Abstract
Tetrahydroxy bile acids (THBAs) are hydrophilic and are present at minimal or undetectable levels in healthy human adults, but are present at high levels in bile salt export pump (abcb11)-knockout mice. The roles of THBAs in human cholestatic diseases are unclear. We aimed to investigate the presence of THBAs in patients with infantile intrahepatic cholestasis and its correlation with outcome. Urinary bile acids (BAs) were analyzed by GC-MS. Data were compared between good (n = 21) (disease-free before 1 year old) and poor prognosis groups (n = 19). Good prognosis patients had a higher urinary THBA proportion than poor prognosis patients [25.89% (3.45–76.73%) vs. 1.93% (0.05–48.90%)]. A urinary THBA proportion >7.23% predicted good prognosis with high sensitivity (95.24%), specificity (84.21%), and area under the curve (0.91) (P < 0.0001). A THBA proportion ≲7.23% was an independent factor for decreased transplant-free survival (hazard ratio = 7.16, confidence interval: 1.24–41.31, P = 0.028). Patients with a confirmed ABCB11 or tight junction protein 2 gene mutation (n = 7) had a minimally detectable THBA proportion (0.23–2.99% of total BAs). Three patients with an ATP8B1 mutation had an elevated THBA proportion (7.51–37.26%). In conclusion, in addition to disease entity as a major determinant of outcome, a high THBA level was associated with good outcome in the infantile intrahepatic cholestasis patients.
Keywords: bile salts, bile acid metabolism, clinical studies, liver, diseases, cytochrome P450, progressive familial intrahepatic cholestasis
Bile acids (BAs) are naturally occurring molecules that are essential for the digestion of lipids and lipid-soluble vitamins. BA homeostasis is tightly regulated by nuclear receptors and is closely associated with lipid and glucose metabolism (1–3). In humans, two primary BAs, cholic acid (CA) and chenodeoxycholic acid (CDCA), are secreted into the duodenum, where they are then converted into secondary BAs (deoxycholic acid and lithocholic acid) by gut microbiota. Other BAs are also present in humans, but at much lower concentrations. Hepatic bile formation and secretion depend on sinusoidal and canalicular transporters, BA metabolic enzymes, and associated membrane proteins. The BA profile and BA metabolism are altered in cholestasis patients (4), resulting in the accumulation of high concentrations of BAs with cytotoxic effects, leading to severe liver injury or even death. The cytotoxicity of BAs is attributable to their structures; BAs with greater hydrophobicity have higher toxicity (5). Both conjugation and hydroxylation detoxify BAs by increasing their solubility (6).
Infantile intrahepatic cholestasis has a wide variety of etiologies, including infection-related neonatal hepatitis (NH), progressive familial intrahepatic cholestasis (PFIC), inborn errors of BA metabolism (IEBAM), and other metabolic or toxic insults (7–9). Prognosis of cholestasis patients is highly variable among the different disease entities and is difficult to predict during the early stage of disease. Bile salt export pump (BSEP) deficiency is one of the major causes of PFIC. It is caused by a mutation in the canalicular bile salt transporter, BSEP [or sister of P-glycoprotein (sPgp)], which is encoded by the ABCB11 gene. BSEP is the major determinant of canalicular bile secretion (10, 11). The rate of biliary bile salt excretion in patients with an ABCB11 mutation is only 1% of that in individuals without a mutation in this gene, and this decreased excretion leads to liver cirrhosis and hepatic failure in children. In contrast, Bsep (abcb11)-knockout mice show only mild nonprogressive cholestasis (12). Interestingly, the BA pool in Bsep-knockout mice contains a high level of a tetrahydroxy BA (THBA), 3α,6β,7β,12α-tetrahydroxy-5β-cholan-24-oic acid, which is highly hydrophilic and less toxic (13). Notably, the polyhydroxylation of BAs has been speculated to be a potential detoxification mechanism that explains the viability of Bsep-knockout mice.
Although THBAs have beneficial roles in Bsep-knockout mice, their potential benefits in human patients remain poorly understood. The detection of THBAs has been reported in healthy neonates. However, data is scarce regarding THBAs in cholestasis patients (14–16). In the present study, we examined BA profiles in infantile cholestasis patients, with a focus on the roles of THBAs in relation to the disease entity and patient outcomes.
MATERIALS AND METHODS
Patients
Patients admitted to the National Taiwan University Hospital for infant-onset intrahepatic cholestasis from January 1999 to April 2014 were included in this study. A modified 3 day protocol that we previously reported was initially used to exclude patients with extrahepatic cholestasis. This protocol included ultrasonography, magnetic resonance cholangiography, liver histological analysis, and assessment of operative findings (17). After the exclusion of patients with extrahepatic cholestasis, thorough assessment of the patients with intrahepatic cholestasis, including analyses of infectious pathogens and metabolic diseases, genetic analyses of specific genes, liver histological analysis, and immunohistochemical staining (for BSEP), was performed on an individual basis.
Urine samples collected before 1 year of age from a total of 40 individuals during this period were sent for analysis of BA profiles for diagnosis of IEBAM. The initial levels of alanine aminotransferase (ALT), total bilirubin, direct bilirubin, and γ-glutamyl transpeptidase (GGT), the age of jaundice disappearance, the age of liver transplantation or biliary diversion, and the follow-up duration were determined by reviewing the medical records. Final diagnoses were reviewed by considering all available clinical and laboratory data from the medical records and were established using uniform criteria (17).
Genetic cholestatic diseases were diagnosed in patients with mutations in both alleles in autosomal recessive genetic disorders [ATP8B1, ABCB11, tight junction protein 2 (TJP2), solute carrier family 25 member 13 (SLC25A13), vacuolar protein sorting 33B (VPS33B), aldo-keto reductase family 1 member D1 (AKR1D1), cytochrome P450 (CYP) family 7 subfamily B polypeptide 1 (CYP7B1)]. Patients with one heterozygous mutation in a cholestatic gene and subsequent recovery before 1 year of age were diagnosed with “transient neonatal cholestasis”. Patients without an identifiable cause of cholestasis after thorough investigation were diagnosed with “idiopathic NH” if they had recovered before 1 year of age or with “phenotypic PFIC” if they had clinical, biochemical, and pathological features compatible with PFIC, but no identifiable genetic mutation.
The patients were followed until total recovery of liver function, death, or receiving liver transplantation or until at least until 1 year of age for those with persistent or progressive disease. The patients who were disease-free (total bilirubin of less than 2.0 mg/dl) before 1 year of age were included in the good prognosis group, and those with persistent jaundice and chronic liver disease were included in the poor prognosis group. Urine BA components were compared between the patients in the good and poor prognosis groups. In addition, the transplant-free survival times of the patients were recorded for analysis of outcomes. This study was approved by the institutional review board.
To further analyze BA profiles in patients with different genetic disorders causing PFIC, patients under 10 years of age (including five patients below 1 year of age and five patients above 1 year of age) with a genetically confirmed mutation in the BSEP (ABCB11), familial intrahepatic cholestasis 1 (FIC1) (ATP8B1), or TJP2 were included in separate analysis.
Urine sample collection and analysis of urine BAs
Random urine samples were collected and stored at −80°C. The urine samples were analyzed by GC-MS as previously described (8, 18, 19). Urine BA concentrations were corrected for the creatinine (Cre) concentration and expressed as micromoles per millimole of Cre. In addition to urinary total BAs (UTBAs), five major subgroups of UTBAs were analyzed, including common BAs (CBAs), THBAs, trihydroxy BAs (TRHBAs), ketonic BAs (KBAs), and unsaturated BAs (UBAs).
mRNA expression analysis by quantitative RT-PCR
To investigate the potential roles of CYP family 3 subfamily A polypeptide 4 (CYP3A4) and CYP family 3 subfamily A polypeptide 5 (CYP3A5) in THBA synthesis, liver samples obtained by diagnostic liver biopsy were analyzed. Liver samples from patients with noncholestatic liver disease were used as controls. Total RNA was extracted from frozen livers using an RNeasy kit (Qiagen GmbH, Hilden, Germany) and was then reverse transcribed (SuperScript II; Invitrogen Life Technologies, Breda, The Netherlands). Real-time PCR was performed using a TaqMan system with a PRISM 7900 HT sequence detection system (Applied Biosystems, Foster City, CA). The primers and probes used were as follows: TBP Hs00427620_m1, CYP3A4 Hs00430021_m1, and CYP3A5 Hs00241417_m1 (TaqMan® Gene Expression Assays; Applied Biosystems).
Statistical analysis
Descriptive statistics, such as the proportion, median, and range, were used to summarize the results for urinary BAs. Differences between the two prognosis groups were assessed with the Wilcoxon rank-sum test or χ2 test. P < 0.05 was considered significant in all analyses. Total urinary BAs, as well as the five major subgroups of urinary BAs, were analyzed by generating a receiver operating characteristic (ROC) curve to determine the cut-off values for predicting prognosis. In addition, univariate and multivariate regression analyses were performed using the Cox proportional hazard model to identify predictive factors for transplant-free survival. We included predictive factors in multivariate analysis that showed a P value of 0.1 or less in univariate analysis. Further, Kaplan-Meier curves were generated to determine the probabilities of liver transplant-free survival of the patients with different levels of urinary BAs. Patients were censored at the time of last follow-up if they no longer had cholestasis and had not received liver transplantation. Statistical analyses were performed using STATA 12.0 (STATA Corp., College Station, TX).
RESULTS
Relationship of urinary BA profile with patient outcome
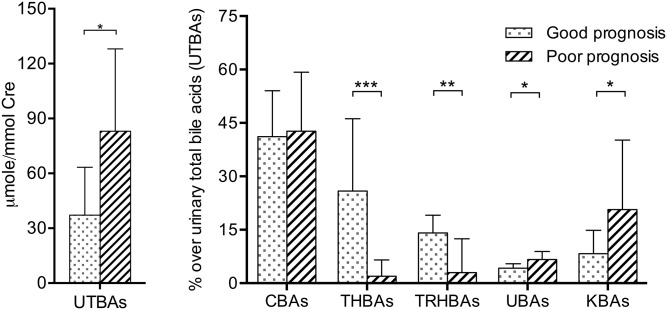
To investigate whether the BA profile can be used as a marker for predicting patient outcome, we analyzed urine samples collected from 40 patients before 1 year of age because BA composition has been shown to differ among patients of different ages (18). The good prognosis group included 21 patients and the poor prognosis group contained 19 patients. There were 14 patients with definite genetic diagnosis. Two patients were confirmed with ABCB11 mutation, one patient with ATP8B1 mutation, two patients with TJP2 mutation, and four patients with IEBAM. In the good prognosis group, two patients with SLC25A13 heterozygous mutation and one patient with VPS33B heterozygous mutation had transient neonatal cholestasis. The patient demographic data are listed in Table 1. The median age of urinary BA examination was 2.5 months (range 1.0–8.8 months) for the patients with a good prognosis and 6.3 months (range 0.7–11.0 months) for those with a poor prognosis. We compared the urinary BA profiles between these two groups of patients (Fig. 1). The patients with a poor prognosis had a median total BA level of 87.26 μmol/mmol Cre (16.17–571.06 μmol/mmol Cre), which was significantly higher than that of the patients with a good prognosis of 37.16 μmol/mmol Cre (7.08–93.14 μmol/mmol Cre; P < 0.05). In addition, the patients with a good prognosis had a significantly higher proportion of uncommon urinary BAs with a higher number of hydroxyl groups, including THBAs and TRHBAs, whereas those with a poor prognosis tended to have significantly higher proportions of KBAs and UBAs in the urine. Detailed data on the levels of individual BAs are provided in supplemental Table S1. There were no differences in the concentration or proportion of total common BAs between the two prognosis groups. Moreover, the CA:CDCA ratio was significantly higher for the patients in the good prognosis group than for those in the poor prognosis group (2.3, range 0.26–20.69; and 0.82, range 0.05–11.29, respectively; P = 0.04).
TABLE 1.
Basic characteristics of patients in the good prognosis and poor prognosis groups
| Good Prognosis (n = 21) | Poor Prognosis (n = 19) | P | |
| Gender (male:female) | 15:6 | 10:9 | 0.23 |
| Diagnosis | |||
| CMV infection | 8 | — | — |
| Urinary tract infection | 2 | — | — |
| Transient neonatal cholestasisa | 3 | — | — |
| Idiopathic NH | 8 | — | — |
| Phenotypic PFIC | — | 7 | — |
| FIC1 deficiency | — | 1 | — |
| BSEP deficiency | — | 2 | — |
| TJP2 deficiency | — | 2 | — |
| NICCD | — | 2 | — |
| IEBAM (CYP7B1 and AKR1D1 mutations) | — | 4 | — |
| PNAC | — | 1 | — |
| Disease onset (months) | 1.9 (0.6–8.9) | 2.2 (0.2–5.7) | 0.54 |
| Follow-up duration (years) | 1.6 (0.1–8.0) | 0.6 (0.2–11.7) | 0.08 |
| Initial biochemistry | |||
| Total bilirubin (mg/dl) | 8.55 (0.68–14.64) | 10.90 (2.65–23.6) | 0.13 |
| Direct bilirubin (mg/dl) | 5.32 (0.29–8.28) | 6.30 (1.72–15.40) | 0.17 |
| ALT (U/l) | 232 (18–814) | 200 (39–400) | 0.34 |
| GGT (U/l) | 67 (4–391) | 48 (14–117) | 0.03 |
| Serum BAsb | 166 (41–166) | 122 (36–400) | 0.27 |
The good prognosis group was jaundice-free before 1 year of age and the poor prognosis group had a persistence of jaundice after 1 year of age. CMV, cytomegalovirus; NICCD, neonatal cholestasis caused by citrin deficiency; PNAC, parenteral nutrition-associated cholestasis; .
Transient neonatal cholestasis, SLC25A13 or VPS33B heterozygous mutation.
Serum BA data for 27 patients (16 in the good prognosis group and 11 in the poor prognosis groups) were included.
Fig. 1.
UTBA levels and percentages of different BAs in the good (jaundice-free before 1 year of age) versus poor prognosis (persistence of jaundice after 1 year of age) patient groups. *P < 0.05; **P < 0.01; and ***P < 0.0001.
THBAs.
The THBAs measured included 1β,3α,7α,12α-tetrahydroxy-5β-cholan-24-oic acid (CA-1β-ol), 2β,3α,7α,12α-tetrahydroxy-5β-cholan-24-oic acid, 3β,4β,7α,12α-tetrahydroxy-5β-cholan-24-oic acid, 3α,4β,7α,12α-tetrahydroxy-5β-cholan-24-oic acid, and 3α,6α,7α,12α-tetrahydroxy-5β-cholan-24-oic acid (CA-6α-ol). The proportion of THBAs was 1.93% (0.05–48.90%) of total BAs for the patients in the poor prognosis group, which was significantly lower than that for the patients in the good prognosis group (25.89%, 3.45–76.73%) (P < 0.0001). Among the THBAs, CA-1β-ol was the most abundant in the urine, followed by CA-6α-ol (supplemental Table S1). CA-1β-ol and CA-6α-ol were also significantly more abundant in the patients with a good prognosis than in those with a poor prognosis (P < 0.0001).
We further demonstrated the THBA proportions in patients with different disease entities, as shown in supplemental Fig. S1. The patients with genetic cholestatic disease tended to have a lower proportion of THBAs than those with cholestasis caused by cytomegalovirus, urinary tract infection, idiopathic NH, etc.
TRHBAs.
Four uncommon TRHBAs detected in the patients’ urine samples were 1β,3α,7α-trihydroxy-5β-cholan-24-oic acid, 2β,3α,7α-trihydroxy-5β-cholan-24-oic acid, 4β,3α,7α-trihydroxy-5β-cholan-24-oic acid, and 3α,6α,7α-dihydroxy-5β-cholan-24-oic acid (hyocholic acid). The proportion of urinary TRHBAs was significantly higher for the patients in the good prognosis group than for those in the poor prognosis group (14.1%, range 4.48–36.58%; and 3.97%, range 0.1–31.99%, respectively). The two neonatal intrahepatic cholestasis caused by citrin deficiency patients were noted to have high TRHBA proportions (26.7%, range 21.4–32.0%). In addition, the proportions of urinary TRHBAs were 8.0% (range 0.37–22.85%) and 0.66% (range 0.1–3.0%) for the PFIC and IEBAM patients, respectively.
KBAs and UBAs.
KBAs and UBAs were detected in all patients, but they were more abundant in the patients in the poor prognosis group, as shown in Fig. 1. Notably, the IEBAM patients had very distinct urinary BA profiles. The two patients with Δ4-3-oxosteroid-5β-reductase deficiency (due to an AKR1D1 mutation) had KBA proportions of 91.7% (76.0 μmol/mmol Cre) and 95.0% (115.0 μmol/mmol Cre), respectively, with a large amount of 7α,12α-dihydroxy-3-oxo-chol-4-en-24-oic acid. In addition, the two patients with 7α-hydroxylase deficiency (due to a CYP7B1 mutation) had UBA proportions of 97.7% (89.5 μmol/mmol Cre) and 77.6% (94.3 μmol/mmol Cre), respectively, with a predominance of 3β-hydroxy-5-cholen-24-oic acid.
ROC curve analysis
The values of UTBAs and each of the different BA components for predicting patient prognosis were assessed by ROC curve analysis. The THBA proportion, with a cut-off value of 7.23%, was the best predictor of poor prognosis [sensitivity = 95.24%, specificity = 84.21%, area under the curve (AUC) = 0.91, and P < 0.0001]. The UTBA concentration and TRHBA, KBA, and UBA proportions, with their respective cut-off values, were also found to have predictive value, but lower sensitivities, specificities, and AUC values than the THBA proportion (Table 2).
TABLE 2.
The optimal cut-off values using different BA compositions for predicting good prognosis
| Criterion | AUC | Sensitivity | Specificity | +LR | −LR | P | |
| UTBAsa | ≤71.25 | 0.73 | 85.71 | 63.16 | 2.33 | 0.23 | 0.009 |
| CBAs | >16.32% | 0.53 | 95.24 | 31.58 | 1.39 | 0.15 | 0.750 |
| THBAs | >7.23% | 0.91 | 95.24 | 84.21 | 6.03 | 0.057 | <0.0001 |
| TRHBAs | >4.98% | 0.76 | 95.24 | 52.63 | 2.01 | 0.09 | 0.0019 |
| UBAs | ≤6.15% | 0.71 | 90.48 | 57.89 | 2.15 | 0.16 | 0.013 |
| KBAs | ≤18.56% | 0.72 | 100.00 | 57.89 | 2.37 | 0.00 | 0.016 |
A good prognosis is described as jaundice-free before 1 year of age. +LR, positive likelihood ratio; −LR, negative likelihood ratio.
Data expressed as micromoles per millimole Cre.
Univariate and multivariate Cox proportional hazard model
The patient characteristics, UTBA concentration, and biochemical data (ALT, GGT, and bilirubin levels) were analyzed using the Cox proportional hazard model to assess their associations with transplant-free survival of the patients. Univariate analysis showed that the total bilirubin, direct bilirubin, and UTBA concentrations affected transplant-free survival. Multivariate analysis revealed that the UTBA concentration was the single independent factor affecting transplant-free survival (Table 3). We included age (months) at urine sample collection in univariate and multivariate analyses and found that it had no effect on patient outcome.
TABLE 3.
Univariate and multivariate Cox proportional analysis of the factors associated with reduced transplant-free survival of infantile intrahepatic cholestasis patients
| Univariate Analysis | Multivariate Analysis | |||
| Hazard Ratio | P | Hazard Ratio | P | |
| UTBAs >71.25a | 6.11 (1.86–20.14) | 0.003 | 10.43 (1.57–69.09) | 0.015 |
| Male versus female | 1.62 (0.49–5.42) | 0.431 | — | — |
| Disease onset (months) | 0.83 (0.57–1.20) | 0.319 | — | — |
| Age at urine sample analysis | 1.16 (0.98–1.37) | 0.07 | 1.15 (0.94–1.41) | 0.165 |
| Initial biochemistry | ||||
| ALT (U/l) | 1.00 (0.99–1.00) | 0.755 | — | — |
| GGT (U/l) | 0.99 (0.97–1.00) | 0.113 | — | — |
| Total bilirubin (mg/dl) | 1.17 (1.02–1.34) | 0.024 | 1.45 (0.95–2.21) | 0.089 |
| Direct bilirubin (mg/dl) | 1.37 (1.10–1.71) | 0.006 | 0.87 (0.53–1.44) | 0.606 |
Data expressed as micromoles per millimole Cre.
We next investigated which of the five major BA subgroups had the most significant effect on transplant-free survival of the patients in this study. Univariate analysis showed that the CBA, THBA, TRHBA, and UBA concentrations were associated with patient outcome. Multivariate analysis revealed that the THBA concentration was the only independent factor affecting transplant-free survival (Table 4). We also performed analysis excluding the four IEBAM patients, and the results still showed that the THBA concentration was the only independent factor affecting survival (P = 0.010).
TABLE 4.
Univariate and multivariate Cox proportional analysis of the different BA cut-off values associated with reduced transplant-free survival of infantile intrahepatic cholestasis patients
| Univariate Analysis | Multivariate Analysis | |||
| Hazard Ratio | P | Hazard Ratio | P | |
| CBAs ≤16.32% | 4.90 (1.52–15.76) | 0.008 | 2.53 (0.68–9.49) | 0.168 |
| THBAs ≤7.23% | 10.78 (2.36–49.18) | 0.002 | 7.16 (1.24–41.31) | 0.028 |
| TRHBAs ≤4.98% | 5.47 (1.68–16.85) | 0.005 | 1.12 (0.27–4.67) | 0.875 |
| UBAs >6.15% | 1.75 (0.58–5.26) | 0.320 | — | — |
| KBAs >18.56% | 3.87 (1.28–11.69) | 0.016 | 1.53 (0.45–5.17) | 0.491 |
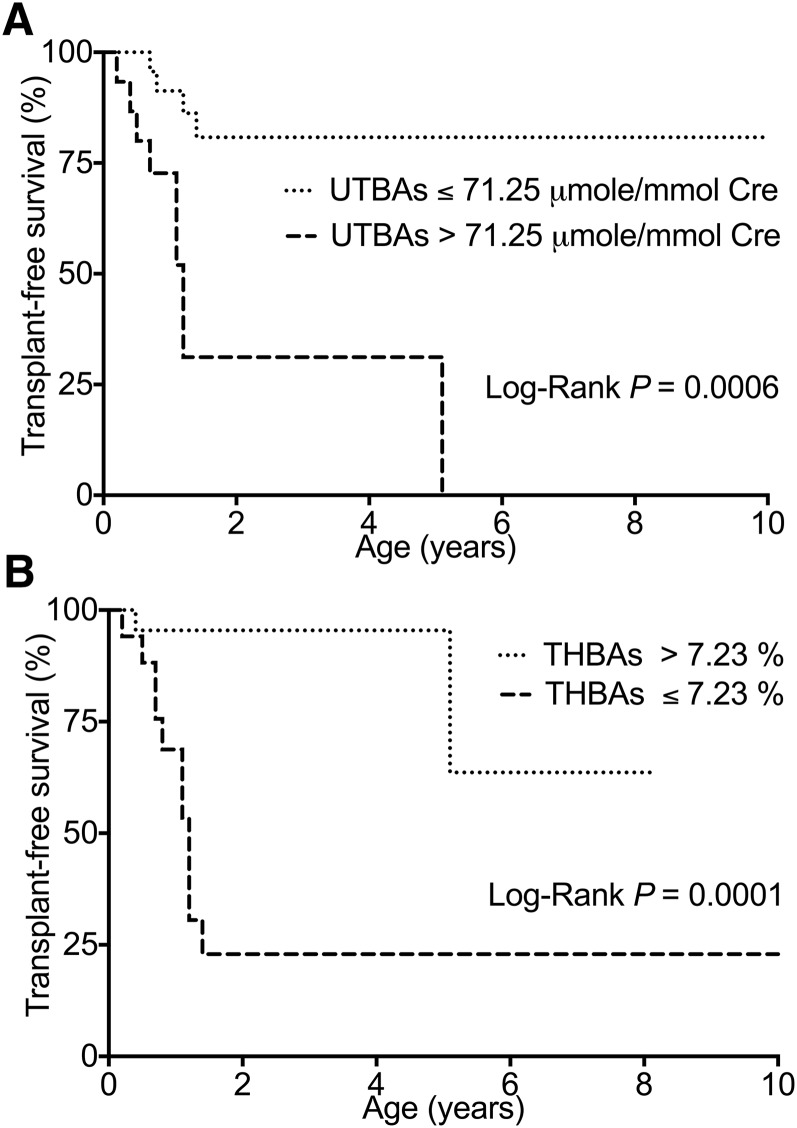
The patients with a UTBA concentration of higher than 71.25 μmol/mmol Cre had a shorter survival time than those with a concentration of lower than 71.25 μmol/mmol Cre (P = 0.0006), as determined by Kaplan-Meier survival analysis (Fig. 2A), and none of these patients survived without liver transplantation. In addition, the patients with a urinary THBA proportion of less than or equal to 7.23% of UTBAs had markedly reduced transplant-free survival, and they all underwent liver transplantation or died at a median age of 1.1 years (confidence interval: 3.3–32.0, P = 0.0001; Fig. 2B). Most of the patients with a high urinary THBA proportion survived without liver transplantation, although there were some exceptions. One patient with a FIC1 deficiency and a high urinary THBA proportion (26.0%) received liver transplantation at 5 years of age. In addition, one patient with a heterozygous SLC25A13 mutation and a urinary THBA proportion of 3.45% was jaundice free at 4 months of age.
Fig. 2.
A: Kaplan-Meier curves showing increased transplant-free survival of the patients with a UTBA level of ≤71.25 μmol/mmol Cre. B: Increased transplant-free survival was observed for the patients with a urinary THBA proportion of above 7.23% of UTBAs.
Gene expression levels
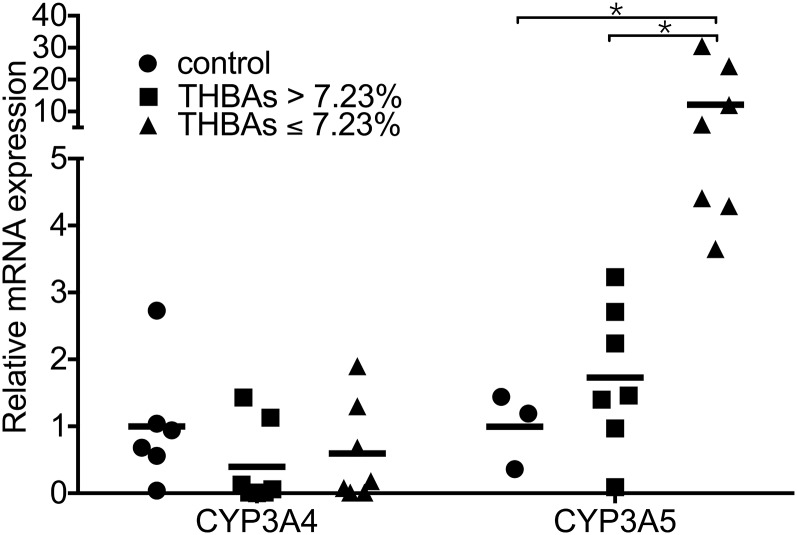
To determine whether the increased THBA concentration was accompanied by the increased expression of CYP enzymes, we compared the mRNA expression of CYP3A4 and CYP3A5 in liver specimens from infantile intrahepatic cholestasis patients with high and low THBA proportions, using 7.23% as a cut-off. A total of 14 liver specimens were available from 40 patients, including seven patients each with low and high THBA proportions. An additional seven age-matched patients with noncholestatic liver disease were included as controls. CYP3A4 mRNA expression was not found to be positively correlated with different THBA proportions (Fig. 3). In contrast, CYP3A5 expression was significantly higher in the patients with a THBA level of less than 7.23%.
Fig. 3.
Relative mRNA expression of CYP3A4 and CYP3A5 in infantile intrahepatic cholestasis patients with different THBA levels compared with those in controls (infants without cholestasis).
BA profiles in patients with ATP8B1, ABCB11, or TJP2 mutations
The THBA concentration has been shown to be increased in Bsep-knockout mice; however, we detected a low THBA concentration in the patients with a poor prognosis, as described above. Therefore, we were interested in determining whether this level was also elevated in human PFIC patients with a specific genetic diagnosis. We further analyzed the BA profiles of the patients with a genetically confirmed ATP8B1 (FIC1 deficiency), ABCB11 (BSEP deficiency) (20), or TJP2 mutation. The BA profiles of five patients from the above-mentioned patient group were analyzed, in addition to those of five patients whose urine samples had been collected after 1 year of age (two with an ATP8B1 mutation, two with an ABCB11 mutation, and one with a TJP2 mutation). The BA profiles are presented according to the genetic diagnosis in supplemental Table S2. Interestingly, the patients with an ABCB11 or TJP2 mutation had low THBA proportions (0.23–5.06%), and those with an ATP8B1 mutation tended to have higher proportions (7.51–37.26%).
DISCUSSION
To our knowledge, the present study is the first to demonstrate an association of the concentration of THBAs, which represent a significant proportion of urinary BAs in patients with infantile cholestatic liver disease, with patient prognosis. Among the various types of BAs with altered levels, THBAs had the highest AUC and the best sensitivity and specificity in predicting prognosis, i.e., the disappearance of jaundice before 1 year of age. Survival analysis revealed that a urinary THBA proportion of higher than 7.23% was associated with relatively high transplant-free survival of the patients.
THBAs, along with other polyhydroxylated BAs, are preferentially excreted into the urine (21) and are present in humans mainly during the neonatal period (18). Previous studies of healthy infants have shown that 1-β THBA is the predominant THBA during early infancy. THBAs have been detected among urinary BAs between 7 days and 11–12 months after birth, with a peak in concentration at 1 month, and their concentration remains relatively stable from 2 to 12 months (approximately 10% of total BAs), after which it gradually decreases to a minimal level at 1 year of age (18), and THBAs are undetectable in adults.
Neonates and infants have relatively high serum BA concentrations, inefficient biliary excretion, and immature enterohepatic circulation, which characterize the developmental stage of physiological cholestasis (22). The polyhydroxylation and urinary excretion of THBAs may serve as a metabolic mechanism to enhance BA excretion in neonates (23). The 1-β-hydroxylated BAs have been detected in fetal, neonatal, and infant livers, and they may participate in a detoxification mechanism during liver development. This hypothesis is supported by the abundance of THBAs in preterm infants with physiologic cholestasis. In the current study, the levels of CA-1β-ol in the patients with a good prognosis reached 10.13 μmol/mmol Cre (0.51–65.26 μmol/mmol Cre), which is higher than the reported levels in healthy infants of 0.24–4.20 μmol/mmol Cre (18). In contrast, the patients with a poor prognosis had a relatively low mean CA-1β-ol concentration of 0.86 μmol/mmol Cre (0.03–67.64 μmol/mmol Cre).
Few previous studies and limited case reports have described the presence of CA-1β-ol (a major type of THBA) in cholestatic patients. Four neonatal patients (ages not specified) with paucity of the interlobular bile ducts have been reported with urinary CA-1β-ol proportions of 2.4–37.1% (24). In addition, two studies have reported CA-1β-ol proportions of 0–11.6% of total urinary BAs in adult patients with cholestatic liver disease and liver cirrhosis (21, 25). Our findings indicated that many of the patients with infantile cholestasis were capable of synthesizing a significant amount of THBAs, which are more hydrophilic, have low toxicity, and may be associated with a good outcome.
Notably, we detected a very low THBA concentration in the BSEP-deficient patients, in contrast with that previously reported in the animal model (Bsep-knockout mice), which synthesizes a large amount of THBAs. It has been speculated that the milder phenotype of Bsep-knockout mice may be due to their ability to synthesize THBAs, which neutralize the toxic effects of accumulated BAs. Additionally, the THBA level was also relatively low in patients with IEBAM in our study. Interestingly, the patients with an ATP8B1 mutation had a higher THBA level than those with an ABCB11 or a TJP2 mutation. It is known that patients with an ATP8B1 mutation tend to have slower disease progression than those with an ABCB11 mutation (10). Taken together, these findings support the notion that THBA synthesis may be associated with less severe liver injury.
The reasons for the variation in the ability of patients to synthesize THBAs are unknown. Aside from the metabolic changes that occur during different developmental stages, this variation may be related to differences in BA metabolism in the different disease entities, differences in BA metabolic enzyme activities due to genetic variation, or differences in the extent and type of injury to hepatocytes. CYPs belong to a superfamily of enzymes that catalyze a broad range of reactions, including the biotransformation of BA. The most abundant CYP enzyme family in the human liver is the CYP3A subfamily, which includes three isoforms: CYP3A4, CYP3A5, and CYP3A7 (26). Cyp3a11 (human homolog of CYP3A5) has been proposed to be involved in THBA formation in mice (27). However, no studies have examined the relationship between CYP3A4 or CYP3A5 and THBAs in humans. CYP3A4 is the predominant enzyme responsible for the 25-hydroxylation of 5β-cholestane-3α,7α,12α-triol in human liver microsomes (28). An in vivo experiment in which deoxycholic acid was incubated with recombinant CYP3A4 and human liver microsomes demonstrated conversion of the substrate to 1β-hydroxyl-deoxycholic acid. Although CYP3A4 is involved in the 1-β-hydroxylation of BAs, the incubation of CA with CYP3A4 has been shown to yield only 3-dehydro-CA (29). CYP3A5 also participates in BA hydroxylation (28), but it has not been found to perform tetrahydroxylation. In the present study, we did not detect a positive correlation between CYP3A4 or CYP3A5 expression and the THBA level.
The clinical implications of our results affect patient diagnosis and treatment. First, although urinary BA analysis is not performed in the laboratories of most hospitals, it should be included in routine investigational assays of chronic cholestatic patients, as expanded lists of phenotypic and metabolic defects associated with IEBAM have been recently reported (30). In clinical practice, urine sample collection is easy to perform, the data are not affected by fasting conditions after correction by Cre, and analyses have low variability and high stability compared with analyses of serum BAs levels (31, 32).
Second, our findings suggest a potential therapeutic role of THBAs. THBA synthesis was reported to be inducible in humans by administration of adrenocorticotropic hormones half a century ago (33). Some evidence indicates that drugs, such as taurine (34), phenobarbital, (35, 36), and rifampicin (37), induce THBA production in humans, but most of them have transient effects. These data suggest that humans are capable of producing THBAs under certain circumstances. Further investigation of inducible or exogenous THBAs as therapeutic agents would be of great clinical interest.
There are several limitations of our study. First, the specific disease entity and its pathophysiology have major impacts on patient outcome, while the THBA proportion may only partly contribute to outcome, or it may reflect an effect of liver injury caused by a specific disease. For example, the patients with FIC1 deficiency in our study had a high THBA proportion, but required liver transplantation at a later age due to disease progression. The FIC1 protein is expressed in multiple organs, including the liver, small intestine, kidneys, and pancreas, but the BSEP protein is mainly expressed in the liver. The association of the disease entity with patient outcome should first be considered. In addition, we noticed that the FIC1-deficient patients had a higher THBA level than the BSEP-deficient patients, as well as slower disease progression. These findings might be due to the fact that liver function was more preserved initially in the FIC1-deficient patients. We propose that the patients with more severe liver disease or less preserved liver function were unable to produce THBAs during cholestasis as a compensatory and protective mechanism. Determining the actual mechanism of THBA production in cholestasis patients is important for understanding its causal relationship with the disease and patient outcome. Second, our patients were a highly heterogeneous group with many different diagnoses. Only a small number of patients were included with each genetic defect because of the rarity of the mutations. Thus, evaluation of more patients is required to confirm the BA profile changes specific to each genetic disease. Nevertheless, our patient population reflects the real-world situation encountered by clinicians in which the common presentation of infantile cholestasis is observed in highly heterogeneous patients with a long list of differential diagnoses. Usually only a preliminary diagnosis can be made initially, and it takes weeks or even months until all of the comprehensive investigations are completed and a final diagnosis is made. Our findings suggest that the urinary THBA level may be an important marker for predicting patient outcome, even when a genetic diagnosis is not available yet. Third, THBA levels were not measured in normal controls in this study. Instead, we used previously published data for normal infant controls, with detailed descriptions for each age group (18).
In conclusion, we have reported the presence of urinary THBAs in patients with infantile intrahepatic cholestasis. Patients with a good prognosis had a higher THBA level than those with a poor prognosis. The roles of THBAs in clinical management and treatment, as well as their potential protective effects against liver injury, warrant further investigation.
Supplementary Material
Acknowledgments
The authors thank Ms. Chien-I Chao, Ms. Chih-Hsuan Jia, and Mr. Bang-Yu Liou for assisting with sample preparation and laboratory techniques.
Footnotes
Abbreviations:
- AKR1D1
- aldo-keto reductase family 1 member D1
- ALT
- alanine aminotransferase
- AUC
- area under the curve
- BA
- bile acid
- BSEP
- bile salt export pump
- CA
- cholic acid
- CA-1β-ol
- 1β,3α,7α,12α-tetrahydroxy-5β-cholan-24-oic acid
- CA-6α-ol
- 3α,6α,7α,12α-tetrahydroxy-5β-cholan-24-oic acid
- CBA
- common bile acid
- CDCA
- chenodeoxycholic acid
- Cre
- creatinine
- CYP
- cytochrome P450
- CYP3A4
- cytochrome P450 family 3 subfamily A polypeptide 4
- CYP3A5
- cytochrome P450 family 3 subfamily A polypeptide 5
- CYP7B1
- cytochrome P450 family 7 subfamily B polypeptide 1
- FIC1
- familial intrahepatic cholestasis 1
- GGT
- γ-glutamyl transpeptidase
- IEBAM
- inborn errors of bile acid metabolism
- KBA
- ketonic bile acid
- NH
- neonatal hepatitis
- PFIC
- progressive familial intrahepatic cholestasis
- ROC
- receiver operating characteristic
- SLC25A13
- solute carrier family 25 member 13
- THBA
- tetrahydroxy bile acid
- TJP2
- tight junction protein 2
- TRHBA
- trihydroxy bile acid
- UBA
- unsaturated bile acid
- UTBA
- urinary total bile acid
- VPS33B
- vacuolar protein sorting 33B
This study was supported by Ministry of Science and Technology, Taiwan, Grant NSC-102-2628-B-002-025-MY3, and partially supported by National Taiwan University Hospital Grant NTUH 105-S3133.
The online version of this article (available at http://www.jlr.org) contains a supplement.
REFERENCES
- 1.Nathanson M. H., and Boyer J. L.. 1991. Mechanisms and regulation of bile secretion. Hepatology. 14: 551–566. [PubMed] [Google Scholar]
- 2.Trauner M., and Boyer J. L.. 2003. Bile salt transporters: molecular characterization, function, and regulation. Physiol. Rev. 83: 633–671. [DOI] [PubMed] [Google Scholar]
- 3.Halilbasic E., Claudel T., and Trauner M.. 2013. Bile acid transporters and regulatory nuclear receptors in the liver and beyond. J. Hepatol. 58: 155–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hofmann A. F. 2002. Cholestatic liver disease: pathophysiology and therapeutic options. Liver. 22(Suppl 2): 14–19. [DOI] [PubMed] [Google Scholar]
- 5.Hofmann A. F. 1999. The continuing importance of bile acids in liver and intestinal disease. Arch. Intern. Med. 159: 2647–2658. [DOI] [PubMed] [Google Scholar]
- 6.Alnouti Y. 2009. Bile acid sulfation: a pathway of bile acid elimination and detoxification. Toxicol. Sci. 108: 225–246. [DOI] [PubMed] [Google Scholar]
- 7.Bezerra J. A., and Balistreri W. F.. 2001. Cholestatic syndromes of infancy and childhood. Semin. Gastrointest. Dis. 12: 54–65. [PubMed] [Google Scholar]
- 8.Nittono H., Takei H., Unno A., Kimura A., Shimizu T., Kurosawa T., Tohma M., and Une M.. 2009. Diagnostic determination system for high-risk screening for inborn errors of bile acid metabolism based on an analysis of urinary bile acids using gas chromatography-mass spectrometry: results for 10 years in Japan. Pediatr. Int. 51: 535–543. [Erratum. 2009. Pediatr. Int. 51: 851.] [DOI] [PubMed] [Google Scholar]
- 9.Cuperus F. J., Claudel T., Gautherot J., Halilbasic E., and Trauner M.. 2014. The role of canalicular ABC transporters in cholestasis. Drug Metab. Dispos. 42: 546–560. [DOI] [PubMed] [Google Scholar]
- 10.Pawlikowska L., Strautnieks S., Jankowska I., Czubkowski P., Emerick K., Antoniou A., Wanty C., Fischler B., Jacquemin E., Wali S., et al. . 2010. Differences in presentation and progression between severe FIC1 and BSEP deficiencies. J. Hepatol. 53: 170–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Karlsen T. H., Lammert F., and Thompson R. J.. 2015. Genetics of liver disease: from pathophysiology to clinical practice. J. Hepatol. 62: S6–S14. [DOI] [PubMed] [Google Scholar]
- 12.Wang R., Salem M., Yousef I. M., Tuchweber B., Lam P., Childs S. J., Helgason C. D., Ackerley C., Phillips M. J., and Ling V.. 2001. Targeted inactivation of sister of P-glycoprotein gene (spgp) in mice results in nonprogressive but persistent intrahepatic cholestasis. Proc. Natl. Acad. Sci. USA. 98: 2011–2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Perwaiz S., Forrest D., Mignault D., Tuchweber B., Phillip M. J., Wang R., Ling V., and Yousef I. M.. 2003. Appearance of atypical 3 alpha,6 beta,7 beta,12 alpha-tetrahydroxy-5 beta-cholan-24-oic acid in spgp knockout mice. J. Lipid Res. 44: 494–502. [DOI] [PubMed] [Google Scholar]
- 14.Nakagawa M., and Setchell K. D.. 1990. Bile acid metabolism in early life: studies of amniotic fluid. J. Lipid Res. 31: 1089–1098. [PubMed] [Google Scholar]
- 15.Kimura A., Yamakawa R., Ushijima K., Fujisawa T., Kuriya N., Kato H., Inokuchi T., Mahara R., Kurosawa T., and Tohma M.. 1994. Fetal bile acid metabolism during infancy: analysis of 1 beta-hydroxylated bile acids in urine, meconium and feces. Hepatology. 20: 819–824. [DOI] [PubMed] [Google Scholar]
- 16.Yousef I. M., Perwaiz S., Lamireau T., and Tuchweber B.. 2003. Urinary bile acid profile in children with inborn errors of bile acid metabolism and chronic cholestasis; screening technique using electrospray tandem mass-spectrometry (ES/MS/MS). Med. Sci. Monit. 9: MT21–MT31. [PubMed] [Google Scholar]
- 17.Lu F. T., Wu J. F., Hsu H. Y., Ni Y. H., Chang M. H., Chao C. I., and Chen H. L.. 2014. gamma-Glutamyl transpeptidase level as a screening marker among diverse etiologies of infantile intrahepatic cholestasis. J. Pediatr. Gastroenterol. Nutr. 59: 695–701. [DOI] [PubMed] [Google Scholar]
- 18.Kimura A., Mahara R., Inoue T., Nomura Y., Murai T., Kurosawa T., Tohma M., Noguchi K., Hoshiyama A., Fujisawa T., et al. . 1999. Profile of urinary bile acids in infants and children: developmental pattern of excretion of unsaturated ketonic bile acids and 7beta-hydroxylated bile acids. Pediatr. Res. 45: 603–609. [DOI] [PubMed] [Google Scholar]
- 19.Mizuochi T., Kimura A., Ueki I., Takahashi T., Hashimoto T., Takao A., Seki Y., Takei H., Nittono H., Kurosawa T., et al. . 2010. Molecular genetic and bile acid profiles in two Japanese patients with 3beta-hydroxy-DELTA5-C27-steroid dehydrogenase/isomerase deficiency. Pediatr. Res. 68: 258–263. [DOI] [PubMed] [Google Scholar]
- 20.Chen H. L., Liu Y. J., Su Y. N., Wang N. Y., Wu S. H., Ni Y. H., Hsu H. Y., Wu T. C., and Chang M. H.. 2008. Diagnosis of BSEP/ABCB11 mutations in Asian patients with cholestasis using denaturing high performance liquid chromatography. J. Pediatr. 153: 825–832. [DOI] [PubMed] [Google Scholar]
- 21.Shoda J., Tanaka N., Osuga T., Matsuura K., and Miyazaki H.. 1990. Altered bile acid metabolism in liver disease: concurrent occurrence of C-1 and C-6 hydroxylated bile acid metabolites and their preferential excretion into urine. J. Lipid Res. 31: 249–259. [PubMed] [Google Scholar]
- 22.Balistreri W. F., Heubi J. E., and Suchy F. J.. 1983. Immaturity of the enterohepatic circulation in early life: factors predisposing to “physiologic” maldigestion and cholestasis. J. Pediatr. Gastroenterol. Nutr. 2: 346–354. [PubMed] [Google Scholar]
- 23.Obinata K., Nittono H., Yabuta K., Mahara R., and Tohma M.. 1992. 1 beta-hydroxylated bile acids in the urine of healthy neonates. J. Pediatr. Gastroenterol. Nutr. 15: 1–5. [DOI] [PubMed] [Google Scholar]
- 24.Kimura A., Mahara R., Tohma M., Ushijima K., Yuge K., Ono E., and Yamashita F.. 1989. Unusual 1 beta-hydroxylated bile acids in children with a paucity in interlobular bile ducts. Clin. Chim. Acta. 185: 215–217. [DOI] [PubMed] [Google Scholar]
- 25.Bremmelgaard A., and Sjovall J.. 1979. Bile acid profiles in urine of patients with liver diseases. Eur. J. Clin. Invest. 9: 341–348. [DOI] [PubMed] [Google Scholar]
- 26.de Wildt S. N., Kearns G. L., Leeder J. S., and van den Anker J. N.. 1999. Cytochrome P450 3A: ontogeny and drug disposition. Clin. Pharmacokinet. 37: 485–505. [DOI] [PubMed] [Google Scholar]
- 27.Hrycay E., Forrest D., Liu L., Wang R., Tai J., Deo A., Ling V., and Bandiera S.. 2014. Hepatic bile acid metabolism and expression of cytochrome P450 and related enzymes are altered in Bsep (-/-) mice. Mol. Cell. Biochem. 389: 119–132. [DOI] [PubMed] [Google Scholar]
- 28.Furster C., and Wikvall K.. 1999. Identification of CYP3A4 as the major enzyme responsible for 25-hydroxylation of 5beta-cholestane-3alpha,7alpha,12alpha-triol in human liver microsomes. Biochim. Biophys. Acta. 1437: 46–52. [DOI] [PubMed] [Google Scholar]
- 29.Bodin K., Lindbom U., and Diczfalusy U.. 2005. Novel pathways of bile acid metabolism involving CYP3A4. Biochim. Biophys. Acta. 1687: 84–93. [DOI] [PubMed] [Google Scholar]
- 30.Setchell K. D., and Heubi J. E.. 2006. Defects in bile acid biosynthesis–diagnosis and treatment. J. Pediatr. Gastroenterol. Nutr. 43(Suppl 1): S17–S22. [DOI] [PubMed] [Google Scholar]
- 31.Simko V., Michael S., and Kelley R. E.. 1987. Predictive value of random sample urine bile acids corrected by creatinine in liver disease. Hepatology. 7: 115–121. [DOI] [PubMed] [Google Scholar]
- 32.Bathena S. P., Thakare R., Gautam N., Mukherjee S., Olivera M., Meza J., and Alnouti Y.. 2015. Urinary bile acids as biomarkers for liver diseases I. Stability of the baseline profile in healthy subjects. Toxicol. Sci. 143: 296–307. [DOI] [PubMed] [Google Scholar]
- 33.Schneider J. J., and Bhacca N. S.. 1966. 1-beta-Hydroxylation of 3-alpha,20-beta,21-tetrahydroxy 5-beta-pregnan-11-one and of other 5-beta-steroids in a man and by surviving liver slices of the guinea pig. J. Biol. Chem. 241: 5313–5324. [PubMed] [Google Scholar]
- 34.Kimura A., Ushijima K., Yamakawa R., Inokuchi T., Kage M., Mahara R., and Tohma M.. 1992. Large amounts of 1 beta-hydroxylated bile acids in urine during taurine therapy. Kurume Med. J. 39: 105–111. [DOI] [PubMed] [Google Scholar]
- 35.Back P. 1982. Phenobarbital-induced alterations of bile acid metabolism in cases of intrahepatic cholestasis. Klin. Wochenschr. 60: 541–549. [DOI] [PubMed] [Google Scholar]
- 36.Kimura A., Ushijima K., Suzuki M., Tohma M., Inokuchi T., and Kato H.. 1995. Profile of urinary bile acids in familial intrahepatic cholestasis with Coombs’ negative haemolytic anaemia. Acta Paediatr. 84: 1119–1124. [DOI] [PubMed] [Google Scholar]
- 37.Mizuochi T., Kimura A., Tanaka A., Muto A., Nittono H., Seki Y., Takahashi T., Kurosawa T., Kage M., Takikawa H., et al. . 2012. Characterization of urinary bile acids in a pediatric BRIC-1 patient: effect of rifampicin treatment. Clin. Chim. Acta. 413: 1301–1304. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.