Abstract
Rat spermatogenic cells contain sphingomyelins (SMs) and ceramides (Cers) with very long-chain PUFAs (VLCPUFAs) in nonhydroxylated (n-V) and 2-hydroxylated (h-V) forms. How these atypical species distribute among membrane fractions during differentiation was investigated here using a detergent-free procedure to isolate a small light raft-like low-density fraction and a large heavy fraction, mostly derived from the plasma membrane of spermatocytes, round spermatids, and late spermatids. The light fraction contained cholesterol, glycerophospholipids (GPLs), and SM with the same saturated fatty acids in all three stages. In the heavy fraction, as PUFA increased in the GPL and VLCPUFA in SM from spermatocytes to spermatids, the concentration of cholesterol was also augmented. The heavy fraction had mostly n-V SM in spermatocytes, but accumulated h-V SM and h-V Cer in spermatids. A fraction containing intracellular membranes had less SM and more Cer than the latter, but in both fractions SM and Cer species with h-V increased over species with n-V with differentiation. This accretion of h-V was consistent with the differentiation-dependent expression of fatty acid 2-hydroxylase (Fa2h), as it increased significantly from spermatocytes to spermatids. The non-raft region of the plasma membrane is thus the main target of the dynamic lipid synthesis and remodeling that is involved in germ cell differentiation.
Keywords: 2-hydroxy fatty acids, fatty acid hydroxylase, membrane domains, spermatogenesis, sphingomyelins, very long-chain polyunsaturated fatty acids
The glycerophospholipids (GPLs) and sphingolipids of rat testis are known to be rich in long-chain PUFAs (C18–C22) and very long-chain PUFAs (VLCPUFAs) (C24–C32) of the n-6 series. In spermatogenic cells from the adult rat, major ester-bound PUFAs of GPLs are docosapentaenoic acid (22:5n-6) and arachidonic acid (20:4n-6) (1); whereas major amide-bound fatty acids of sphingomyelins (SMs) and ceramides (Cers) are 28:4n-6, 30:5n-6, and 32:5n-6 (2). Previous work has shown that in rat and boar testes, such fatty acids occur in SM in nonhydroxylated (n-V) and 2-hydroxylated (h-V) versions (3). Focus on the fatty acids of rat testis Cer showed it to contain even higher percentages of such fatty acids than SM (4, 5). The atypical 2-hydroxy-VLCPUFAs (e.g., h-30:5n-6) were also found by Sandhoff et al. (6) to be components of a novel series of glycosphingolipids (GSLs) that contain fucose in their head groups (FGSLs), which they discovered and thoroughly characterized in the rodent testis (mouse, rat). In the mouse, these complex sphingolipids were shown to be required in spermatogenic cells for a proper completion of meiosis (7). More recently, by conditionally knocking out (endoplasmic reticulum-located) Cer synthase 3 (CerS3) and glucosyl-Cer synthase specifically in mouse germ cells, the essentiality of both simple and complex sphingolipids that contain VLCPUFAs has been demonstrated, not only for meiosis, but also for the stability of spermatids (8). Neither Cer with VLCPUFA, nor the species of SM or FGSL that contain such fatty acids are produced in the testis in the absence of CerS3, resulting in spermatogenic arrest, increased apoptosis, and formation of multinuclear giant cells.
With normal postnatal development, SM and Cer with nonhydroxy fatty acids (n-V SM, n-V Cer) appear in the rat testis in temporal concomitance with pachytene spermatocytes (PtSs), while species containing 2-hydroxy-VLCPUFA (h-V SM, h-V Cer) only become present when spermatids do (4). The opposite occurs when spermatogonia are damaged in the adult rat testis by an antineoplastic drug (4) or by X-rays (5), as the disappearance of spermatocytes (concomitantly with n-V SM and n-V Cer) temporally precedes that of spermatids (together with h-V SM and h-V Cer) from the testis.
Classical studies comparing lipids of spermatocytes and spermatids isolated from adult rats showed that a hallmark of germ cell differentiation was an increase in the 22:5n-6/20:4n-6 ratio in GPLs and triacylglycerols (1). By the same token, a similar comparison focused on the VLCPUFA of rat SM and Cer showed that the ratio between h-V and n-V VLCPUFAs also increases significantly in both lipids when comparing premeiotic PtSs with postmeiotic round spermatids (RSs) (9). Recently, different lipid classes and species of membrane lipids have been localized in adult mouse testis sections directly in situ, for the first time, using high-resolution mass spectrometric imaging (8). In agreement with our studies in isolated cells (9), these authors found major n-V SM species, like 30:5 SM, in the region of spermatocytes, and major h-V SM species, like h-30:5 SM, in the center of the seminiferous tubules where spermatids are located.
At present, there is no information on the localization of the n-V- and h-V-containing species of SM and Cer in the membranes of rat spermatogenic cells. In general, SM molecules are accepted to associate with cholesterol in the laterally segregated discrete membrane microdomains commonly known as “lipid rafts” or “membrane rafts”. These structures are envisaged to function as platforms for the attachment of specific proteins when membranes move around the cell and during signal transduction (10). In an international meeting bringing together biophysicists, biochemists, and cell biologists to elaborate consensus about membrane rafts, these structures were defined as “small (10–200 nm) heterogeneous highly dynamic sterol- and sphingolipid-enriched domains that compartmentalize cellular processes” (11).
As compared with well-characterized SM species, such as the “typical” 16:0 SM, some of the biophysical properties of n-V SM or h-V SM species are quite atypical when placed in bilayers (12) or Langmuir monolayers (13). Their low transition temperatures and, especially, the lack of propensity to form laterally segregated domains when interacting with cholesterol in giant unilamellar vesicles (12), suggest that the inclusion of such SMs in the classical cholesterol-rich membrane rafts is unlikely in the case of spermatogenic cells. The present study was undertaken to assess this possibility and to answer the question of how membrane components, like cholesterol and species of GPL, SM, and Cer with such unusual fatty acids, are laterally distributed among cell membranes, including raft-like and the corresponding non-raft-like membrane domains, as spermatogenic cells differentiate.
The insolubility of rafts in nonionic detergents, such as Triton X-100, at 4°C has been the most common means of raft isolation, being represented by the low-density “detergent-resistant membranes” (DRMs) obtained after ultracentrifugation in a sucrose gradient (14, 15). In the present study, some technical difficulties were encountered when DRMs were isolated from spermatogenic cells with this detergent, one of which was the impossibility of recovering the non-raft fraction of membranes, which is dissolved and merged with the rest of the detergent-soluble membranes. This and other practical reasons regarding lipids (see supplemental Fig. S1) led us to use a detergent-free procedure. A method previously described for thymocytes (16) and further applied with modifications to Xenopus laevis oocytes (17) was used to isolate two membrane fractions expected to mostly derive from the plasma membrane: a light low-density fraction and a heavy fraction from rat spermatogenic cells at three specific maturational stages: PtS, RS, and late spermatid (LS). A third fraction collecting an assortment of membranes of intracellular origin, including the endoplasmic reticulum, Golgi, mitochondria, and lysosomes (16), here given the name of “extra-heavy” membrane fraction because it was denser than the heavy fraction, was also appraised, just for quantitative purposes and for qualitative comparison with the light and heavy fractions.
Our results provide evidence that the SM and Cer with VLCPUFA (n-V and h-V) are virtually excluded from the low-density raft-like domains, at the cell maturation stages surveyed, and that differentiation entails a remodeling of the species located in the non-raft portion of the plasma membrane, where such unique SM and Cer molecular species find their ultimate location. Because the atypical h-V chains of these lipids are expected to be produced by the introduction of a hydroxyl group in a preexisting n-V Cer species, the expression (mRNA) of the fatty acid 2-hydroxylase (Fa2h) gene was surveyed and our results pointed to a significant intensification of its transcription as cells differentiated from spermatocytes to spermatids.
MATERIALS AND METHODS
Preparation of spermatogenic cells
Three-month-old Wistar male rats were employed. The protocol for animal use was approved by the Committee for the Care of Laboratory Animals (CICUAE) of the Universidad Nacional del Sur, Argentina, whose rules agree with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. The seminiferous tubules were obtained from the testes immediately after the animals were euthanized by CO2 inhalation. A Krebs-Henseleit medium supplemented with 0.5 mM of CaCl2 and 10 mM of DL (+)-lactate, type I collagenase (0.5 mg/ml), and DNase (20 μg/ml) was used to separate the interstitial cells from the tubules. The latter were digested with trypsin (0.4 mg/ml) and DNase (20 μg/ml) to release the intratubular cells, and the resulting cell suspensions were gently aspired with Pasteur pipettes. The cells were filtered successively through 250 and 60 μm nylon meshes to remove spermatozoa and aggregates of Sertoli and germ cells. The resultant suspension of total spermatogenic cells (TCs) was then separated, by means of the STA-PUT procedure (18), into three major cellular fractions corresponding to PtS, RS, and LS in an acceptable level of purity (84%, 90%, and 76%, respectively). The cells were identified by size and by the typical aspect of their nuclei, which were stained with H33342 (19).
Cell membrane fractionation
The detergent-free method, described by Luria et al. (17), was employed to obtain membrane fractions from TCs, PtSs, RSs, and LSs. The cells were centrifuged and the pellets were lysed in ice-cold TNE buffer [50 mM Tris-HCl, 70 mM NaCl, and 5 mM EDTA (pH 7.4)] in the presence of the Sigma P-8340 protease inhibitor cocktail. The cell lysates were homogenized by passing them repeatedly through a 27 gauge needle and then subjecting them for 3 min in a cold bath to sonication at 50 Hz (Branson Sonifier B-220, Smithkline Co.). Each lysate was mixed with an equal volume of 80% sucrose in TNE to obtain a final equivalent density of 40% sucrose. A discontinuous sucrose gradient was generated by placing 3 ml of this cell homogenate at the bottom of ultracentrifuge tubes and overlaying 3 ml each of sucrose at decreasing concentrations of 35%, 22.5%, and 10% (Fig. 1A). The tubes were centrifuged for 3 h at 100,000 g at 4°C in a SW41 Beckman rotor using a Beckman Coulter Optima L-90K ultracentrifuge. Three bands corresponding to a light (at the 10–22.5% sucrose interface), a heavy (22.5–35% sucrose interface), and an extra-heavy (35–40% sucrose interface) membrane fraction were obtained. The membrane fractions obtained from each spermatogenic cell type were separately collected, suspended in TNE buffer, and centrifuged for 45 min at 200,000 g at 4°C in a 90Ti Beckman rotor to remove excess sucrose from membrane samples. The membrane pellets were suspended in TNE buffer containing protease inhibitors, aliquots were taken for protein quantitation and enzyme assays, and the remainder were used for lipid extraction and analysis. The DC Protein Assay (BIO-RAD Life Science Group, FL) was used to determine the protein content.
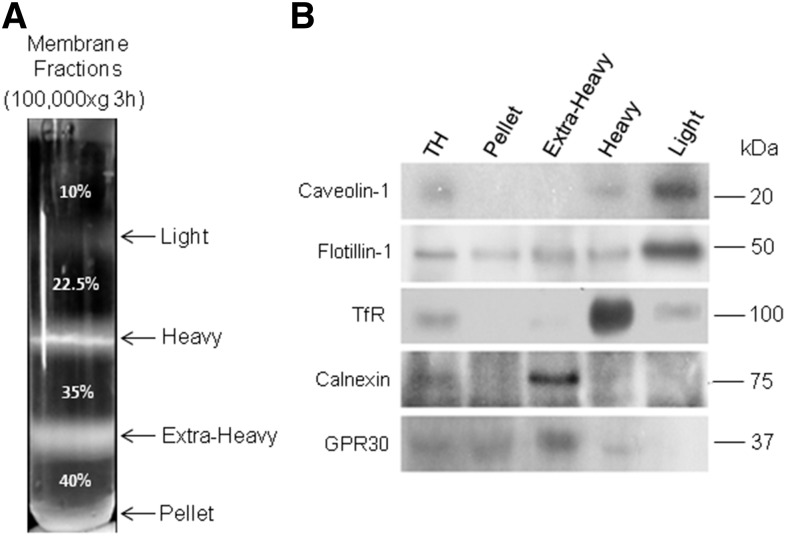
Fig. 1.
Isolation and features of the membrane fractions obtained from adult rat spermatogenic cells by a detergent-free method. The same procedures illustrated here for the TCs were applied to the PtS, RS, and LS populations. Total homogenates (TH) were prepared in each case and subjected to ultracentrifugation using a discontinuous sucrose gradient to separate the membrane fractions. A: Membrane fractions isolated from the TH of TCs. The arrows point to the separation between the sucrose concentrations shown in the tube (35%, 22.5%, and 10%), the arrows indicating the interphases at which the light, heavy, and extra-heavy membrane fractions were collected (at the bottom of the tube, was an insoluble pellet). B: Representative immunoblots showing the distribution of the indicated proteins in the TH before fractionation and among membrane fractions separated as shown in (A). The amount of protein loaded in each lane was 25 μg. The proteins shown are two raft/caveolae markers (caveolin-1 and flotillin-1), a non-raft plasma membrane marker protein (TfR), and two endoplasmic reticulum markers (calnexin and GPR30 protein).
Membrane characterization
Western blot assays.
Aliquots from cell membrane fractions were denatured with Laemmli sample buffer at 100°C for 5 min (20). Equivalent amounts of proteins (25 μg) from different samples were resolved by SDS-PAGE on 10% polyacrylamide gels and transferred to PVDF membranes (Millipore, Bedford, MA). After being blocked for 2 h at room temperature with 5% BSA in TBS-T buffer [20 mM Tris-HCl (pH 7.4), 100 mM NaCl, and 0.1% (w/v) Tween 20], the PVDF membranes were incubated overnight at 4°C with primary rabbit polyclonal antibodies against caveolin-1 (1:1,000; sc-894), flotillin-1 (1:1,000; sc-25506), calnexin (1:1,000; sc-11397), and GPR30 (1:500; sc-48524), all from Santa Cruz Biotechnology, and with a mouse monoclonal antibody against transferrin receptor (TfR) (H68.4, 13-6800) from Thermo Fisher Scientific. After washing three times with TBS-T, PVDF membranes were exposed for 2 h at room temperature to the appropriate HRP-conjugated secondary antibodies (1:5,000 dilution, for anti-rabbit sc-20049 and 1:2,000 dilution for anti-mouse sc-2005, both from Santa Cruz Biotechnology). After extensive washing with TBS-T, immunoreactive bands were detected by ECL (Amersham Biosciences) using standard X-ray films (Kodak X-Omat AR).
5′ nucleotidase.
The activity of this enzyme, considered a marker of plasma membrane, was determined in all membrane fractions according to Widnell and Unkeless (21). This method measures the release of inorganic phosphate from AMP.
Lipid separation and analysis
After collecting membrane fractions from cells, lipid extracts were prepared and partitioned according to Bligh and Dyer (22). The organic phases containing the lipids were recovered, the organic solvents were evaporated under N2, and the samples were dissolved in chloroform-methanol (2:1 v/v). Aliquots from the lipid extracts were taken for lipid analysis. Lipid phosphorus (P) was determined as described by Rouser, Fkeischer, and Yamamoto (23) and cholesterol was measured using an enzymatic assay (Wiener Laboratories, Rosario, Argentina). Phospholipid (PL) class composition was determined after separation on HPTLC plates (Merck, Germany) using chloroform:methanol:acetic acid:water (50:37.5:3.5:2 by volume) (24) as solvent. After locating the PLs with iodine vapors, the silica gel containing each class was transferred to tubes and lipids were quantified by phosphorus analysis (23).
The rest of the lipid extracts, after being spotted on TLC plates (500 μm, silica gel G), were used to preparatively isolate SM and Cer, essentially as described previously (9). Briefly, the n-V- and h-V-containing Cer bands were resolved directly (and from other neutral lipids) using two solvent mixtures run successively. Chloroform:methanol:aqueous ammonia (90:10:2 v/v) was run up to the middle of the plates and, after drying under N2, hexane:ether (80:20, v/v) was run up to the top. The total polar lipid fraction remaining at the origin of these plates was recovered and used to isolate the total GPL and total SM in samples. Silica gel H plates and chloroform:methanol:aqueous ammonia (65:25:5 v/v) as solvent were used. The lipid bands were located on the plates under UV light after spraying with 2′,7′-dichlorofluorescein, and the zones containing the lipids were scraped into tubes for elution. This was performed by three successive extractions with chloroform:methanol:water (5:5:1 v/v), thorough mixing, centrifuging, collecting the solvents, and partitioning with 4 vol of water to recover the lipids in the chloroform phase.
After elution of Cer and SM, a mild alkali treatment (exposure 0.5 N NaOH in anhydrous methanol at 50°C for 10 min), followed by a second TLC, was routinely performed to remove any potential lipid contaminant containing ester-bound fatty acids (9). This procedure, involving alkaline methanolysis, was also used to obtain, as methyl esters, the fatty acids ester-bound to the GPL present in the total polar lipid fraction (thus excluding the fatty acids amide-bound to SM and other sphingolipids) for GPL fatty acid analysis.
Fatty acid analysis of SM and Cer in membrane fractions (light, heavy, and extra-heavy) was performed in those derived from TCs (using 0.5 N H2SO4 in anhydrous methanol under N2 at 45°C, overnight, for methanolysis) (25). The methyl esters of n-V and h-V fatty acids [fatty acid methyl esters (FAMEs)] were separated by TLC [on silica gel G plates that had been prewashed with methanol:ethyl ether (75:25 v/v) and dried]. Hexane:ether (80:20 v/v) was run up to the middle of the plates, dried, and then hexane:ether (95:5 v/v) was run up to the top. The bands containing nonhydroxy- and 2-hydroxy-FAME were located and scraped into tubes, where the silica was thoroughly mixed with water:methanol:hexane (1:1:1 v/v) and centrifuged. The upper hexane layer was recovered apart, the hexane extraction of the methanol:water-containing phase was repeated twice more, and the three hexane upper phases were combined. After drying, the nonhydroxy-FAMEs were analyzed directly by GC and the 2-hydroxy-FAMEs were analyzed after being converted into O-TMS derivatives (4, 9). Briefly, this involved dissolving the N2-dried samples (in small conical silanized tubes with Teflon-lined caps) with 50 μl hexane, adding 100 μl of a mixture of N,O-bis (TMS) trifluoroacetamide and 5% trimethylchlorosilane (Sigma-Aldrich, Fluka reagent), adding N2 gas, capping the tubes, and keeping them overnight at 45°C with the reactants. The latter were evaporated and the O-TMS derivatives, taken up into hexane, were injected into the GC.
Gas chromatographic analysis of all fatty acids was performed as described in previous work (4, 9). Before methanolysis and O-trimethylsilylation, methyl heneicosanoate (21:0) and methyl 2-OH lignocerate (h24:0) had been added as internal standards for quantification of nonhydroxy- and 2-hydroxy-FAMEs, respectively. The column oven temperature was programmed from 150 to 230°C for nonhydroxy-FAMEs and from 180 to 230°C for the O-TMS of 2-hydroxy-FAMEs of SM and Cer. Injector and detector temperatures were 220 and 230°C, respectively, and N2 (30 ml/min) was the carrier gas in both cases.
MALDI-TOF/MS of SM and Cer species
The vast differences in amounts and proportions of these two lipids among the membrane fractions of this study precluded their comparison on the same basis using classical GC-based analysis. For this reason, the SM and Cer recovered from the present fractions were compared using MALDI-TOF/MS. Analyses were performed on a 4800 MALDI TOF/TOF Plus mass spectrometer (AB-Sciex), using 2,5-dihydroxy benzoic acid saturated with ammonium sulfate as the matrix in the LANAIS PROEM-CONICET, Buenos Aires, Argentina. Aliquots of SM or Cer samples from membrane fractions (10 μl) were acidified with 1 μl of 0.1% trifluoroacetic acid and 1 μl of the resultant solution was combined with 1 μl of matrix and these mixtures were spotted on the MALDI plate. Highly purified n-V- and h-V-containing SM and Cer had been previously employed for optimization of MALDI-TOF/MS parameters. All spectra were acquired in the positive ion reflection mode using a mass spectrometer equipped with a neodymium YAG laser (355 nm). All MS data were acquired and analyzed using the MALDI-MS Data Explorer® software.
Fa2h mRNA levels
The total RNA from preparations containing the populations of spermatogenic cells, PtSs, RSs, and LSs, was isolated using TRIzol reagent (Invitrogen, Carlsbad, CA) according to the manufacturer’s instructions. The RNA was resuspended in RNase-free water and its concentration assessed from the A260:A280 absorbance ratio using a PicoDrop spectrophotometer. Samples were stored at −80°C until use. Aliquots containing 2 μg total RNA were used to synthesize cDNA in reactions containing 1 μg Random Primers hexamers (Biodynamics), 1× M-MLV RT reaction buffer, 0.5 mM each of dNTP, 25 UI RNase inhibitor (Promega), and 200 UI M-MLV RT (Promega). The cDNA resulting from RT was amplified by real-time quantitative PCR (qPCR). Gene expression levels were determined using Rotor-Gene 6000 (Corbett Research, Australia).
The RT-qPCR was performed in a final volume of 10 μl using KAPA SYBR FAST Master Mix (Kapa Biosystems) and 0.2 μM of each primer. The gene-specific primer sequences designed for RT-qPCR to rat Fa2h were: forward, agtactatgtgggcgaactgc and reverse, caatagcagcatctgtcttctga; and to a reference gene, hypoxanthine-guanine phosphoribosyl transferase (Hprt), were: forward, ctcatggactgattatggacaggac and reverse, gcaggtcagcaaagaacttatagcc. All primer pairs were from Invitrogen Life Technologies. The PCR conditions were as follows: 35 cycles of denaturation at 94°C for 20 s, annealing and extension at 58°C for 30 s, and a final extension step at 72°C for 30 s. Ct values of Fa2h mRNA obtained from three different experiments were normalized according to the 2−ΔΔCt method (26), using the Hprt as reference gene and RNA from adult rat testis samples as the calibrator for each comparison to be made. At the end of the amplification phase, a melting curve analysis was carried out on the product formed, and agarose gel electrophoresis was performed to confirm the product size. The level of Fa2h expression in each cell type (PtS, RS, and LS) was calculated as the relative change in gene expression compared with that in whole testis.
Statistical analysis
One-way ANOVA was used to determine differences among mean values, which were compared using the Tukey’s multiple comparison test. Data in figures and tables are presented as mean values ± SD from at least four different samples obtained in independent isolations of germ cells and membrane fractions.
RESULTS
Membrane fractions from spermatogenic cells
The detergent-free method employed in this study, optimized by Luria et al. (17), enabled reproducible and consistent isolation of membrane fractions from homogenates of TCs before and after their further separation into populations of PtSs, RSs, and LSs. In all cases, three membrane fractions were recovered at the sucrose interphases described in the Material and Methods and illustrated in Fig. 1A. The comparative Western blot analyses shown in Fig. 1B revealed that two raft/caveolae markers, caveolin-1 and flotillin-1, were concentrated in the light membrane fraction, a result that is consistent with this fraction being rich in raft-like membrane domains. Caveolin-1 and flotillin-1 were only faintly represented in the heavy membrane fraction. The latter, in turn, showed to be extremely rich in the archetypal non-raft marker, TfR protein.
Rafts and non-raft markers were absent altogether from the extra-heavy membrane fractions and the pellets collecting at the bottom of the tubes. Using a series of enzyme markers and microscopy, the heterogeneous extra-heavy fraction had been previously shown to contain a mixture of membranes derived from intracellular organelles, including endoplasmic reticulum, Golgi, mitochondria, and lysosomes (16, 27). In our membrane preparations, two endoplasmic reticulum-resident proteins, calnexin and GPR30 (28, 29), were well represented in this extra-heavy membrane fraction, as expected from a fraction that mainly contains membranes of intracellular origin. The scarce representation of these two proteins in the light and heavy membrane fractions supports our view that the latter were isolated with an acceptable degree of purity.
The glycosylphosphatidylinositol-anchored enzyme, 5′ nucleotidase, is a feature of caveolae domains (30). This enzyme was highly active in the light fraction isolated from spermatogenic cells at the three differentiation stages studied (Table 1). Although the specific activity was the highest in this membrane fraction, the enzyme was also present in the heavy fraction. Interestingly, the light:heavy ratio of the enzyme activity was similar, around 4:1, in the three cell differentiation stages studied. The specific activity and the relative abundance of 5′ nucleotidase were, in comparison, negligible in the extra-heavy membrane fraction: not only lower than in the light and heavy membrane fractions, but also lower than in the total homogenates before fractionation in all cells. Taken together, the data in Table 1 and Fig. 1 support our interpretation that the light and heavy membrane fractions were mostly contributed by membrane fragments derived from the plasma membrane of the cells under study.
TABLE 1.
Activity of 5′ nucleotidase in total homogenates and in membrane fractions from spermatogenic cells at progressive stages of differentiation
| Total Homogenate | Light | Heavy | Extra Heavy | |
| TCs | 3.2 ± 0.7a (1.0) | 28.9 ± 1.5b (9.0) | 8.6 ± 0.7c (2.7) | 2.37 ± 0.3a (0.7) |
| PtSs | 2.5 ± 0.1a (1.0) | 31.4 ± 4.0b (12.8) | 8.2 ± 1.6c (3.4) | <2 |
| RSs | 4.3 ± 0.7a (1.0) | 41.4 ± 5.3b (9.6) | 10.8 ± 0.6c (2.5) | 2.60 ± 0.4d (0.6) |
| LSs | 2.3 ± 0.2a (1.0) | 23.4 ± 3.0b (10.2) | 6.4 ± 1.2c (2.8) | <2 |
Total spermatogenic cells (TCs) were isolated from rat seminiferous tubules and separated into pachytene spermatocytes, round spermatids, and late spermatids (PtSs, RSs, and LSs, respectively). Total homogenates were prepared and subjected to membrane fractionation. Membrane fractions (light, heavy, and extra-heavy) were obtained as shown in Fig. 1A in all cases. The enzyme activity is expressed as nanomoles of inorganic phosphate per milligram protein per minute. Data represent mean values ± SD from three independent preparations. Samples were compared using Tukey’s statistics; different letter superscripts (a–d) in the rows indicate significant differences (P < 0.01) between total homogenates and each membrane fraction in each cell type. Numbers in parenthesis indicate the ratio between the enzyme activity in the membrane fraction and that in the corresponding total homogenate.
Total PL, cholesterol, and SM distribution among membrane fractions
When the content of total lipid phosphorus in the membrane fractions under study (proportional to the amount of membranes) was expressed in relation to the amount of total lipid phosphorus in the homogenates initially loaded on the sucrose gradients (Table 2), the light fractions represented as little as 0.9%, 1.0%, and 1.5% of the total membrane fractions recovered from PtS, RS, and LS, respectively. On the same basis and in the same order, the heavy membrane fractions concentrated 21.7%, 18.8%, and 19.6% of the total cell lipid phosphorus. Adding up these values, and admitting that most of the light and heavy fractions derive from the plasma membranes of the cells under study, such membranes contributed with a similar percentage to the total lipid phosphorus (22.6%, 19.8%, and 21.1% on average) in the three cell types. In contrast, the extra-heavy membranes showed significant differences among cells, as PtS, RS, and LS, collected large, but decreasing, amounts of lipid P (40%, 31%, and 23% of the total membranes recovered, respectively). This difference could have been expected, considering that the volume and size of spermatogenic cells decrease with differentiation (PtS > RS > LS), and that PtSs contain more voluminous endoplasmic reticulum and Golgi systems than RSs, and these, in turn, more voluminous than LSs (31). After adding up the lipid phosphorus content of the light, heavy, and extra-heavy fractions for each cell type and that from the cell structures and remnants (including nuclei) recovered as a sediment at the bottom of the tubes as the residual pellet fraction, the recovery of lipid phosphorus after, with respect to before, membrane fractionation was around 80% in all cases (Table 2).
TABLE 2.
Amounts of lipid phosphorus recovered in membrane fractions from differentiating spermatogenic cells with respect to the amounts present in their corresponding total homogenates
| Light | Heavy | Extra Heavy | Pellet | |
| μg of lipid P/100 μg of P from TH | ||||
| TCs | 1.1 ± 0.1 (5.7%) | 17.4 ± 1.4 (94.3%) | 25.0 ± 4.7 | 36.5 ± 3.9 |
| PtSs | 0.9 ± 0.1 (2.3%) | 21.7 ± 4.2 (97.7%) | 39.5 ± 2.8 | 18.3 ± 5.9 |
| RSs | 1.0 ± 0.2 (5.3%) | 18.8 ± 5.7 (94.7%) | 31.5 ± 2.3 | 28.6 ± 3.1 |
| LSs | 1.5 ± 0.2 (7.0%) | 19.6 ± 2.6 (93.0%) | 23.4 ± 1.2 | 35.5 ± 1.1 |
Total spermatogenic cells (TC) were obtained from adult rat seminiferous tubules and pachytene spermatocytes, round spermatids, and late spermatids (PtSs, RSs, and LSs, respectively), were isolated therefrom. Total homogenates (TH) were prepared from each of the cell populations and subjected to membrane fractionation as illustrated in Fig. 1A. Lipids were extracted from each TH and from the membrane fractions of each cell population. The results compare the amount of lipid phosphorus (P) contained in each membrane fraction with respect to a fixed amount (100 μg) of lipid phosphorus present in the corresponding TH. Note that the lipid phosphorus recovered in the three membrane fractions plus pellets, added up, amounted on average to 80%, 80.4%, 79.9%, and 80% of the content in each of the initial TH from TCs, PtSs, RSs and LSs, respectively. Figures in parenthesis indicate the mole percent distribution of lipid phosphorus between the light and heavy fractions.
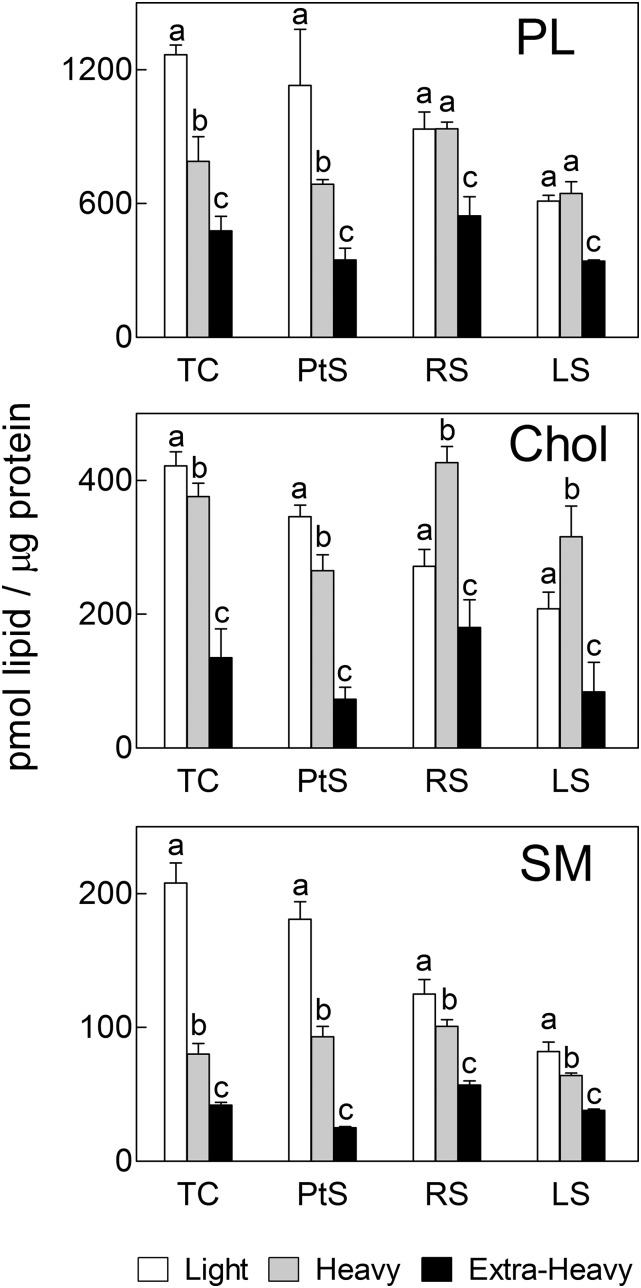
The content of PL, cholesterol, and SM in each membrane fraction from the three cell stages under study, expressed on the basis of total protein, is compared in Fig. 2. In PtS, the light membranes concentrated more phospholipid per microgram protein than the heavy fraction. Interestingly, such concentration was lower and quantitatively similar in the light and heavy fractions from both spermatid stages (RS and LS). In all three developmental stages, the L and H membrane fractions, together, were, in turn, considerably richer in the amount of PL per microgram protein than the corresponding EH membranes (Fig. 2).
Fig. 2.
Comparison of the concentrations of total PL, cholesterol (Chol), and sphingomyelin (SM) in membrane fractions from differentiating spermatogenic cells. Total spermatogenic cells, pachytene spermatocytes, round spermatids, and late spermatids (TC, PtS, RS, and LS, respectively) were isolated from adult rat seminiferous tubules. The white, gray, and black bars represent the light, heavy, and extra-heavy membrane fractions, respectively, isolated from each cell population. Values with different letters (a–c) represent significant differences among membrane fractions within each cell (P < 0.05).
Regarding the concentration of cholesterol, in PtS the light membranes concentrated more cholesterol per microgram of protein than their heavy counterparts, as could have been expected from a raft-like type of domain. It was then quite unanticipated that in round and elongated spermatids, the heavy membranes were the ones to contain the highest concentration of cholesterol (Fig. 2). From these results, it follows that the concentration of phospholipids plus cholesterol per microgram of protein increased significantly in plasma membranes from spermatocytes to spermatids. As would be expected from intracellular membranes, the cholesterol concentration was the lowest in the extra-heavy fraction in all cases.
The total SM concentration, also on a protein basis, was significantly higher in the light than in the heavy membrane fraction in the three types of spermatogenic cells: there was twice as much SM per microgram of protein in the light as in the heavy of spermatocytes and ∼1.2-fold more SM in the light as in the heavy of the two spermatid stages (Fig. 2). The corresponding extra-heavy fractions, in turn, contained significantly less SM per microgram of protein than the heavy fractions.
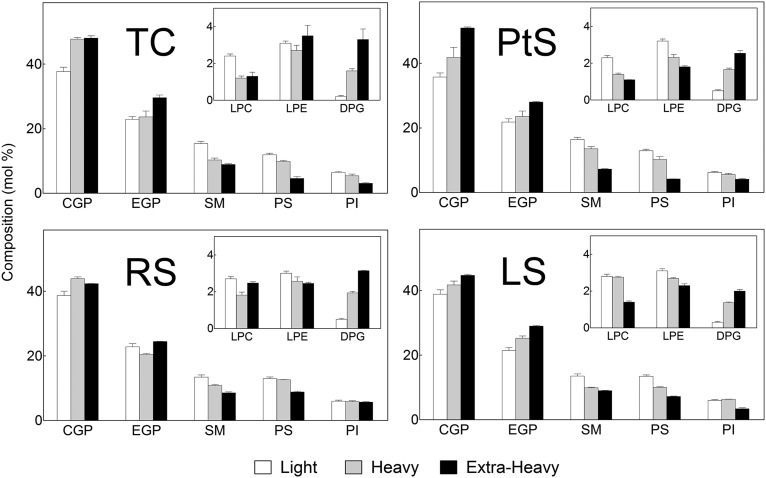
PL classes
The choline GPLs (CGPs) and ethanolamine GPLs (EGPs), followed by SM, were major phospholipid classes in all membrane fractions (Fig. 3). However, the proportions among PLs differed among membranes. In the three cell populations (PtS, RS, and LS) the light membranes contained, on a percentage basis, more GPL and less SM than the heavy membranes. The mole ratio between the two PLs that contained phosphocholine as their head group, CGP/SM, was around two in light and three in heavy fractions from spermatocytes, in comparison with nearly three in light and four in heavy fractions from both spermatids.
Fig. 3.
Comparison of the PL composition of membrane fractions from differentiating spermatogenic cells. Light, heavy, and extra-heavy membrane fractions were obtained from total spermatogenic cells, pachytene spermatocytes, round spermatids, and late spermatids (TC, PtS, RS, and LS, respectively). CGP, choline glycerophospholipids, DPG, diphosphatidylglycerol; EGP, ethanolamine glycerophospholipids; LPC, lysophosphatidylcholine; LPE, lysophosphatidylethanolamine; PI, phosphatidylinositol; PS, phosphatidylserine; SM, sphingomyelin.
In whole cells, whether TCs or any of the cell types analyzed here, the percentages of SM and phosphatidylserine (PS) were, on average, 5% and 4%, respectively (9). Interestingly, after fractionation, the percentages of SM and PS were much higher in the light membranes of total cells (15% and 12%), of spermatocytes (16% and 13%), and of both spermatids (13% and 13%) (Fig. 3). In the PL component of the heavy membranes, the percentages of SM and PS were, in all cases, significantly lower than in the light fraction (Fig. 3).
Usually, spermatogenic cells contain a small amount of lysophospholipids. It is worth noting that, in the present cell preparations, the starting cell homogenates contained less than 1% and the three membrane fractions from the three cell types under study had measurable, yet relatively low (2–3%), proportions of lysophospholipids (lysophosphatidylcholine and lysophosphatidylethanolamine; Fig. 3), expected products of partial PL hydrolysis during membrane fraction isolation procedures. A common feature of extra-heavy membranes from the three cell stages was that they contained the highest CGP/SM ratios, the lowest percentages of SM and PS, and the highest proportion of cardiolipin (diphosphatidylglycerol, Fig. 3), the latter mostly ascribable to mitochondrial membranes.
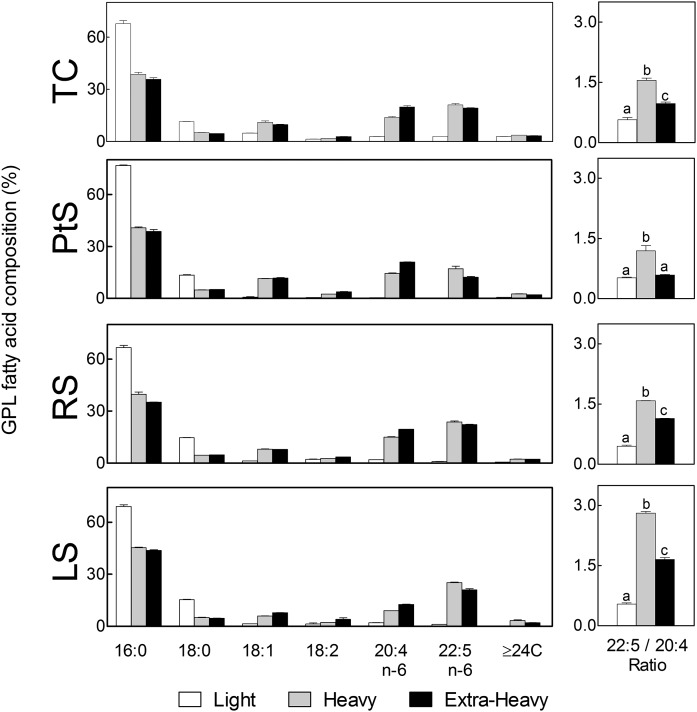
GPL fatty acids
The GPLs were far richer in species with saturated fatty acids in the light than in the heavy fraction of the plasma membrane in all spermatogenic cells surveyed, almost exclusively 16:0 followed by 18:0 (Fig. 4), their high percentages (>90%) being compatible with an abundance of disaturated species of GPLs. By contrast, the GPLs of the heavy fraction contained nearly 50% PUFAs, virtually all of them being 20:4n-6 and 22:5n-6, the typical PUFAs of rat germ cell GPLs (1), in turn, compatible with an abundance in this fraction of tetraenoic and pentaenoic molecular species of GPLs. The latter were virtually absent from the GPLs of the raft-like membranes.
Fig. 4.
Main fatty acids of the total GPLs present in membrane fractions from differentiating spermatogenic cells. Light, heavy, and extra-heavy membrane fractions from the spermatogenic cells under study are compared. The panels on the right show the 22:5n-6/20:4n-6 ratio in the total GPLs of each membrane fraction. Cell differentiation caused little change in the fatty acid composition of the light fraction, while it entailed a notable increase in the proportion of 22:5n-6 in the heavy fraction. Values with different letters (a–c) represent significant differences (P < 0.05).
The 22:5n-6/20:4n-6 mole ratio increased with differentiation, with more intensity in the heavy fraction (1.2, 1.6, and 2.8 in PtS, RS, and LS, respectively) than in the extra-heavy membranes (0.6, 1.1, and 1.6, respectively, in the same order of cells). This concurs with the increase in this ratio in major GPL classes that accompanies spermatogenic cell development in whole cells in the rat (1, 9) and implies that metabolic energy is spent in these cells to enrich their heavy membranes with 22:5n-6-containing GPL species.
SM and Cer fatty acids
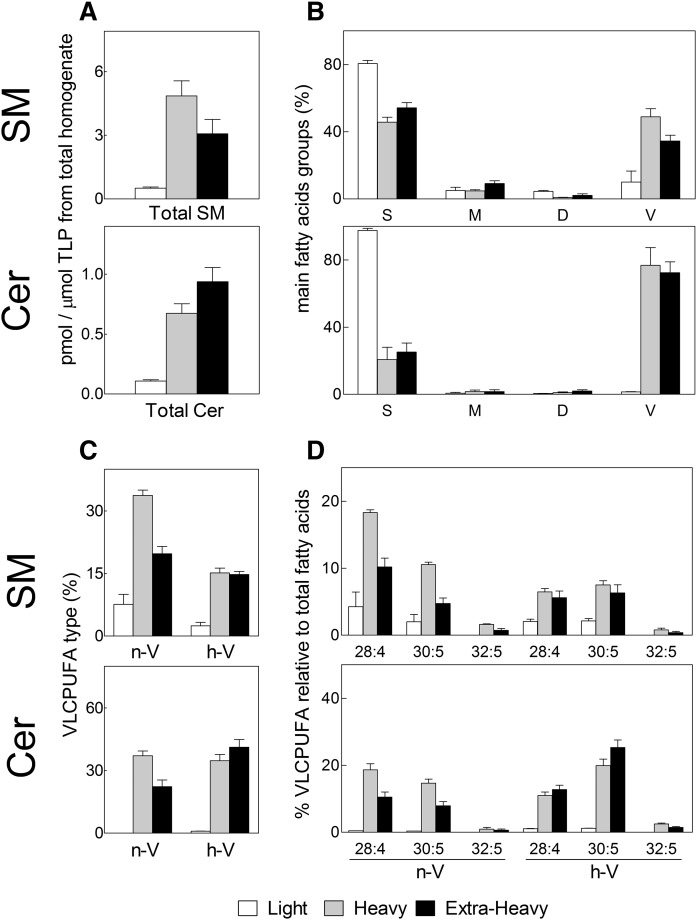
The SM and Cer species from membrane fractions of TCs were quantified from the amounts of their fatty acids to facilitate comparisons on the same basis. Most of the SM present was contributed by the heavy fraction of cell plasma membranes (Fig. 5A). The amount of SM contributed by the light membrane fraction was very low, as this minute fraction constituted a small portion of the cell plasma membrane (0.9–1.5% of the cell PLs, Table 2). As expected of a raft-like membrane domain, the SM from the light fraction contained mainly saturated fatty acids (mostly 16:0) and less than 10% VLCPUFAs. By contrast, the SM from the heavy fraction had predominantly VLCPUFAs, nearly 50% of its acyl groups (Fig. 5B). This 50% was made up of nearly 35% n-V and 15% h-V in this fraction from TCs (Fig. 5C). The main n-V components of this SM were 28:4n-6, 30:5n-6, and 32:5n-6, in that order; whereas among the h-V components, the order of abundance was h-30:5n-6, h-28:4n-6, and h-32:5n-6 (Fig. 5D).
Fig. 5.
SM and Cer contents and their main fatty acids in membrane fractions from adult rat TCs. The SM and Cer were quantified by their fatty acids to facilitate comparison on the same basis. A: Amounts of SM and Cer expressed as picomoles per micromole of the total lipid phosphorus (TLP) originally present in the germ cell total homogenates before fractionation. B: Percentages of the main fatty acids grouped into saturated (S), monoenoic (M), dienoic (D), and VLCPUFA (V). C: Contribution of the n-V and h-V to the total VLCPUFA group of fatty acids. D: Relative abundances of the major individual fatty acids composing the n-V and h-V groups.
The extra-heavy membranes contained significantly less SM than that present in the plasma membrane (Fig. 5A). The heavy and extra-heavy SMs were similar in fatty acid composition, except for a smaller percentage of total VLCPUFAs (35%) in the latter (Fig. 5B), of which 19% were n-V and 16% h-V (Fig. 5C). Interestingly, the extra-heavy fraction contained less n-V (28:4n-6, 30:5n-6) than the heavy fraction, but similar h-V (h-28:4n-6, h-30:5n-6) to the heavy fraction (Fig. 5D).
The minute Cer of the small light fraction was hard to locate and analyze. Only traces appeared in the Cer area of the TLC chromatograms, compatible with a negligible concentration of Cer in this fraction (Fig. 5A). Most of the Cer present in TC membrane preparations was contributed by the extra-heavy fraction, the heavy fraction coming next (Fig. 5A). The Cer:SM mole ratio was around 1:3 in the extra-heavy fraction and nearly 1:7 in the heavy fraction.
The Cer of extra-heavy and heavy membranes was far richer than the corresponding SM species in VLCPUFAs (63% and 72% of the Cer fatty acids in extra-heavy and heavy, respectively) (Fig. 5B). Of the 72% VLCPUFAs of the heavy-associated Cer, nearly equal proportions were contributed by n-V Cer and h-V Cer species (Fig. 5C). Of the 63% VLCPUFAs of the extra-heavy-associated Cer, there were twice as many h-V as n-V species of Cer, with h-30:5n-6 followed by h-28:4n-6 being the major Cer VLCPUFAs on a percentage basis (Fig. 5D) and also on a concentration basis, considering that the amount of Cer was the largest in this extra-heavy fraction (Fig. 5A). Taken together, the enrichment in h-V SM and h-V Cer in the extra-heavy membranes with differentiation is consistent with this fraction including the intracellular membranous site(s) of spermatogenic cells where these species are biosynthesized.
MALDI-TOF/TOF MS of SM and Cer species
To study the distribution of SM and Cer molecular species in samples so different in size and lipid composition as PtSs, RSs, and LSs, each giving rise to three membrane fractions also so broadly different in size and lipid content and composition as the light, heavy, and extra-heavy fractions, we resorted to MALDI-TOF/TOF MS detection (Figs. 6, 7).
Fig. 6.
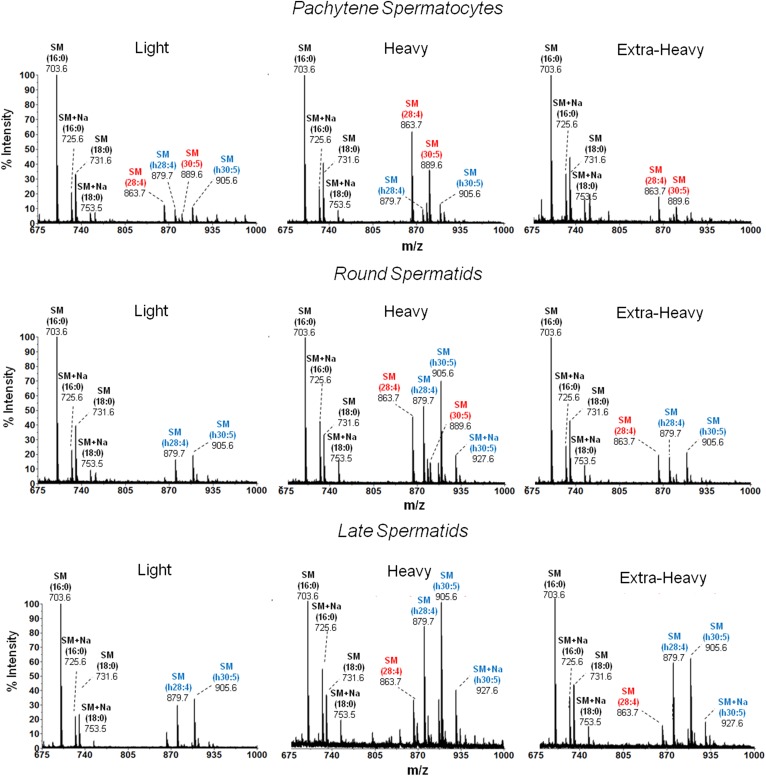
MALDI-TOF MS analysis of the SM species from membrane fractions of differentiating spermatogenic cells. The SM of the indicated membrane fractions from the spermatogenic cells under study were isolated by TLC and purified as explained in the text. All SM molecules contained sphingosine (d18:1) as the sphingoid base. Molecular species of SM were identified by the product ion scan m/z +184. The n-V SM and h-V SM species are colored in red and blue, respectively. Signals at m/z 863.6 and 889.6 are (d18:1/28:4n-6 + H+)SM and (d18:1/30:5n-6 + H+)SM, and those at m/z 879.6 and 905.6 are (d18:1/h28:4n-6 + H+)SM and (d18:1/h30:5n-6 + H+)SM, respectively. The intensities detected from these molecular species were low in light compared with heavy membrane fractions. In the latter, differentiation is shown to entail a notable increase in the proportion of h-V species, (d18:1/h28:4n-6)SM and (d18:1/h30:5n-6)SM. Occasionally, sodium (Na) adducts were observed for some species (d18:1/16:0 + Na+)SM and (d18:1/h30:5n-6 + Na+)SM, m/z 725.6 and 927.6, respectively. Amplified mass spectra in the m/z 915–935 region for the three cells are shown in supplemental Fig. S2, where the peak intensities at m/z 917.7 and 933.7 were identified as (d18:1/32:5n-6)SM and (d18:1/h32:5n-6)SM, respectively.
Fig. 7.
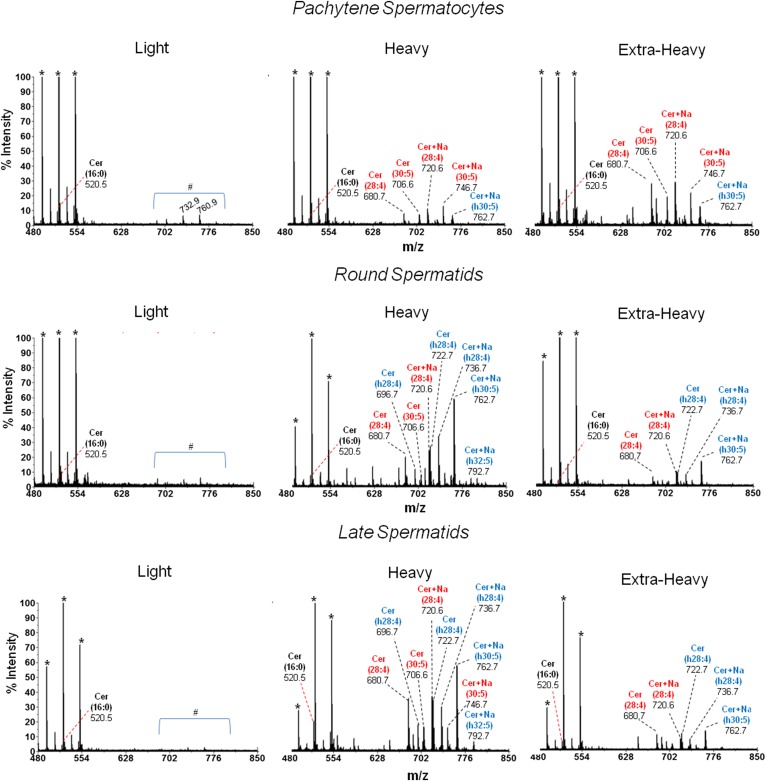
MALDI-TOF MS analysis of the Cer species from membrane fractions of differentiating spermatogenic cells. The Cers of the indicated membrane fractions from the spermatogenic cells under study were isolated by TLC and purified as described in the text. All molecular species of Cer were identified by the product ion scan m/z +264 that corresponds to twice-dehydrated sphingosine (d18:1). The n-V Cer and h-V Cer species are colored in red and blue, respectively. Signals at m/z 680.7 and 706.6 are [(M+H)+ − H2O] of (d18:1/28:4n-6)Cer and (d18:1/30:5n-6)Cer, respectively. The latter two species were also detected as sodium adducts (Cer + Na+) at m/z 720.6 and 746.7, respectively. The intensities detected at m/z 696.7 and 722.7 are dehydrated species [(M+H)+ − H2O] of (d18:1/h28:4n-6)Cer and (d18:1/h30:5n-6)Cer, respectively. The latter two molecular species of Cer were also detected as sodium adducts (Cer + Na+) at m/z 736.7 and 762.7, respectively. In light membranes from the three cell types, no Cers with VLCPUFA were present and only minor amounts of 16:0 Cer (m/z 520.5) were detected. The peaks marked with an asterisk indicate matrix ions signals. The area under the # symbol corresponds to unidentified signals. The heavy membranes from spermatids showed a signal at m/z 792.7 that corresponds to (d18:1/h32:5n-6 + Na+)Cer.
As previously shown (2), the SM and Cer from spermatogenic cells contain sphingosine (d18:1) as their sphingoid base. In the present MALDI-TOF MS analysis, SM was identified by the characteristic m/z +184 product ion, corresponding to phosphocholine. Abundant H+ adducts and only moderate Na+ adducts were generated. On the other hand, Cer was identified by the product ion scan at m/z +264, corresponding to the molecular weight of sphingosine + H+ (−2 H2O) (twice dehydrated species). This indicated that Cer dehydrates to ionize [Cer + H+ (−H2O)]. In addition, [Cer + Na+] adducts were generated in abundance; for this reason, the sum of the signals [Cer + H+ (−H2O)] + [Cer + Na+] was calculated to estimate the relative intensities of Cer species.
In the three membrane fractions from the three germ cell maturation stages under study, PtS, RS, and LS, the predominance of saturated SM species (d18:1/16:0 and d18:1/18:0) (main signals at m/z 703.6 and 731.6, respectively) in the raft-like light fractions was confirmed (Fig. 6). The non-raft heavy region of the plasma membrane from PtSs contained predominantly n-V SM species (colored in red in Fig. 6), the major ones being d18:1/28:4n-6 and d18:1/30:5n-6 (main signals at m/z 863.6 and 889.6, respectively). On the other hand, the heavy fractions from RSs and LSs had, in addition to these, a high proportion of the h-V SM counterparts (colored in blue), the major ones being d18:1/h28:4n-6 and d18:1/h30:5n-6 (m/z 879.6 and 905.6, respectively). Magnification of the SM spectra from the heavy fraction of PtSs, RSs, and LSs allowed the observation of two peaks at m/z 917.7 and 933.7, ascribable to the relatively minor d18:1/32:5n-6 and d18:1/h32:5n-6 SM species (see supplemental Fig. S2).
Regarding Cer, the light fraction from the plasma membrane of PtSs, RSs, and LSs lacked Cer with VLCPUFA altogether, and displayed only minute amounts of a signal at m/z 520.5, corresponding to traces of the (d18:1/16:0) Cer species (Fig. 7). Remarkably, the heavy and the extra-heavy membrane fractions from cells in the three cell stages contained little of this Cer and plenty of Cer species with VLCPUFA. In PtSs, high relative intensities of n-V Cer species (d18:1/28:4n-6 and d18:1/30:5n-6) were found, more intense in extra-heavy than in heavy membranes. In contrast, in both spermatids, the heavy domains of the plasma membrane showed higher relative intensities than the corresponding extra-heavy fractions of n-V Cer (mainly d18:1/28:4n-6 and d18:1/30:5n-6) and h-V Cer (mainly d18:1/h28:4n-6 and d18:1/h30:5n-6).
Expression (mRNA) of the Fa2h gene
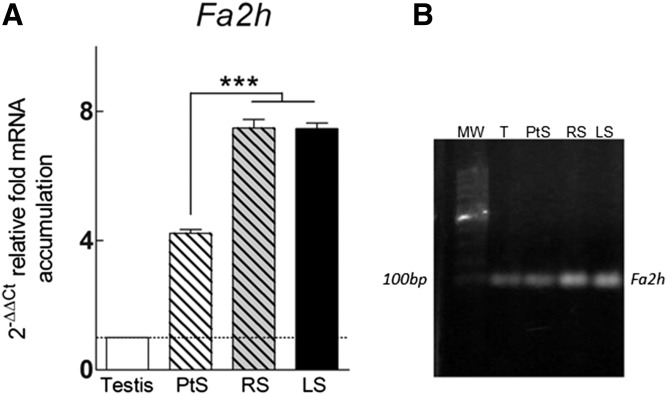
Because the proportion of h-V VLCPUFAs increases in SM and Cer with the progression of differentiation from spermatocytes to spermatids, and considering that most of this increase occurs in the extra-heavy fraction, prompts the question about the enzyme responsible for the synthesis of such fatty acids. An antecedent was found in a study published by Eckhardt et al. (32), motivated by the galacto-sphingolipids rich in 2-hydroxy fatty acids of myelin. It was observed that, in the mouse, the Fa2h gene is expressed in the brain, the skin, and, intriguingly to us, even more intensely in the testes. In the present study the gene was also found to be actively expressed in the adult rat testis (Fig. 8). The Fa2h expression was concentrated in spermatogenic cells, as compared with whole testis, and increased significantly (almost twice) with the progression of differentiation from spermatocytes to RSs, remaining at similarly high levels in later stages.
Fig. 8.
Fa2h transcripts in adult rat testis (T) and differentiating spermatogenic cells. PtSs, RSs, and LSs were isolated from the seminiferous epithelium of adult rats. A: Data were obtained by RT-qPCR and normalized to Hprt as internal reference gene using the 2−ΔΔCt method, as described in the text. The relative expression of Fa2h in adult rat testis (set to equal 1) was used as a calibrator for comparison among the cell populations. The asterisks point to a significantly different value (P < 0.01). B: Analysis of RT-PCR products on a 2% agarose gel. After reverse transcription using random primers, the corresponding cDNAs were amplified using specific primers for rat Fa2h. In all samples, the products were in agreement with the predicted size of 96 bp. MW, molecular weight markers.
DISCUSSION
In the present study, the distribution of SM and Cer species among membrane fractions obtained from spermatogenic cells in three stages of their differentiation was characterized. It was mainly focused on two membrane fractions obtained from such cells using a detergent-free method (light and heavy). The small low-density light fraction contained the highest concentration of protein markers, like caveolin-1 and flotillin-1, and the highest activity of 5′ nucleotidase, indicating that it was rich in raft-like structures mostly derived from the plasma membrane. The several-fold larger heavy fraction was practically the only one, among the present membrane fractions, to contain the TfR, a protein that is a typical marker of non-raft plasma membrane domains. Interestingly, this protein is known to play an important role in spermatogenic cells, receiving and incorporating the transferrin that is provided to them by Sertoli cells. Because the light and heavy fractions virtually lacked proteins known to be located in the endoplasmic reticulum, one of the major contributors to the membranes sedimenting in the large extra-heavy fraction, these two membrane fractions may be considered to mostly derive from the plasma membrane of the cells under study.
Independently of the maturation stage, a finding that was common to spermatogenic cells was that most of the SM and Cer that contain VLCPUFAs (n-V and h-V) in their plasma membrane were concentrated in the heavy fraction, i.e., they were virtually excluded from the low-density raft-like light domains. The SM and the negligible amounts of Cer present in the raft fraction contained, by contrast, 16:0 as a main component. A similar concentration of saturated fatty acid-containing SM was obtained when DRMs were obtained by the classical procedure of solubilizing the isolated cells with Triton X-100 at 4°C (see supplemental Fig. S3). Our data confirmed that “conventional” molecular species of SM, namely those with saturated fatty acids, “prefer” the raft-like fraction;, whereas the SM species with n-V and h-V fatty acids do not, thus becoming part of the detergent-soluble fraction of the cells. Exclusion from the raft domains of the germ cell FGSL that contain VLCPUFAs was also previously demonstrated by Sandhoff et al. (6). After solubilizing mouse testes at 4°C with Triton X-100 followed by ultracentrifugation to isolate a caveolin-containing detergent-resistant raft membrane fraction, these authors detected the presence of saturated fatty acid-containing GSL classes in the raft fraction; the VLCPUFA-containing FGSLs, by contrast, stayed in the detergent-soluble fraction.
In cells in the three stages surveyed in the present study, the lateral distribution of GPL species followed a similar trend as those of SM, as the GPLs of the heavy fraction concentrated the main PUFAs (20:4n-6 and 22:5n-6), which were virtually absent from the GPLs of the light domains, which were, in turn, rich in species with saturated fatty acids. Thus, the present raft-like fractions, independently of the differentiation stage, were “typical” in their lipid composition, in all cases being rich in cholesterol and saturated fatty acid-containing SM and GPL species.
By comparing the light and heavy membrane fractions in the order PtS → RS → LS, it was clear that the changes that are hallmarks of spermatogenic cell differentiation in the rat, namely, the increase of the 22:5/20:4 ratio in GPL and that of the h-V/n-V ratio in SM and Cer (1, 9) took place mainly in the non-raft portion of the plasma membrane. The light raft-like structures remained quite constant in lipid and fatty acid composition (percent) during cell maturation. This constancy is reasonable, if one envisages rafts as lipid assemblies forming platforms that function in cellular processes as common and essential among cells as it is, for example, signal transduction, which may be expected to be conserved independently of age and differentiation state. Our results suggest that the non-raft portion of the plasma membrane is the one that undergoes the highest dynamism regarding lipid remodeling during spermatogenic cell maturation.
An initially intriguing finding when comparing the light with the heavy fraction in each of the cell stages was that the concentration of cholesterol per microgram of protein was, as expected, higher in the light than in the heavy fraction in PtSs, but changed to become the opposite, significantly higher in the heavy than in the light fraction, in the two spermatids (Fig. 2). Although less marked than cholesterol, the concentrations per microgram protein of GPL and SM within the heavy fraction followed a similar trend. Thus, the three lipid components increased in concentration per microgram of protein from PtSs to RSs and then decreased again from RSs to LSs. It must be borne in mind, however, that the amount of protein, which goes in the denominator in these expressions, far from being invariable, also changes (at higher, lower, or similar rates as the lipids) with cell differentiation.
Another finding contrary to our initial expectations was that, in the heavy fraction, the proportion of cholesterol increased more than that of GPL and more than that of SM with cell differentiation. In PtSs, RSs, and LSs, the cholesterol/GPL ratios in the light fraction were 0.35, 0.34, and 0.39 and in the heavy fraction were 0.45, 0.51 and 0.54, respectively. In the same order of cells, the cholesterol/SM ratio was 1.91, 2.18, and 2.53 in the light fraction and 2.85, 4.23, and 4.92 in the heavy fraction. As after the PtS stage the amounts per cell of cholesterol, GPL, and SM decrease with differentiation because of cell size reduction (9), these ratios indicate that cholesterol decreases relatively less than GPL and SM during this process in the heavy fraction. The ordering effect of cholesterol is probably needed to compensate, in part, for the large increase in polar lipid acyl chain unsaturation the membrane undergoes.
With the present detergent-free isolation approach, in addition to the light and heavy fractions mostly ascribed to the plasma membrane, a membrane fraction, here named extra-heavy, containing membranes from intracellular structures can be obtained. This extra-heavy membrane fraction was useful because: i) it served as a control for membrane purity (Fig. 1) and lipid recovery (Table 2); and ii) it showed that the heavy fraction accumulated more cholesterol, GPL, and SM (the latter two rich in PUFA and VLCPUFA), but much less Cer, than the extra-heavy fraction in the three developmental stages studied. The Cers with VLCPUFA of the extra-heavy fraction are likely the species of Cer that are synthesized, mostly in the endoplasmic reticulum, as precursors of the simple and complex sphingolipids with VLCPUFA that will find their final location in the membranes of spermatids and nascent spermatozoa.
The present observation that the extra-heavy fraction of spermatocytes and spermatids contains, in each case, an important proportion of the n-V Cer and the h-V Cer of the whole cell, implies that these Cers are the major products of CerS3, one of the six CerS genes whose expression was shown to be specific of spermatogenic cells in the rodent testis (7). Such Cer species must be precursors of the n-V SM and the h-V SM that compose the main part of the plasma membrane (the heavy fraction) of spermatocytes and spermatids, respectively. The essentiality of this enzyme and its lipid products in spermatogenesis has elegantly been demonstrated (8) by knocking out the (endoplasmic reticulum-located) mouse CerS3 specifically in germ cells. No Cers, SMs, or FGSLs with VLCPUFA are produced, the consequence being the loss of fertility.
The finding that the expression of the Fa2h gene, starting in PtSs, was twice as active in spermatids is consistent with the fatty acid composition change that Cer, and concomitantly SM, as lipid classes, undergo with differentiation in these cells (9), with 2-hydroxy versions of VLCPUFAs being low in both lipids of spermatocytes and eventually predominating in those of spermatids and spermatozoa. Using high-resolution mass spectrometric imaging, lipid classes, including phosphatidyl- and plasmenyl-choline and several species of SMs, have been localized, for the first time, directly in situ in adult mouse testis sections (8). Thus, major n-V SM species, like 30:5-SM, are detected in the region of spermatocytes; whereas major h-V SM species, like h-30:5 SM, abound in the area where spermatids are located. Interestingly, after subtraction of accompanying ions, like K+, the m/z values were 888.6 and 904.6, respectively, for the mentioned SM species, in perfect concordance with the present data (Fig. 6).
The present results show that Fa2h is differentially expressed among spermatogenic cells, with a significant upregulation taking place from spermatocytes to spermatids (Fig. 8). Such cells are thus responsible for the high expression of this gene in the mouse testis in comparison with other tissues (brain, skin, stomach) shown in the article by Eckhardt and coworkers (32). Researchers from this group generated mice lacking the Fa2h (−/−) gene, with the aim of investigating its effects on myelin formation. Unexpectedly, these mice developed structurally and functionally normal myelin up to early adulthood, the myelin defects appearing only in aged animals, despite the decrease observed in myelin galactosyl-Cers and sulfatides with 2-hydroxy saturated and monoenoic fatty acids (33). Moreover, males and females were fertile. It would not be surprising that, in rodents, the enzyme Fa2h expressed by oligodendrocytes and that expressed by spermatids were not identical at the protein level, considering the difference of the corresponding final sphingolipid products that result in each case.
Although the expression of Fa2h among membrane fractions of spermatids was not done in the present study, Fa2h is known to be an enzyme with four transmembrane domains anchored to the endoplasmic reticulum (34). This membrane location is consistent with the present results showing that h-V Cer, a main product of the activity of this enzyme, was most highly concentrated in the extra-heavy fraction of spermatids.
Our results suggest that, in the rat testis, the enzyme Fa2h is involved in the biosynthesis of the h-V Cer species that are produced by the CerS3 of spermatids. In addition, these h-V Cer species are not only intermediates in the biosynthesis of the corresponding SM and FGSL with 2-hydroxy VLCPUFA, but are also, themselves, final membrane products. Here we show that they are minor in the plasma membrane of spermatocytes, but definitely compose the heavy fraction of the membrane in spermatids. In testicular mouse spermatozoa, 2-hydroxy VLCPUFA-rich FGSLs were detected by antibody staining to be located on the sperm tail (6). Previous fractionation of the heads and tails from rat spermatozoa obtained from cauda epididymis, aimed at localizing SM and Cer species and GPL, showed that the long voluminous tail contains h-V Cer, while it lacks n-V or h-V species of SM, in contrast to the minuscule hook-shaped head, which contains such SM species almost exclusively, but lacks Cer (35). The h-V Cer species shown in this study to be located in the heavy fraction of rat membrane spermatids may be the ones that find their final destination in (non-raft domains of) the plasma membrane that covers the rat sperm tail. The mechanisms that promote such specific localization within the seminiferous epithelium on the one hand, and its final functional or structural role in the mature gametes during their activation to become able to fertilize an oocyte on the other, remain to be elucidated.
Supplementary Material
Acknowledgments
The authors thank Dr. Lorena Milanesi and Dr. José Luis Daniotti for generously providing some of the antibodies we employed.
Footnotes
Abbreviations:
- Cer
- ceramide
- CerS3
- ceramide synthase 3
- CGP
- choline glycerophospholipid
- DRM
- detergent-resistant membrane
- EGP
- ethanolamine glycerophospholipid
- Fa2h
- fatty acid 2-hydroxylase
- FAME
- fatty acid methyl ester
- FGSL
- glycosphingolipid with fucose in its headgroup
- GPL
- glycerophospholipid
- GSL
- glycosphingolipid
- Hprt
- hypoxanthine-guanine phosphoribosyltransferase
- h-V
- 2-hydroxylated
- LS
- late spermatid
- n-V
- nonhydroxylated
- P
- pellet
- PL
- phospholipid
- PS
- phosphatidylserine
- PtS
- pachytene spermatocyte
- qPCR
- quantitative PCR
- RS
- round spermatid
- TC
- total spermatogenic cell
- TfR
- transferrin receptor
- VLCPUFA
- very long-chain PUFA
This work was supported by funds granted by ANPCyT (Agencia Nacional de Promoción Científica y Tecnológica, PICT2013-2533 and PICT2013-1356), CONICET (Consejo Nacional de Investigaciones Científicas y Técnicas, PIP112-201101-00843), and UNS (Universidad Nacional del Sur, PGI 24/B218). The authors declare that they have no conflicts of interest with the contents of this article.
The online version of this article (available at http://www.jlr.org) contains a supplement.
REFERENCES
- 1.Beckman J. K., Gray M. E., and Coniglio J. G.. 1978. The lipid composition of isolated rat spermatids and spermatocytes. Biochim. Biophys. Acta. 530: 367–374. [DOI] [PubMed] [Google Scholar]
- 2.Furland N. E., Zanetti S. R., Oresti G. M., Maldonado E. N., and Aveldaño M. I.. 2007. Ceramides and sphingomyelins with high proportions of very long-chain polyunsaturated fatty acids in mammalian germ cells. J. Biol. Chem. 282: 18141–18150. [DOI] [PubMed] [Google Scholar]
- 3.Robinson B. S., Johnson D. W., and Poulos A.. 1992. Novel molecular species of sphingomyelin containing 2-hydroxylated polyenoic very-long-chain fatty acids in mammalian testes and spermatozoa. J. Biol. Chem. 267: 1746–1751. [PubMed] [Google Scholar]
- 4.Zanetti S. R., Monclus M. A., Rensetti D. E., Fornes M. W., and Aveldaño M. I.. 2010. Ceramides with 2-hydroxylated, very long-chain polyenoic fatty acids in rodents: From testis to fertilization-competent spermatozoa. Biochimie. 92: 1778–1786. [DOI] [PubMed] [Google Scholar]
- 5.Oresti G. M., Ayuza Aresti P. L., Gigola G., Reyes L. E., and Aveldaño M. I.. 2010. Sequential depletion of rat testicular lipids with long-chain and very long-chain polyenoic fatty acids after X-ray-induced interruption of spermatogenesis. J. Lipid Res. 51: 2600–2610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sandhoff R., Geyer R., Jennemann R., Paret C., Kiss E., Yamashita T., Gorgas K., Sijmonsma T. P., Iwamori M., Finaz C., et al. 2005. Novel class of glycosphingolipids involved in male fertility. J. Biol. Chem. 280: 27310–27318. [DOI] [PubMed] [Google Scholar]
- 7.Rabionet M., van der Spoel A. C., Chuang C. C., von Tumpling-Radosta B., Litjens M., Bouwmeester D., Hellbusch C. C., Korner C., Wiegandt H., Gorgas K., et al. 2008. Male germ cells require polyenoic sphingolipids with complex glycosylation for completion of meiosis: a link to ceramide synthase-3. J. Biol. Chem. 283: 13357–13369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rabionet M., Bayerle A., Jennemann R., Heid H., Fuchser J., Marsching C., Porubsky S., Bolenz C., Guillou F., Grone H. J., et al. 2015. Male meiotic cytokinesis requires ceramide synthase 3-dependent sphingolipids with unique membrane anchors. Hum. Mol. Genet. 24: 4792–4808. [DOI] [PubMed] [Google Scholar]
- 9.Oresti G. M., Reyes J. G., Luquez J. M., Osses N., Furland N. E., and Aveldaño M. I.. 2010. Differentiation-related changes in lipid classes with long-chain and very long-chain polyenoic fatty acids in rat spermatogenic cells. J. Lipid Res. 51: 2909–2921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Simons K., and Ikonen E.. 1997. Functional rafts in cell membranes. Nature. 387: 569–572. [DOI] [PubMed] [Google Scholar]
- 11.Pike L. J. 2006. Rafts defined: a report on the Keystone Symposium on Lipid Rafts and Cell Function. J. Lipid Res. 47: 1597–1598. [DOI] [PubMed] [Google Scholar]
- 12.Peñalva D. A., Furland N. E., Lopez G. H., Aveldaño M. I., and Antollini S. S.. 2013. Unique thermal behavior of sphingomyelin species with nonhydroxy and 2-hydroxy very-long-chain (C28-C32) PUFAs. J. Lipid Res. 54: 2225–2235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Peñalva D. A., Wilke N., Maggio B., Aveldaño M. I., and Fanani M. L.. 2014. Surface behavior of sphingomyelins with very long chain polyunsaturated fatty acids and effects of their conversion to ceramides. Langmuir. 30: 4385–4395. [DOI] [PubMed] [Google Scholar]
- 14.Brown D. A., and Rose J. K.. 1992. Sorting of GPI-anchored proteins to glycolipid-enriched membrane subdomains during transport to the apical cell surface. Cell. 68: 533–544. [DOI] [PubMed] [Google Scholar]
- 15.Brown D. A., and London E.. 2000. Structure and function of sphingolipid- and cholesterol-rich membrane rafts. J. Biol. Chem. 275: 17221–17224. [DOI] [PubMed] [Google Scholar]
- 16.Monneron A., and d’Alayer J.. 1978. Isolation of plasma and nuclear membranes of thymocytes. I. Enzymatic composition and ultrastructure. J. Cell Biol. 77: 211–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Luria A., Vegelyte-Avery V., Stith B., Tsvetkova N. M., Wolkers W. F., Crowe J. H., Tablin F., and Nuccitelli R.. 2002. Detergent-free domain isolated from Xenopus egg plasma membrane with properties similar to those of detergent-resistant membranes. Biochemistry. 41: 13189–13197. [DOI] [PubMed] [Google Scholar]
- 18.Romrell L. J., Bellve A. R., and Fawcett D. W.. 1976. Separation of mouse spermatogenic cells by sedimentation velocity. A morphological characterization. Dev. Biol. 49: 119–131. [DOI] [PubMed] [Google Scholar]
- 19.Reyes J. G., Diaz A., Osses N., Opazo C., and Benos D. J.. 1997. On stage single cell identification of rat spermatogenic cells. Biol. Cell. 89: 53–66. [DOI] [PubMed] [Google Scholar]
- 20.Laemmli U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 227: 680–685. [DOI] [PubMed] [Google Scholar]
- 21.Widnell C. C., and Unkeless J. C.. 1968. Partial purification of a lipoprotein with 5′-nucleotidase activity from membranes of rat liver cells. Proc. Natl. Acad. Sci. USA. 61: 1050–1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bligh E. G., and Dyer W. J.. 1959. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 37: 911–917. [DOI] [PubMed] [Google Scholar]
- 23.Rouser G., Fkeischer S., and Yamamoto A.. 1970. Two dimensional then layer chromatographic separation of polar lipids and determination of phospholipids by phosphorus analysis of spots. Lipids. 5: 494–496. [DOI] [PubMed] [Google Scholar]
- 24.Holub B. J., and Skeaff C. M.. 1987. Nutritional regulation of cellular phosphatidylinositol. Methods Enzymol. 141: 234–244. [DOI] [PubMed] [Google Scholar]
- 25.Christie W. W., and Han X.. 2012. Lipid Analysis. Woodhead Publishing Limited, Cambridge, UK. [Google Scholar]
- 26.Livak K. J., and Schmittgen T. D.. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 25: 402–408. [DOI] [PubMed] [Google Scholar]
- 27.Monneron A., and d’Alayer J.. 1978. Isolation of plasma and nuclear membranes of thymocytes. II. Biochemical composition. J. Cell Biol. 77: 232–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Williams D. B. 2006. Beyond lectins: the calnexin/calreticulin chaperone system of the endoplasmic reticulum. J. Cell Sci. 119: 615–623. [DOI] [PubMed] [Google Scholar]
- 29.Otto C., Rohde-Schulz B., Schwarz G., Fuchs I., Klewer M., Brittain D., Langer G., Bader B., Prelle K., Nubbemeyer R., et al. 2008. G protein-coupled receptor 30 localizes to the endoplasmic reticulum and is not activated by estradiol. Endocrinology. 149: 4846–4856. [DOI] [PubMed] [Google Scholar]
- 30.Kittel A., and Bacsy E.. 1994. Ecto-ATPases and 5′-nucleotidases in the caveolae of smooth muscle. Enzyme-histochemical evidence may indicate a role for caveolae in neurotransmission. Cell Biol. Int. 18: 875–879. [DOI] [PubMed] [Google Scholar]
- 31.Russell L. D., and de Franca L. R.. 1995. Building a testis. Tissue Cell. 27: 129–147. [DOI] [PubMed] [Google Scholar]
- 32.Eckhardt M., Yaghootfam A., Fewou S. N., Zoller I., and Gieselmann V.. 2005. A mammalian fatty acid hydroxylase responsible for the formation of alpha-hydroxylated galactosylceramide in myelin. Biochem. J. 388: 245–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zöller I., Meixner M., Hartmann D., Büssow H., Meyer R., Gieselmann V., and Eckhardt M.. 2008. Absence of 2-hydroxylated sphingolipids is compatible with normal neural development but causes late-onset axon and myelin sheath degeneration. J. Neurosci. 28: 9741–9754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhu G., Koszelak-Rosenblum M., Connelly S. M., Dumont M. E., and Malkowski M. G.. 2015. The crystal structure of an integral membrane fatty acid alpha-hydroxylase. J. Biol. Chem. 290: 29820–29833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Oresti G. M., Luquez J. M., Furland N. E., and Aveldaño M. I.. 2011. Uneven distribution of ceramides, sphingomyelins and glycerophospholipids between heads and tails of rat spermatozoa. Lipids. 46: 1081–1090. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.