 utilizes the phage antireceptor (Orf18) to adsorb onto the surface of S. thermophilus, although the receptor on the bacteria has not yet been defined (34). Ssal_phage00001 of YMC-2011 shares 75% similarity with Orf18 of DT1, suggesting that YMC-2011 is able to infect S. thermophilus. However, multiple attempts to detect any plaques in S. thermophilus ATCC BAA-250 (35) and S. salivarius ATCC 25975 (36) infected with purified YMC-2011 phage particles failed. This observation has three possible explanations, i.e., (i) Ssal_phage0001 may not be responsible for infection, (ii) the expression of pR may not be induced in these two strains, or (iii) the holin-lysin system may not be active enough to lyse the cell walls, as seen with S. salivarius 57.I.
A lysogenic state can be maintained with either an integrated prophage or a plasmid with a low copy number. In general, prophages result from integration of the phage genome into the host chromosome at a specific site through the activity of a phage integrase. For instance, a 40-bp region, known as attP, in O1205 (37) and Sfi21 (38) is the site of recombination between the phage and the host chromosome. This region is quite conserved, as the prophage of S. salivarius strain JIM8777 is also integrated at this site. While this core sequence is found in YMC-2011 and the S. salivarius 57.I chromosome, at locations analogous to those seen in S. thermophilus and S. salivarius JIM8777, plasmid YMC-2011 could also exist as an integrated prophage, as seen in strain JIM8777. When we examined, by PCR, the distance between ORFs flanking the putative attB locus in S. salivarius 57.I, we obtained an amplicon whose size was indicative of a genome-derived origin (data not shown), confirming that YMC-2011 is not integrated, at least not at the putative attB locus. When we compared the putative integrase (Ssal_phage00010) of YMC-2011 with the integrases of phages O1205 and Sfi21 and the prophage of S. salivarius JIM8777, we found that, first, Ssal_phage00010 shared the least sequence identity at the deduced amino acid level with the other three integrases and, second, most of the variations were located at the N terminus. Based on the analysis of the conserved domains, this region is proposed to harbor an AP2-like DNA-binding integrase domain. Thus, it is possible that YMC-2011 lacks an active integrase.
In contrast to phages isolated from L. lactis, which consist of at least 10 genetically distinct groups (39), the phages from S. thermophilus are derived from a single ancestor (11); thus, YMC-2011 is likely to be derived from the same ancestor, based on the sequence homology between YMC-2011 and S. thermophilus phages. Comparative sequence analysis revealed that two distinct processes, i.e., recombination and the accumulation of point mutations, created diversity among S. thermophilus phages. Recombination is apparently the basis for observed differences in lifestyle (virulent versus temperate phages), host range, and DNA packaging systems, whereas point mutations contribute approximately 10% of the sequence diversity observed among S. thermophilus phages (40). It is possible that the observed divergence between YMC-2011 and the known cos-type phages may be derived from both recombination events and point mutations.
The mechanisms for the lytic-lysogenic switch are quite similar among temperate phages. Similar to λ, two promoters, responsible for lytic and lysogenic growth, are arranged adjacently in opposite orientations in several Gram-positive bacteria phages, including Tuc2009, TP901-1, BK5-T, r1t, and φLC3 of L. lactis and Sfi21 of S. thermophilus (41), although only two operators, instead of three in λ, with palindromic sequences are observed between pL and pR in all of those phages. The repressor and the antirepressor compete for overlapping operator sites, which regulate the switch between the lysogenic state and the lytic state in most temperate lactic acid phages (42). Furthermore, the two CI repressor proteins of YMC-2011 share 52% and 81% identity with ORF127 and ORF122 of Sfi21, respectively, suggesting that the sequence of the operator in YMC-2011 is similar to that in Sfi21. Therefore, it is likely that the expression of pL and pR of YMC-2011 determines the switch between the lysogenic stage and the lytic stage, as seen in Sfi21.
In conclusion, we demonstrated that YMC-2011 could generate phage particles upon mitomycin C induction. The presence of YMC-2011 may provide immunity for S. salivarius 57.I against infection with related phages.
MATERIALS AND METHODS
Bacterial strains, plasmids, and culture conditions.
The bacterial strains and plasmids used in this work are listed in Table 2. S. salivarius 57.I was cultivated in brain heart infusion (BHI) medium (Difco) at 37°C in a 10% CO2 atmosphere. Recombinant streptococcal strains were maintained in BHI medium containing spectinomycin (Sp) at 750 μg ml−1, erythromycin (Em) at 5 μg ml−1, or kanamycin (Km) at 800 μg ml−1, as needed. Recombinant Escherichia coli strains were cultured in LB broth containing ampicillin (Ap) at 100 μg ml−1 or Sp at 100 μg ml−1, as needed.
TABLE 2.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Relevant phenotypea | Description | Source |
|---|---|---|---|
| S. salivarius strains | |||
| 57.I | Wild-type strain | 59 | |
| ΔcodY | Emr, CodY− | Strain 57.I codY::erm | 52 |
| ΔcodY_pL-cat | Emr, Spr, CodY− | Strain ΔcodY harboring spe-pL-cat fusion at lacZ | This study |
| ΔcodY_pR-cat | Emr, Spr, CodY− | Strain ΔcodY harboring spe-pR-cat fusion at lacZ | This study |
| CodY+/ΔcodY_pL-cat | Spr, Kmr, Ems, CodY+ | codY::erm in strain ΔcodY_pL-cat replaced by Ωkan-tagged codY | This study |
| CodY+/ΔcodY_pR-cat | Spr, Kmr, Ems, CodY+ | codY::erm in strain ΔcodY_pR-cat replaced by Ωkan-tagged codY | This study |
| Plasmid | |||
| pMC300 | Apr, Spr | Integration vector for S. salivarius harboring pureI-cat fusion | 52 |
r, resistant; s, sensitive.
In silico analysis of YMC-2011.
The complete sequence of YMC-2011 (GenBank accession number CP002889.1) was obtained from GenBank. The basic characteristics of ORFs of ≥30 amino acids in YMC-2011 were analyzed using Vector NTI Advance 11 (Invitrogen). The annotation of the ORFs was verified using BLASTX (http://blast.ncbi.nlm.nih.gov/Blast.cgi). A DnaA box consensus sequence, derived from the putative DnaA box sequences of S. salivarius strains in DoriC (http://tubic.tju.edu.cn/doric/search1.php) (43, 44), was used to search for the putative ori of YMC-2011 using Ori-Finder (45). Sequence alignment was analyzed using ClustalW at the San Diego Supercomputer Center Biology Workbench (http://workbench.sdsc.edu).
The whole-genome sequences of phages O1205 (GenBank accession number U88974.1), 858 (GenBank accession number EF529515.1), ALQ13.2 (GenBank accession number FJ226752.1), Abc2 (GenBank accession number NC_013645.1), DT1 (GenBank accession number AF085222.2), Sfi19 (GenBank accession number AF115102.1), Sfi21 (GenBank accession number AF115103.1), 2972 (GenBank accession number NC_007019.1), Sfi11 (GenBank accession number NC_002214.1), 7201 (GenBank accession number NC_002185.1), 9871 (GenBank accession number KU678389), 9872 (GenBank accession number KU678390), 9873 (GenBank accession number KU678391), 9874 (GenBank accession number KU678392), and 5093 (GenBank accession number NC_012753.1) of S. thermophilus were obtained from GenBank. The sequences of the putative prophages of S. salivarius were extracted from the genomes of S. salivarius strains JF (GenBank accession number NZ_CP014144.1), NCTC8618 (GenBank accession number NZ_CP009913.1), and JIM8777 (GenBank accession number NC_017595.1), respectively. The evolutionary relationships of the aforementioned phages and prophages and YMC-2011 were analyzed using MEGA 6.0 (46).
Southern hybridization.
Total cellular DNA (47) and plasmid DNA (48) were isolated from S. salivarius 57.I as described previously. Purified DNA was separated on a 0.5% agarose gel and subjected to Southern hybridization analysis. Probes specific for chromosome-borne ureC and Ssal_phage00042 of YMC-2011 were generated by PCR using the primer pairs ureC_p_S/ureC_p_AS and phage_24328_S/phage_25698_AS, respectively. All primers used in this study are listed in Table 3. The PCR products were purified and then labeled with digoxigenin (DIG) using a DIG DNA labeling and detection kit (Roche). Hybridization was carried out at 52°C for 16 h in hybridization buffer (4× Denhardt's solution, 3× SSC [standard saline citrate] [1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate], 0.5% SDS, 100 μg ml−1 salmon sperm DNA). The unbound probes were removed with two 5-min washes at 30°C at low stringency (1× SSC, 0.1% SDS), followed by two 15-min washes at 50°C at high stringency (0.1× SSC, 0.1% SDS). The bound probe was detected using the DIG detection kit, according to the manufacturer's instruction (Roche).
TABLE 3.
Primers used in this study
| Primer | Sequencea | Purpose |
|---|---|---|
| ureC_p_S | GACATCAAAGGTCTGTGGATCG | Generation of probes used in Southern analysis |
| ureC_p_AS | CTGGTCCTGCTGATGGAAGTAA | |
| phage_24328_S | GAGCCGCCACAAGGTGTTCTTT | |
| phage_25698_AS | CGCACTCACAATAACCACCTCC | |
| phage_1_S | TGGCATGTTGCCATACTCAAACAGT | Conformation of YMC-2011 as circular molecule by PCR |
| phage_5000_AS | CTGTAGTTCCTTCTTCTACGACGTT | |
| phage_4776_S | ACACAAAGAATGCGCAGTGTGGCAT | |
| phage_9776_AS | ATGTCATTTATTGGGGATATCACCG | |
| phage_9531_S | CACAGATAGAGACATTGAACTGCTC | |
| phage_14550_AS | TGTTCCCAATCGATTGAATCCAAGG | |
| phage_14326_S | TTGACGGCTCGGACTTTGAACGCAC | |
| phage_19390_AS | TGTAATCGTCTTTGATTGCCCAATT | |
| phage_19140_S | GTACAAGCTGGTATCTACGTGGATG | |
| phage_24170_AS | GTAGCTCGGTGTTCTGTTTCGTTCT | |
| phage_23871_S | ACCGAGATGATGAGAGTAAGGAACG | |
| phage_29000_AS | TACCATTGGCATTTAGGGCGTTCTT | |
| phage_28800_S | CGTCACTTTTGATGATCCACGCATT | |
| phage_33865_AS | AGAGATTTCTTGTGTGCGGACATTG | |
| phage_33651_S | CAAGCAACCGTAATGTGTTGCGTAT | |
| phage_38666_AS | AACCGTTACGGATATTGTTGTGCCC | |
| phage_38431_S | CGATGGCGTGATTCGGTTGTTCGAG | |
| phage_2600_AS | AGCGCTCTGACATATCGCTCAGATT | |
| phage_3019_S_002 | GGTTACGTCGATGGTTTTGGGC | qPCR for YMC-2011 copy number estimation |
| phage_3245_AS_002 | CCTCTTGGGATAGTTGTCAGTG | |
| phage_18928_S_031 | TGGCAAGTCATTTCTGCCAGCC | |
| phage_19142_AS_031 | ACATCGTCACCAATAATGACGG | |
| phage_36884_S_063 | GGGATAACGTCAAGAAATTCGC | |
| phage_37122_AS_063 | GGTCAATGCGTCGGTGATTAGC | |
| codY_1170_S | GGCTGCTCAATTGGCGGATATCAT | |
| codY_1280_AS | CTCCTCCACACGATCATTGTTGGTT | |
| lacZ_1241_S | GGGATATCAAAGTGATGAAAC | |
| lacZ_1465_AS | GGCACGATCCAAACAAGC | |
| ureC_6301_S | GGACCAACTGTAGGTGATAGCGTAC | |
| ureC_6460_AS | TGGATTGTCACGTGTTTCCGTAGC | |
| lacZ_1241_S | GGGATATCAAAGTGATGAAAC | Negative control for Fig. 4 |
| lacZ_1465_AS | GGCACGATCCAAACAAGC | |
| phage_9787_013_S | ATTCTCAGCTTTTGCGTTCATG | RT-PCR for transcription organization study |
| phage_10755_017_AS | ACTGATTACTGGCTCATGATTT | |
| phage_10134_014_S | CAGACTTGCTCGGAGTTGATT | |
| phage_20582_016_AS | CAACGAATCTGTTAGCGAAGC | |
| phage_24765_042_S | AGCCATGCAACTAGGTCTCA | |
| phage_25034_043_AS | ATGGCAAAAAAACGTATGACGT | |
| phage_25162_045_AS | TGTGTCTTCCGTCTGCTCAT | |
| phage_10757_R1_AS | GCACTGATTACTGGCTCATG | 5′ RACE for Ssal_phage00013 |
| phage_10653_R2_AS | GGAGAGACAAGCCATGATTGAA | |
| phage_10514_R3_AS | TTGCTATTTCAAGTGCTATCGC | |
| phage_9251_L1_S | CTCATCACAAATCGTGGTGAG | 5′ RACE for Ssal_phage00014 |
| phage_9369_L2_S | TTTCACGCAACCGTTCATAGTC | |
| phage_9479_L3_S | CTGTTTACAGGGTCGATAAAGC | |
| lacZ_S | GGTTTTGGTTCTCCACAATATGTG | Construction of pL-cat and pR-cat fusions |
| pureI_481SmaI_AS | AATCCCGGGGACCATATGGGAGTCGGTAC | |
| pureI_958SalI_S | GAGGTCGACATGAACTTTAATAAAATTGATTT | |
| lacZ_AS | CAGCAACATATTGACCGCGAAC | |
| phage_9943SmaI_PR_S | GTACCCGGGATTTCTCTTTCTTTCTATATAT | |
| phage_10067SalI_PR_AS | ATTGTCGACCTCCTTCCTTAAGCTTGATTTAAGTA | |
| phage_10067SmaI_PL_S | GCTCCCGGGTTCCTTAAGCTTGATTTAAGTA | |
| phage_9943SalI_PL_AS | TTTGTCGACCTCCATTTCTCTTTCTTTCTATATAT | |
| codY+_815BamHI_S | TTGGATCCCTGGACAAAAAGGCTTGTCC | PCR for establishing intact codY in strain ΔcodY |
| codY+_3606SphI_AS | ATAGCATGCCTTGTGACATTCTTTGAAGAGG | |
| codY+_518SacI_S_1 | GGTGAGCTCGTTTCAGAGATGATTTCCATGT | |
| codY+_824BamHI_AS | AGGGATCCAAGAAATTAACGCTTGTAATATG |
Inserted restriction recognition sites are underlined.
Confirmation of the circular structure and determination of the copy number of YMC-2011.
The conformation of YMC-2011 as a circular molecule was verified by PCR with primers generating overlapping fragments around YMC-2011. The copy number of YMC-2011 was determined by real-time qPCR, based on the method described by Chen et al. (49), with minor modifications. The reactions were conducted using iQ SYBR green supermix (Bio-Rad) and a 7500 fast real-time PCR system (Applied Biosystems). Primers specific for Ssal_phage00002 (phage_3019_S_002 and phage_3245_AS_002), Ssal_phage00031 (phage_18928_S_031 and phage_19142_AS_031), and Ssal_phage00063 (phage_36884_S_063 and phage_37122_AS_063) from YMC-2011 and for the chromosomal genes lacZ (lacZ_1241_S and lacZ_1465_AS), codY (codY_1170_S and codY_1280_AS), and ureC (ureC_6301_S and ureC_6460_AS) were designed and used for qPCR. All primers were designed to have a predicted melting temperature of ∼55°C and a product of approximately 250 bp. The copy numbers were calculated as the mean quantification cycle (Cq) values of the chromosomal genes, compared to the phage-specific genes, using the formula 2ΔCq, where ΔCq is the Cq obtained with primers specific for chromosome-borne genes minus the Cq obtained with primers specific for the ORFs of YMC-2011.
Induction and examination of phage particles.
An early-exponential-phase culture (an OD600 of 0.25 to 0.3) of S. salivarius 57.I in BHI medium was treated for 3 h with mitomycin C (Sigma) at a final concentration of 0.625 μg ml−1. At the end of the treatment, the culture was subjected to centrifugation at 3,500 rpm for 10 min at 4°C, and the supernatant was recovered. Cell debris in the supernatant was removed by filtration through a 0.45-μm-pore-size membrane (Pall). Phage particles in the supernatants were collected by ultracentrifugation at 25,000 rpm for 2 h at 4°C. The purified phage particles were suspended in 10 mM Tris-HCl (pH 8) and stored at 4°C until use. An aliquot of the phage suspension (5 μl) was deposited on top of a Formvar/carbon-coated 200-mesh grid, and the grid was set at room temperature for 1 min. Excess liquid on the grid was removed with a piece of Whatman paper. The grid was then negatively stained for 1 min with 2% phosphotungstic acid (PTA) (pH 7). The staining solution was removed with a piece of Whatman paper. The grid was dried in a chamber under low vacuum pressure. The morphology of the phage particles was observed using a JEOL 1230 transmission electron microscope, at a magnification of ×200,000. The size of phage YMC-2011 was estimated using AxioVision LE (Zeiss).
Isolation of YMC-2011 phage DNA.
DNA was isolated from the phage particles by the method described by Zinno et al. (50), with minor modifications. Briefly, the phage preparation was treated initially with DNase I and RNase A to remove free DNA and RNA in the preparation. The resulting suspension was then treated at 65°C for 30 min with 100 μl of 2.5% SDS in 0.5 M Tris-HCl, 0.25 M EDTA (pH 8), to remove the protein coat. The released DNA was further purified by phenol-chloroform (1:1) extraction and ethanol precipitation. The purity of the phage DNA was verified by PCR with two primer pairs, i.e., phage_3019_S_002/phage_3245_AS_002 (specific for Ssal_phage00002) and lacZ_1241_S/lacZ_1465_AS (specific for chromosome-borne lacZ).
RNA isolation, RT-PCR, and 5′ RACE.
Total cellular RNA was isolated from S. salivarius 57.I by the method described by Chen et al. (51). The residual DNA in the RNA preparation was removed with DNase I. The final product was further purified using an RNeasy minikit (Qiagen). To investigate the transcriptional organization of YMC-2011 by RT-PCR, 2 μg of purified RNA was reverse transcribed using avian myeloblastosis virus (AMV) reverse transcriptase and random primers (Promega). The cDNA was used in the PCR with primers specific for YMC-2011.
The transcriptional start sites of Ssal_phage00013 and Ssal_phage00014 from YMC-2011 were determined using the 5′ RACE system (Invitrogen); 5 μg of total RNA from S. salivarius 57.I was used in the 5′-RACE reaction. Ssal_phage00013- and Ssal_phage00014-specific cDNAs were synthesized from the total RNA using primers phage_9251_L1_S and phage_10757_R1_AS, respectively. After removal of the mRNA template and addition of the poly(C) tail to the cDNA pieces, the abridged anchor primer (AAP) was paired with primers phage_9369_L2_S and phage_10653_R2_AS to amplify Ssal_phage00013- and Ssal_phage00014-specific cDNA, respectively; 0.1% of the final products was then reamplified by nested PCR using the abridged universal amplification primer (AUAP) paired with the primers phage_9479_L3_S and phage_10514_R3_AS, respectively. Notably, the AUAP contains an adapter region that is complementary to the adapter region of the AAP. The final PCR products were separated by agarose gel electrophoresis and purified for sequencing analysis.
Construction of recombinant S. salivarius strains.
S. salivarius 57.I is not naturally competent; therefore, it is rather difficult to generate recombinant strains. Our recent studies revealed that a spontaneous mutation had occurred in the 5′-flanking region of comX in a codY-deficient derivative of S. salivarius 57.I (strain ΔcodY) (52). The mutation resulted in enhanced comX expression and a naturally competent phenotype (unpublished results by Y.-Y. Kao and Y.-S. Yen). Thus, S. salivarius ΔcodY was used to generate recombinant strains harboring a Ssal_phage00013 promoter (pL)-cat fusion and a Ssal_phage00014 promoter (pR)-cat fusion in the lacZ locus, by using PCR ligation mutagenesis (53). Briefly, three DNA fragments were prepared by PCR. First, the intergenic region between Ssal_phage00013 and Ssal_phage00014 was amplified from S. salivarius 57.I with two sets of primers, i.e., phage_9943SmaI_PR_S/phage_10067SalI_PR_AS and phage_10067SmaI_PL_S/phage_9943SalI_PL_AS. The second DNA fragment, containing the 5′ portion of lacZ fused to a Sp resistance gene (spe), was amplified from pMC300 (52) with the primers lacZ_S and pureI_481SmaI_AS. The third fragment, containing a promoterless cat (54) fused with the 3′ portion of lacZ, was amplified from pMC300 with the primers pureI_958SalI_S and lacZ_AS. Notably, a RBS (5′-GGAGG) was located 5 bases 5′ to the translational start site of cat. Restriction sites were included in the primers to facilitate cloning. All PCR products were digested with SmaI and/or SalI to allow for ligation in the following order: lacZ-spe followed by pL-cat/pR-cat and the 3′ portion of lacZ. The resulting ligation mixture was introduced into S. salivarius ΔcodY by natural transformation (55), and the correct recombination event in the Sp-resistant transformants was verified by colony PCR using lacZ-specific primers. The recombinant strains harboring the pL-cat and pR-cat transcriptional fusions in the lacZ locus were designated ΔcodY_pL-cat and ΔcodY_pR-cat, respectively. Once the desired constructs were established in strain ΔcodY, an intact codY was reestablished in the host using a similar approach. To accomplish this, a 2-kbp amplicon containing the promoter and coding sequence of codY and its 3′-flanking region of 550 bp was amplified from S. salivarius 57.I with primers codY+_815BamHI_S and codY+_3606_SphI_AS. An amplicon containing the 5′-flanking gene of codY, alaA, was generated from S. salivarius 57.I by using primers codY+_518SacI_S_1 and codY+_824BamHI_AS. Both PCR products were digested with BamHI and then ligated to the Ωkan cassette (56) on a BamHI fragment. The resulting ligation mixture was used to transform strains ΔcodY_pL-cat and ΔcodY_pR-cat, and transformants were selected on agar containing Km. The correct recombination event was verified by colony PCR using codY-specific primers. The resulting recombinant strains were designated CodY+/ΔcodY_pL-cat and CodY+/ΔcodY_pR-cat, respectively.
CAT assay.
Recombinant S. salivarius pL-cat and pR-cat fusion strains were cultivated in BHI medium to an OD600 of 0.25 to 0.3, followed by a 1-hour treatment with mitomycin C; control cultures were grown and not treated. At the end of the treatment, the cultures were harvested, washed once with 10 mM Tris-HCl (pH 7.8), and resuspended in the same buffer in 2.5% of the original culture volume. Total lysates were prepared as described previously (57). The protein concentrations of the lysates were measured using the Bio-Rad protein assay. CAT activities were determined by the method described by Shaw (58), and specific activity values were calculated as nanomoles of Cm acetylated per minute per milligram of total protein. All reactions were performed in triplicate, and negative-control reactions were performed in the absence of the substrates. Statistical analysis between mitomycin C-treated and untreated samples was performed using the unpaired two-tailed Student's t test.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by the Ministry of Science and Technology, Republic of China (grant NSC102-2320-B-182_031-MY3 to Y.-Y.M.C.).
We thank R. Faustoferri and S. T. Liu for review of the manuscript.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/AEM.03186-16.
REFERENCES
- 1.Brussow H, Hendrix RW. 2002. Phage genomics: small is beautiful. Cell 108:13–16. doi: 10.1016/S0092-8674(01)00637-7. [DOI] [PubMed] [Google Scholar]
- 2.Hitch G, Pratten J, Taylor PW. 2004. Isolation of bacteriophages from the oral cavity. Lett Appl Microbiol 39:215–219. doi: 10.1111/j.1472-765X.2004.01565.x. [DOI] [PubMed] [Google Scholar]
- 3.Bachrach G, Leizerovici-Zigmond M, Zlotkin A, Naor R, Steinberg D. 2003. Bacteriophage isolation from human saliva. Lett Appl Microbiol 36:50–53. doi: 10.1046/j.1472-765X.2003.01262.x. [DOI] [PubMed] [Google Scholar]
- 4.Delisle AL, Guo M, Chalmers NI, Barcak GJ, Rousseau GM, Moineau S. 2012. Biology and genome sequence of Streptococcus mutans phage M102AD. Appl Environ Microbiol 78:2264–2271. doi: 10.1128/AEM.07726-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Siboo IR, Bensing BA, Sullam PM. 2003. Genomic organization and molecular characterization of SM1, a temperate bacteriophage of Streptococcus mitis. J Bacteriol 185:6968–6975. doi: 10.1128/JB.185.23.6968-6975.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maal KB, Bouzari M, Zavareh A. 2010. Identification of Streptococcus salivarius bacteriophage isolated from Persian Gulf as potential agent for dental caries phage therapy. Afr J Microbiol Res 20:2127–2132. [Google Scholar]
- 7.Guedon E, Delorme C, Pons N, Cruaud C, Loux V, Couloux A, Gautier C, Sanchez N, Layec S, Galleron N, Almeida M, van de Guchte M, Kennedy SP, Ehrlich SD, Gibrat JF, Wincker P, Renault P. 2011. Complete genome sequence of the commensal Streptococcus salivarius strain JIM8777. J Bacteriol 193:5024–5025. doi: 10.1128/JB.05390-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Quiberoni A, Moineau S, Rousseau GM, Reinheimer J, Ackermann H-W. 2010. Streptococcus thermophilus bacteriophages. Int Dairy J 20:657–664. doi: 10.1016/j.idairyj.2010.03.012. [DOI] [Google Scholar]
- 9.Mercenier A. 1990. Molecular genetics of Streptococcus thermophilus. FEMS Microbiol Rev 7:61–77. [DOI] [PubMed] [Google Scholar]
- 10.Mahony J, van Sinderen D. 2014. Current taxonomy of phages infecting lactic acid bacteria. Front Microbiol 5:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Le Marrec C, van Sinderen D, Walsh L, Stanley E, Vlegels E, Moineau S, Heinze P, Fitzgerald G, Fayard B. 1997. Two groups of bacteriophages infecting Streptococcus thermophilus can be distinguished on the basis of mode of packaging and genetic determinants for major structural proteins. Appl Environ Microbiol 63:3246–3253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tremblay DM, Moineau S. 1999. Complete genomic sequence of the lytic bacteriophage DT1 of Streptococcus thermophilus. Virology 255:63–76. doi: 10.1006/viro.1998.9525. [DOI] [PubMed] [Google Scholar]
- 13.Desiere F, Lucchini S, Brussow H. 1998. Evolution of Streptococcus thermophilus bacteriophage genomes by modular exchanges followed by point mutations and small deletions and insertions. Virology 241:345–356. doi: 10.1006/viro.1997.8959. [DOI] [PubMed] [Google Scholar]
- 14.Guglielmotti DM, Deveau H, Binetti AG, Reinheimer JA, Moineau S, Quiberoni A. 2009. Genome analysis of two virulent Streptococcus thermophilus phages isolated in Argentina. Int J Food Microbiol 136:101–109. doi: 10.1016/j.ijfoodmicro.2009.09.005. [DOI] [PubMed] [Google Scholar]
- 15.Bruttin A, Desiere F, Lucchini S, Foley S, Brussow H. 1997. Characterization of the lysogeny DNA module from the temperate Streptococcus thermophilus bacteriophage ϕSfi21. Virology 233:136–148. doi: 10.1006/viro.1997.8603. [DOI] [PubMed] [Google Scholar]
- 16.Ali Y, Koberg S, Hessner S, Sun X, Rabe B, Back A, Neve H, Heller KJ. 2014. Temperate Streptococcus thermophilus phages expressing superinfection exclusion proteins of the Ltp type. Front Microbiol 5:98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lucchini S, Desiere F, Brussow H. 1999. Comparative genomics of Streptococcus thermophilus phage species supports a modular evolution theory. J Virol 73:8647–8656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Levesque C, Duplessis M, Labonte J, Labrie S, Fremaux C, Tremblay D, Moineau S. 2005. Genomic organization and molecular analysis of virulent bacteriophage 2972 infecting an exopolysaccharide-producing Streptococcus thermophilus strain. Appl Environ Microbiol 71:4057–4068. doi: 10.1128/AEM.71.7.4057-4068.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Deveau H, Barrangou R, Garneau JE, Labonte J, Fremaux C, Boyaval P, Romero DA, Horvath P, Moineau S. 2008. Phage response to CRISPR-encoded resistance in Streptococcus thermophilus. J Bacteriol 190:1390–1400. doi: 10.1128/JB.01412-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mills S, Griffin C, O'Sullivan O, Coffey A, McAuliffe OE, Meijer WC, Serrano LM, Ross RP. 2011. A new phage on the ‘Mozzarella’ block: bacteriophage 5093 shares a low level of homology with other Streptococcus thermophilus phages. Int Dairy J 21:963–969. doi: 10.1016/j.idairyj.2011.06.003. [DOI] [Google Scholar]
- 21.McDonnell B, Mahony J, Neve H, Hanemaaijer L, Noben JP, Kouwen T, van Sinderen D. 2016. Identification and analysis of a novel group of bacteriophages infecting the lactic acid bacterium Streptococcus thermophilus. Appl Environ Microbiol 82:5153–5165. doi: 10.1128/AEM.00835-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brussow H, Desiere F. 2001. Comparative phage genomics and the evolution of Siphoviridae: insights from dairy phages. Mol Microbiol 39:213–222. doi: 10.1046/j.1365-2958.2001.02228.x. [DOI] [PubMed] [Google Scholar]
- 23.Prentki P, Chandler M, Caro L. 1977. Replication of prophage P1 during the cell cycle of Escherichia coli. Mol Gen Genet 152:71–76. doi: 10.1007/BF00264942. [DOI] [PubMed] [Google Scholar]
- 24.Zhong L, Cheng Q, Tian X, Zhao L, Qin Z. 2010. Characterization of the replication, transfer, and plasmid/lytic phage cycle of the Streptomyces plasmid-phage pZL12. J Bacteriol 192:3747–3754. doi: 10.1128/JB.00123-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Little JW, Mount DW. 1982. The SOS regulatory system of Escherichia coli. Cell 29:11–22. doi: 10.1016/0092-8674(82)90085-X. [DOI] [PubMed] [Google Scholar]
- 26.Atsumi S, Little JW. 2006. Role of the lytic repressor in prophage induction of phage λ as analyzed by a module-replacement approach. Proc Natl Acad Sci U S A 103:4558–4563. doi: 10.1073/pnas.0511117103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Geng J, Huang SC, Li S, Hu S, Chen YY. 2011. Complete genome sequence of the ureolytic Streptococcus salivarius strain 57.I. J Bacteriol 193:5596–5597. doi: 10.1128/JB.05670-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stanley E, Walsh L, van der Zwet A, Fitzgerald GF, van Sinderen D. 2000. Identification of four loci isolated from two Streptococcus thermophilus phage genomes responsible for mediating bacteriophage resistance. FEMS Microbiol Lett 182:271–277. doi: 10.1111/j.1574-6968.2000.tb08907.x. [DOI] [PubMed] [Google Scholar]
- 29.Lamothe G, Levesque C, Bissonnette F, Cochu A, Vadeboncoeur C, Frenette M, Duplessis M, Tremblay D, Moineau S. 2005. Characterization of the cro-ori region of the Streptococcus thermophilus virulent bacteriophage DT1. Appl Environ Microbiol 71:1237–1246. doi: 10.1128/AEM.71.3.1237-1246.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shi Y, Yan Y, Ji W, Du B, Meng X, Wang H, Sun J. 2012. Characterization and determination of holin protein of Streptococcus suis bacteriophage SMP in heterologous host. Virol J 9:70. doi: 10.1186/1743-422X-9-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sheehan MM, Stanley E, Fitzgerald GF, van Sinderen D. 1999. Identification and characterization of a lysis module present in a large proportion of bacteriophages infecting Streptococcus thermophilus. Appl Environ Microbiol 65:569–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Huang WM, Ao SZ, Casjens S, Orlandi R, Zeikus R, Weiss R, Winge D, Fang M. 1988. A persistent untranslated sequence within bacteriophage T4 DNA topoisomerase gene 60. Science 239:1005–1012. doi: 10.1126/science.2830666. [DOI] [PubMed] [Google Scholar]
- 33.Herr AJ, Wills NM, Nelson CC, Gesteland RF, Atkins JF. 2001. Drop-off during ribosome hopping. J Mol Biol 311:445–452. doi: 10.1006/jmbi.2001.4899. [DOI] [PubMed] [Google Scholar]
- 34.Duplessis M, Moineau S. 2001. Identification of a genetic determinant responsible for host specificity in Streptococcus thermophilus bacteriophages. Mol Microbiol 41:325–336. doi: 10.1046/j.1365-2958.2001.02521.x. [DOI] [PubMed] [Google Scholar]
- 35.Bolotin A, Quinquis B, Renault P, Sorokin A, Ehrlich SD, Kulakauskas S, Lapidus A, Goltsman E, Mazur M, Pusch GD, Fonstein M, Overbeek R, Kyprides N, Purnelle B, Prozzi D, Ngui K, Masuy D, Hancy F, Burteau S, Boutry M, Delcour J, Goffeau A, Hols P. 2004. Complete sequence and comparative genome analysis of the dairy bacterium Streptococcus thermophilus. Nat Biotechnol 22:1554–1558. doi: 10.1038/nbt1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hamilton IR. 1968. Synthesis and degradation of intracellular polyglucose in Streptococcus salivarius. Can J Microbiol 14:65–77. doi: 10.1139/m68-011. [DOI] [PubMed] [Google Scholar]
- 37.Stanley E, Fitzgerald GF, Le Marrec C, Fayard B, van Sinderen D. 1997. Sequence analysis and characterization of ϕO1205, a temperate bacteriophage infecting Streptococcus thermophilus CNRZ1205. Microbiology 143:3417–3429. doi: 10.1099/00221287-143-11-3417. [DOI] [PubMed] [Google Scholar]
- 38.Bruttin A, Foley S, Brussow H. 1997. The site-specific integration system of the temperate Streptococcus thermophilus bacteriophage phiSfi21. Virology 237:148–158. doi: 10.1006/viro.1997.8769. [DOI] [PubMed] [Google Scholar]
- 39.Deveau H, Labrie SJ, Chopin MC, Moineau S. 2006. Biodiversity and classification of lactococcal phages. Appl Environ Microbiol 72:4338–4346. doi: 10.1128/AEM.02517-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Brussow H, Bruttin A, Desiere F, Lucchini S, Foley S. 1998. Molecular ecology and evolution of Streptococcus thermophilus bacteriophages: a review. Virus Genes 16:95–109. doi: 10.1023/A:1007957911848. [DOI] [PubMed] [Google Scholar]
- 41.Kenny JG, Leach S, de la Hoz AB, Venema G, Kok J, Fitzgerald GF, Nauta A, Alonso JC, van Sinderen D. 2006. Characterization of the lytic-lysogenic switch of the lactococcal bacteriophage Tuc2009. Virology 347:434–446. doi: 10.1016/j.virol.2005.11.041. [DOI] [PubMed] [Google Scholar]
- 42.Bruttin A, Foley S, Brussow H. 2002. DNA-binding activity of the Streptococcus thermophilus phage Sfi21 repressor. Virology 303:100–109. doi: 10.1006/viro.2002.1574. [DOI] [PubMed] [Google Scholar]
- 43.Gao F, Luo H, Zhang CT. 2013. DoriC 5.0: an updated database of oriC regions in both bacterial and archaeal genomes. Nucleic Acids Res 41:D90–D93. doi: 10.1093/nar/gks990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gao F, Zhang CT. 2007. DoriC: a database of oriC regions in bacterial genomes. Bioinformatics 23:1866–1867. doi: 10.1093/bioinformatics/btm255. [DOI] [PubMed] [Google Scholar]
- 45.Gao F, Zhang CT. 2008. Ori-Finder: a web-based system for finding oriCs in unannotated bacterial genomes. BMC Bioinformatics 9:79. doi: 10.1186/1471-2105-9-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tamura K, Stecher G, Peterson D, Filipski A, Kumar S. 2013. MEGA6: Molecular Evolutionary Genetics Analysis version 6.0. Mol Biol Evol 30:2725–2729. doi: 10.1093/molbev/mst197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chen YY, Clancy KA, Burne RA. 1996. Streptococcus salivarius urease: genetic and biochemical characterization and expression in a dental plaque streptococcus. Infect Immun 64:585–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Anderson DG, McKay LL. 1983. Simple and rapid method for isolating large plasmid DNA from lactic streptococci. Appl Environ Microbiol 46:549–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chen YY, Shieh HR, Lin CT, Liang SY. 2011. Properties and construction of plasmid pFW213, a shuttle vector with the oral Streptococcus origin of replication. Appl Environ Microbiol 77:3967–3974. doi: 10.1128/AEM.02828-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zinno P, Janzen T, Bennedsen M, Ercolini D, Mauriello G. 2010. Characterization of Streptococcus thermophilus lytic bacteriophages from mozzarella cheese plants. Int J Food Microbiol 138:137–144. doi: 10.1016/j.ijfoodmicro.2009.12.008. [DOI] [PubMed] [Google Scholar]
- 51.Chen YY, Weaver CA, Mendelsohn DR, Burne RA. 1998. Transcriptional regulation of the Streptococcus salivarius 57.I urease operon. J Bacteriol 180:5769–5775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Huang SC, Burne RA, Chen YY. 2014. The pH-dependent expression of the urease operon in Streptococcus salivarius is mediated by CodY. Appl Environ Microbiol 80:5386–5393. doi: 10.1128/AEM.00755-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lau PC, Sung CK, Lee JH, Morrison DA, Cvitkovitch DG. 2002. PCR ligation mutagenesis in transformable streptococci: application and efficiency. J Microbiol Methods 49:193–205. doi: 10.1016/S0167-7012(01)00369-4. [DOI] [PubMed] [Google Scholar]
- 54.Horinouchi S, Weisblum B. 1982. Nucleotide sequence and functional map of pC194, a plasmid that specifies inducible chloramphenicol resistance. J Bacteriol 150:815–825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Perry D, Kuramitsu HK. 1981. Genetic transformation of Streptococcus mutans. Infect Immun 32:1295–1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Perez-Casal J, Caparon MG, Scott JR. 1991. Mry, a trans-acting positive regulator of the M protein gene of Streptococcus pyogenes with similarity to the receptor proteins of two-component regulatory systems. J Bacteriol 173:2617–2624. doi: 10.1128/jb.173.8.2617-2624.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chen YY, Betzenhauser MJ, Burne RA. 2002. cis-Acting elements that regulate the low-pH-inducible urease operon of Streptococcus salivarius. Microbiology 148:3599–3608. doi: 10.1099/00221287-148-11-3599. [DOI] [PubMed] [Google Scholar]
- 58.Shaw WV. 1975. Chloramphenicol acetyltransferase from chloramphenicol-resistant bacteria. Methods Enzymol 43:737–755. doi: 10.1016/0076-6879(75)43141-X. [DOI] [PubMed] [Google Scholar]
- 59.Sissons CH, Hancock EM, Perinpanayagam HE, Cutress TW. 1988. The bacteria responsible for ureolysis in artificial dental plaque. Arch Oral Biol 33:727–733. doi: 10.1016/0003-9969(88)90006-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.