Abstract
Background
Vascular complications are a major concern for patients with diabetes. Endothelial cells (ECs) play a key role in vascular function. MicroRNAs (miRNAs) have been shown to play an important role in mediating EC function; miRNAs are vulnerable to hyperglycemic conditions. Previous reports verified that Fas apoptotic inhibitory molecule 2 (FAIM2) can inhibit cell apoptosis through repressing the FAS-associated death domain protein (FADD) pathway. This current study was designed to explore the potential involvement of miR-3202 in the pathogenesis of ECs in high-glucose conditions.
Material/Methods
The aim of this study was to investigate the role of miR-3202 in regulating hyperglycemia-induced ECs by targeting FAIM2. The endothelial cell line H5V was cultured in a high-glucose condition to induce damage to FAIM2 expression in ECs; mimic and inhibition of miR-3202 were used to enhance and depress miR-3202’s function to explore its function on FAIM2.
Results
Our study showed that FAIM2 was inhibited by high-glucose conditions, and miRNA-3202 was induced by high-glucose conditions. FAIM2 was identified as the target gene of miRNA-3202; luciferase reporter assays confirmed that FAIM2 was downregulated by miR-3202 directly, that is, miR-3202 can upregulate Fas/FADD through inhibiting FAIM2.
Conclusions
MiR-3202 can promote EC apoptosis in hyperglycemic conditions, which demonstrated that EC apoptosis induced by high-glucose conditions partly depends on miR-3202 targeting FAIM2.
MeSH Keywords: Apoptosis, Diabetic Angiopathies, MicroRNAs
Background
Nowadays diabetes is a major health problem. A report that collected and studied diabetes data from 130 countries in 2013 showed that 382 million people had diabetes, and the number is expected to rise to 592 million by 2035 [1]. Different types of complications, such as retinopathy, nephropathy, or diabetic foot syndrome (DFS), are caused by prolonged hyperglycemia and are the main harms and chief contributors to death of diabetic patients [2]. Enlarged blood vessels and microvascular damage are major pathological changes and the pathological mechanism of many diabetes-related complications [3]. Endothelial cells (ECs) are the first barrier that ensures the integrity of the vascular endothelium, playing an important role in the function of blood vessels. Abnormal changes in EC function can play a key role in the development of diabetic complications. Hyperglycemia is considered a significant cause of vascular endothelium damage, which is a major contributor to the diabetes-related complications process [2].
MiRNAs are non-coding small RNA molecules, which participate in various pathophysiological regulation processes. They also play a vital role in EC development and function. For example, miR-125b, miR-146a-5p, and miR-29a-3p are involved in impaired endothelial function and altered pro-inflammatory gene expression, including nuclear factor-B (NF-B) subunit p65 [4]. At the same time, mRNA expression and function are susceptible to environmental factors, especially hyperglycemic conditions, which usually further result in disease complications. In addition, platelet and plasma miR-144 and miR-223 have been shown to be significantly altered in type 2 diabetes mellitus (T2DM) patients [5]; changes in the expression of miR-155 may play a role in the pathogenesis of diabetes-related complications [6]. Studies have shown that miR-3202 is downregulated in patients with early-onset post-stroke depression [7], that its expression is upregulated by radon in the lung BEAS-2B cell line [8], and that it is upregulated as secreted miRNAs isolated from LNCaP prostate cancer cells compared with normal human prostate epithelial cells (PrECs) [9]. However, there are few published studies involving miR-3202 function. To explore the role of miR-3202 we used computer software to predict the actions of its target gene, Fas apoptotic inhibitory molecule 2 (FAIM2), in order to study how miR-3202 might affect cell function through FAIM2.
The Fas-associated death domain protein (FADD) interacts with the death domain of Fas and initiates apoptosis [10]. It has been demonstrated that Fas plays an important role in epigenetic regulation of gene expression and function in a study of chronic diabetic polyneuropathy in mice [11]. FAIM2, also called neural membrane protein 35 (NMP35) or LFG (lifeguard), is known to codify an anti-apoptotic protein highly expressed in the brain that antagonizes the Fas pathway [12]. It is also known as an obesity-related gene [13]. However, the mechanisms by which FAIM2 is involved in vascular endothelium in hyperglycemia are not well understood. Soluble Fas (sFas) levels have been positively associated with plasma glucose levels recorded at hospital admission [14]; however, whether a relationship exists between Fas levels and glycometabolism risk factors among diabetic patients remains unclear. This study aimed to explore how miR-3202 functions in high-glucose induced H5V cells by targeting the FAIM2/Fas pathway.
Material and Methods
Cell lines and cell culture
H5V cells, murine heart endothelial immortalized cells (kindly gifted by Fudan Medical University, Shanghai, China), were grown to 80% confluence in DMEM containing fetal calf serum 10% (v/v) and penicillin/streptomycin 100 U/mL/100 lg/mL, then the cells were washed three times with PBS and incubated as described later.
miRNA overexpression
All oligonucleotides were purchased from GenePharma (Shanghai, China). Oligonucleotides used in this study: Ctttaatgattggagtc-miR-3202-F; Atggaagggagaagagc-miR-3202-R; Gctcgtctctgatctcgcg-Fas-F; Caacccgcgggagaag-Fas-R; Gtccgcggcgccgccag-FADD-F; Caggtctcgccgcttg-FADD-R. H5V cells (1×105 per well) were cultured in six-well plates the day before transfection. According to the manufacturer’s instructions, oligonucleotides were transfected into H5V cells by Lipofectamine 2000 (Invitrogen) at a final concentration of 50 nM/mimics or 100 nM/inhibitors. Six hours later, the medium was changed, and cells continued to be cultured for 48 or 72 hours. Samples were collected for real-time PCR and Western blot assays.
MTT colorimetric analysis method
H5V cells in logarithmic growth phase were prepared for cell transfection with miRNA-3202 mimic or its non-specific control (GenePharma, Shanghai, China). Four hours before the end of transfection, 20 μL MTT (5 mg/mL, PBS) was added to each well, and the culture continued for another four hours. Then the cells were removed from the culture plate and centrifuged at 1,500 rpm for five minutes. The supernatant was discarded and 200 uL DMSO added; oscillation blending was used to make formazan particles completely dissolve; OD value was measured at 560 nm wavelength.
Western blot
H5V cells were lysed in lysis buffer. Total protein was separated by SDS-PAGE in 12% acrylamide gels and electroblotted onto polyvinylidene difluoride membranes. The membrane was blocked and incubated with primary antibodies. It was then incubated with horseradish peroxidase-conjugated anti-rabbit or anti-mouse secondary immunoglobulins. The signal was then detected by enhanced chemiluminescence (Pierce, Rockford, IL, USA), according to the manufacturer’s protocol.
RNA isolation and analysis
Total RNA (miRNA and mRNA) was extracted from H5V cells using TRIzol Reagent (Invitrogen). Then 2 μg of total RNA was reverse transcribed using the miRNA cDNA synthesis kit or PrimeScript®RT reagent kit (TaKaRa, Dalian, China). Real-time PCR of mRNA or miRNA was performed using SYBR Green (TaKaRa) using the 7300 Sequence Detection System (Applied Biosystems, Foster City, CA, USA). All hsa-miRNA primers were synthesized by RiboBio (Guangzhou, China). Then U6 small nuclear RNA and β-actin were used for normalization. The fold-change in miRNA and mRNA levels in the sample group (T) compared to control group (NT) was calculated using the 2−ΔΔCT method according to the manufacturer’s protocol.
Immunocytochemistry
H5V cells were fixed for 20 minutes using PBS containing 4% paraformaldehyde (Guangzhou, China), and then blocked for 30 minutes using blocking buffer (RiboBio). Cells were incubated with primary antibody rat anti-mouse FAIM2 (Abcam) at 4°C overnight, then incubated with fluorescently-tagged secondary antibodies (Cell Signaling Technology) for 30 minutes at 37°C. Nuclei were stained using 4′, 6-diamidino-2-phenylindole (DAPI) (Sigma-Aldrich, St. Louis, MO, USA). Images were captured by a 50i Nikon fluorescence microscope (Nikon, Melville, NY, USA), and processed with Adobe Photoshop CS4 software (San Jose, CA, USA). The number of Fas, FADD, or Caspase3 positive cells was determined by the number of DAPI-stained positive cells.
Luciferase reporter assay
H5V cells were seeded at a suitable density in 12-well plates and cultured until reaching a sub-confluence. The cells were then transiently co-transfected with 50 nM pre-miR-3202 or preNC and with luciferase reporter plasmid (1 ug/well) containing 3′UTR sequences for FAIM2 (GeneCopoeia, Inc., Rockville, MD, USA), using PureFection transfection reagent. The mutant 3′UTR of FAIM2 had a mutated sequence in the miR-3202-complementary region. Firefly and Renilla luciferase activities were measured after 48 hours of transfection using the Dual-Luciferase Reporter Assay System (Promega, Madison, WI, USA) according to the manufacturer’s instructions. Firefly luciferase activity was normalized to Renilla luciferase activity, and data of each group are presented as mean ±SD (standard deviation) of three experiments performed in duplicate and compared with a ratio of cells transfected with preNC independently.
Statistical analysis
All qRT-PCR results are expressed as the mean ±SE. Comparisons between data sets with two groups were evaluated using an unpaired Student’s t test. A p value of ≤0.05 was considered statistically significant. The correlation plots were performed using GraphPad Prism.
Results
High glucose inhibited miR-3202 expression
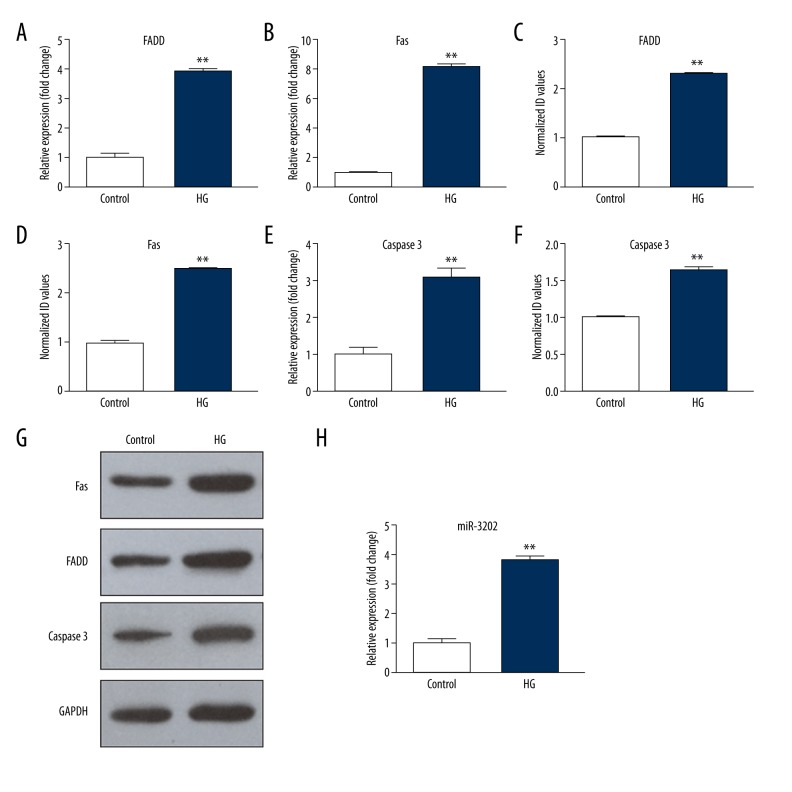
We first validated the miR-3202 expression of ECs exposed to high glucose. H5V cells were cultured in normal glucose (5 mM) and high glucose (25 mM). Taking normal glucose as the control, the H5V cells were cultured for eight hours and miRNAs were then extracted to determine the expression level through quantitative PCR. The result showed that miR-3202 was upregulated in high-glucose medium compared with the normal glucose medium (Figure 1H).
Figure 1.
FADD, Fas, and miR-3202 were induced by high glucose. HG – high glucose (25 mM). (A, B, E) The mRNA expression; (C, D, F, G) The protein expression. (H) the miR-3202 level. Data were presented as mean ±SD. ** p<0.05 vs. the control. HG – high glucose (25 mM).
FADD and Fas gene was induced by high glucose
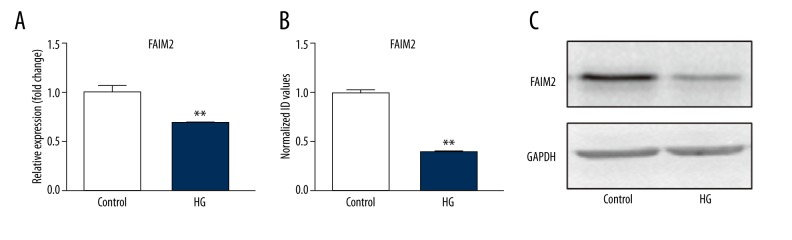
To determine how hyperglycemic stress affects FADD and Fas, we exposed H5V cells to high-glucose (25 mM) conditions, with normal glucose (5 mM) culture medium used as the control. Western blot was used to detect FAIM2, FADD, and Fas protein expression in cells. Quantitative PCR was used to detect FAIM2, FADD, and Fas mRNAs expression. We can see from Figure 1 and Figure 2 that when cells were stimulated by a high-glucose medium, FAIM2 was downregulated; meanwhile FADD and Fas were significantly increased, compared with normal culture medium; the differences were statistically significant (p<0.05).
Figure 2.
FAIM2 was inhibited by high glucose. HG – high glucose (25 mM). (A) The mRNA expression; (B, C) The protein expression. Data were presented as mean ±SD. ** p<0.05 vs. the control. HG – high glucose (25 mM).
miR-3202 triggered FADD and Fas by targeting FAIM2
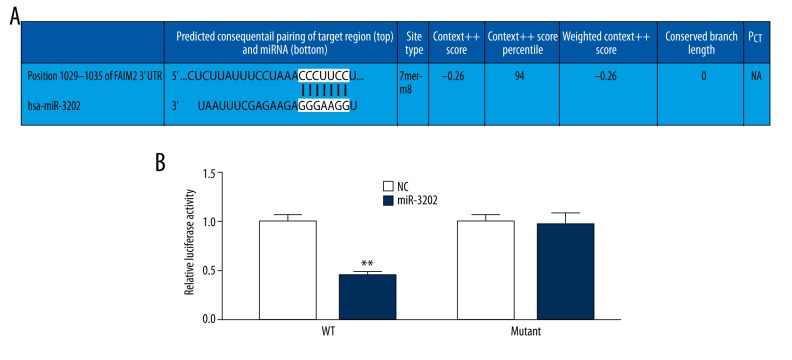
To determine the function of miR-3202, we used computational algorithms (Targetscan and Miranda) to identify the potential target gene of miR-3202. It was predicted that FAIM2 was miR-3202’s potential target gene (Figure 3A). To verify the real relationship between them, we performed luciferase reporter assays. The full-length FAIM2 was cloned at the downstream side of the firefly luciferase gene and co-transfected with miR-3202 mimics or scrambled oligonucleotide controls. Luciferase activity was measured at 48 hours. The results showed that the luciferase expression was decreased in H5V cells that were co-transfected with miR-3202 and the wild-type FAIM2 3′-UTR, compared to the controls. However, no decrease could be detected in cells co-transfected with miR-3202 and the mutated FAIM2 3′-UTR (Figure 3B). These results suggest that miR-3202 can directly target FAIM2.
Figure 3.
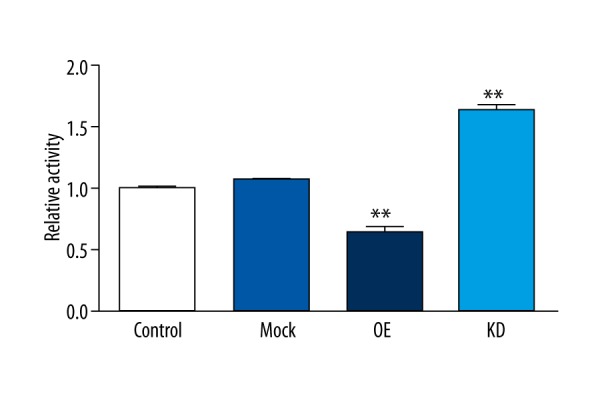
(A, B) The luciferase activity of NC and miR-3202. The luciferase expression was decreased in H5V cells that were co-transfected with miR-3202 and the wild-type FAIM2 3′UTR, compared to controls. NC – negative control. Data were presented as mean ±SD. ** p<0.05 vs. the NC.
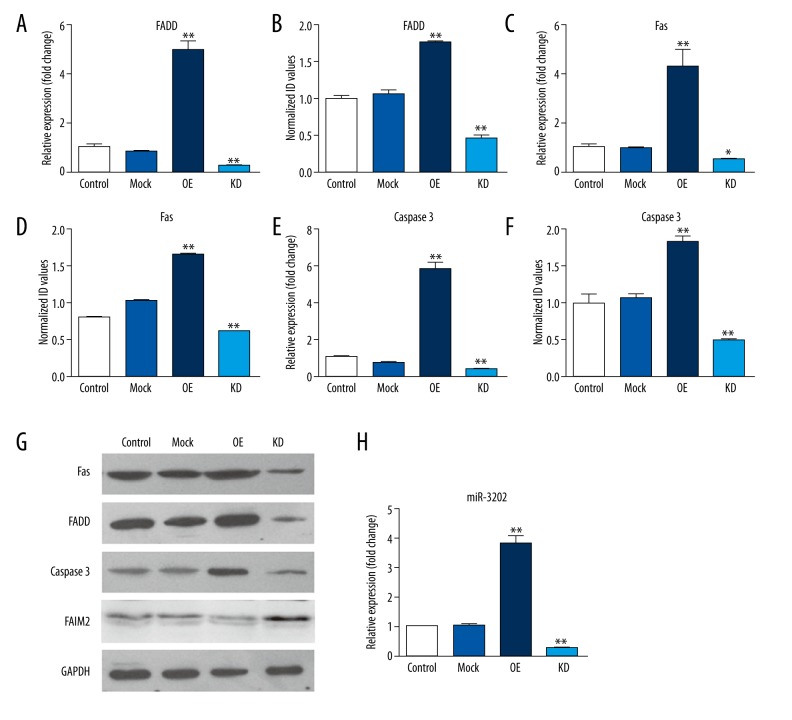
Next, to further determine the degree to which miR-3202 inhibits FAIM2 expression, we examined the expression of FAIM2 mRNA after transfection with miR-3202 mimics or scrambled oligonucleotide controls in H5V cells. MiR-3202 mimics’ transfection efficiency can be seen in Figure 4H; the Western blotting results showed that the expression of FAIM2 in the mimic group was lower than in the control group (Figure 4G); the results also verified the effect of miR-3202 on Fas or FADD expression (Figure 4G); the overexpression of miR-3202 effectively increased Fas and FADD protein levels (Figure 4G). The quantitative PCR showed similar results (Figure 4). These results provide evidence that miR-3202 directly recognizes the 3′-UTR of FAIM2 mRNA and inhibits FAIM2 translation, and increases Fas and FADD expression.
Figure 4.
Overexpression of miR-3202 in H5V cells increased the mRNA and protein expression of FADD, Fas, and Caspase3 in H5V cells. (A, C, E) The mRNA expression; (B, D, F, G) The protein expression; (H) Transfection efficiency of miR-3202 in H5V cells. Data were presented as mean ±SD. ** p<0.05 vs. the mock group.
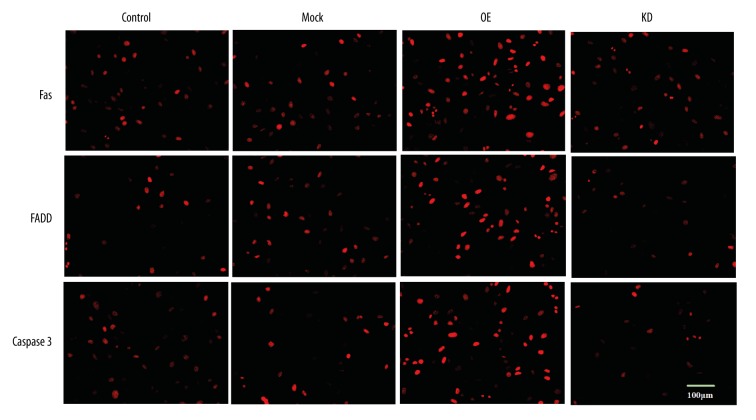
Fas/FADD is an apoptosis-related pathway, and FAIM2 can restrict the pathway. Therefore, we assumed that miR-3202 might regulate that pathway by targeting FAIM2. We then upregulated miR-3202 levels via miR-3202 mimics in H5V cells. Western blotting results demonstrated that overexpression of miR-3202 decreased the FAIM2 levels (Figure 4), which reduced the inhibition of Fas or FADD, that then increased the expression of Fas and FADD. From these results we can see that Fas and FADD were increased at the level of mRNA and protein; similar results were verified by immunocytochemistry assays (Figure 5).
Figure 5.
MiR-3202 increased FADD, Fas, and Caspase3, confirmed by immunocytochemistry assay. The level of fluorescence of the cells transfected with miR-3202 was higher than that of the control. ** p<0.05 vs. the mock group.
Subsequently, we treated H5V cells with miR-3202 or scramble control. At 24 hours, we transfected them with FAIM2-encoding vector (without an endogenous 3′-UTR) or mock vector (Figure 4H). With the expression of FAIM2 inhibited by miR-3202 mimic, an adverse alteration was observed in the expression levels of its downstream proteins Fas or FADD, so that the expressions of Fas or FADD were increased by adding miR-3202 in H5V cells (Figure 4). All these results suggested that miR-3202 could stimulate the Fas/FADD pathways by targeting FAIM2.
Overexpression of miR-3202 in H5V promoted H5V apoptosis
To find the biological effect of the miR-3202-driven repression of FAIM2 to H5V cells, we conducted an experiment to determine if increasing or decreasing the expression of miR-3202 would have an impact on cell proliferation in H5V cells. We transfected H5V cells with miR-3202 mimics, miR-3202 inhibitors, or their scrambled oligonucleotide controls, respectively. Then 24 hours later, the CCK8 proliferation assay showed that the increased expression of miR-3202 in H5V cells inhibited the cell proliferation compared with the control cells, while the decreased expression of miR-3202 promoted the proliferation (Figure 6), which was in accordance with Western blotting results for Caspase3 (Figure 4).
Figure 6.
MiR-3202 inhibited the proliferation of VEC H5V by targeting FAIM2. The proliferation rate of H5V cells was decreased when treated with miR-3202 mimic compared with controls. ** p<0.05 vs. the mock group.
These results demonstrated that Fas or FADD expression was induced by high-glucose conditions, partly dependent on miR-3202, which was also increased by high-glucose conditions. In addition, this effect promoted apoptosis of H5V cells, which are involved in vasculopathy induced by hyperglycemia. For this purpose, H5V cells were transfected with mock vector, scrambled oligonucleotide controls, FAIM2-encoding vector + miR-3202 or mock vector + scrambled oligonucleotide controls, respectively. The results showed that increased expression of miR-3202 promoted H5V cell apoptosis by repressing FAIM2 and increasing Fas/FADD and Caspase3 expression in high-glucose conditions (Figure 4).
Discussion
The pathogenesis of diabetic vascular complications is undoubtedly multifactorial. Nevertheless, impaired angiogenesis is the major cause. Angiogenesis is a multi-stage process involving the endothelium, growth factors and their inhibitors, cytokines, endothelial progenitor cells (EPCs), and enzymes [15]. Insufficient angiogenesis contributes to non-healing ulcers, which accounts for the majority of non-traumatic lower limb amputations.
A hyperglycemic environment is destructive to cells, particularly for T2DM patients; hyperglycemia-induced oxidative stress has been shown to induce apoptosis in glial cells [19]. High-glucose-induced increases in production of reactive oxygen/nitrogen species (ROS/RNS) is recognized as a major cause of clinical complications associated with diabetes, causing a series of cells to be damaged [20], including ECs [14]. It also results in hypercontractility of vascular smooth muscle [21], increases DNA damage [22], and induces human brain microvascular EC apoptosis [23]. Beyond that, hyperglycemia can lead to miRNA profiling of different tissues and organs to change abnormally [16–18,24] including miR-3202 [7]. In this study, we exposed H5V to high-glucose conditions to mimic a diabetic hyperglycemic environment; the results showed that miR-3202 was upregulated.
The FADD/Fas signal pathway plays a critical role in the apoptosis processes [25], it also plays an important role in vascular endothelial disease [26] and has been implicated in T2DM and cardiovascular disease [27]. It was suggested that sFas levels appeared to be associated with overweight, hyperlipedema, and clusters of metabolic risk factors among men [28]. It is not surprising that aberrant gain of FADD/Fas signaling components has been directly linked to vascular endothelial cell apoptosis. Our study showed that FADD/Fas was upregulated by a high-glucose environment in H5V cells.
The present study showed that miR-3202 levels were elevated in high-glucose conditions. We could not find published reports related to this effect; however, several articles have reported upregulated expression induced by radon [8,29,30] (which is a radioactive element in the form of a gas). One report found decreased expression of miR-3202 in patients with early-onset post-stroke depression [7]. To some extent, high glucose and radon may have a similar oxidative stress effect, and perhaps it was because of an oxidative stress effect that miR-3202 was upregulated. Our study first demonstrated that miR-3202 inhibited FAIM2 by directly binding 3′UTR of FAIM2’s promoter. A previous study demonstrated that FAIM2 inhibited Fas-mediated apoptosis [31], which was also verified by our study. Thus, miR-3202 mediating FADD/Fas by targeting FAIM2 may involve vascular damage caused by hyperglycemia, and the method of inhibiting miR-3202 may provide some basis for a novel therapeutic approach for patients with vasculopathy in the diabetic foot.
However, a previous study showed FADD regulated Notch signaling during muscle regeneration [25], and Notch signaling plays a significant role in cell differentiation, primarily determining and regulating cell survival [32]. In our study we did not explore Notch signaling. So it is probable that multiply factors are involved in EC apoptosis and cell damage, including damage induced by hyperglycemia, which is itself a complex process.
One limitation of our study is that it was an in vitro study, which did not provide a chance to observe the whole process of vascular impairement in hyperglycemic conditions, or explore morphology-related events. Further study using diabetic animal models should be done to explore miR-3202’s function on ECs and angiogenesis.
Conclusions
Our findings revealed that miR-3202 can promote EC apoptosis in high-glucose conditions, which demonstrated that EC apoptosis induced by high glucose partly depends on miR-3202 targeting FAIM2. In addition, our findings provided some basis for consideration of novel therapies for diabetic vascular complications.
Footnotes
Source of support: This study was financially supported by Shuofei Yang, MD, PhD, Department of General Surgery, Jinling Hospital
Competing interests
We declare that we have no conflicts of interest.
References
- 1.Guariguata L, Whiting DR, Hambleton I, et al. Global estimates of diabetes prevalence for 2013 and projections for 2035. Diabetes Res Clin Pract. 2014;103:137–49. doi: 10.1016/j.diabres.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 2.Silambarasan M, Tan JR, Karolina DS, et al. MicroRNAs in hyperglycemia induced endothelial cell dysfunction. Int J Mol Sci. 2016;17:518–40. doi: 10.3390/ijms17040518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Klein R. Hyperglycemia and microvascular and macrovascular disease in diabetes. Diabetes Care. 1995;18:258–68. doi: 10.2337/diacare.18.2.258. [DOI] [PubMed] [Google Scholar]
- 4.Zhong X, Liao Y, Chen L, et al. The MicroRNAs in the pathogenesis of metabolic memory. Endocrinology. 2015;156:3157–68. doi: 10.1210/en.2015-1063. [DOI] [PubMed] [Google Scholar]
- 5.Yang S, Zhao J, Chen Y, Lei M. Biomarkers associated with ischemic stroke in diabetes mellitus patients. Cardiovasc Toxicol. 2016;16:213–22. doi: 10.1007/s12012-015-9329-8. [DOI] [PubMed] [Google Scholar]
- 6.Khamaneh AM, Alipour MR, Sheikhzadeh Hesari F, Ghadiri Soufi F. A signature of microRNA-155 in the pathogenesis of diabetic complications. J Physiol Biochem. 2015;71:301–9. doi: 10.1007/s13105-015-0413-0. [DOI] [PubMed] [Google Scholar]
- 7.Zhang Y, Cheng L, Chen Y, et al. Clinical predictor and circulating microRNA profile expression in patients with early onset post-stroke depression. J Affect Disord. 2016;193:51–58. doi: 10.1016/j.jad.2015.12.061. [DOI] [PubMed] [Google Scholar]
- 8.Cui FM, Li JX, Chen Q, et al. Radon-induced alterations in micro-RNA expression profiles in transformed BEAS2B cells. J Toxicol Environ Health A. 2013;76:107–19. doi: 10.1080/15287394.2013.738176. [DOI] [PubMed] [Google Scholar]
- 9.Korzeniewski N, Tosev G, Pahernik S, et al. Identification of cell-free microRNAs in the urine of patients with prostate cancer. Urol Oncol. 2015;33:16e17–22. doi: 10.1016/j.urolonc.2014.09.015. [DOI] [PubMed] [Google Scholar]
- 10.Xu YR, Dong HS, Yang WX. Regulators in the apoptotic pathway during spermatogenesis: Killers or guards? Gene. 2016;582:97–111. doi: 10.1016/j.gene.2016.02.007. [DOI] [PubMed] [Google Scholar]
- 11.Cheng C, Kobayashi M, Martinez JA, et al. Evidence for epigenetic regulation of gene expression and function in chronic experimental diabetic neuropathy. J Neuropathol Exp Neurol. 2015;74:804–17. doi: 10.1097/NEN.0000000000000219. [DOI] [PubMed] [Google Scholar]
- 12.Reich A, Spering C, Gertz K, et al. Fas/CD95 regulatory protein Faim2 is neuroprotective after transient brain ischemia. J Neurosci. 2011;31:225–33. doi: 10.1523/JNEUROSCI.2188-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wu L, Zhao X, Shen Y, et al. Promoter methylation of fas apoptotic inhibitory molecule 2 gene is associated with obesity and dyslipidaemia in Chinese children. Diab Vasc Dis Res. 2015;12:217–20. doi: 10.1177/1479164114565630. [DOI] [PubMed] [Google Scholar]
- 14.Chang J, Zhang G, Zhang L, et al. High admission glucose levels increase Fas apoptosis and mortality in patients with acute ST-elevation myocardial infarction: A prospective cohort study. Cardiovasc Diabetol. 2013;12:159–71. doi: 10.1186/1475-2840-12-171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McQuilling JP, Arenas-Herrera J, Childers C, et al. New alginate microcapsule system for angiogenic protein delivery and immunoisolation of islets for transplantation in the rat omentum pouch. Transplant Proc. 2011;43:3262–64. doi: 10.1016/j.transproceed.2011.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Costantino S, Paneni F, Luscher TF, Cosentino F. MicroRNA profiling unveils hyperglycaemic memory in the diabetic heart. Eur Heart J. 2016;37:572–76. doi: 10.1093/eurheartj/ehv599. [DOI] [PubMed] [Google Scholar]
- 17.Santovito D, De Nardis V, Marcantonio P, et al. Plasma exosome microRNA profiling unravels a new potential modulator of adiponectin pathway in diabetes: Effect of glycemic control. J Clin Endocrinol Metab. 2014;99:E1681–85. doi: 10.1210/jc.2013-3843. [DOI] [PubMed] [Google Scholar]
- 18.Peng J, Li X, Zhang D, et al. Hyperglycemia, p53, and mitochondrial pathway of apoptosis are involved in the susceptibility of diabetic models to ischemic acute kidney injury. Kidney Int. 2015;87:137–50. doi: 10.1038/ki.2014.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kumar P, Rao GN, Pal BB, Pal A. Hyperglycemia-induced oxidative stress induces apoptosis by inhibiting PI3-kinase/Akt and ERK1/2 MAPK mediated signaling pathway causing downregulation of 8-oxoG-DNA glycosylase levels in glial cells. Int J Biochem Cell Biol. 2014;53:302–19. doi: 10.1016/j.biocel.2014.05.038. [DOI] [PubMed] [Google Scholar]
- 20.Kumar P, Swain MM, Pal A. Hyperglycemia-induced inflammation caused down-regulation of 8-oxoG-DNA glycosylase levels in murine macrophages is mediated by oxidative-nitrosative stress-dependent pathways. Int J Biochem Cell Biol. 2016;73:82–98. doi: 10.1016/j.biocel.2016.02.006. [DOI] [PubMed] [Google Scholar]
- 21.Hien TT, Turczynska KM, Dahan D, et al. Elevated glucose levels promote contractile and cytoskeletal gene expression in vascular smooth muscle via Rho/protein kinase C and actin polymerization. J Biol Chem. 2016;291:3552–68. doi: 10.1074/jbc.M115.654384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xavier DJ, Takahashi P, Evangelista AF, et al. Assessment of DNA damage and mRNA/miRNA transcriptional expression profiles in hyperglycemic versus non-hyperglycemic patients with type 2 diabetes mellitus. Mutat Res. 2015;776:98–110. doi: 10.1016/j.mrfmmm.2015.01.016. [DOI] [PubMed] [Google Scholar]
- 23.Shao B, Bayraktutan U. Hyperglycaemia promotes human brain microvascular endothelial cell apoptosis via induction of protein kinase C-ssI and prooxidant enzyme NADPH oxidase. Redox Biol. 2014;2:694–701. doi: 10.1016/j.redox.2014.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Costantino S, Paneni F, Lüscher TF, Cosentino F. MicroRNA profiling unveils hyperglycaemic memory in the diabetic heart. Eur Heart J. 2016;37(6):572–76. doi: 10.1093/eurheartj/ehv599. [DOI] [PubMed] [Google Scholar]
- 25.Zhang R, Wang L, He L, et al. Fas-associated protein with death domain regulates notch signaling during muscle regeneration. Cells Tissues Organs. 2014;200:253–64. doi: 10.1159/000437258. [DOI] [PubMed] [Google Scholar]
- 26.Williams KP, Steinle JJ. Maintenance of beta-adrenergic receptor signaling can reduce Fas signaling in human retinal endothelial cells. Exp Eye Res. 2009;89:448–55. doi: 10.1016/j.exer.2009.04.015. [DOI] [PubMed] [Google Scholar]
- 27.Corella D, Sorli JV, Gonzalez JI, et al. Novel association of the obesity risk-allele near Fas Apoptotic Inhibitory Molecule 2 (FAIM2) gene with heart rate and study of its effects on myocardial infarction in diabetic participants of the PREDIMED trial. Cardiovasc Diabetol. 2014;13:5. doi: 10.1186/1475-2840-13-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tamakoshi A, Suzuki K, Lin Y, et al. Relationship of sFas with metabolic risk factors and their clusters. Eur J Clin Invest. 2010;40:527–33. doi: 10.1111/j.1365-2362.2010.02293.x. [DOI] [PubMed] [Google Scholar]
- 29.Fu Y, Yi Z, Li J, Li R. Deregulated microRNAs in CD4+ T cells from individuals with latent tuberculosis versus active tuberculosis. J Cell Mol Med. 2014;18:503–13. doi: 10.1111/jcmm.12205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Izzotti A, Pulliero A. The effects of environmental chemical carcinogens on the microRNA machinery. Int J Hyg Environ Health. 2014;217:601–27. doi: 10.1016/j.ijheh.2014.01.001. [DOI] [PubMed] [Google Scholar]
- 31.Besirli CG, Zheng Q-D, Reed DM, Zacks DN. ERK-mediated activation of Fas apoptotic inhibitory molecule 2 (Faim2) prevents apoptosis of 661W cells in a model of detachment-induced photoreceptor cell death. PLoS One. 2012;7:e46664. doi: 10.1371/journal.pone.0046664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kopan R, Ilagan MX. The canonical Notch signaling pathway: Unfolding the activation mechanism. Cell. 2009;137:216–33. doi: 10.1016/j.cell.2009.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]