Abstract
Aging with HIV poses unique and complex challenges, including avoidance of neurocognitive disorder. Our objective here is to identify the prevalence and predictors of successful cognitive aging (SCA) in a sample of older adults with HIV. One hundred three HIV-infected individuals aged 50 and older were recruited from the Modena HIV Metabolic Clinic in Italy. Participants were treated with combination antiretroviral therapy for at least 1 year and had suppressed plasma HIV viral load. SCA was defined as the absence of neurocognitive impairment (as defined by deficits in tasks of episodic learning, information processing speed, executive function, and motor skills) depression, and functional impairment (instrumental activities of daily living). In cross-sectional analyses, odds of SCA were assessed in relation to HIV-related clinical data, HIV-Associated Non-AIDS (HANA) conditions, multimorbidity (≥2HANA conditions), and frailty. A frailty index was calculated as the number of deficits present out of 37 health variables. SCA was identified in 38.8% of participants. Despite no differences in average chronologic age between groups, SCA participants had significantly fewer HANA conditions, a lower frailty index, and were less likely to have hypertension. In addition, hypertension (odds ratio [OR] = 0.40, p = .04), multimorbidity (OR = 0.35, p = .05), and frailty (OR = 0.64, p = .04) were significantly associated with odds of SCA. Frailty is associated with the likelihood of SCA in people living with HIV. This defines an opportunity to apply knowledge from geriatric population research to people aging with HIV to better appreciate the complexity of their health status.
Keywords: : neuropsychological assessment, AIDS dementia, depression, frail elderly, aging
Introduction
Due to the success of combined antiretroviral therapies (cART), many individuals with HIV can expect to live longer lives and the population of people aged 50 and older with HIV is growing.1 Despite increased longevity, people aging with HIV appear to be at greater risk for poor health outcomes compared with their uninfected peers, including HIV-associated non-AIDS (HANA) conditions such as cancer, cardiovascular disease, liver disease, and osteoporosis.1,3–5 People aging with HIV also appear to be at excess risk for neurocognitive impairment, a phenomenon that has been termed HIV-associated neurocognitive disorders (HAND).6–8
Compared with people of the same age without HIV, people living with HIV also appear to exhibit greater severity of frailty.9,10 Frailty is a state of increased vulnerability to adverse outcomes, commonly seen in older adults.11 People aging with HIV appear to exhibit vulnerability across multiple physiologic systems, evidenced by HANA conditions. Frailty has been proposed as a useful marker of overall biological health and aging in this context, especially among people with immune recovery and suppressed viral loads.9,12 As frailty is a holistic measure of health, it might be able to more sensitively discriminate risk among people aging with HIV than individual disease markers or comorbidity diagnoses.12 Although early cross-sectional data have associated frailty with vulnerability for multimorbidity and mortality among people with HIV,13 the relationship between frailty and cognitive aging has not yet been established.
The absence of cognitive impairment can be considered a measure of successful cognitive aging (SCA) and an important clinical outcome among older adults with HIV. SCA has been defined as aging without any subjective or objective cognitive impairment, depressive symptoms, or functional impairment.14,15 SCA prevalence estimates range between 19%14 and 32%15 among middle-aged and older adults with HIV. Among people aging with HIV, SCA is associated with better mental quality of life,14 medication adherence, and capacity to interact with health professionals.15 However, predictors of SCA are unknown, with one cross-sectional study identifying no relationship between odds of SCA and individual markers of HIV-disease severity, including current CD4 cell count and HIV RNA viral load, or with individual comorbidities, including diabetes or dyslipidemia.15
The objectives for the present study were to assess the prevalence of SCA in a cohort of people with HIV aged 50 years and older and to identify health factors associated with odds of SCA in this cohort, including whether SCA is associated with individual HANA condition diagnoses, multimorbidity, or frailty.
Materials and Methods
Participants
Participants were recruited from the Modena HIV Metabolic Clinic (MHMC) between July 1st and December 31st, 2013. The multidisciplinary MHMC, at the University of Modena and Reggio Emilia, Italy, was initiated in 2004 to assess metabolic changes among people with HIV and has been described elsewhere.16 The current study is a cross-sectional secondary analysis of existing data; criteria for inclusion in the present study were HIV-infected individuals aged 50 years or older, on cART for at least 1 year, with suppressed HIV viral load (<40copies/mL). Criteria for exclusion were inability to speak Italian fluently and the presence of acute psychotic disorders or severe neurological disease. All procedures followed were in accordance with the ethical standards of comitato Etico provinciale di Modena and with the Helsinki Declaration of 1975, as revised in 2000.
Successful cognitive aging
SCA was defined by the absence of depressive symptoms, cognitive impairment, and functional impairment.15,17 Assessments of these domains are described next.
Depressive symptoms assessment
Depressive symptoms were assessed by using the 20-item self-report Centre for Epidemiologic Studies Depression scale. Items ask how often in the past week the participant has experienced depressive symptoms, such as restless sleep, feelings of loneliness, and poor appetite. Higher scores on this measure indicate greater depressive symptoms; it is not focused on somatic symptoms that could overlap between depression and HIV infection itself.18 It has been validated in many cohorts, including people living with HIV.18,19 Participants with a score of 16 or above were identified as not achieving SCA; scores above this cut-point indicate the presence of many depressive symptoms and are found at a much higher rate in depressed populations than the general population.20
Neurocognitive assessment
Neurocognitive functioning was assessed with the Italian version (cultural translation) of a validated abbreviated neuropsychological battery.21 The battery took ∼25 min and was designed to assess the cognitive domains most frequently impacted by HIV-associated neurocognitive disorders, including episodic learning (Rey Auditory Verbal Learning test), information processing speed (Trail Making Test part A), executive functions (Trail Making Test part B), and motor skills (Non-Dominant grooved pegboard test).14 Cut-points were age- and education specific and based on Italian normative data.22 Impairment was defined as at least one deficit in the following:
• Rey Auditory Verbal Learning Test (<28 was considered a deficit and, thus, did not meet criteria for SCA),
• Non-Dominant Grooved Pegboard Test (1 standard deviation [SD] above the age-specific mean was considered a deficit and, thus, did not meet criteria for SCA), and the
• Trail Making Test parts A (≥94 was considered a deficit and, thus, did not meet criteria for SCA) and B (≥282 was considered a deficit and, thus, did not meet criteria for SCA).23–25
Functional assessment
Function was assessed with the Lawton-Brody Instrumental Activities of Daily Living (IADL) scale.26 Current difficulty in one or more of the eight domains was considered a functional impairment. IADL impairment was used, as it was considered to be a more sensitive measure than impairment in basic ADLs in this relatively young sample.27
HANA conditions
Current and nadir CD4+ T-cell lymphocyte counts were categorized into clinically relevant groups as follows: >500, 351–500, 101–350, ≤100. Eight HANA conditions were examined: cardiovascular disease, end-stage kidney disease, cancer, osteoporosis, hypertension, type 2 diabetes mellitus, liver cirrhosis, and chronic obstructive pulmonary disease. Cardiovascular disease was identified by clinical diagnosis with history of myocardial infarction, stroke, revascularization, or peripheral artery disease. End-stage kidney disease was clinically diagnosed with estimated glomerular filtration rate <60 mL/min as measured with Modification of Diet in Renal Disease [MDRD] study equation.28 Cancer was identified as clinical diagnosis with biopsy confirmation. Osteoporosis was defined by dual-energy x-ray absorptiometry T- or Z-score less than −2.5 or history of fragility fracture. Hypertension was identified by clinical diagnosis with blood pressure measured twice ≥140 systolic or ≥90 disatolic mmHg or taking antihypertensive medicine. Type 2 diabetes mellitus was defined as clinical diagnosis with measured fasting glucose ≥126 or oral glucose tolerance test >200 or on treatment. Liver cirrhosis was defined by FIB-4 score >3.25.29 Chronic obstructive pulmonary disease was defined by FEV1/FVC ratio <0.7. Multimorbidity was defined as the presence of two or more of these conditions.
Frailty index
A frailty index was calculated based on the established deficit accumulation approach that has been described in depth elsewhere.30 Briefly, the frailty index quantifies overall physiologic vulnerability of an individual or organism.9,31,32 This model has shown that the combination of many different health deficits can produce larger system effects, even if none of the individual deficits are predictive in isolation. The frailty index approach has been previously applied, and its validity has been assessed in HIV-infected cohorts.13 Based on current criteria, variables included in the frailty index should number at least 30, be age related, and be associated with adverse health, and as a group represent multiple physiologic systems.30 The frailty index that we constructed here included 37 variables that span multiple systems. First, each variable was coded as 1, indicating a health deficit or 0, indicating no deficit. The index score was then calculated as the proportion of deficits present out of the total number of variables considered31; this means that each individual in the sample had a calculated frailty index score between 0 and 1, representing the number of health problems they experience and in effect, quantifying their health state. To assess the effect of frailty independently from variables that are related to HIV (markers of infection or immune function) or HANA conditions, we did not include these variables as items in the frailty index (see Supplementary Table S1; Supplementary Data are available online at www.liebertpub.com/aid for list of variables included). Further, we excluded variables from the frailty index if they comprised the outcome (SCA), that is, any cognitive, functional, or psychiatric variables.
Statistical analysis
Descriptive statistics were used to characterize the sample. Individual logistic regression models were used to examine the relationship between each of the current and nadir CD4 count, individual HANA conditions, multimorbidity, and frailty with SCA as the primary outcome. All regression models were adjusted for age and sex.
For each variable that was significantly associated with SCA based on regression models, we tested its ability to discriminate likelihood of SCA with receiver-operating characteristic curves and evaluated the area under the curve (AUC). Given the inverse relationship between SCA and the explanatory variables, for ease of interpretation we used “unsuccessful cognitive aging” (those participants who did not achieve SCA = 1-SCA) as the outcome, which is the same binary variable but it allows us to look at positive relationships with covariates (i.e., the presence of a condition, multimorbidity, or frailty) and allows for a more straightforward interpretation of AUC statistics.
All analyses were conducted by using SPSS (version 21.0, SPSS, Inc.). All reported confidence intervals were 95%, and statistical significance was set at a p value of .05.
Results
A total of 103 participants were included in our analyses after giving informed consent. The mean age of the sample at baseline was 56.4 years (SD = 6.1; range 50–81) (Table 1). No participants were identified as having functional impairment on self-report scales of IADL. The mean number of HANA conditions for all participants was 1.2 (SD = 1.0), and 33% of participants had multimorbidity. The mean frailty index was 0.26 (SD = 0.10), corresponding to 9.6 deficits of a possible 37 deficits (Table 1). In total, 38.8% (n = 40) of participants were identified as meeting criteria for research-defined SCA. Despite no differences in average chronologic age between groups, SCA participants had significantly fewer HANA conditions, a lower frailty index, and were less likely to have hypertension (Table 1).
Table 1.
Characteristics of the Study Sample (Modena HIV Metabolic Clinic)
| Whole sample | SCA | Non-SCA | |
|---|---|---|---|
| Sample size (n [%]) | 103 [100] | 40 [38.8] | 63 [61.2] |
| Age (mean ± SD) | 56.4 ± 6.1 | 57.0 ± 6.7 | 56.0 ± 5.7 |
| Sex (female; n [%]) | 28 [27.2] | 13 [33] | 15 [24] |
| Nadir CD4 (mean ± SD) | 178.9 ± 141.3 | 179.7 ± 129.7 | 178.1 ± 149.2 |
| Current CD4 (mean ± SD) | 611.0 ± 212.9 | 604.2 ± 171.1 | 615.4 ± 237.3 |
| Functional impairmenta | 0 | 0 | 0 |
| Number of HANAb conditions | 1.2 ± 1.0 | 0.9 ± 1.0 | 1.3 ± 1.0* |
| Frailty index (mean ± SD) | 0.26 ± 0.10 | 0.23 ± 0.09 | 0.28 ± 0.10* |
| Multi-morbidity (n [%]) | 34 [33.0] | 9 [22.5] | 25 [39.7]* |
| Successful cognitive aging (n [%]) | 40 [38.8] | — | — |
| Cardiovascular disease (n [%]) | 9 [8.7] | 2 [5.0] | 7 [11.1] |
| Hypertension (n [%]) | 57 [55.3] | 17 [42.5] | 40 [63.5]* |
| Diabetes (type II; n [%]) | 16 [15.5] | 5 [12.5] | 11 [17.5] |
| End-stage kidney failure (n [%]) | 2 [1.9] | 2 [5.0] | 0 |
| Liver cirrhosis (n [%]) | 5 [4.9] | 2 [5.0] | 3 [4.8] |
| Chronic obstructive pulmonary disease (n [%]) | 7 [6.8] | 3 [7.5] | 4 [6.3] |
| Osteoporosis (n [%]) | 24 [23.3] | 6 [15.0] | 18 [28.6] |
| Cancer (any; n [%]) | 1 [1.0] | 0 | 1 [1.6] |
As measured by difficulty with instrumental activities of daily living.
HIV-Associated Non-AIDS.
p < 0.05.
SCA, successful cognitive aging; SD, standard deviation.
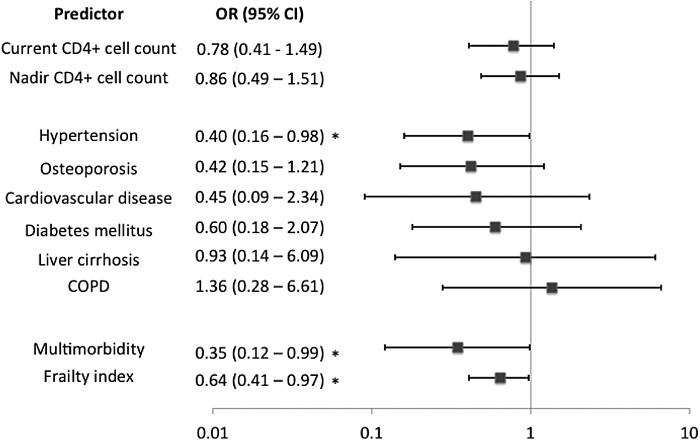
Neither nadir nor current CD4 counts were significantly associated with SCA in individual models adjusted for age and sex (p = .60, p = .45, respectively; Fig. 1). Hypertension was the only HANA condition that was significantly associated with decreased odds for SCA (Fig. 1). Multimorbidity was marginally statistically significant; specifically, the presence of more than one HANA condition decreased the odds of SCA by 65% [odds ratio (OR) 0.35, p = .05] (Fig. 1). Frailty was significantly associated with SCA. Specifically, for each 0.1 point increase in the frailty index, the odds of SCA were reduced by 36% [OR 0.64, p = .04] (Fig. 1).
FIG. 1.
Odds ratios and confidence intervals based on separate logistic regression models (adjusted for age and sex) for each predictor and its relationship to successful cognitive aging (outcome). *p < .05.
Receiver operating characteristic curves were employed to assess the discriminative ability of factors found to be statistically associated with SCA in the individual logistic regression analyses: hypertension, multimorbidity, and the frailty index. The frailty index was the only variable found to be statistically significant in its ability to discriminate the SCA from those who did not meet criteria (AUC = 0.63, p = .02). Hypertension and multimorbidity were not able to discriminate the outcome in a statistically significant manner (p = .08 and p = .14, respectively; Fig. 2).
FIG. 2.
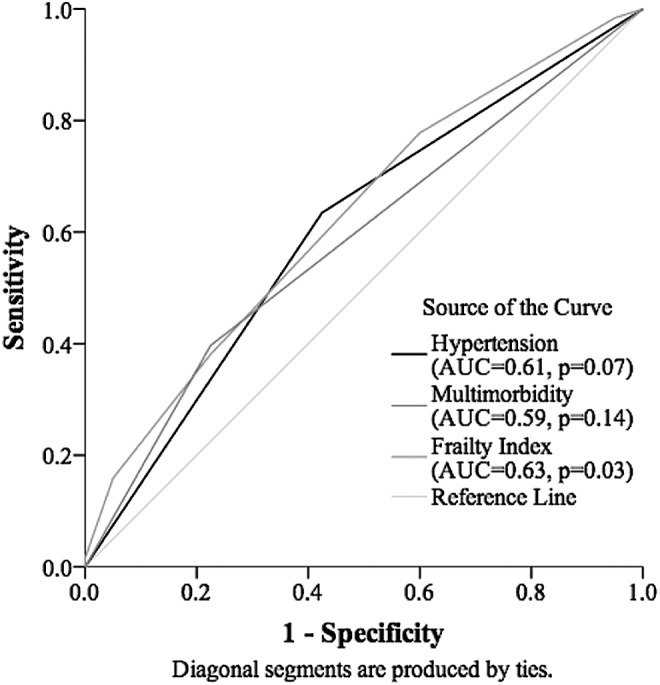
Receiver operating characteristic curve for discriminative ability of risk factors for successful cognitive aging.
Discussion
In this cross-sectional analysis of 103 people with aviremic HIV aged 50 years and older, we found that frailty was independently, inversely associated with odds of SCA; whereas CD4 counts, multimorbidity, and most individual HANA conditions were not. Frailty, a comprehensive measure of vulnerability, appears to be able to more sensitively discriminate odds of SCA among people aging with HIV than individual disease diagnoses or the presence of multimorbidity. Each additional health deficit decreased study participants' odds of meeting criteria for SCA by 12%, on average (OR = 0.88, p = .04). This suggests that frailty might have a meaningful impact on the ability to experience SCA in people living with HIV.
Our findings are consistent with the few previous investigations of SCA in individuals aging with HIV. The prevalence of SCA in our sample (38.8%) is similar to estimates from previous studies of SCA that used similar criteria: Average prevalence of SCA was 32% in a sample of community-dwelling middle-aged and older adults,15 and 19% in a sample made up of young and older community-dwelling adults living with HIV.14 These previous studies also identified no relationship between SCA and markers of HIV disease severity or comorbidities (including diabetes and hypercholesterolemia).14,15 Although we found no significant relationships between CD4 counts or comorbid HANA conditions (besides hypertension) and odds of SCA, frailty was significantly associated with odds of SCA. By incorporating information across multiple physiologic systems, frailty might be describing important health information over and above that of specific illnesses, demonstrating a potential role for assessing frailty in older HIV-positive adults. Further, although the relationship between frailty and SCA has not been previously assessed, a recent cross-sectional study found a relationship between neurocognitive impairment and the Veterans Aging Cohort Study (VACS; another summary health measure) score among people aging with HIV.33 Frailty, as measured by the frailty index, has also been associated with dementia in the general population.34,35
Relationships between frailty and adverse health outcomes are not yet well understood among people with HIV in the cART era, although the few investigations of this relationship produce consistent and compelling results that suggest that frailty is useful in understanding the complexity of risk for adverse health outcomes in those living with HIV.9,12 The 37-item frailty index used in the current study was previously found to independently predict survival and incident multimorbidity in the MHMC cohort.13 Further, a 31-item frailty index predicted mortality and transitions in frailty severity.36 Frailty as measured with a five-item frailty phenotype was predictive of mortality in a cohort of injection drug users,37 and frail individuals with HIV are also more likely to have been hospitalized in the past year and to have longer hospital stays.38 The VACS score has also been associated with adverse health outcomes such as mortality39; greater severity of frailty has been also been associated with higher VACS scores.40 Frailty appears to be a promising approach to measuring health status and vulnerability among people with HIV, and larger, longitudinal studies of the relationship between frailty and cognitive outcomes are warranted.
Frailty has been described as physiologic vulnerability as a consequence of reduced capacity to respond to stressors, acknowledging that frailty is multifactorial and dynamic.41 The reduced capacity to respond to stressors is frequently associated with increasing age, as there is more time with which to accumulate deficits, but not everyone of the same age experiences the same degree of frailty. This is a product of multiple factors, including processes that are intrinsic to the aging process itself and social and environmental exposures that affect both the rate of insults and the body's ability to repair them.32 In people living with HIV, chronic inflammation resulting from the virus, cumulative drug toxicity, and excess rates of social and behavioral risk factors associated with HIV disease may all contribute to frailty.9 For example, frailty is associated with both current and nadir CD4 count, but it appears to describe vulnerability independent from these markers of HIV disease severity.13 As frailty is a phenomenon that is not limited to one physiologic system, but instead accounts for interaction and redundancy across multiple systems, frailty might be valuable in describing the complexity of multiple interacting health problems among people aging with HIV.9,12
Rates of severe HIV-associated dementia have declined substantially since the introduction of cART, but less severe neurocognitive impairment still persists at high rates.6,7 Studies suggest that up to half of people living with HIV in the United States might experience some form of cognitive impairment despite cART,6 even with suppression of serum HIV RNA viral load to undetectable levels.42 HAND in older adults with HIV have been associated with both somatic and psychiatric comorbidities2,6,7 as well as the VACS Index.33 Determination of predictors of SCA in HIV-positive individuals is a useful way to inform health promotion, manage care in this population, and assist individuals in preserving function, independence, and age successfully, despite their HIV status. This study identifies the utility of the frailty index in the prediction of SCA and proposes that a holistic approach to physiologic vulnerability may be a useful approach to capture the complexity of aging and HIV on cognition.
Our results should be interpreted with caution. Our analysis was cross-sectional, and so temporal and causative relationships cannot be established. In the future, we hope to extend this to examine longitudinal data to understand what modifiable factors might be predictive of SCA earlier in the lifespan. In addition, our sample size was relatively small and our analysis will need to be replicated in larger cohorts to confirm the generalizability of our findings and inform estimates of SCA prevalence. Research on SCA among middle-aged and older adults with HIV is limited, however, and our sample of 103 individuals aged 50 and older living with HIV compares with previously published studies (n = 74 and n = 107),14,15 though it should be noted that our sample appears to be less functionally impaired than other samples. Further complicating our interpretation of the findings are other important risk factors in people living with HIV, particularly chronic inflammation, drug toxicity, and factors associated with the virus itself that may all contribute to frailty. Further research is needed to elucidate these mechanisms.
It is possible that these results are an under-representation of the true potential of the frailty index; this is because our analysis plan excluded any HIV-related or HANA condition variables from inclusion in the frailty index. To test whether including these variables would increase the ability of the frailty index to predict adverse outcomes related to HIV, we re-ran our analysis by using a comprehensive frailty index including all variables (CD4 counts, HANA conditions) and found that it had no more or less predictive ability than our original frailty index. This is a phenomenon noted in other investigations of the frailty index; specifically, it has been demonstrated that the predictive ability of the frailty index is optimized at the inclusion of 30 variables, after which predictive ability is not additive with additional variables.36 Our rationale for leaving these variables out of the frailty index in this article was to clearly demonstrate that the frailty index captures a state of physiologic vulnerability that is independent of these potentially disease-specific variables.
It is also important to consider whether blood pressure is a “good enough” proxy for health status, given these results. Although blood pressure may be readily available, the advantage of the frailty index is that it can be operationalized by using any and all routinely collected data. Further, the frailty index has a much smaller confidence interval than hypertension (Fig. 1), leading us to believe that frailty may be a more precise measure of health status, rather than just a proxy.
In summary, we found that frailty is associated with the likelihood of SCA in people living with HIV. This defines an opportunity to apply knowledge from geriatric population research to people aging with HIV to better appreciate the complexity of their health status. We hope that this will inform future research on treatment, management, and health service access for people aging with HIV and highlight the need to consider how to best support these individuals in their unique aging experience. Specific mechanisms by which frailty, HIV infection, and cognition are related remain poorly understood, and these are motivating future inquiry by our group.
Supplementary Material
Acknowledgments
The authors would like to gratefully acknowledge F. Carli, C. Stentarelli, A. Santoro, and M. Menozzi for their contribution in patient evaluation, and B. Beghetto, G. Nardini, and E. Roncaglia for collecting the data. This study is supported by the Gilead Fellowship Program in SCA. T.D.B. was supported by a Dalhousie University Internal Medicine Research Foundation award and a Canadian Institutes of Health Research summer studentship. G.G. has received research grants from Bristol-Myers Squibb, Gilead Sciences, Jansen. He has received honoraria as speaker and/or advisor from Gilead Sciences, BMS, Merck and Jansen.
Results from this study were presented in part at the following conferences: The 5th Annual Workshop on HIV & Aging, held in Washington, US, in October 2014. The 8th International Symposium on Neuropsychiatry and HIV, held in Barcelona, Spain, in June 2015.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Vance DE, Robinson FP: Reconciling successful aging with HIV: A biopsychosocial overview. J HIV/AIDS Soc Serv 2004;3:59–78 [Google Scholar]
- 2.Becker JT, Lopez OL, Dew MA, Aizenstein HJ: Prevalence of cognitive disorders differs as a function of age in HIV virus infection. AIDS 2004;18 (suppl 1):S11–S18 [PubMed] [Google Scholar]
- 3.Guaraldi G, Orlando G, Zona S, et al. : Premature age-related comorbidities among HIV-infected persons compared with the general population. Clin Infect Dis 2011; cir627. doi: 10.1093/cid/cir627 [DOI] [PubMed] [Google Scholar]
- 4.Schouten J, Wit FW, Stolte IG, et al. and AGEhIV Cohort Study Group: Cross-sectional comparison of the prevalence of age-associated comorbidities and their risk factors between HIV-infected and uninfected individuals: The AGEhIV Cohort Study. Clin Infect Dis 2014;59:1787–1797 [DOI] [PubMed] [Google Scholar]
- 5.Althoff KN, McGinnis KA, Wyatt CM, et al. : Comparison of risk and age at diagnosis of myocardial infarction, end-stage renal disease, and non-AIDS-defining cancer in HIV-infected versus uninfected adults. Clin Infect Dis 2015;60:627–638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Heaton RK, Clifford DB, Franklin DR, et al. : HIV-associated neurocognitive disorders persist in the era of potent antiretroviral therapy CHARTER study. Neurology 2010;75:2087–2096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Heaton RK, Franklin DR, Ellis RJ, et al. : HIV-associated neurocognitive disorders before and during the era of combination antiretroviral therapy: Differences in rates, nature, and predictors. J NeuroVirol 2010;17:3–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ku N, Lee Y, Ahn J, et al. : HIV-associated neurocognitive disorder in HIV-infected Koreans: The Korean NeuroAIDS Project. HIV Med 2014;15:470–477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brothers TD, Kirkland SA, Guaraldi G, et al. : Frailty in people aging with human immunodeficiency virus (HIV) infection. J Infect Dis 2014;210:1170–1179 [DOI] [PubMed] [Google Scholar]
- 10.Althoff KN, Jacobson LP, Cranston RD, et al. : Age, comorbidities, and AIDS predict a frailty phenotype in men who have sex with men. J Gerontol Ser A Biol Sci Med Sci 2014;69A:189–198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Clegg A, Young J. Iliffe S, et al. : Frailty in elderly people. Lancet 2013;381:752–762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brothers TD, Rockwood K: Biologic aging, frailty, and age-related disease in chronic HIV infection. Curr Opin HIVAIDS 2014;9:412–418 [DOI] [PubMed] [Google Scholar]
- 13.Guaraldi G, Brothers TD, Zona S, et al. : A frailty index predicts survival and incident multimorbidity independent of markers of HIV disease severity. AIDS 2015; doi: 10.1097/QAD.0000000000000753 [DOI] [PubMed] [Google Scholar]
- 14.Moore RC, Fazeli PL, Jeste DV, et al. . and The HIV Neurobehavioral Research Program (HNRP) Group: Successful cognitive aging and health-related quality of life in younger and older adults infected with HIV. AIDS Behav 2014;18:1186–1197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Malaspina L, Woods SP, Moore DJ, et al. . and The HIV Neurobehavioral Research Programs (HNRP) Group: Successful cognitive aging in persons living with HIV infection. J Neurovirol 2011;17:110–119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guaraldi G, Orlando G, Squillace N, et al. : Multidisciplinary approach to the treatment of metabolic and morphologic alterations of HIV-related lipodystrophy. HIV Clin Trials 2006;7:97–106 [DOI] [PubMed] [Google Scholar]
- 17.Moore RC, Moore DJ, Thompson W, et al. : A case-controlled study of successful aging in older adults with HIV. J Clin Psychiatry 2013;74:e417–e423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang W, O'Brien N, Forrest JI, et al. : Validating a shortened depression scale (10 Item CES-D) among HIV-positive people in British Columbia, Canada. PLoS One 2012;7:e40793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Natamba BK, Achan J, Arbach A, et al. : Reliability and validity of the center for epidemiologic studies-depression scale in screening for depression among HIV-infected and -uninfected pregnant women attending antenatal services in Northern Uganda: A cross-sectional study. BMC Psychiatry 2014;14:303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Radloff LS: The CES-D scale a self-report depression scale for research in the general population. App Psychol Measurement 1977;1:385–401 [Google Scholar]
- 21.Blackstone K, Moore DJ, Franklin DR, et al. : Defining neurocognitive impairment in HIV: Deficit scores versus clinical ratings. Clin Neuropsychol 2012;26:894–908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Spinnler H, Tognoni G, and MLA, Gruppo italiano per lo studio neuropsicologico dell'invecchiamento. Standardizzazione E Taratura Italiana Di Test Neuropsicologici. Masson Italia Periodici; 1987 [PubMed] [Google Scholar]
- 23.Brandt J: The hopkins verbal learning test: Development of a new memory test with six equivalent Forms. Clin Neuropsychol 1991;5:125–142 [Google Scholar]
- 24.Merker B, Podell K: Grooved Pegboard Test. In:Encyclopedia of Clinical Neuropsychology (Kreutzer , DeLuca , Caplan , eds.) Springer, New York, 2011, pp. 1176–1178. http://link.springer.com/referenceworkentry/10.1007/978-0-387-79948-3_187 [Google Scholar]
- 25.Reitan RM: Validity of the trail making test as an indicator of organic brain damage. Percept Mot Skills 1958;8:271–276 [Google Scholar]
- 26.Physical Self-maintenance: Assessment of Older People: Self-Maintaining and Instrumental Activities of Daily Living 1969; www.eurohex.eu/bibliography/pdf/Lawton_Gerontol_1969-1502121986/Lawton_Gerontol_1969.pdf [PubMed]
- 27.Vance DE, McGuinness T, Musgrove K, et al. : Successful aging and the epidemiology of HIV. Clin Interventions Aging 2011;6:181–192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Levey AS, Bosch JP, Lewis JB, et al. : A more accurate method to estimate glomerular filtration rate from serum creatinine: A new prediction equation. Ann Intern Med 1999;130:461–470 [DOI] [PubMed] [Google Scholar]
- 29.Sterling RK, Lissen E, Clumeck N, et al. : Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV Coinfection. Hepatology 2006;43:1317–1325 [DOI] [PubMed] [Google Scholar]
- 30.Searle SD, Mitnitski A, Gahbauer EA, et al. : A standard procedure for creating a frailty index. BMC Geriatr 2008;8:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wallace LMK, Theou O, Kirkland SA, et al. : Accumulation of non-traditional risk factors for coronary heart disease is associated with incident coronary heart disease hospitalization and death. PLoS One 2014;9:e90475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mitnitski A, Song X, Rockwood K: Assessing biological aging: The origin of deficit accumulation. Biogerontol 2013;14:709–717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Marquine MJ, Umlauf A, Rooney AS, et al. : The veterans aging cohort study index is associated with concurrent risk for neurocognitive impairment. JAIDS 2014;65:190–197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Song X, Mitnitski A, Rockwood K: Nontraditional risk factors combine to predict alzheimer disease and dementia. Neurology 2011;77:227–234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Song X, Mitnitski A, Rockwood K: Age-related deficit accumulation and the risk of late-life dementia. Alzheimer's Res Ther 2014;6:54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brothers TD, Kirkland SA, Theou O, et al. : Health transitions among people aging with HIV: A multistate modeling approach [Submitted 2015]
- 37.Piggott DA, Muzaale AD, Mehta SH, et al. : Frailty, HIV infection, and mortality in an aging cohort of injection drug users. PLoS One 2013;8:e54910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Önen NF, Agbebi A, Shacham E, et al. : Frailty among HIV-infected persons in an Urban outpatient care setting. J Infect 2009;59:346–352 [DOI] [PubMed] [Google Scholar]
- 39.Justice A, McGinnis K, Skanderson M, et al. : Towards a combined prognostic index for survival in HIV infection: The role of “non-HIV” Biomarkers. HIV Med 2010;11:143–151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Escota GV, Patel P, Brooks JT, et al. : The veterans aging cohort study index is an effective tool to assess baseline frailty status in a contemporary cohort of HIV-infected persons. AIDS Res Human Retroviruses 2014; doi: 10.1089/aid.2014.0225 [DOI] [PubMed] [Google Scholar]
- 41.Rockwood K, Mitnitski A: Frailty defined by deficit accumulation and geriatric medicine defined by frailty. Clin Geriatr Med 2011;27:17–26 [DOI] [PubMed] [Google Scholar]
- 42.Simioni S, Cavassini M, Annoni JM, et al. : Cognitive dysfunction in HIV patients despite long-standing suppression of Viremia. AIDS 2009. doi: 10.1097/QAD.0b013e3283354a7b [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.