To the editor:
Allogeneic bone marrow (BM) transplantation (alloBMT) is a unique curative therapy for diverse diseases. However, its use is limited by the development of severe treatment-related complications, most importantly, the occurrence of acute graft-versus-host-disease (aGVHD). Despite the use of modern immunosuppressive drugs, aGVHD remains the major cause of morbidity and mortality.1-3 aGVHD manifestation depends on the degree of HLA differences between recipients and donors, the T-cell fraction in the graft, patient’s age, and prophylaxis/therapy regimens.1,2,4 In addition, GVHD development is influenced by microbiota in the gut.5-7 Beelen et al demonstrated that mice raised in germ-free conditions did not develop GVHD, whereas control animals raised in conventional conditions died early after transplantation.5 Other studies presented evidence for an association between the GVHD outcome and elimination of Lactobacillales before BMT or reintroduction of probiotic Lactobacillus after BMT.4,8,9 In light of these results, we hypothesized that orally applied antibodies produced in hens to capture and eliminate bacteria and/or bacterial products in the gut might influence the bacterial composition and lead to improvement of GVHD outcome.
Using a haploidentical murine model, B6D2F1 mice conditioned with total-body irradiation received BM cells and splenocytes (SCs) from either syngeneic (B6D2F1) or allogeneic (C57BL/6) donors. Starting 2 days before transplantation (day −2) through day 28 after transplantation, animals received feed pellets containing immunoglobulin yolk (IgY) antibodies from hens immunized with heat-inactivated Escherichia coli, Clostridium perfringens, and Salmonella typhimurium (IgNova GmbH, Oberursel, Germany). In an alternative protocol, animals received identical pellets from day −2 to day 15 for subsequent stool analyses. Thereafter, severity of aGVHD, microbial composition, and cytokine levels were analyzed and compared with control animals receiving feed pellets without IgY.
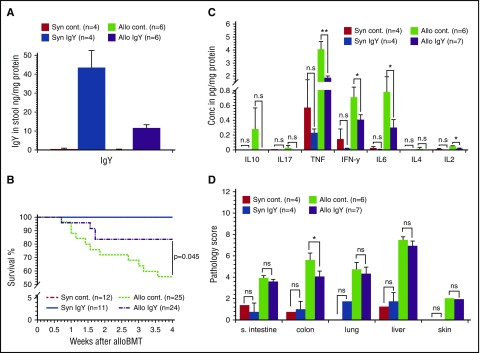
After transplantation, we determined the presence of IgY antibodies in stool and serum samples. IgY antibodies were present in stool samples (Figure 1A), but not in the serum (data not shown). One week after BMT, syngeneic recipients demonstrated minor weight changes due to radiation toxicity-related tissue damage but continuously recovered, and all animals survived until day 28 (Figure 1B). In contrast, allogeneic recipients developed severe clinical symptoms within the first week after transplantation. However, treatment of allogeneic recipients with IgY antibodies resulted in reduced weight loss and decreased clinical GVHD scores when compared with control animals. Furthermore, only 12.5% of the animals receiving IgY feed pellets died within 4 weeks after transplantation (Figure 1B) compared with 40% in the control group (P = .045). On day 28 after alloBMT, animals receiving IgY antibodies exhibited significantly reduced tumor necrosis factor (TNF) serum levels (1.86 ± 0.13 pg/mg protein vs 4.04 ± 0.63 pg/mg protein; P < .01) (Figure 1C). Similarly, levels of interleukin 2 (IL2) and IL6 were significantly reduced in treated animals (0.02 ± 0.003 pg/mg protein vs 0.05 ± 0.01 pg/mg protein [P < .05] and 0.3 ± 0.1 pg/mg protein vs 0.78 ± 0.2 pg/mg protein [P < .05], respectively). However, the difference in interferon γ (IFNγ) levels did not reach statistical significance (0.41 ± 0.07 pg/mg protein vs 0.7 ± 0.14 pg/mg protein; P = .073). Serum analysis on day 7 did not reveal a significant difference in relevant cytokine levels (data not shown).
Figure 1.
IgY antibodies in the stool, survival rates, cytokine levels, and histopathology scores. Lethally irradiated B6D2F1 mice received BM cells supplemented with SCs from either syngeneic (Syn; B6D2F1) or allogeneic (Allo; C57BL/6) donors. Thereafter, animals received feed pellets with IgY or control (cont) pellets without IgY. (A) On day 15 after transplantation, stool samples were isolated and the presence of IgY antibodies was determined by enzyme-linked immunosorbent assay. (B) Survival of syngeneic and allogeneic recipients. (C) Cytokine levels on day 28 after alloBMT. Serum cytokine levels were determined on day 28 after alloBMT using cytokine bead assay. (D) Pathology score 28 days after alloBMT. Histopathology scores for small intestine (s. intestine), colon, liver, lung, and skin at day 28 after BMT. Data are presented as mean ± standard error of the mean (SEM); *P < .05, **P < .01. Conc, concentration; n.s, not significant.
The pathology score, performed 28 days after transplantation, showed less organ injury in syngeneic animals compared with allogeneic animals, indicating the inferior effect of the conditioning regimen on organ damage.10 In contrast, allogeneic mice displayed a high pathology score in liver, lung, small intestine, colon, and skin, whereby the difference between IgY-fed animals and controls was significant for the colon only (4.07 ± 0.57 vs 5.58 ± 0.51; P < .05). The impact of IgY on colon pathology is expected because the colon hosts >1500 bacterial species11,12 and also because of the association between the gut microbiome and GVHD outcome.4,5 In this context, we analyzed the stool microflora at day 15 and detected a significantly reduced bacterial diversity in syngeneic mice compared with allogeneic mice (supplemental Figure 1 [see supplemental Data, available on the Blood Web site]; syngeneic vs allogeneic controls, 0.87 ± 0.02 vs 0.94 ± 0.01 [P = .019] and syngeneic vs allogeneic IgY, 0.8 ± 0.05 vs 0.89 ± 0.01 [P = .038], respectively). Interestingly, there was also a significant difference between allogeneic controls and allogeneic IgY-fed mice (0.94 ± 0.01 vs 0.89 ± 0.01; P = .009). Moreover, the bacterial load differed between allogeneic controls and allogeneic IgY-fed mice, reaching significance at day 15 (4.08 × 109 ± 0.54 × 109 vs 1.1 × 109 ± 0.56 × 109; P = .04).
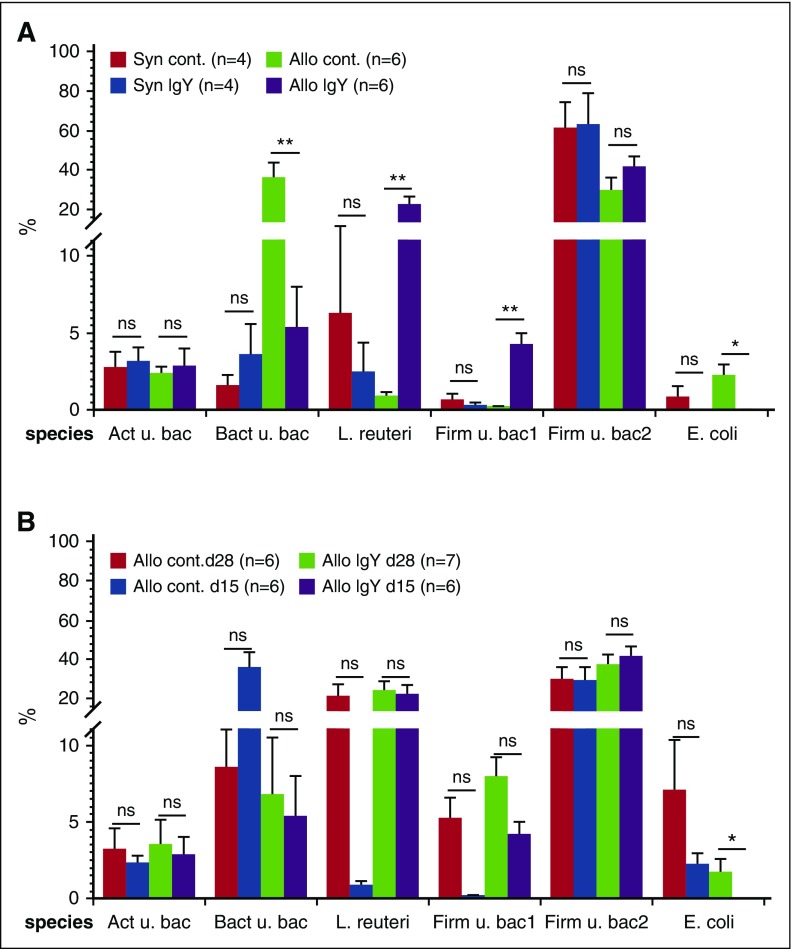
Previous studies demonstrated extensive microbial change in consequence of a decreased production of antimicrobial peptides due to the loss of Paneth cells during aGVHD development.9,13,14 The loss of Paneth cells may account for the higher bacterial load in allogeneic animals compared with syngeneic controls. Analyzing the bacterial composition, we identified bacteria from 10 different phyla and 19 classes, whereby only 5 phyla (Firmicutes, Bacteroidetes, Actinobacteria, Proteobacteria, and Verrucomicrobia) containing 8 classes presented >98% of the whole microbiota (supplementals Figure 2-3). Using analysis of variance, we found significant differences in 4 classes (supplemental Figure 3). Additional Mann-Whitney U test analysis between allogeneic animals receiving IgY pellets and controls showed similar levels at day 28 (data not shown), but at day 15 after transplantation (Figure 2A), we found a significant increase of Lactobacillus reuteri (21.95% ± 4.18% vs 0.85% ± 0.3%) and another uncultured bacterium from the phylum Firmicutes (4.18% ± .08% vs 0.17% ± 0.05%) in allogeneic IgY animals vs controls. These results are in line with a previous study demonstrating the important role of Lactobacillales in the GVHD outcome.8,9 Contrary to the increase of L reuteri and uncultured bacterium from the phylum Firmicutes, we found a decreased level of E coli (0.05% ± 0.02% vs 2.18% ± 0.76%) and another uncultured bacterium from the phylum Bacteroidetes (5.35% ± 2.62% vs 35.65% ± 8.13%) in stool samples of allogeneic IgY-fed animals compared with allogeneic controls. Interestingly, only E coli showed a significant difference between levels at day 15 and day 28 (0.05% ± 0.02% vs 1.63% ± 0.09%; P < .05) in IgY-fed animals (Figure 2B). Our findings are in line with previous studies demonstrating that administration of IgY leads to reduction of the viral load.15,16 The exact mechanisms remain unknown; so far, however, several mechanisms have been proposed in earlier publications, including agglutination, opsonization, and toxin neutralization.17,18 Furthermore, previous studies revealed a correlation between high organ damage, population shift toward E coli, and the abundance of adherent-invasive E coli in patients with Crohn disease compared with healthy subjects.19,20 Of further interest, stool samples of animals receiving IgY pellets presented high levels of Akkermansia compared with controls. Recently, Li and coworkers confirmed the anti-inflammatory role of this genus in atherosclerosis apolipoprotein E–deficient mice (Apoe−/−),21 whereas in another study, the authors showed that this genus is pathogenic.22
Figure 2.
Microbiota composition in stool samples. After transplantation, animals received feed pellets with hen antibodies (IgY) or control pellets (without IgY). The microbiota composition was determined in stool samples using a new generation sequencing method. (A) Microbiome composition on day 15 after transplantation. (B) Microbiome composition of allogeneic mice on days 15 and 28 after transplantation. Data are presented as mean ± SEM; *P < .05, **P < .01. Act, Actinobacteria; Bact, Bacteroidetes; Firm, Firmicutes; u. bac, uncultured bacteria.
In summary, the improvement of aGVHD outcome in animals treated with IgY may be mediated by reducing pathogenic bacteria such as E coli and by increasing the amount of probiotic bacteria such as L reuteri. This microbial shift may account for the reduction of indoleamine 2,3-dioxygenase, Toll-like receptors 2 and 4, and nucleotide-binding oligomerization domain-containing protein 2 expression and a subsequent decrease in chemokine and cytokine expression in the colon (data not shown).
In conclusion, this study confirmed that oral immunoglobulin administration can be considered as an attractive strategy for passive bacterial modification in the gut to improve the GVHD outcome. Combination of this treatment strategy with other approaches23 (antibiotic, probiotic, postbiotic, and prebiotic approaches) in order to manipulate the microbiota-host interactions can improve the GVHD outcome significantly.
Footnotes
The online version of this article contains a data supplement.
Authorship
Acknowledgment: This work was supported by IgNova GmbH, Oberursel, Germany.
Contribution: A.B. designed and performed experiments, analyzed data, and wrote the manuscript; E. Huber performed experiments and analyzed data; J.K. performed experiments; A.H. performed experiments, analyzed data, and wrote the manuscript; A.G. provided reagents and designed experiments; A.D., F.A.A.-A., J.P., G.S., and W.H. provided reagents and wrote the manuscript; and E. Holler provided reagents, designed experiments, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Ernst Holler, Medical Clinic 3–Hematology/Oncology, University of Regensburg Medical Center, Franz-Josef-Strauss Allee 11, D-93053 Regensburg, Germany; e-mail: ernst.holler@ukr.de; and Abdellatif Bouazzaoui, Medical Clinic 3–Hematology/Oncology, University of Regensburg Medical Center, Franz-Josef-Strauss Allee 11, D-93053 Regensburg, Germany; e-mail: abdellatif.bouazzaoui@ukr.de or alazzauoi@uqu.edu.sa.
References
- 1.Ferrara JL, Levine JE, Reddy P, Holler E. Graft-versus-host disease. Lancet. 2009;373(9674):1550-1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Paczesny S, Choi SW, Ferrara JL. Acute graft-versus-host disease: new treatment strategies. Curr Opin Hematol. 2009;16(6):427-436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Armitage JO. Bone marrow transplantation. N Engl J Med. 1994;330(12):827-838. [DOI] [PubMed] [Google Scholar]
- 4.van Bekkum DW, Knaan S. Role of bacterial microflora in development of intestinal lesions from graft-versus-host reaction. J Natl Cancer Inst. 1977;58(3):787-790. [DOI] [PubMed] [Google Scholar]
- 5.Beelen DW, Haralambie E, Brandt H, et al. . Evidence that sustained growth suppression of intestinal anaerobic bacteria reduces the risk of acute graft-versus-host disease after sibling marrow transplantation. Blood. 1992;80(10):2668-2676. [PubMed] [Google Scholar]
- 6.Jenq RR, Taur Y, Devlin SM, et al. . Intestinal blautia is associated with reduced death from graft-versus-host disease. Biol Blood Marrow Transplant. 2015;21(8):1373-1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Taur Y, Jenq RR, Perales MA, et al. . The effects of intestinal tract bacterial diversity on mortality following allogeneic hematopoietic stem cell transplantation. Blood. 2014;124(7):1174-1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gerbitz A, Schultz M, Wilke A, et al. . Probiotic effects on experimental graft-versus-host disease: let them eat yogurt. Blood. 2004;103(11):4365-4367. [DOI] [PubMed] [Google Scholar]
- 9.Jenq RR, Ubeda C, Taur Y, et al. . Regulation of intestinal inflammation by microbiota following allogeneic bone marrow transplantation. J Exp Med. 2012;209(5):903-911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bouazzaoui A, Dickhöfer S, Kreuz M, Huber E, Holler E, Wolff D. Cytostatic conditioning in experimental allogeneic bone marrow transplantation: Busulfan causes less early gastrointestinal toxicity but Treosulfan results in improved immune reconstitution. Immunopharmacol Immunotoxicol. 2014;36(2):158-164. [DOI] [PubMed] [Google Scholar]
- 11.Palmer C, Bik EM, DiGiulio DB, Relman DA, Brown PO. Development of the human infant intestinal microbiota. PLoS Biol. 2007;5(7):e177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xu J, Gordon JI. Honor thy symbionts. Proc Natl Acad Sci USA. 2003;100(18):10452-10459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Holler E, Butzhammer P, Schmid K, et al. . Metagenomic analysis of the stool microbiome in patients receiving allogeneic stem cell transplantation: loss of diversity is associated with use of systemic antibiotics and more pronounced in gastrointestinal graft-versus-host disease. Biol Blood Marrow Transplant. 2014;20(5):640-645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eriguchi Y, Takashima S, Oka H, et al. . Graft-versus-host disease disrupts intestinal microbial ecology by inhibiting Paneth cell production of α-defensins. Blood. 2012;120(1):223-231. [DOI] [PubMed] [Google Scholar]
- 15.Rahman S, Van Nguyen S, Icatlo FC Jr, Umeda K, Kodama Y. Oral passive IgY-based immunotherapeutics: a novel solution for prevention and treatment of alimentary tract diseases. Hum Vaccin Immunother. 2013;9(5):1039-1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vega CG, Bok M, Vlasova AN, et al. . IgY antibodies protect against human Rotavirus induced diarrhea in the neonatal gnotobiotic piglet disease model. PLoS One. 2012;7(8):e42788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xu Y, Li X, Jin L, et al. . Application of chicken egg yolk immunoglobulins in the control of terrestrial and aquatic animal diseases: a review. Biotechnol Adv. 2011;29(6):860-868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li X, Jing K, Wang X, et al. . Protective effects of chicken egg yolk antibody (IgY) against experimental Vibrio splendidus infection in the sea cucumber (Apostichopus japonicus). Fish Shellfish Immunol. 2016;48:105-111. [DOI] [PubMed] [Google Scholar]
- 19.Heimesaat MM, Fischer A, Siegmund B, et al. . Shift towards pro-inflammatory intestinal bacteria aggravates acute murine colitis via Toll-like receptors 2 and 4. PLoS One. 2007;2(7):e662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Martinez-Medina M, Aldeguer X, Lopez-Siles M, et al. . Molecular diversity of Escherichia coli in the human gut: new ecological evidence supporting the role of adherent-invasive E. coli (AIEC) in Crohn’s disease. Inflamm Bowel Dis. 2009;15(6):872-882. [DOI] [PubMed] [Google Scholar]
- 21.Li J, Lin S, Vanhoutte PM, Woo CW, Xu A. Akkermansia muciniphila protects against atherosclerosis by preventing metabolic endotoxemia-induced inflammation in Apoe-/- mice. Circulation. 2016;133(24):2434-2446. [DOI] [PubMed] [Google Scholar]
- 22.Shono Y, Docampo MD, Peled JU, et al. . Increased GVHD-related mortality with broad-spectrum antibiotic use after allogeneic hematopoietic stem cell transplantation in human patients and mice. Sci Transl Med. 2016;8(339):339ra71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Peled JU, Jenq RR, Holler E, van den Brink MR. Role of gut flora after bone marrow transplantation. Nat Microbiol. 2016;1:16036. [DOI] [PMC free article] [PubMed] [Google Scholar]