Abstract
Injury to the central nervous system (CNS) includes both traumatic brain and spinal cord injury (TBI and SCI, respectively). These injuries, which are heterogeneous and, therefore, difficult to treat, result in long-lasting functional, cognitive, and behavioral deficits. Severity of injury is determined by multiple factors, and is largely mediated by the activity of the CNS inflammatory system, including the primary CNS immune cells, microglia. The nicotinamide adenine dinucleotide phosphate (NADPH) oxidase (NOX) family of enzymes is a primary source of reactive oxygen species (ROS), key inflammatory mediators after CNS injury. ROS play a central role in inflammation, contributing to cytokine translation and release, microglial polarization and activation, and clearance of damaged tissue. NOX has been suggested as a potential therapeutic target in CNS trauma, as inhibition of this enzyme family modulates inflammatory cell response and ROS production. The purpose of this review is to understand how the different NOX enzymes function and what role they play in the scope of CNS trauma.
Keywords: : inflammation, oxidative stress, TBI, traumatic SCI
Introduction
With roughly 1,700,000 cases of traumatic brain injury (TBI) and 200,000 cases of spinal cord injury (SCI) in the United States alone,1–3 trauma to the central nervous system (CNS) is a serious medical concern. Unfortunately, because of the heterogeneity of injury by age, location, and severity, finding appropriate treatments or cures has proved a complicated task.
The inflammatory response after CNS injury is a common target for therapy, because of the wide-reaching and well-documented effects of post-injury inflammation. The primary injury results in damage to the CNS, leading to a robust inflammatory response, including activation of microglia, invasion of peripheral immune cells, and inflammatory mediator release, which contribute to tissue loss and scarring.4 This secondary injury, caused by biochemical and redox reactions, leads to necrosis and apoptosis of both the initially damaged tissue and healthy peripheral tissue over the hours to months following the injury, and contributes to functional deterioration.5
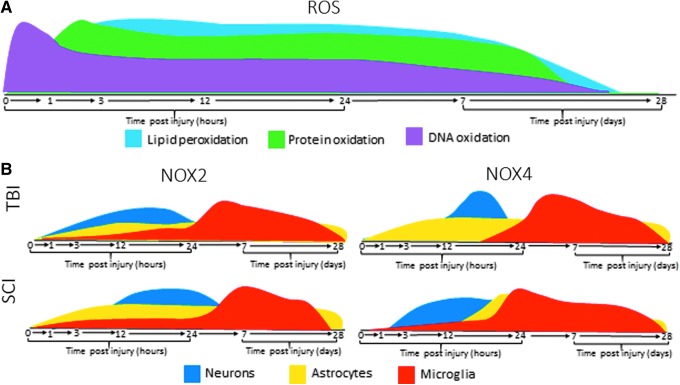
Reactive oxygen species (ROS) are one such inflammatory mediator. These highly reactive oxygen-derived small molecules, which include peroxides, superoxides, hydroxyl radicals, and singlet oxygen, can significantly impact severity of injury, lesion size, and temporal progression of TBI and SCI. Microglia are a primary source of ROS in the CNS, but these reactive molecules can also be produced by neurons and astrocytes. A certain basal level of these molecules is necessary for normal cellular function, and the body produces antioxidants to counter these ROS. However, when there is an overproduction of these unstable molecular byproducts, the balance is lost, leading to oxidative damage in the cellular environment caused by detrimental ROS interactions with proteins, lipids, carbohydrates, and nucleic acids.6,7 Observations of oxidative damage have been observed after traumatic injury to the CNS, including TBI and SCI.8 Figure 1A provides a summary of oxidative damage expression after injury.
FIG. 1.
Expression profiles of reactive oxygen species (ROS) and nicotinamide adenine dinucleotide phosphate (NADPH) oxidase (NOX) 2/4 post-traumatic central nervous system (CNS) injury. (A) DNA, protein, and lipids undergo oxidation acutely after injury, leading to increased ROS and oxidative stress in the cellular environment. DNA oxidation (pink) increases rapidly within the first 15 min after injury, and protein oxidation (green) and lipid peroxidation (light blue) are increased by 3 h post-injury, with both remaining elevated over normal conditions out to at least 96 h post-injury, with evidence of oxidation remaining within 2 weeks post-injury. (B) The NOX enzyme family is primary a producer of oxidative stress, and both produces and is influenced by the expression of ROS. Expression of the two enzymes most involved in injury, NOX 2 and 4, differs by injury, cell type, and time post-injury. NOX2 expression in traumatic brain injury (TBI): NOX2 is upregulated between 6 and 12 h post-injury in astrocytes (yellow), 12–24 h post-injury in neurons (blue), and peaks between 1 and 7 days post-injury (dpi) in microglia (red). NOX2 expression in spinal cord injury (SCI): NOX2 is seen consistently post-injury in astrocytes, more acutely in neurons, and peaks by 7 dpi in microglia. In both injury types, some upregulation in both microglia and astrocytes maintains out to 28 dpi. NOX4 expression in TBI: NOX4 upregulation is seen acutely in neurons, and in microglia and astrocytes it is seen by 24 h post-injury, maintaining out to 28 dpi. NOX4 expression in SCI: NOX4 is seen in microglia and astrocytes by 24 h, peaking by 7 dpi and maintaining through 28 dpi. Color image is available online at www.liebertpub.com/neu
Markers of oxidative damage, including DNA oxidation, protein oxidation, lipid peroxidation, and protein carbonylation, have been correlated with impaired function after TBI (for review see Mendez and coworkers9). Interaction of ROS with the lipid bilayer of membranes results in lipid peroxidation, causing damage and cell death.10 In clinical samples after moderate to severe TBI, protein oxidation and lipid peroxidation were noted in both the contusion and pericontusion regions within 2 weeks post-injury.11 In rats after a controlled cortical impact (CCI), lipid peroxidation was elevated by 3 h post-injury, and remained elevated through 96 h.12 Similarly, protein oxidation in rats after a moderate TBI was elevated by 3 h post-injury.13 Further damage can result in the form of DNA oxidation when ROS react with cellular DNA.9,14 Further, oxidative stress can result in protein carbonylation, an underlying factor of protein function alteration.15 Previous studies have shown that after TBI and SCI, mitochondrial proteins become oxidatively modified, which can lead to reduction in mitochondrial enzyme activity and mitochondrial bioenergetic abilities.13,16,17 It is important to note that at low levels, these modifications are the result of signaling or beneficial responses to stress;18,19 however, excessive modification can lead to enzyme dysfunction. Proteins that appear to be most sensitive to oxidative damage following CNS injury include glial fibrillary acidic protein (GFAP), dihydropyrimidase-related protein 2, fructose-bisphosphate aldolase C, and fructose-bisphosphate aldolase A.20
At the cellular level, excess ROS can cause endothelial, oligodendrocyte, neuronal, and glial damage. Increased ROS exposure in endothelial cells in vitro increases contractile and adhesion molecule function, leading to impaired blood–brain barrier (BBB) function.21 Increased ROS also directly induce oligodendrocyte cell death, which reduces remyelination of axons at the injury site.22 The accumulation of ROS can lead to oxidative damage to neurons, resulting in diminished neuronal health and cell death.23 And as part of the inflammatory response, excess ROS and ROS-induced damage can amplify the activation of microglia and astrocytes.24,25
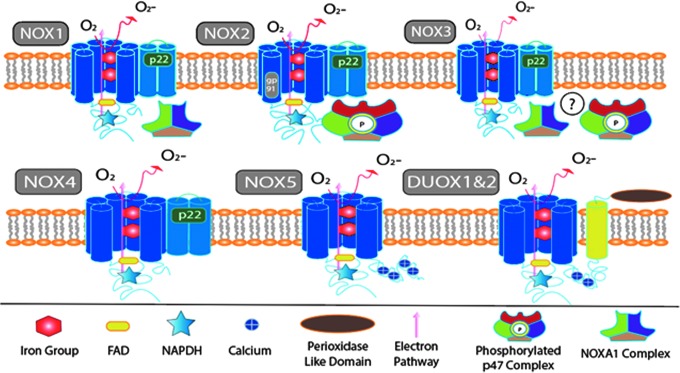
One of the primary sources of ROS is the nicotinamide adenine dinucleotide phosphate (NADPH) oxidase (NOX) family of enzymes (Fig. 2). This family includes 7 isoforms: NOX1, NOX2, NOX3, NOX4, NOX5, dual oxidase 1 (DUOX1), and DUOX2.26 Each isoform is composed of several subunits that exist either within the membrane or in the cytosol. Upon phosphorylation, the cytosolic subunits cause the assembly of the active enzyme, which translocates to the membrane, where they generate ROS.27 The purpose of this review is to understand the different NOX enzymes and their functions in the scope of a TBI and SCI.
FIG. 2.
Structural illustration of all major isoforms of nicotinamide adenine dinucleotide phosphate (NADPH) oxidase (NOX). In all isoforms, an electron is transferred from NADPH (star) on the C terminus and transferred through flavin adenine dinucleotide (FAD) (yellow oval) and 2 Heme groups, which deliver the electron to awaiting molecular oxygen. The movement of the electron is denoted by the pink arrow. NOX1 is represented with its activating trimetric complex NOX organizer 1 (NOXO1), NOX activator 1 (NOXA1), and Ras-related C3 botulinum toxin substrate 1 (Rac1). NOX2 is represented with activating quaternary complex interacting with both gp91phox and p22phox. Phosphorylation (green circle) of p47phox must occur to facilitate recruitment of P67phox and p40phox and subsequent translocation toward the p22phox and gp91phox complex. NOX3 is seen with all known activating subunits which, when present, accentuate function, although the exact mechanism is still unclear (illustrated by a circled question mark). NOX4 is considered a constitutive enzyme, because of its lack of additional subunits, although p22phox is still necessary for stabilization of the primary six transmembrane structure. NOX5 is unique in that it lacks the requirement for p22phox, but needs calcium interaction with four EF hand domains on the N terminus. Dual oxidase (DUOX) 1 and 2 are the least relevant of the NOX enzymes within central nervous system (CNS) injury and are significantly different in that they have an additional trans-membrane helix with a peroxidase like domain. Color image is available online at www.liebertpub.com/neu
Overview of NOX Isoforms
Currently, there are seven known isoforms of the NOX enzyme. All isoforms possess a highly conserved 6 trans-membrane domains that encompasses 2 heme groups and both flavin adenine dinucleotide (FAD) and NADPH binding sites on the C terminus, which mediate electron transfer from NADPH to molecular oxygen.26 The differences between the isoforms predominantly involve the subunit proteins required for activation.
NOX1, 2, 3, and 4, and DUOX 2 are reported to be present in detectable quantities in the parenchyma of the brain and spinal cord.28–30 NOX1, 2, 3, and 4 are present in neurons, whereas astrocytes and microglia are reported to only express NOX2 and NOX4.31,32 DUOX2 is reported in the vestibular system and cerebellum, although the cellular source is unclear.33,34 In 2013, we reported that NOX 2 and 4 were upregulated after traumatic CNS injury.29 Figure 1B provides a summary of NOX2 and 4 expression profiles after CNS injury.
NOX2 is predominantly found on phagocytes.26 It was the first NOX isoform identified and is currently the most studied isoform of NOX. Like all NOX family members, NOX2 consists of the conserved membrane enzymatic core, termed gp91phox, two heme groups and the FAD and NADPH binding sites on its C terminus. In addition, it requires the p22phox subunit protein to dimerize and form a heterodimeric flavocytochrome (cyt b558) which is the catalytic core of the oxidase.35 Once stabilized, NOX2 remains quiescent until phosphorylation of the cytoplasmic subunits p47phox, p40phox, and p67phox. These subunits seem to exist as a complex in the cytosol upon stimulation although the resting state of the cytosolic components is still a controversial area of research.35–37 After phosphorylation on the p47phox C-terminus serines,38 the trimeric complex translocates to the membrane and binds to the flavocytocrome via p22phox.39,40 Although the chronology of the p47phox phosphorylation events are not completely understood, it has been suggested that some events happen after the binding of the flavocytochrome which would be further steps towards activation and possibly even membrane binding (for review see Groemping and coworkers35). Additionally, a guanosine triphosphate (GTP)ase, RAC2, is necessary for activation and acts directly in electron transfer by NOX2.41 Once assembled, the components reduce oxygen to superoxide.42 Figure 2 provides detailed diagram of component assembly.
Unlike NOX2, NOX4 does not require multiple subunits or translocation in order to function. NOX4 retains ∼40% homology with NOX2, and consists of the ix conserved transmembrane domains and p22phox.41,43 It is suspected to be constitutively active following heterodimerization with p22phox.44,45 Dense concentrations of NOX4 have been revealed within the endoplasmic reticulum.46 This observation may be the result of the hypothesized homeostatic function of NOX4 within the endoplasmic reticulum, or may be merely a coincidental finding related to protein synthesis.47 NOX4 was initially detected in the kidneys, and is suspected to aide in the regulation of electrolytes and water.48 Hydrogen peroxide is considered the primary product of NOX4; however, it has been suggested that NOX4 generates superoxide, which is quickly converted to hydrogen peroxide by antioxidant enzymes such as superoxide dismutase.26,48 It has also been suggested that the dismutation activity is intrinsic of NOX4.49–51
The remaining NOX isoforms, NOX1, NOX3, NOX5, DUOX1, and DUOX2, have limited expression, if any, in the CNS and their expression after injury is unclear. NOX1 was the first identified homolog of NOX2. This isoform possesses 56% homology with NOX2.52 It is predominantly found in the colon, but is also reported to be present in the brain within the vasculature, microglia, astrocytes, and neurons.53–56 NOX1 has been associated with ROS production in hypoxic injury models, Parkinson's disease and cancers.55,57,58 NOX1 also requires cytosolic subunits for optimal function, the NOX organizer 1 (NOXO1) and NOX activator 1 (NOXA1) complexes.36 These subunits are homologs of p47phox and p67phox, respectively, although their role seems to be less dependent on cell stimulation, suggesting that NOX1 is constitutively active.59 Phosphorylation of NOXA1 reduces the activity of NOX1, suggesting a potential deactivation mechanism.59 RAC activation is also essential for NOX1 function.60
NOX3 was identified in the early 2000s.61 Initially it was only identified within the vestibular apparatus of the inner ear, and is essential for proper development.62 NOX3 has been located in other cell types, including neurons.29,61,62 Conflicting evidence suggests that the subunit p22phox is required for function.63 In addition, active NOXO1, NOXA1, p47phox, and p67phox were all found to potentiate NOX3 function.64 Because of this unclear evidence of activation, it has been suggested that NOX3 may also be constitutively active, like NOX1 and 4.64
NOX5 retains 27% homology with NOX2.26,43 This difference can be attributed to the fact that NOX5 seems to function without the presence of any subunits, including p22phox. Research toward structure and function have been stymied because of the absence of NOX5 within rodent genomes.65 Five splice variants of NOX5 have been identified, leading to five isoforms.65 Calcium modulates all isoforms of NOX5 via its calcium-binding domain on the N terminus.66
DUOX1 and DUOX2 are less commonly studied isoforms of NOX that were identified initially in thyroid tissue.67,68 DUOX1-2 has been described in vestibular cells of the ear and immortalized neuroblastoma cells, oligodendrocytes, and rat brain.69 These enzymes consist of the primary conserved transmembrane structure except for an additional transmembrane protein with an extracellular N terminus that contains 2 EF domains and a peroxidase domain.70 Calcium and platelet derived growth factor are known facilitators of DUOX1 and 2.71 Little research exists concerning DUOX1 and 2 involvement in secondary TBI or SCI.
NOX enzymes play a large role in overall oxidative activity in the CNS. The subtle differences in locations and roles in normal function that these enzymes perform vary, because of the large number of isoforms.
NOX and Inflammation
ROS are known to play an important role in post-injury inflammation. NOX-generated ROS can affect the type and amount of cytokines produced in the body, propagating the inflammatory response.72 ROS can act on the NFκB transcription factor to induce further NOX expression.73 Further, pro-inflammatory cytokines, such as tumor necrosis factor alpha (TNF-α), interleukin-1 beta (IL-1β) and interferon gamma (IFNγ), can increase the NOX activity.72
Inhibition of NOX has helped to clarify the role of this enzyme family in inflammation in the CNS. In work by Choi and coworkers,74 wild type (WT) mice treated with apocynin, a nonspecific plant-derived NOX antagonist, or gp91phox−/− transgenic mice were investigated to determine if microglial activation phenotype and inflammatory response in mice was influenced by NOX activity. WT mice were given an intraperitoneal (i.p.) treatment of apocynin (5 mg/kg) 30 min prior to inflammatory stimulation with lipopolysaccharide (LPS). These mice showed enhanced expression of anti-inflammatory microglial mRNA (Ym1 and mitochondrial antisense RNA for cytochrome C oxidase [MARCO]), and decreased expression of pro-inflammatory mRNA (TNF-α and chemokine [C-C motif] ligand 2 [CCL2]) 24 h after LPS injection. The same findings were seen in gp91phox−/− mice, although the trend toward pro-inflammatory cytokine decreases was not significant.
Inhibition of NOX with the nonspecific flavoprotein inhibitor diphenyleneiodinium (DPI) showed neuroprotective and anti-inflammatory effects in primary midbrain cell cultures.75 Further, treatment with DPI in a mouse model of inflammation showed reduced microglial activation.76 DPI similarly reduced both ROS formation and the pro-inflammatory cytokines TNF-α, IL-1β, and monocyte chemoattractant protein-1 (MCP-1). Mechanistically, these findings suggests that inhibition of NOX prevents the production of microglial ROS and the release of pro-inflammatory cytokines.
NOX and TBI
TBI increases the expression and activity of many of the NOX isoforms. NOX2 and NOX4 have been identified in clinical brain tissue samples from 6 to 24 h and 24–48 h after injury, respectively.77 Positive immunostaining for both NOX2 and NOX4 was negatively correlated with Glasgow Coma Score (GCS) in this study. Unfortunately, to date, no study has examined NOX expression beyond 48 h in clinical samples. However, pre-clinical work in rodent models has shown similar acute elevations in NOX expression. Blast-induced brain injury in adult rats resulted in an increase in NOX4 expression at 24 h post-injury.78 With a CCI model in rats, there is a similar expression pattern, wherein NOX2 and NOX4 expression peaks between 1 and 7 days post-injury (dpi).29 This same pattern was observed after weight drop injury in rats, with increased NOX2 expression in the hippocampus with a peak between 2 and 3 dpi.79 In mice after CCI, the time course appears to be similar, with NOX2 expression observed with a peak at 2 dpi, particularly in microglia.80
NOX2 and NOX4 appear to be primarily expressed by microglia and macrophages after TBI. This expression in animal models appears to peak between 1 and 7 dpi, but remains elevated through 28 days.29 In addition to playing a role in microglial activation, NOX activity may also play a role in microglial phenotype.74 Kumar and coworkers31 found that microglia that were either fully or partially polarized toward the M1, or pro-inflammatory phenotype, expressed high levels of NOX2 at 7 days after CCI injury in mice. This coexpression was mostly found in the lesion penumbra, and was not observed in sites distal from the lesion. Microglia polarized toward the M2 or anti-inflammatory phenotype were infrequently positive for NOX2. In aged mice, a significant increase in NOX2 gene and protein expression has been correlated with a shift in microglial polarization toward the M1 phenotype.81
Although NOX has been primarily characterized as an inflammatory cell enzyme, isoforms have also been identified in astrocytes and neurons (see Fig. 1B for expression pattern summary). NOX2 and NOX4 have been specifically co-labeled within neurons and astrocytes after brain injury in human patients.77 In this study, NOX2 was found to be upregulated in astrocytes acutely (6–12 h) and in neurons at a somewhat delayed time course (12–24 h). Rats show a similar pattern, with acute elevations in neuronal NOX2 and NOX4.29 Although the functional significance is not yet clear, neurons also appear to be NOX3 positive after TBI.29 In contrast, NOX2 and NOX4 are upregulated acutely (24 h) and chronically (28 days) in astrocytes after CCI in rats. In mice after CCI, NOX2 has been observed in both neurons and astrocytes as well as microglia.80
To assess activity, researchers have investigated both NADPH consumption and ROS production, as well as phosphorylation of cytosolic components and translocation of components to the membrane. Following CCI injury in adult male Sprague–Dawley rats, NOX activity was elevated in the cortex by 6 h and remains elevated through 72 h.82 This activity was assessed by both NADPH consumption and translocation of p67phox and p47phox to the membrane.
NOX and TBI: Inhibition
The most convincing evidence that NOX plays a role in TBI progression is data from studies demonstrating that inhibition of members of this enzyme family improves recovery and reduces inflammation. Apocynin, a nonspecific plant-derived NOX antagonist, has been shown to reduce NOX activation in TBI models.79 Apocynin reduces NOX expression for as long as 24 h after TBI.83 Studies have shown that ROS production can induce expression of NOX components, typically via activation of the NFκB transcription factor pathway.84,85
Oxidative stress was also reduced by administration of apocynin prior to a CCI or a weight drop injury.86,87 These effects were found to be both immediate and long lasting. Whereas Choi and coworkers87 found that pretreatment led to reductions in ROS production as early as 3 h after injury, Zhang and coworkers86 assessed oxidative stress markers 4-hydroxy-2-nonenal (4-HNE), 8-hydroxy-2'-deoxyguanosine (8-OHdG), and phosphorylated H2A histone family, member X (p-H2AX) in the cortex and hippocampus and observed significant reductions in these markers at 48 h after TBI. Post-treatment paradigms have been equally successful in reducing oxidative stress markers. Apocynin administered 30 min after a moderate lateral fluid percussion injury in mice was found to reduce both protein carbonylation and lipid peroxidation by 24 h post-injury.88
NOX inhibition has also been shown to reduce inflammation and neuronal damage. For example, pretreatment with apocynin markedly reduced microglial and macrophage numbers, reduced brain edema, and improved cognitive function after weight drop or CCI injury.74,79,86 This may be related to a reduction in oxidative stress-induced pro-inflammatory cytokine release.89 Apocynin has been shown to significantly reduce both IL-1β and TNF-α production 3 and 24 h after TBI.88 In addition, apocynin administration to rats 15 min prior to weight drop injury significantly reduced Fluoro-Jade B staining at 24 h after injury.87
In contrast to the pretreatment studies, post-treatment administration of apocynin has been met with conflicting results. For example, administration of apocynin (5 mg/kg) at 30 min or 24 h after a moderate lateral fluid percussion injury in mice had no effect on brain edema or motor function.88 However, in this same study, a significant improvement in novel object recognition and a reduction in lesion volume was observed at 7 dpi with apocynin treatment.88 Similarly, lesion volume was found to be significantly reduced 21 days after a moderate CCI injury in mice with apocynin (5 mg/kg, 30 min post-injury administration).90 However, with CCI injury, apocynin was found to significantly improve motor function, but to have a nonsignificant effect on cognitive function in the Morris Water Maze test.90 It is unclear why similar dosages, administration times, and models would have such significantly different results, but may be the result of slight differences in location of the primary impact (motor cortex88 vs. parietal cortex90). NOX2 in particular has been targeted for therapeutic inhibition after TBI, most likely because of its acute upregulation profile after TBI and its involvement in microglial- and macrophage-related inflammation. Knockout of the NOX2 gene itself, CYBB, significantly reduces lesion volume and neuronal apoptosis after CCI in mice.80
Pretreatment with the NOX2-specific inhibitor gp91 ds-tat at 250 mg/mouse 20 min prior to a CCI injury increased cortical neuron viability,86 demonstrating that NOX2 inhibition has significant effects on outcome after TBI. However, in the most clinically significant treatment protocol, Kumar and coworkers31 demonstrated that i.p. administration of gp91 ds-tat (5 mg/kg) for 3 days beginning 24 h after a moderate CCI injury in mice significantly reduced lesion volume and improved cognitive function. This treatment also led to a shift in microglial polarization toward the M2 state. This furthers the idea that NOX2 plays an important role in post-injury polarization of microglia.
To date, only one study has been published using the gp91phox−/− transgenic mouse model for TBI. In a mouse model of surgical brain injury, Lo and coworkers91 examined the influence of NOX on neurological outcome measures. After injury, sensorimotor deficits were observed in both WT and gp91phox−/− mice that decreased neurological scores, however, outcomes for gp91phox−/− mice were significantly better than those for WT mice.91 Further, when gp91phox−/− mice were compared with a group of mice pretreated with the nonspecific NOX inhibitor apocynin (5 mg/kg), there was no significant different in performance between the two treatment groups.91 These findings suggest that the lack of gp91phox, the functional subunit of NOX, results in performance similar to suppression of activity of the NOX enzymes, and that performance is improved compared with no treatment at all.
Further, the same study assessed effect of acute and chronic inhibition of NOX on oxidative stress. Acute inhibition with apocynin pretreatment significantly decreased oxidative stress (indicated by lipid peroxidation) at 3 h after injury; however, this decrease was not observed at 24 h, suggesting that pharmacological interventions may require multiple dosages for therapeutic effects.91 Interestingly, oxidative stress levels increased significantly in both the gp91phox−/− and WT mice 24 h after injury, although the transgenic mice showed lower levels than WT mice, which did not reach statistical significance.91 These findings suggest that although NOX is a major source of ROS, other sources may compensate or be more active at chronic stages post-injury, and inhibition of NOX alone may not be sufficient to reverse these effects.
NOX and SCI
Work by Vaziri and coworkers92 found an abundance of NOX activity at acute (1 day) and subacute (14 days) time points post-SCI in a rodent model. Their results demonstrated an elevation in protein expression of the NOX subunits gp91phox and p67phox at the injury site at both time points.92 This finding of prolonged upregulation of NOX after SCI suggests that there is an increased baseline oxidative stress at the injury site that does not change with time. Further, inducible nitric oxide synthase (iNOS) was found to be upregulated concurrently with NOX, suggesting that elevated NO and O- were present in the environment.
The upregulation of NOX in the spinal cord after SCI is both cell type and isoform specific. Work by Cooney and coworkers93 delineated this expression profile and found that expression of the various isoforms is not only cell dependent but also dependent on injury status (see Fig. 1B for expression summary). This study demonstrated that whereas NOX2, 3, and 4 were present in spinal cord before and after injury, NOX2 was found to be present in all cell types. NOX3 was only found in neurons and NOX4 was only found acutely in glial cells.93
NOX2 is the most responsive to injury, with a large increase in expression in glial cells after injury. Cooney and coworkers93 found that cells positive for the neuronal marker NeuN were also positive for both NOX2 and 3 before and after injury. Microglia (or cells positive for the microglial marker ionized calcium-binding adaptor molecule 1 [Iba-1]) show NOX2 only after injury and not in naïve tissue.93 Co-labeling for Iba1 and NOX2 revealed an increase in concentration, which peaked at 7 days and continued out to 28 days.93 NOX4, on the other hand, was present on microglia in small amounts in naïve tissue, but was only found at very acute time points post-injury (up to 24 h), and not at chronic time points.93 Interestingly, in other glial cells such as astrocytes (noted by the astrocyte marker GFAP), NOX2 and 4 were present both before and after injury.93 Further, NOX4 maintained a consistent presence in astrocytes throughout, showing an increase at 24 h and 7 days, although decreasing somewhat by 28 days.93
NOX and SCI: Inhibition
Inhibition of NOX has shown promise in pre-clinical SCI research as a therapeutic treatment option, with research examining a variety of treatment paradigms. Both general NOX inhibitors and NOX2-specific inhibitors have been employed in an attempt to differentiate the influence of NOX by a specific isoform.
Work by Byrnes and coworkers94 in a contusion rat model used DPI administered at 30 min post-injury and continuing for 7 days to determine the role of NOX in chronic SCI (28 dpi outcome). Both MRI and histology at 28 dpi revealed significant reductions in lesion volume in DPI treated rats.94 Further, immunohistochemical and Western blotting showed a reduction in chronically expressed inflammatory proteins progranulin and galectin-3.94 These findings demonstrate that nonspecific inhibition of NOX can be used as a therapeutic target for modulation of inflammatory proteins at chronic time points post-injury.
Specific inhibition of the NOX2 isoform has demonstrated that acute (15 min post-injury) and slightly delayed (6 h post-injury) i.p. administration of gp91 ds-tat reduced several markers of inflammation in a rat SCI model, including pro-inflammatory cytokine and CD11b expression at 24 h post-injury.93 Recent work by our laboratory demonstrated that acute inhibition of NOX2 in a mouse SCI model can result in long-term alterations in function and microglial activity.95 Mice received an intrathecal treatment of either gp91 ds-tat or a scrambled peptide control immediately following contusion SCI.95 Our results showed significant reductions in inflammatory cell numbers in the spinal cord as early as 24 h and lasting up to 7 dpi, as well as reductions in NOX2 activation and oxidative stress markers by 7 dpi.95 These acute improvements were reflected in improvements in general locomotor scores in the treated mice compared with controls at 14 and 28 dpi.95 However, no difference in any inflammatory marker was observed at 28 dpi, suggesting that acute treatment does not have long-lasting effects on the inflammatory response, despite sustained functional improvement.95 In addition, specific inhibition of NOX2 after SCI resulted in no effect on neuron or astrocyte populations at 24 h post-injury in a rat SCI model.93
Combined therapeutic treatments have been investigated as well. Work by Johnstone and coworkers96 used a novel combined therapeutic treatment in a mouse model of SCI. The axonal α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) glutamate receptor activation has been identified for axon dysfunction and degeneration in SCI.96 The combined use of the nonspecific apocynin and the AMPA receptor inhibitor 2,3-dihydroxy-6-nitro-7-sulfamoyl-benzo[f]quinoxaline-2,3-dione (NBQX) resulted in reduced lesion volume and increased preservation of white matter and a greater overall improvement to functional recovery out to 6 weeks post-injury.96 These findings support the potential for combined treatment targets as the result of an additive effect of both AMPA receptor and NOX inactivation.
No studies to date have been performed in SCI using a NOX knockout mouse model. However, based on previous work in inflammation and promising results in TBI,91 it would be worthwhile to pursue this line of research to determine the effects of elimination NOX2 enzyme activity after SCI.
Conclusion
Research on NOX demonstrates a role in the progression and severity of traumatic CNS injury. It is important to proceed with a note of caution, however, as NOX may also serve an important positive role in injury progression and resolution. For example, NOX-produced ROS contribute to microglial phagocytosis21; microglia are essential in clearing myelin debris and preparing tissue for potential regeneration. This phagocytosis of myelin also causes a burst of ROS in microglia that leads to reduced pro-inflammatory cytokine production.21 Further, NOX plays an essential role in cerebrovascular regulation, as peroxynitrite activity can be involved in normalization of blood flow after stroke.97 NOX as a therapeutic target is, therefore, somewhat complicated. However, in counter to these concerns, recent work and studies explored in this review have shown that NOX inhibition studies show examples of reduced inflammation that ultimately result in improved outcome.74 Therefore, there is likely a balance that NOX activity is involved in following traumatic CNS injury, and therapeutic approaches will have to carefully navigate this road to achieve optimal responses.
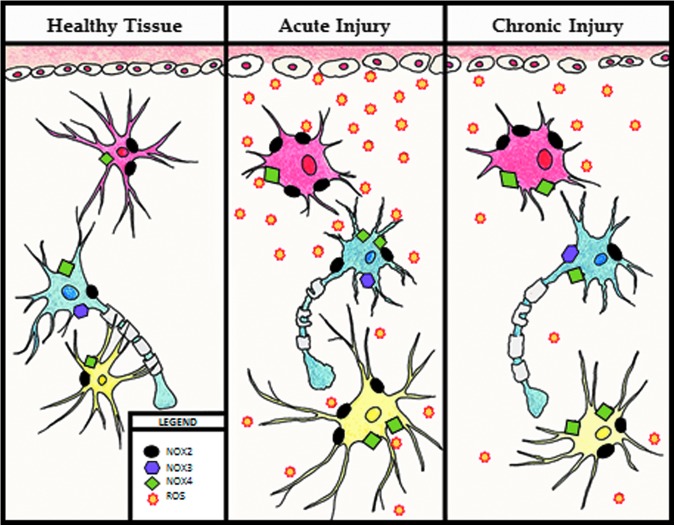
In summary, the NOX family of enzymes, particularly NOX2 and NOX4, clearly plays a central role in oxidative stress after CNS injury, and inhibition of these enzymes may be useful as future therapeutic approaches. Both direct and consequential evidence exist to show that NOX2 and 4 expression and activity correlate with oxidative stress and secondary damage after brain and spinal cord trauma, and contribute to post-injury neuronal loss, chronic inflammation, and functional impairment. The activity and expression of these enzymes changes through the acute and chronic phase, affecting both the level of oxidative stress and inflammatory response post-injury (Fig. 3).
FIG. 3.
Changes to the cellular environment after injury. Healthy tissue: in healthy tissue, microglia (in red) have a resting phenotype represented by a small body with long processes with nicotinamide adenine dinucleotide phosphate (NADPH) oxidase (NOX)4 (green boxes) and NOX2 (black ovals) enzymes present. Neurons (in blue) have an equal representation of NOX2, NOX3 (purple hexagon), and NOX4. Astrocytes (in yellow) have a small body and long processes, and show low expression of NOX2 and NOX4. Acute injury: Immediately post-injury and up to 3 days post-injury, activated microglia bodies become larger and processes shrink. NOX2 activation is increased, and significantly more reactive oxygen species (ROS) (orange starbursts) are produced. Neurons show demyelination and an increase in NOX2 and NOX4 expression. Astrocyte cell bodies are enlarged and processes are elongated, with increased expression of both NOX2 and NOX4. Chronic injury: Microglial phenotype remains enlarged with small processes out to 28 days post-injury, with NOX2 expression and production of ROS remaining elevated, although diminished from the acute phase. An increase in NOX4 expression is noted. In neurons, NOX enzymes return to baseline levels, and re-myelination slowly occurs, although not completely restored to baseline levels. Astrocyte cell bodies remain slightly enlarged and processes retract back to standard length, although some elevation in NOX2 and 4 expression remains. Color image is available online at www.liebertpub.com/neu
Inhibition of NOX activity can alleviate some of these concerns; however, it is necessary to determine a more accurate therapeutic efficacy window and time course. Studies discussed in this review in both TBI and SCI show that pretreatment and post-treatment with both general NOX inhibitors and NOX2 specific inhibitors show improvements in outcomes post-injury, although no one time point of inhibition appears to be ideal for treatment. Pretreatment with NOX inhibitors show improvements post-injury, though these improvements are seen only within the first 48 h post-injury.86,87 Further, it is unlikely that there will be instances in clinical practice in which it would be possible to provide treatment before an injury is sustained. Post- injury, acute treatments result in acute to subacute improvements in outcome,31,91,94,95 but these changes in post-injury inflammation do not appear to be sustained, in part because of the accumulation of ROS at the injury site as treatments wear off and because other producers of ROS continue to produce unimpeded. The novel combined treatment assessed by Johnstone and coworkers96 does present an interesting alternative to the more traditional single therapeutic approach, and demonstrates that improved long-term outcome may require the inhibition of multiple factors in the post-injury cellular environment. Based on the findings of the studies reviewed here, likely the most effective therapeutic window for improved long-term outcomes will require multiple doses, beginning acutely (within the first 24 h after injury), and maintaining at least through the first week post-injury, when peak inflammatory activity is seen. Future research will be needed to both further explore the established and potential inhibitory targets as a therapy and, as findings have demonstrated effectiveness for both NOX2 and nonspecific NOX inhibition, to further investigate the role of the entire NOX family in post-injury outcomes.
Acknowledgments
All figures in this manuscript are original work. The authors acknowledge the contributions in preparing each figure: Ramona von Leden for Figure 1, Young Yauger for Figure 2, and Guzal Khayrullina for Figure 3.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Faul M., Xu L, Wald M., and Coronado V. (2010). Traumatic Brain Injury in the United States: Emergency Department Visits, Hospitalizations and Deaths 2002–2006. Centers for Disease Control and Prevention, National Center for Injury Prevention and Control: Atlanta [Google Scholar]
- 2.Finkelstein E., Corso P., and Miller T. (2006). The Incidence and Economic Burden of Injuries in the United States. Oxford Univerisity Press: New York [Google Scholar]
- 3.Centers for Disease Control and Prevention, National Center for Injury Prevention and Control (2013). Report to Congress on Mild Traumatic Brain Injury in the United States: Steps to Prevent a Serious Public Health Problem. Centers for Disease Control and Prevention, National Center for Injury Prevention and Control: Atlanta [Google Scholar]
- 4.Trivedi A., Olivas A.D., and Noble-Haeusslein L.J. (2006). Inflammation and spinal cord injury: infiltrating leukocytes as determinants of injury and repair processes. Clin. Neurosci. Res. 6, 283–292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Borgens R.B., and Liu-Snyder P. (2012). Understanding secondary injury. Q. Rev. Biol. 87, 89–127 [DOI] [PubMed] [Google Scholar]
- 6.Ali Z.A., de Jesus Perez V., Yuan K., Orcholski M., Pan S., Qi W., Chopra G., Adams C., Kojima Y., Leeper N.J., Qu X., Zaleta-Rivera K., Kato K., Yamada Y., Oguri M., Kuchinsky A., Hazen S.L., Jukema J.W., Ganesh S.K., Nabel E.G., Channon K., Leon M.B., Charest A., Quertermous T., and Ashley E.A. (2014). Oxido-reductive regulation of vascular remodeling by receptor tyrosine kinase ROS1. J. Clin. Invest. 124, 5159–5174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huang J., and Brumell J.H. (2009). NADPH oxidases contribute to autophagy regulation. Autophagy 5, 887–889 [DOI] [PubMed] [Google Scholar]
- 8.Lane T., Carson M., Bergmann C., and Wyss-Coray T. (2008). Central Nervous System Disease and Inflammation. Springer Science and Business Media, New York, NY [Google Scholar]
- 9.Mendez Arent A., de Souza L.F., Walz R., and Dafre A.L. (2014). Perspectives on molecular biomarkers of oxidative stress and antioxidant strategies in traumatic brain injury. BioMed Res. Int. 2014, 723060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Borza C., Muntean D., Dehelean C., Savoiu G., Serban C., Simu G., Andoni M., Butur M., andDragan S. (2013). Oxidative stress and lipid peroxidation - a lipid metabolism dysfunction, in: Lipid Metabolism. Baez R.V. (ed.). InTech, Rijeka, Croatia, pp. 23–38 [Google Scholar]
- 11.Harish G., Mahadevan A., Pruthi N., Sreenivasamurthy S.K., Puttamallesh V.N., Keshava Prasad T.S., Shankar S.K., and Srinivas Bharath M.M. (2015). Characterization of traumatic brain injury in human brains reveals distinct cellular and molecular changes in contusion and pericontusion. J. Neurochem. 134, 156–172 [DOI] [PubMed] [Google Scholar]
- 12.Ansari M.A., Roberts K.N., and Scheff S.W. (2008). Oxidative stress and modification of synaptic proteins in hippocampus after traumatic brain injury. Free Radic. Biol. Med. 45, 443–452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Opii W.O., Nukala V.N., Sultana R., Pandya J.D., Day K.M., Merchant M.L., Klein J.B., Sullivan P.G., and Butterfield D.A. (2007). Proteomic identification of oxidized mitochondrial proteins following experimental traumatic brain injury. J. Neurotrauma 24, 772–789 [DOI] [PubMed] [Google Scholar]
- 14.Cooke M.S., Evans M.D., Dizdaroglu M., and Lunec J. (2003). Oxidative DNA damage: mechanisms, mutation, and disease. FASEB J. 17, 1195–1214 [DOI] [PubMed] [Google Scholar]
- 15.Frohnert B.I., and Bernlohr D.A. (2013). Protein carbonylation, mitochondrial dysfunction, and insulin resistance. Adv. Nutr. 4, 157–163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jia Z.-Q., Li G., Zhang Z.-Y., Li H.-T., Wang J.-Q., Fan Z.-K., and Lv G. (2016). Time representation of mitochondrial morphology and function after acute spinal cord injury. Neural Regen. Res. 11, 137–143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Greco T., Glenn T.C., Hovda D.A., and Prins M.L. (2015). Ketogenic diet decreases oxidative stress and improves mitochondrial respiratory complex activity. J. Cereb. Blood Flow Metab. PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gutierrez J., Ballinger S.W., Darley-Usmar V.M., and Landar A. (2006). Free radicals, mitochondria, and oxidized lipids: the emerging role in signal transduction in vascular cells. Circ. Res. 99, 924–932 [DOI] [PubMed] [Google Scholar]
- 19.Cai Z., and Yan L.-J. (2013). Protein oxidative modifications: beneficial roles in disease and health. J. Biochem. Pharmacol. Res. 1, 15–26 [PMC free article] [PubMed] [Google Scholar]
- 20.Lazarus R.C., Buonora J.E., Jacobowitz D.M., and Mueller G.P. (2015). Protein carbonylation after traumatic brain injury: cell specificity, regional susceptibility, and gender differences. Free Radic. Biol. Med. 78, 89–100 [DOI] [PubMed] [Google Scholar]
- 21.Pajoohesh-Ganji A., and Byrnes K.R. (2011). Novel neuroinflammatory targets in the chronically injured spinal cord. Neurotherapeutics 8, 195–205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ransom B., and Kettenmann H. (2012). Neuroglia. Oxford University Press, New York, NY [Google Scholar]
- 23.Demaurex N., and Scorrano L. (2009). Reactive oxygen species are NOXious for neurons. Nat. Neurosci. 12, 819–820 [DOI] [PubMed] [Google Scholar]
- 24.Block M.L., Zecca L., and Hong J.-S. (2007). Microglia-mediated neurotoxicity: uncovering the molecular mechanisms. Nat. Rev. Neurosci. 8, 57–69 [DOI] [PubMed] [Google Scholar]
- 25.Zhu D., Hu C., Sheng W., Tan K.S., Haidekker M.A., Sun A.Y., Sun G.Y., and Lee J.C.-M. (2009). NAD(P)H oxidase-mediated reactive oxygen species production alters astrocyte membrane molecular order via phospholipase A2. Biochem. J. 421, 201–210 [DOI] [PubMed] [Google Scholar]
- 26.Bedard K., and Krause K.-H. (2007). The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol. Rev. 87, 245–313 [DOI] [PubMed] [Google Scholar]
- 27.Panday A., Sahoo M.K., Osorio D., and Batra S. (2015). NADPH oxidases: an overview from structure to innate immunity-associated pathologies. Cell. Mol. Immunol. 12, 5–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Choi D.-H., Lee K.-H., Kim J.-H., Seo J.-H., Kim H.Y., Shin C.Y., Han J.-S., Han S.-H., Kim Y.-S., and Lee J. (2014). NADPH oxidase 1, a novel molecular source of ROS in hippocampal neuronal death in vascular dementia. Antioxid. Redox Signal. 21, 533–550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cooney S.J., Bermudez-Sabogal S.L., and Byrnes K.R. (2013). Cellular and temporal expression of NADPH oxidase (NOX) isotypes after brain injury. J. Neuroinflammation 10, 155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Krause K.-H. (2004). Tissue distribution and putative physiological function of NOX family NADPH oxidases. Jpn. J. Infect. Dis. 57, S28–29 [PubMed] [Google Scholar]
- 31.Kumar A., Alvarez-Croda D.-M., Stoica B.A., Faden A.I., and Loane D.J. (2015). Microglial/macrophage polarization dynamics following traumatic brain injury. J. Neurotrauma [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Choi D.-H., Kim J.-H., Lee K.-H., Kim H.-Y., Kim Y.-S., Choi W.S., and Lee J. (2015). Role of neuronal NADPH oxidase 1 in the peri-infarct regions after stroke. PloS One 10, e0116814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vlajkovic S.M., Lin S.C.-Y., Wong A.C.Y., Wackrow B., and Thorne P.R. (2013). Noise-induced changes in expression levels of NADPH oxidases in the cochlea. Hear. Res. 304, 145–152 [DOI] [PubMed] [Google Scholar]
- 34.Hill A.J., Drever N., Yin H., Tamayo E., Saade G., and Bytautiene E. (2014). The role of NADPH oxidase in a mouse model of fetal alcohol syndrome. Am. J. Obstet. Gynecol. 210, 466..e1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Groemping Y., and Rittinger K. (2005). Activation and assembly of the NADPH oxidase: a structural perspective. Biochem. J. 386, 401–416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bánfi B., Clark R.A., Steger K., and Krause K.-H. (2003). Two novel proteins activate superoxide generation by the NADPH oxidase NOX1. J. Biol. Chem. 278, 3510–3513 [DOI] [PubMed] [Google Scholar]
- 37.Touyz R.M., Chen X., Tabet F., Yao G., He G., Quinn M.T., Pagano P.J., and Schiffrin E.L. (2002). Expression of a functionally active gp91phox-containing neutrophil-type NAD(P)H oxidase in smooth muscle cells from human resistance arteries: regulation by angiotensin II. Circ. Res. 90, 1205–1213 [DOI] [PubMed] [Google Scholar]
- 38.el Benna J., Faust L.P., and Babior B.M. (1994). The phosphorylation of the respiratory burst oxidase component p47phox during neutrophil activation. Phosphorylation of sites recognized by protein kinase C and by proline-directed kinases. J. Biol. Chem. 269, 23,431–23,436 [PubMed] [Google Scholar]
- 39.Sumimoto H., Hata K., Mizuki K., Ito T., Kage Y., Sakaki Y., Fukumaki Y., Nakamura M., and Takeshige K. (1996). Assembly and activation of the phagocyte NADPH oxidase. Specific interaction of the N-terminal Src homology 3 domain of p47phox with p22phox is required for activation of the NADPH oxidase. J. Biol. Chem. 271, 22,152–22,158 [DOI] [PubMed] [Google Scholar]
- 40.Groemping Y., Lapouge K., Smerdon S.J., and Rittinger K. (2003). Molecular basis of phosphorylation-induced activation of the NADPH oxidase. Cell 113, 343–355 [DOI] [PubMed] [Google Scholar]
- 41.Geiszt M., Kopp J.B., Várnai P., and Leto T.L. (2000). Identification of renox, an NAD(P)H oxidase in kidney. Proc. Natl. Acad. Sci. U. S. A. 97, 8010–8014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Diebold B.A., and Bokoch G.M. (2001). Molecular basis for Rac2 regulation of phagocyte NADPH oxidase. Nat. Immunol. 2, 211–215 [DOI] [PubMed] [Google Scholar]
- 43.Kawahara T., Quinn M.T., and Lambeth J.D. (2007). Molecular evolution of the reactive oxygen-generating NADPH oxidase (Nox/Duox) family of enzymes. BMC Evol. Biol. 7, 109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Martyn K.D., Frederick L.M., von Loehneysen K., Dinauer M.C., and Knaus U.G. (2006). Functional analysis of Nox4 reveals unique characteristics compared to other NADPH oxidases. Cell. Signal. 18, 69–82 [DOI] [PubMed] [Google Scholar]
- 45.Nisimoto Y., Jackson H.M., Ogawa H., Kawahara T., and Lambeth J.D. (2010). Constitutive NADPH-dependent electron transferase activity of the Nox4 dehydrogenase domain. Biochemistry (Mosc.) 49, 2433–2442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Van Buul J.D., Fernandez-Borja M., Anthony E.C., and Hordijk P.L. (2005). Expression and localization of NOX2 and NOX4 in primary human endothelial cells. Antioxid. Redox Signal. 7, 308–317 [DOI] [PubMed] [Google Scholar]
- 47.Wu R.-F., Ma Z., Liu Z., and Terada L.S. (2010). Nox4-derived H2O2 mediates endoplasmic reticulum signaling through local Ras activation. Mol. Cell. Biol. 30, 3553–3568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shiose A., Kuroda J., Tsuruya K., Hirai M., Hirakata H., Naito S., Hattori M., Sakaki Y., and Sumimoto H. (2001). A novel superoxide-producing NAD(P)H oxidase in kidney. J. Biol. Chem. 276, 1417–1423 [DOI] [PubMed] [Google Scholar]
- 49.Nisimoto Y., Diebold B.A., Cosentino-Gomes D., Constentino-Gomes D., and Lambeth J.D. (2014). Nox4: a hydrogen peroxide-generating oxygen sensor. Biochemistry (Mosc.) 53, 5111–5120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ellmark S.H.M., Dusting G.J., Fui M.N.T., Guzzo-Pernell N., and Drummond G.R. (2005). The contribution of Nox4 to NADPH oxidase activity in mouse vascular smooth muscle. Cardiovasc. Res. 65, 495–504 [DOI] [PubMed] [Google Scholar]
- 51.Takac I., Schröder K., Zhang L., Lardy B., Anilkumar N., Lambeth J.D., Shah A.M., Morel F., and Brandes R.P. (2011). The E-loop is involved in hydrogen peroxide formation by the NADPH oxidase Nox4. J. Biol. Chem. 286, 13304–13313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Suh Y.A., Arnold R.S., Lassegue B., Shi J., Xu X., Sorescu D., Chung A.B., Griendling K.K., and Lambeth J.D. (1999). Cell transformation by the superoxide-generating oxidase Mox1. Nature 401, 79–82 [DOI] [PubMed] [Google Scholar]
- 53.Rump T.J., Abdul Muneer P.M., Szlachetka A.M., Lamb A., Haorei C., Alikunju S., Xiong H., Keblesh J., Liu J., Zimmerman M.C., Jones J., Donohue T.M., Persidsky Y., and Haorah J. (2010). Acetyl-L-carnitine protects neuronal function from alcohol-induced oxidative damage in the brain. Free Radic. Biol. Med. 49, 1494–1504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fischer M.T., Sharma R., Lim J.L., Haider L., Frischer J.M., Drexhage J., Mahad D., Bradl M., van Horssen J., and Lassmann H. (2012). NADPH oxidase expression in active multiple sclerosis lesions in relation to oxidative tissue damage and mitochondrial injury. Brain J. Neurol. 135, 886–899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Choi D.-H., Cristóvão A.C., Guhathakurta S., Lee J., Joh T.H., Beal M.F., and Kim Y.-S. (2012). NADPH oxidase 1-mediated oxidative stress leads to dopamine neuron death in Parkinson's disease. Antioxid. Redox Signal. 16, 1033–1045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chéret C., Gervais A., Lelli A., Colin C., Amar L., Ravassard P., Mallet J., Cumano A., Krause K.-H., and Mallat M. (2008). Neurotoxic activation of microglia is promoted by a nox1-dependent NADPH oxidase. J. Neurosci. 28, 12039–12051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kahles T., Kohnen A., Heumueller S., Rappert A., Bechmann I., Liebner S., Wittko I.M., Neumann-Haefelin T., Steinmetz H., Schroeder K., and Brandes R.P. (2010). NADPH oxidase Nox1 contributes to ischemic injury in experimental stroke in mice. Neurobiol. Dis. 40, 185–192 [DOI] [PubMed] [Google Scholar]
- 58.Laurent E., McCoy J.W., Macina R.A., Liu W., Cheng G., Robine S., Papkoff J., and Lambeth J.D. (2008). Nox1 is over-expressed in human colon cancers and correlates with activating mutations in K-Ras. Int. J. Cancer 123, 100–107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kroviarski Y., Debbabi M., Bachoual R., Périanin A., Gougerot-Pocidalo M.-A., El-Benna J., and Dang P.M.-C. (2010). Phosphorylation of NADPH oxidase activator 1 (NOXA1) on serine 282 by MAP kinases and on serine 172 by protein kinase C and protein kinase A prevents NOX1 hyperactivation. FASEB J. 24, 2077–2092 [DOI] [PubMed] [Google Scholar]
- 60.Miyano K., Ueno N., Takeya R., and Sumimoto H. (2006). Direct involvement of the small GTPase Rac in activation of the superoxide-producing NADPH oxidase Nox1. J. Biol. Chem. 281, 21,857–21,868 [DOI] [PubMed] [Google Scholar]
- 61.Cheng G., Cao Z., Xu X., van Meir E.G., and Lambeth J.D. (2001). Homologs of gp91phox: cloning and tissue expression of Nox3, Nox4, and Nox5. Gene 269, 131–140 [DOI] [PubMed] [Google Scholar]
- 62.Bánfi B., Malgrange B., Knisz J., Steger K., Dubois-Dauphin M., and Krause K.-H. (2004). NOX3, a superoxide-generating NADPH oxidase of the inner ear. J. Biol. Chem. 279, 46,065–46,072 [DOI] [PubMed] [Google Scholar]
- 63.Cheng G., Ritsick D., and Lambeth J.D. (2004). Nox3 regulation by NOXO1, p47phox, and p67phox. J. Biol. Chem. 279, 34250–34255 [DOI] [PubMed] [Google Scholar]
- 64.Ueno N., Takeya R., Miyano K., Kikuchi H., and Sumimoto H. (2005). The NADPH oxidase Nox3 constitutively produces superoxide in a p22phox-dependent manner: its regulation by oxidase organizers and activators. J. Biol. Chem. 280, 23,328–23,339 [DOI] [PubMed] [Google Scholar]
- 65.Fulton D.J.R. (2009). Nox5 and the regulation of cellular function. Antioxid. Redox Signal. 11, 2443–2452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bánfi B., Molnár G., Maturana A., Steger K., Hegedûs B., Demaurex N., and Krause K.H. (2001). A Ca(2+)-activated NADPH oxidase in testis, spleen, and lymph nodes. J. Biol. Chem. 276, 37,594–37,601 [DOI] [PubMed] [Google Scholar]
- 67.Björkman U., and Ekholm R. (1984). Generation of H2O2 in isolated porcine thyroid follicles. Endocrinology 115, 392–398 [DOI] [PubMed] [Google Scholar]
- 68.Dupuy C., Pomerance M., Ohayon R., Noël-Hudson M.S., Dème D., Chaaraoui M., Francon J., and Virion A. (2000). Thyroid oxidase (THOX2) gene expression in the rat thyroid cell line FRTL-5. Biochem. Biophys. Res. Commun. 277, 287–292 [DOI] [PubMed] [Google Scholar]
- 69.Damiano S., Fusco R., Morano A., De Mizio M., Paternò R., De Rosa A., Spinelli R., Amente S., Frunzio R., Mondola P., Miot F., Laccetti P., Santillo M., and Avvedimento E.V. (2012). Reactive oxygen species regulate the levels of dual oxidase (Duox1-2) in human neuroblastoma cells. PloS One 7, e34405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Pachucki J., Wang D., Christophe D., and Miot F. (2004). Structural and functional characterization of the two human ThOX/Duox genes and their 5′-flanking regions. Mol. Cell. Endocrinol. 214, 53–62 [DOI] [PubMed] [Google Scholar]
- 71.Dupuy C., Dème D., Kaniewski J., Pommier J., and Virion A. (1988). Ca2+ regulation of thyroid NADPH-dependent H2O2 generation. FEBS Lett. 233, 74–78 [DOI] [PubMed] [Google Scholar]
- 72.Yang D., Elner S.G., Bian Z.-M., Till G.O., Petty H.R., and Elner V.M. (2007). Pro-inflammatory cytokines increase reactive oxygen species through mitochondria and NADPH oxidase in cultured RPE cells. Exp. Eye Res. 85, 462–472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Naik E., and Dixit V.M. (2011). Mitochondrial reactive oxygen species drive proinflammatory cytokine production. J. Exp. Med. 208, 417–420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Choi S.-H., Aid S., Kim H.-W., Jackson S.H., and Bosetti F. (2012). Inhibition of NADPH oxidase promotes alternative and anti-inflammatory microglial activation during neuroinflammation. J. Neurochem. 120, 292–301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Qian L., Gao X., Pei Z., Wu X., Block M., Wilson B., Hong J.-S., and Flood P.M. (2007). NADPH oxidase inhibitor DPI is neuroprotective at femtomolar concentrations through inhibition of microglia over-activation. Parkinsonism Relat. Disord. 13, Suppl. 3, S316–320 [DOI] [PubMed] [Google Scholar]
- 76.Qin L., Liu Y., Hong J.-S., and Crews F.T. (2013). NADPH oxidase and aging drive microglial activation, oxidative stress, and dopaminergic neurodegeneration following systemic LPS administration. Glia 61, 855–868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Li Z., Tian F., Shao Z., Shen X., Qi X., Li H., Wang Z., and Chen G. (2015). Expression and clinical significance of non-phagocytic cell oxidase 2 and 4 after human traumatic brain injury. Neurol. Sci. 36, 61–71 [DOI] [PubMed] [Google Scholar]
- 78.Lucke-Wold B.P., Naser Z.J., Logsdon A.F., Turner R.C., Smith K.E., Robson M.J., Bailes J.E., Lee J.M., Rosen C.L., and Huber J.D. (2015). Amelioration of nicotinamide adenine dinucleotide phosphate-oxidase mediated stress reduces cell death after blast-induced traumatic brain injury. Transl. Res. J. Lab. Clin. Med. 166, 509–528.e1. [DOI] [PubMed] [Google Scholar]
- 79.Song S.-X., Gao J.-L., Wang K.-J., Li R., Tian Y.-X., Wei J.-Q., and Cui J.-Z. (2013). Attenuation of brain edema and spatial learning deficits by the inhibition of NADPH oxidase activity using apocynin following diffuse traumatic brain injury in rats. Mol. Med. Rep. 7, 327–331 [DOI] [PubMed] [Google Scholar]
- 80.Dohi K., Ohtaki H., Nakamachi T., Yofu S., Satoh K., Miyamoto K., Song D., Tsunawaki S., Shioda S., and Aruga T. (2010). Gp91phox (NOX2) in classically activated microglia exacerbates traumatic brain injury. J. Neuroinflammation 7, 41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kumar A., Stoica B.A., Sabirzhanov B., Burns M.P., Faden A.I., and Loane D.J. (2013). Traumatic brain injury in aged animals increases lesion size and chronically alters microglial/macrophage classical and alternative activation states. Neurobiol. Aging 34, 1397–1411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ansari M.A., Roberts K.N., and Scheff S.W. (2014). A time course of NADPH-oxidase up-regulation and endothelial nitric oxide synthase activation in the hippocampus following neurotrauma. Free Radic. Biol. Med. 77, 21–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lu X.-Y., Wang H.-D., Xu J.-G., Ding K., and Li T. (2014). NADPH oxidase inhibition improves neurological outcome in experimental traumatic brain injury. Neurochem. Int. 69, 14–19 [DOI] [PubMed] [Google Scholar]
- 84.Brown G.C. (2010). Nitric oxide and neuronal death. Nitric Oxide 23, 153–165 [DOI] [PubMed] [Google Scholar]
- 85.Zinkevich N.S., and Gutterman D.D. (2011). ROS-induced ROS release in vascular biology: redox-redox signaling. Am. J. Physiol. Heart Circ. Physiol. 301, H647–653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zhang Q.-G., Laird M.D., Han D., Nguyen K., Scott E., Dong Y., Dhandapani K.M., and Brann D.W. (2012). Critical role of NADPH oxidase in neuronal oxidative damage and microglia activation following traumatic brain injury. PloS One 7, e34504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Choi B.Y., Jang B.G., Kim J.H., Lee B.E., Sohn M., Song H.K., and Suh S.W. (2012). Prevention of traumatic brain injury-induced neuronal death by inhibition of NADPH oxidase activation. Brain Res. 1481, 49–58 [DOI] [PubMed] [Google Scholar]
- 88.Ferreira A.P.O., Rodrigues F.S., Della-Pace I.D., Mota B.C., Oliveira S.M., Velho Gewehr C., de C., Bobinski F., de Oliveira C.V., Brum J.S., Oliveira M.S., Furian A.F., de Barros C.S.L., Ferreira J., Santos A.R.S.D., Fighera M.R., and Royes L.F.F. (2013). The effect of NADPH-oxidase inhibitor apocynin on cognitive impairment induced by moderate lateral fluid percussion injury: role of inflammatory and oxidative brain damage. Neurochem. Int. 63, 583–593 [DOI] [PubMed] [Google Scholar]
- 89.Bruce-Keller A.J. (1999). Microglial-neuronal interactions in synaptic damage and recovery. J. Neurosci. Res. 58, 191–201 [DOI] [PubMed] [Google Scholar]
- 90.Loane D.J., Stoica B.A., Byrnes K.R., Jeong W., and Faden A.I. (2013). Activation of mGluR5 and inhibition of NADPH oxidase improves functional recovery after traumatic brain injury. J. Neurotrauma 30, 403–412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lo W., Bravo T., Jadhav V., Titova E., Zhang J.H., and Tang J. (2007). NADPH oxidase inhibition improves neurological outcomes in surgically-induced brain injury. Neurosci. Lett. 414, 228–232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Vaziri N.D., Lee Y.-S., Lin C.-Y., Lin V.W., and Sindhu R.K. (2004). NAD(P)H oxidase, superoxide dismutase, catalase, glutathione peroxidase and nitric oxide synthase expression in subacute spinal cord injury. Brain Res. 995, 76–83 [DOI] [PubMed] [Google Scholar]
- 93.Cooney S.J., Zhao Y., and Byrnes K.R. (2014). Characterization of the expression and inflammatory activity of NADPH oxidase after spinal cord injury. Free Radic. Res. 48, 929–939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Byrnes K.R., Washington P.M., Knoblach S.M., Hoffman E., and Faden A.I. (2011). Delayed inflammatory mRNA and protein expression after spinal cord injury. J. Neuroinflammation 8, 130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Khayrullina G., Bermudez S., and Byrnes K.R. (2015). Inhibition of NOX2 reduces locomotor impairment, inflammation, and oxidative stress after spinal cord injury. J. Neuroinflammation 12, 172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Johnstone J.T., Morton P.D., Jayakumar A.R., Johnstone A.L., Gao H., Bracchi-Ricard V., Pearse D.D., Norenberg M.D., and Bethea J.R. (2013). Inhibition of NADPH oxidase activation in oligodendrocytes reduces cytotoxicity following trauma. PloS One 8, e80975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kunz A., Park L., Abe T., Gallo E.F., Anrather J., Zhou P., and Iadecola C. (2007). Neurovascular protection by ischemic tolerance: role of nitric oxide and reactive oxygen species. J. Neurosci. 27, 7083–7093 [DOI] [PMC free article] [PubMed] [Google Scholar]