Abstract
Migraine is among the most common diseases on earth and one of the most disabling, the latter due in large part to poor treatment efficacy. Development of new therapeutics is dependent on the identification of mechanisms contributing to migraine and discovery of targets for new drugs. Numerous genome-wide association studies (GWAS) have implicated the transient receptor-potential M8 (TRPM8) channel in migraine. This channel is predominantly expressed on peripheral sensory neurons and is known as the sensor for cold temperature in cutaneous tissue but is also expressed on deep visceral afferents where cold is not likely a stimulus. Consequently, a number of alternative endogenous agonists have been proposed. Apart from its role in cold sensation, TRPM8 also contributes to cold allodynia after nerve injury or inflammation, and it is necessary for cooling/menthol-based analgesia. How it might contribute to migraine is less clear. The purpose of this review is to discuss the anatomical and physiological mechanisms by which meningeal TRPM8 may play a role in migraine as well as the potential of TRPM8 as a therapeutic target. TRPM8 is expressed on sensory afferents innervating the meninges, and these neurons are subject to developmental changes that may influence their contribution to migraine. As in viscera, meningeal TRPM8 channels are unlikely to be activated by temperature fluctuations and their endogenous ligands remain unknown. Preclinical migraine studies show that activation of meningeal TRPM8 by exogenous agonists can both cause and alleviate headache behaviors, depending on whether other meningeal afferents concurrently receive noxious stimuli. This is reminiscent of the fact that cold can trigger migraine in humans but menthol can also alleviate headache. We propose that both TRPM8 agonists and antagonists may be potential therapeutics, depending on how migraine is triggered in individual patients. In this regard, TRPM8 may be a novel target for personalized medicine in migraine treatment.
Keywords: dura, meninges, trigeminal, menthol, icilin, TRP
TRANSIENT RECEPTOR POTENTIAL (TRP) CHANNELS
TRP channels are a large family of non-selective cation channels that are expressed on the plasma membrane as well as the membranes of intracellular organelles. Based on their amino acid sequence homology, TRP channels are divided into six subfamilies including TRPC, TRPM, TRPV, TRPA, TRPP, and TRPML.1 They can be activated by a wide variety of stimuli, including changes in temperature, osmolarity, pH as well as various natural products.2 Activation of TRPs allows the influx of Ca2+ and Na+, resulting in membrane depolarization as well as the activation of second messenger signaling cascades.2 It is well established that TRP channels participate in the sensory encoding of pain under both normal and disease states.3 Recent studies suggest that multiple TRP channels, including TRPV1, TRPA1, TRPV1, and TRPM8, contribute to the pathophysiology of headache and may represent novel targets for headache therapeutics.4
TRPM8 POLYMORPHISM AND MIGRAINE SUSCEPTIBILITY
Migraine is one of the most common neurovascular disorders with a strong genetic component. One of the TRP channels, the transient receptor potential melastatin 8 (TRPM8), has been consistently identified in several genome-wide association studies (GWAS) as one of the migraine susceptibility genes in multiple cohorts.5–9 All three TRPM8 single nucleotide polymorphisms (SNPs) are associated with both migraine with and without aura, and show little selectivity for any of the migraine characteristics.10 The SNPs rs75772625 and rs101669426-9 are 7 kb and 950 bp upstream of the transcription start site for TRPM8 mRNA, respectively. Carriers of the rarer allele of rs10166942 (C;C or C;T) have a lower risk of migraine, compared with those carrying two common alleles (T;T).6,8 Another SNP rs17862920 is in the first intron of TRPM8 gene.8 It remains to be tested whether and how the three variants alter the TRPM8 expression level, as suggested by their locations in regions involved in transcriptional regulation. However, a recent study suggests that some TRPM8 SNP variants causing amino acid substitutions affect the expression level as well as the function of TRPM8 channels in cell lines,11 providing support for the idea that the variants identified in migraine patients may indeed impact channel expression/function.
TRPM8 channels are known to be activated by temperatures below 26°C as well as by cooling agents such as menthol and icilin.3,12,13 Recent studies show that TRPM8 also responds to a broad spectrum of endogenous and exogenous ligands (see below). Both pharmacological blockade and genetic knockout experiments have revealed that TRPM8 is essential in the detection of cool to noxious cold temperatures as well as the discrimination between warm and cold temperatures.14–19 Cold temperature is known to be a migraine trigger,20 and more than 50% of migraine patients exhibit cold allodynia, with mean cold pain threshold shifted by about 12°C.21 Conversely, topical application of the TRPM8 agonist menthol offers pain relief in some migraine patients.22 Collectively, these studies strongly suggest a role of TRPM8 channels in migraine pathogenesis.
THE EXPRESSION OF TRPM8 CHANNELS AND THE PROJECTION OF TRPM8-EXPRESSING PRIMARY AFFERENT NEURONS
In mice, the expression of TRPM8 in primary afferent neurons (PANs) in the trigeminal ganglion (TG) and dorsal root ganglion (DRG) starts around embryonic day 14.5 (E14.5) and reaches the level found in adult PANs by E18.5.23 In adult mice, TRPM8 is present in a distinct population of small-diameter PANs. These neurons do not bind to the plant isolectin B4 (IB4), a marker for non-peptidergic PANs in rodents. However, only a small percentage of TRPM8-containing PANs expresses the neuropeptide calcitonin gene-related peptide (CGRP) and TRPV1 channels.14,24–26 The fraction of TG neurons expressing TRPM8 channels is similar between the three trigeminal divisions (~12%,27). Within the TRPM8-expressing TG population, approximately half of the neurons are localized in the V3 division of the TG, and the remaining half are distributed uniformly between the V1 and V2 divisions.25,27 TRPM8 is not expressed in sympathetic neurons in the superior cervical ganglion and is sparsely expressed in the nodose ganglion.28–31 Thus, most TRPM8-containing fibers represent projection of PAN axons to the target tissues.
PANs expressing TRPM8 channels innervate many peripheral tissues that regularly receive cold stimuli, including skin, oral cavity, teeth, taste buds, and cornea.23,24,32–34 Lips, masticatory muscles, and temporomandibular joints are mostly devoid of TRPM8-expressing fibers.34 Interestingly, TRPM8 is also expressed in PANs that project to deep tissues such as colon and bladder.35–37 These nerve endings are usually not exposed to cool or cold temperature that activates TRPM8, raising the possibility that TRPM8 in deep tissues and visceral organs may respond to other endogenous ligands (see below for more detailed discussion).
The projection of TRPM8-expressing fibers to the dura has been studied with a mouse line expressing farnesylated enhanced green fluorescent protein (EGFPf) from one of the TRPM8 loci (TRPM8EGFPf/+). Sparse innervation of the EGFP-positive, TRPM8-expressing fibers are observed in some areas of the dura in adult mice.38 Interestingly, both the density and the number of axonal branches of dural fibers expressing TRPM8 are decreased substantially from postnatal day 2 (P2) to adulthood.39 The reduction occurs before the onset of puberty (P25) in both male and female mice, and is independent of the expression and/or the activation of TRPM8 channels per se. Conversely, the density and the number of branches of CGRP-expressing dural fibers are comparable to those expressing TRPM8 channels at P2 and remain stable to adulthood. The density of TRPM8-expressing fibers innervating the mouse cornea epithelium is significantly increased from P2 to adulthood. These results suggest that TRPM8-expressing dural afferent fibers undergo unique cell- and target tissue-specific axonal pruning during postnatal development. The underlying mechanisms and the functional consequences of these changes are not clear at present.
TRPM8-expressing PANs innervate the dorsal horn of the spinal cord at approximately E16.5 and confine the projection at the superficial laminae as in adult mice by P10.23 However, the neuronal circuits encoding cold sensation are not fully functional until P14 in mice.23 It has been shown that TRPM8-expressing central projecting fibers are selectively coupled to a subpopulation of neurons in the dorsal horn of the spinal cord.40 Both TRPM8-positive DRG neurons and a subset of dorsal horn neurons express cadherin-8 (cad8,40), which mediates subtype-specific cell-cell adhesion. Morphological study reveals synapse formation between the cad8-expressing PAN central terminals and cad8-expressing dorsal horn neurons. Electro-physiological recording indicates that menthol selectively increases the synaptic inputs to cad8-positive dorsal horn neurons and this effect is abolished in cad8 knockout mice, which exhibit a reduced sensitivity to cold temperature. Thus, cad8 is essential for establishing the physiological coupling between TRPM8-expressing PANs and their target neurons in the dorsal horn of the spinal cord. The functional relevance of the connectivity is not yet clear, although TRPM8-expressing PANs play a complex role in sensory signaling (see discussion below) and their connectivity within the dorsal horn may contribute to these effects.
ENDOGENOUS LIGANDS FOR DURAL TRPM8
The most obvious function for TRPM8 on sensory nerve endings is in the detection and transduction of environmental cold temperature. TRPM8 is also expressed on vagal afferent neurons innervating the trachea and activation of the channel leads to afferent signaling.31 Although the trachea is exposed to cold temperature when breathing cold air, which similarly points to a role for the channel in cold detection, these studies nonetheless demonstrate that TRPM8 has functions outside of simple cold sensation and may contribute to cold-induced autonomic responses. But it is not immediately clear why cold would be a relevant stimulus for TRPM8 in the dura as temperature fluctuations inside the skull would rarely reach levels necessary to activate this channel. The same could be said for the colon and bladder but TRPM8 is expressed on afferents innervating these tissues as well.35,37 It could be the case that afferent signaling from the dura is necessary if the dura were to reach cold enough temperatures as this would be a clear signal that one was in a potentially damaging environment (ie, one that is substantially too cold). But this is highly speculative and it is more likely that TRPM8 serves a function in the dura that is independent of cold transduction. Additional functions have been demonstrated for this channel including in the detection of volatile chemicals such as odorants41 or in changes in extracellular osmolarity.42 Detection of the former seems unlikely in the dura although osmolarity changes may be important. Other endogenous ligands have been proposed including various lipids, the growth factor artemin,43,44 and even testosterone.45 The membrane phosphoinositide PIP2 is critical in the proper gating of the channel46,47 and it was recently shown that an endogenous membrane protein known as phosphoinositide interacting regulator of TRP (Pirt) is also an endogenous regulator of TRPM8 function.48 In the case of all of these activators and regulators, there is no clear link to headache with any of the stimuli and the endogenous function of TRPM8 in the dura remains unknown.
THE ROLE OF TRPM8 CHANNELS IN SOMATIC AND VISCERAL PAIN MODELS
Given that TRPM8 is a non-selective cation channel, it is clear that channel activation leads to depolarization of nerve endings and afferent input into the central nervous system. This is undoubtedly the mechanism by which cool temperatures as well as cooling agents such as menthol are sensed on the skin. There are distinct sub-populations of TRPM8-expressing sensory neurons that respond to cool/cold and menthol. Some of these neurons have properties consistent with nociceptors, and are preferentially activated by noxious cold temperatures. The majority of them co-express TRPV1, TRPA1, tetrodotoxin-resistant Na+ channels as well ATP receptors.26,49,50 Others have non-nociceptive properties, and are activated at lower menthol concentrations or at cooling temperature range. Their response properties and intracellular signaling pathway engagement following stimulation are different.31,49,51 Consistent with the physiological data, numerous behavioral studies have shown that activation of TRPM8 is necessary for the responses to cool and cold temperatures as well as cooling agents under normal conditions.14–16,52,53 TRPM8 is also essential for the expression of cold hypersensitivity after inflammation and nerve injury.14,15,52–56 Administration of TRPM8 antagonists, knockout of the TRPM8 gene, or ablation of TRPM8-expressing PANs effectively attenuate cold pain associated with tissue and/or nerve injury.17,19 Conversely, ablation of PANs expressing CGRP enhances TRPM8-mediated behavioral responses to cold in mice.57
But cooling and TRPM8 activators are well known to be analgesic, thus the popularity of menthol-based topical therapeutics for a variety of pain conditions. Preclinical studies have shown that application of menthol to the skin decreases activity of dorsal horn neurons to noxious heat and also decreases behavioral responses to noxious heat.58,59 Behaviorally, menthol effectively diminishes pain behavior elicited by chemical stimuli, noxious heat, and inflammation.16,18 Of note, TRPM8-mediated analgesia in the hot plate test is blocked by naloxone, suggesting activation of endogenous opioid-dependent analgesic pathways.18 In addition, there is anti-nociceptive efficacy of TRPM8 activators in models of nerve injury.17,60,61 The ability of cold temperature to produce anti-nociceptive responses in mice after nerve injury is eliminated in the absence of TRPM8,17 demonstrating a requirement for this channel in the analgesic responses to cold and likely menthol. TRPM8 has also been shown to form complexes with the 5-HT–1B receptor in PANs, and amplifies the analgesic effects of both TRPM8 activators and 5-HT–1B agonists in tissue-and nerve-injury models in rats.62
Taken together, TRPM8-expressing afferent fibers appear to have the ability to both produce and alleviate pain and context may determine which is ultimately produced (Fig. 1). Supporting this notion are preclinical studies using an operant behavioral model showing that activation of TRPM8 alone can produce behavioral signs of nociception but activation of TRPM8 can decrease the nociceptive responses due to concurrent TRPV1 activation.63 These data are consistent with Klein et al above (ie, where menthol decreases responses to noxious heat) and suggest that when TRPM8 is activated alone, the resulting sensation is either cool or cold pain (depending on stimulus magnitude) but when TRPM8 is activated in the presence of other noxious stimuli, the result is analgesia.
Fig. 1.
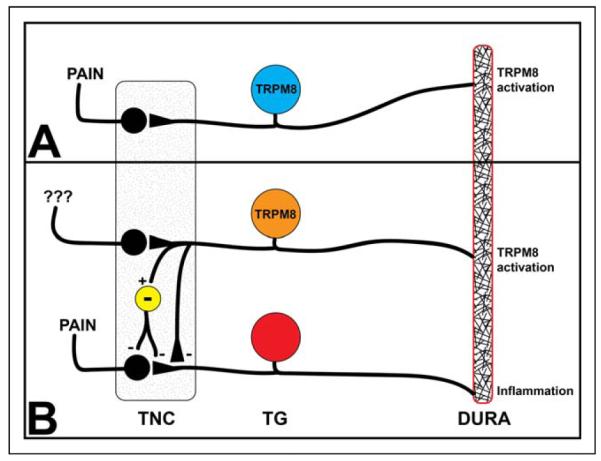
TRPM8 activation in the dura can be pro- or anti-nociceptive. Panel A shows that activation of TRPM8-expressing fibers (blue) in the absence of other stimuli causes afferent signaling into the trigeminal nucleus caudalis (TNC), activation of second-order neurons that project into the brain, and ultimately to the perception of pain. Panel B shows that activation of TRPM8-expressing fibers (orange) in the presence of ongoing activation of other TG nociceptors (red) by stimuli such as inflammation can inhibit nociceptive signaling. The inhibition of nociceptive afferent signaling by TRPM8-expressing fibers can either be via direct inhibition of TG nociceptor central terminals (orange to red) or by activation of inhibitory TNC interneurons (yellow) that synapse onto nociceptor afferents or onto second-order TNC projection neurons. [Color figure can be viewed at wileyonlinelibrary.com]
In visceral pain models, activation of TRPM8 also exhibits both pro- and anti-nociceptive effects. AMTB, a TRPM8 antagonist, significantly attenuates bladder distension-induced nociceptive reflex in rats, suggesting that activation of TRPM8 contributes to overactive bladder and painful bladder syndrome.64 Conversely, activation of TRPM8 by icilin attenuates inflammatory responses in mouse models of colitis.65 Single fiber recordings of colonic afferents show that topical application of icilin desensitizes afferents to mechanical and chemical stimuli,35 suggesting that the analgesic effect of TRPM8 activation may occur at the level of PAN. So in addition to the complex actions of TRPM8 in cutaneous tissues, this channel appears to also have a dual role in nociceptive signaling from deep tissue.
IS ACTIVATION OF TRPM8 WITHIN THE DURA PRO- OR ANTI-NOCICEPTIVE?
One of the biggest questions related to determining a role for TRPM8 in migraine is whether activation of TRPM8 within the meninges leads to an increase or a decrease in pain. Although the endogenous ligand for TRPM8 within the dura is not known, activation of the channel is nonetheless likely to depolarize nerve endings and initiate afferent input. It is not clear whether TRPM8-expressing dural afferent fibers have the properties of nociceptors and/or non-nociceptors. As discussed above, activation of TRPM8-expressing dural afferents is not necessarily pronociceptive (or pro-sensory) despite the initiation of afferent input. Ultimately it is the central connections of these neurons that determines their function and central connections of this population of afferents are unique compared with other sensory afferents.66 Consequently, processing/perception of input from TRPM8-expressing dural afferents may have unique features and may also depend on context (Fig. 1).
Most relevant to migraine are two recent studies examining the effects of TRPM8 activation in experimental preclinical models of this disorder. In one of these studies, the TRPM8 activator icilin was applied to the dura of rats which caused cutaneous allodynia that was blocked by co-administration of sumatriptan or a nitric-oxide synthase inhibitor.67 Consistent with the discussion above, this finding suggests that activation of dural TRPM8 alone produces pro-nociceptive behavior. In contrast, when dural TRPM8 was activated with menthol in mice, the effects were anti-nociceptive.39 Importantly in the latter study, TRPM8 was not activated alone but was activated concurrently with inflammatory mediator application to the dura. So again consistent with the discussion above, when TRPM8 is activated in the presence of other stimuli, the result is anti-nociceptive. These preclinical headache studies, along with pain studies from the spinal system, paint a complex picture of the role of TRPM8-expressing PANs in sensory perception where the final result of activation of these neurons is context dependent. Ultimately, it remains unclear how activation of TRPM8 within the meninges contributes to the pathophysiology of migraine. It is also unclear whether this role is restricted to episodic migraine (<15 headache days/month) or whether it may also contribute to chronic migraine (15 or more headache days/month).
TRPM8 AS A THERAPEUTIC TARGET FOR NON-HEADACHE DISORDERS
Interest in development of TRPM8-modulating drugs is not unique to migraine as either activators or blockers have been suggested as therapeutics for other pain states and disorders. As discussed above, TRPM8 is thought to mediate cold allodynia in a variety of neuropathic pain states so not surprisingly there is interest in TRPM8 antagonists for neuropathic pain.68 It should be noted however that TRPM8 agonists have also been shown to have efficacy in preclinical neuropathic pain models54 and topical menthol was recently reported to provide pain relief to patients with chemotherapy-induced peripheral neuropathy.69 In addition, a recent study found that the TRPM8 antagonist AMG2850 did not have efficacy in either an inflammatory or neuropathic preclinical pain model70 causing some uncertainty for the efficacy of TRPM8 therapeutics (at least antagonists) in non-headache pain.
Outside of pain, one prominent area of interest for TRPM8 as a therapeutic target is in the control of bladder function, specifically in bladder filling and voiding mechanisms. Several studies have shown activation of bladder TRPM8 decreases volume of bladder filling and decreases voiding intervals71,72 while antagonists cause the opposite effects, leading to the possibility of TRPM8 antagonists as therapeutics for conditions such as overactive bladder. Two recent preclinical studies further support this concept, one using menthol as a TRPM8 activator to increase activity of mechanically sensitive bladder afferents while the other used a newly characterized TRPM8 antagonist, DFL23448, to attenuate overactive bladder symptoms.73,74 There is also interest in TRPM8 as a therapeutic target for cancer75 as channel expression has been demonstrated in numerous tumor types.76 However, it remains unclear at this point how to target the channel as activation of TRPM8 can either promote tumor survival or decrease tumor viability, depending on the tumor. Finally, menthol is widely used as an antitussive agent, implicating TRPM8 in cough reflexes.77 Here, it is thought that TRPM8 agonists may have efficacy for cough. However, cold temperature is also known to promote asthmatic symptoms75 and as described above, activation of TRPM8 excited vagal afferents from the airways that may contribute to broncho-constrictive responses.31
Based on the above rationale, new TRPM8-modulating molecules have been developed and characterized in the last several years (for excellent tables summarizing older or more commonly used TRPM8 agonists and antagonists see75,76 and68). Recent examples of novel TRPM8 modulators include compounds 12 (inhibitor) and 21 (activator),78 and the blockers PF-05105679,79 AMG2850,70 compound 4580 (this compound is the same as AMG1161 in67), RQ-00203078,81 another compound 12,82 and DFL23448.73 Interestingly, a recent report from a team at Amgen used commercially available TRPM8 antibodies that recognize epitopes on the extracellular surface as antagonists to block channel function.83 Use of one of these antibodies as an antagonist (ACC-049) was able to block multiple modes of channel activation (eg, cold, menthol, and icilin) in both cell lines and DRG neurons providing proof of concept that antagonist antibodies for TRPM8 can potentially be developed. Together, these studies highlight the continuing interest in drug development for TRPM8.
One important aspect related to the development of TRPM8-based therapeutics, regardless of whether they are tested for migraine, overactive bladder, or other indications, is the concern over adverse effects of these drugs. Little information exists from human clinical trials with TRPM8 modulators (outside of natural products such as menthol) so it is still unclear what, if any, problems will occur with these drugs. But given the role for TRPM8 in the variety of biological/pathological systems described above, one could speculate that a TRPM8 modulator used for migraine may cause adverse effects in the bladder, airways, and possibly a contribution to tumor survival. There would also likely be some impairment in the ability to sense cold temperatures, although for an acute migraine therapeutic that is only taken a few times/month this may not be an issue. In addition, it has been shown preclinically that block of TRPM8 can decrease core body temperature by approximately 1°C84,85 raising concerns of hypothermia with TRPM8 antagonists, but the decrease in body temperature does not occur after repetitive dosing. A recent clinical study in 32 subjects found that PF-05105679 did not raise core body temperature at doses that blocked pain in the cold pressor test.86 While no other major adverse events were reported in this study, minor events included oral hypoesthesia and dysgeusia, and some subjects (23–36%, depending on dose) reported a paradoxical hot sensation in the face and upper body that was intolerable in two cases. It still remains to be determined what adverse events would occur in larger patient populations and with repetitive dosing.
REMAINING QUESTIONS AND FUTURE OF TRPM8 AS A MIGRAINE DRUG TARGET
Many questions still remain surrounding the role of TRPM8 in migraine. These questions cover the following anatomic/functional areas: (1) the postnatal pruning of TRPM8-expressing dural afferent fibers in mice occurs between P16-21, right before the onset of puberty (P25). This coincides with the increase in migraine prevalence from childhood to early adulthood in humans.87 Whether a similar postnatal change of TRPM8-expressing meningeal fibers occur in humans and, if so, whether and how it contributes to the increase in migraine prevalence merits further study. (2) Retrograde-labeling of TRPM8-expressing dural afferent neurons in adult mice has not been successful,27 likely due to the sparse innervation and the lack of extensive axonal branches of these fibers. Efforts need to be made to bypass this technical hurdle to label TRPM8-expressing dural afferent neurons/fibers (possibly using genetic tools) for both physiological and morphological studies. For example, knowing the abundance of nociceptive vs non-nociceptive neurons within the TRPM8-expressing meningeal afferent population will help elucidate the role of TRPM8 in migraine pathophysiology. In addition, studies where TRPM8-expressing meningeal afferents are either selectively activated or silenced (using opto- or chemogenetics) would allow for additional preclinical studies that can better tease apart a role for these fibers in events contributing to migraine. (3) A critical missing piece in uncovering how TRPM8 may contribute to migraine is to know whether channel activation in the meninges occurs alone or in the presence of other events. Whether dural inflammation is present at the time of TRPM8 activation can determine how the afferent fibers influence headache. (4) Uncovering more information on the endogenous activator of TRPM8 within the meninges can help to determine whether it is likely that the channel is activated alone or whether it would only be activated along with other events. This can also help determine the approximate magnitude of the activation, which is important in understanding which sub-population of TRPM8 afferents might be activated. (5) More work is needed to determine whether extracranial TRPM8-expressing fibers play a role in cold-triggered migraine and/or migraine-induced cold allodynia. Some meningeal afferents may issue collaterals that innervate the periosteum through sutures on the skull.88,89 Whether or not these fibers include TRPM8-expressing dural afferents remains to be tested. (6) Whether TRPM8 plays a role in episodic or chronic migraine (or both) should be examined. Fortunately, animal models of chronic migraine exist,90,91 so preclinical experiments can shed light on this question.
Several questions related to drug development for TRPM8 as a migraine therapeutic also remain to be answered. (1) The most important question related to TRPM8 drugs for migraine is whether these drugs would need to be agonists or antagonists. As mentioned above, cold can trigger migraine attacks and patients report cold allodynia during migraines, but there are also reports of cold and menthol application having therapeutic efficacy for migraine. At this point it is largely unclear which direction channel activity should change for efficacy. This determination would be helped by a better understanding of the points raised above, namely whether activation of TRPM8 in the meninges is pro- or anti-nociceptive and what conditions determine these effects. (2) Possible pruning of TRPM8-expressing fibers could impact drug efficacy. If TRPM8 expression within the dura of adult humans is minimal, there may not be sufficient target available for channel agonists/antagonists to modulate. (3) A better understanding of how variants in TRPM8 contribute to channel function/expression is critical. Related to the points above, it will not be clear whether TRPM8-based migraine drugs need to be agonists or antagonists until the effects of these gene variants on channel function/expression/contribution to migraine are known. (4) Determining whether TRPM8-based therapeutics would have efficacy as acute agents or would need to be taken daily as preventative agents is an important question to resolve. Similarly, answering whether these drugs would have efficacy for episodic vs chronic migraine would help determine treatment protocols. (5) Would potential adverse events limit the utility of these drugs as is commonly the case with many current migraine therapeutics.
Taken together, the studies listed above strongly suggest a contribution of TRPM8 to migraine. Given the current lack of efficacious therapeutics for this highly prevalent and extremely disabling disorder, a better understanding of how TRPM8 contributes to migraine may lead to the development of new therapeutics based on modulation of TRPM8 activity.
Acknowledgments
This review was written with support from NIH (NS072204, GD) and (NS083698, YC).
Footnotes
Category 1
(a) Conception and Design
Greg Dussor, Yu-Qing Cao
(b) Acquisition of Data
Greg Dussor, Yu-Qing Cao
(c) Analysis and Interpretation of Data
Greg Dussor, Yu-Qing Cao
Category 2
(a) Drafting the Manuscript
Greg Dussor, Yu-Qing Cao
(b) Revising It for Intellectual Content
Greg Dussor, Yu-Qing Cao
Category 3
(a) Final Approval of the Completed Manuscript
Greg Dussor, Yu-Qing Cao
REFERENCES
- 1.Wu LJ, Sweet TB, Clapham DE. International Union of Basic and Clinical Pharmacology. LXXVI. Current progress in the mammalian TRP ion channel family. Pharmacol Rev. 2010;62:381–404. doi: 10.1124/pr.110.002725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ramsey IS, Delling M, Clapham DE. An introduction to TRP channels. Annu Rev Physiol. 2006;68:619–647. doi: 10.1146/annurev.physiol.68.040204.100431. [DOI] [PubMed] [Google Scholar]
- 3.Julius D. TRP channels and pain. Annu Rev Cell Dev Biol. 2013;29:355–384. doi: 10.1146/annurev-cellbio-101011-155833. [DOI] [PubMed] [Google Scholar]
- 4.Dussor G, Yan J, Xie JY, Ossipov MH, Dodick DW, Porreca F. Targeting TRP channels for novel migraine therapeutics. ACS Chem Neurosci. 2014;5:1085–1096. doi: 10.1021/cn500083e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chasman DI, Anttila V, Buring JE, et al. Selectivity in genetic association with sub-classified migraine in women. PLoS Genetics. 2014;10:e1004366. doi: 10.1371/journal.pgen.1004366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chasman DI, Schurks M, Anttila V, et al. Genome-wide association study reveals three susceptibility loci for common migraine in the general population. Nat Genet. 2011;43:695–698. doi: 10.1038/ng.856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Esserlind AL, Christensen AF, Le H, et al. Replication and meta-analysis of common variants identifies a genome-wide significant locus in migraine. Eur j Neurol. 2013;20:765–772. doi: 10.1111/ene.12055. [DOI] [PubMed] [Google Scholar]
- 8.Freilinger T, Anttila V, de Vries B, et al. Genome-wide association analysis identifies susceptibility loci for migraine without aura. Nat Genet. 2012;44:777–782. doi: 10.1038/ng.2307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sintas C, Fernandez-Morales J, Vila-Pueyo M, et al. Replication study of previous migraine genome-wide association study findings in a Spanish sample of migraine with aura. Cephalalgia. 2015;35:776–782. doi: 10.1177/0333102414557841. [DOI] [PubMed] [Google Scholar]
- 10.Zhao H, Eising E, de Vries B, et al. Gene-based pleiotropy across migraine with aura and migraine without aura patient groups. Cephalalgia. 2015;36:648–657. doi: 10.1177/0333102415591497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Morgan K, Sadofsky LR, Morice AH. Genetic variants affecting human TRPA1 or TRPM8 structure can be classified in vitro as ‘well expressed,’ ‘poorly expressed’ or ‘salvageable. Biosci Rep. 2015;35 doi: 10.1042/BSR20150108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McKemy DD, Neuhausser WM, Julius D. Identification of a cold receptor reveals a general role for TRP channels in thermosensation. Nature. 2002;416:52–58. doi: 10.1038/nature719. [DOI] [PubMed] [Google Scholar]
- 13.Peier AM, Moqrich A, Hergarden AC, et al. A TRP channel that senses cold stimuli and menthol. Cell. 2002;108:705–715. doi: 10.1016/s0092-8674(02)00652-9. [DOI] [PubMed] [Google Scholar]
- 14.Bautista DM, Siemens J, Glazer JM, et al. The menthol receptor TRPM8 is the principal detector of environmental cold. Nature. 2007;448:204–208. doi: 10.1038/nature05910. [DOI] [PubMed] [Google Scholar]
- 15.Colburn RW, Lubin ML, Stone DJ, Jr, et al. Attenuated cold sensitivity in TRPM8 null mice. Neuron. 2007;54:379–386. doi: 10.1016/j.neuron.2007.04.017. [DOI] [PubMed] [Google Scholar]
- 16.Dhaka A, Murray AN, Mathur J, Earley TJ, Petrus MJ, Patapoutian A. TRPM8 is required for cold sensation in mice. Neuron. 2007;54:371–378. doi: 10.1016/j.neuron.2007.02.024. [DOI] [PubMed] [Google Scholar]
- 17.Knowlton WM, Palkar R, Lippoldt EK, et al. A sensory-labeled line for cold: TRPM8-expressing sensory neurons define the cellular basis for cold, cold pain, and cooling-mediated analgesia. J Neurosci. 2013;33:2837–2848. doi: 10.1523/JNEUROSCI.1943-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu B, Fan L, Balakrishna S, Sui A, Morris JB, Jordt SE. TRPM8 is the principal mediator of menthol-induced analgesia of acute and inflammatory pain. Pain. 2013;154:2169–2177. doi: 10.1016/j.pain.2013.06.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pogorzala LA, Mishra SK, Hoon MA. The cellular code for mammalian thermosensation. J Neurosci. 2013;33:5533–5541. doi: 10.1523/JNEUROSCI.5788-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Prince PB, Rapoport AM, Sheftell FD, Tepper SJ, Bigal ME. The effect of weather on headache. Headache. 2004;44:596–602. doi: 10.1111/j.1526-4610.2004.446008.x. [DOI] [PubMed] [Google Scholar]
- 21.Burstein R, Yarnitsky D, Goor-Aryeh I, Ransil BJ, Bajwa ZH. An association between migraine and cutaneous allodynia. Ann Neurol. 2000;47:614–624. [PubMed] [Google Scholar]
- 22.Borhani Haghighi A, Motazedian S, Rezaii R, et al. Cutaneous application of menthol 10% solution as an abortive treatment of migraine without aura: A randomised, double-blind, placebo-controlled, crossed-over study. Int J Clin Pract. 2010;64:451–456. doi: 10.1111/j.1742-1241.2009.02215.x. [DOI] [PubMed] [Google Scholar]
- 23.Takashima Y, Ma L, McKemy DD. The development of peripheral cold neural circuits based on TRPM8 expression. Neuroscience. 2010;169:828–842. doi: 10.1016/j.neuroscience.2010.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dhaka A, Earley TJ, Watson J, Patapoutian A. Visualizing cold spots: TRPM8-expressing sensory neurons and their projections. J Neurosci. 2008;28:566–575. doi: 10.1523/JNEUROSCI.3976-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kobayashi K, Fukuoka T, Obata K, et al. Distinct expression of TRPM8, TRPA1, and TRPV1 mRNAs in rat primary afferent neurons with adelta/c-fibers and colocalization with trk receptors. J Comp Neurol. 2005;493:596–606. doi: 10.1002/cne.20794. [DOI] [PubMed] [Google Scholar]
- 26.Takashima Y, Daniels RL, Knowlton W, Teng J, Liman ER, McKemy DD. Diversity in the neural circuitry of cold sensing revealed by genetic axonal labeling of transient receptor potential melastatin 8 neurons. J Neurosci. 2007;27:14147–14157. doi: 10.1523/JNEUROSCI.4578-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huang D, Li SY, Dhaka A, Story GM, Cao YQ. Expression of the transient receptor potential channels TRPV1, TRPA1 and TRPM8 in mouse trigeminal primary afferent neurons innervating the dura. Mol Pain. 2012;8:66. doi: 10.1186/1744-8069-8-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hondoh A, Ishida Y, Ugawa S, et al. Distinct expression of cold receptors (TRPM8 and TRPA1) in the rat nodose-petrosal ganglion complex. Brain Res. 2010;1319:60–69. doi: 10.1016/j.brainres.2010.01.016. [DOI] [PubMed] [Google Scholar]
- 29.Munns C, AlQatari M, Koltzenburg M. Many cold sensitive peripheral neurons of the mouse do not express TRPM8 or TRPA1. Cell Calcium. 2007;41:331–342. doi: 10.1016/j.ceca.2006.07.008. [DOI] [PubMed] [Google Scholar]
- 30.Staaf S, Franck MC, Marmigere F, Mattsson JP, Ernfors P. Dynamic expression of the TRPM sub-group of ion channels in developing mouse sensory neurons. Gene Expr Patterns. 2010;10:65–74. doi: 10.1016/j.gep.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 31.Xing H, Ling JX, Chen M, et al. TRPM8 mechanism of autonomic nerve response to cold in respiratory airway. Mol Pain. 2008;4:22. doi: 10.1186/1744-8069-4-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Abe J, Hosokawa H, Okazawa M, et al. TRPM8 protein localization in trigeminal ganglion and taste papillae. Brain Res. 2005;136:91–98. doi: 10.1016/j.molbrainres.2005.01.013. [DOI] [PubMed] [Google Scholar]
- 33.Parra A, Madrid R, Echevarria D, et al. Ocular surface wetness is regulated by TRPM8-dependent cold thermoreceptors of the cornea. Nat Med. 2010;16:1396–1399. doi: 10.1038/nm.2264. [DOI] [PubMed] [Google Scholar]
- 34.Yajima T, Sato T, Hosokawa H, et al. Distribution of transient receptor potential melastatin-8-containing nerve fibers in rat oral and craniofacial structures. Ann Anat. 2015;201:1–5. doi: 10.1016/j.aanat.2015.04.003. [DOI] [PubMed] [Google Scholar]
- 35.Harrington AM, Hughes PA, Martin CM, et al. A novel role for TRPM8 in visceral afferent function. Pain. 2011;152:1459–1468. doi: 10.1016/j.pain.2011.01.027. [DOI] [PubMed] [Google Scholar]
- 36.Hayashi T, Kondo T, Ishimatsu M, et al. Expression of the TRPM8-immunoreactivity in dorsal root ganglion neurons innervating the rat urinary bladder. Neurosci Res. 2009;65:245–251. doi: 10.1016/j.neures.2009.07.005. [DOI] [PubMed] [Google Scholar]
- 37.Mukerji G, Yiangou Y, Corcoran SL, et al. Cool and menthol receptor TRPM8 in human urinary bladder disorders and clinical correlations. BMC Urol. 2006;6:6. doi: 10.1186/1471-2490-6-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Newsom J, Holt JL, Neubert JK, Caudle R, Ahn AH. A high density of TRPM8 expressing sensory neurons in specialized structures of the head. Soc Neurosci Meeting Abstr. 2012 Poster #881.09/CC7. [Google Scholar]
- 39.Ren L, Dhaka A, Cao YQ. Function and postnatal changes of dural afferent fibers expressing TRPM8 channels. Mol Pain. 2015;11:37. doi: 10.1186/s12990-015-0043-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Suzuki SC, Furue H, Koga K, et al. Cadherin-8 is required for the first relay synapses to receive functional inputs from primary sensory afferents for cold sensation. J Neurosci. 2007;27:3466–3476. doi: 10.1523/JNEUROSCI.0243-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lubbert M, Kyereme J, Schobel N, Beltran L, Wetzel CH, Hatt H. Transient receptor potential channels encode volatile chemicals sensed by rat trigeminal ganglion neurons. PLoS One. 2013;8:e77998. doi: 10.1371/journal.pone.0077998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Quallo T, Vastani N, Horridge E, et al. TRPM8 is a neuronal osmosensor that regulates eye blinking in mice. Nat Commun. 2015;6:7150. doi: 10.1038/ncomms8150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sousa-Valente J, Andreou AP, Urban L, Nagy I. Transient receptor potential ion channels in primary sensory neurons as targets for novel analgesics. Br J Pharmacol. 2014;171:2508–2527. doi: 10.1111/bph.12532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lippoldt EK, Elmes RR, McCoy DD, Knowlton WM, McKemy DD. Artemin, a glial cell line-derived neurotrophic factor family member, induces TRPM8-dependent cold pain. J Neurosci. 2013;33:12543–12552. doi: 10.1523/JNEUROSCI.5765-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Asuthkar S, Demirkhanyan L, Sun X, et al. The TRPM8 protein is a testosterone receptor: II. Functional evidence for an ionotropic effect of testosterone on TRPM8. J Biol Chem. 2015;290:2670–2688. doi: 10.1074/jbc.M114.610873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zakharian E, Cao C, Rohacs T. Gating of transient receptor potential melastatin 8 (TRPM8) channels activated by cold and chemical agonists in planar lipid bilayers. J Neurosci. 2010;30:12526–12534. doi: 10.1523/JNEUROSCI.3189-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Daniels RL, Takashima Y, McKemy DD. Activity of the neuronal cold sensor TRPM8 is regulated by phospholipase C via the phospholipid phosphoinositol 4,5-bisphosphate. J Biol Chem. 2009;284:1570–1582. doi: 10.1074/jbc.M807270200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tang Z, Kim A, Masuch T, et al. Pirt functions as an endogenous regulator of TRPM8. Nat Commun. 2013;4:2179. doi: 10.1038/ncomms3179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sarria I, Ling J, Xu GY, Gu JG. Sensory discrimination between innocuous and noxious cold by TRPM8-expressing DRG neurons of rats. Mol Pain. 2012;8:79. doi: 10.1186/1744-8069-8-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Teichert RW, Raghuraman S, Memon T, et al. Characterization of two neuronal subclasses through constellation pharmacology. Proc Natl Acad Sci U S A. 2012;109:12758–12763. doi: 10.1073/pnas.1209759109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sarria I, Gu J. Menthol response and adaptation in nociceptive-like and nonnociceptive-like neurons: Role of protein kinases. Mol Pain. 2010;6:47. doi: 10.1186/1744-8069-6-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Knowlton WM, Bifolck-Fisher A, Bautista DM, McKemy DD. TRPM8, but not TRPA1, is required for neural and behavioral responses to acute noxious cold temperatures and cold-mimetics in vivo. Pain. 2010;150:340–350. doi: 10.1016/j.pain.2010.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Knowlton WM, Daniels RL, Palkar R, McCoy DD, McKemy DD. Pharmacological blockade of TRPM8 ion channels alters cold and cold pain responses in mice. PLoS One. 2011;6:e25894. doi: 10.1371/journal.pone.0025894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Patel R, Goncalves L, Leveridge M, et al. Anti-hyperalgesic effects of a novel TRPM8 agonist in neuropathic rats: A comparison with topical menthol. Pain. 2014;155:2097–2107. doi: 10.1016/j.pain.2014.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Xing H, Chen M, Ling J, Tan W, Gu JG. TRPM8 mechanism of cold allodynia after chronic nerve injury. J Neurosci. 2007;27:13680–13690. doi: 10.1523/JNEUROSCI.2203-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zuo X, Ling JX, Xu GY, Gu JG. Operant behavioral responses to orofacial cold stimuli in rats with chronic constrictive trigeminal nerve injury: Effects of menthol and capsazepine. Mol Pain. 2013;9:28. doi: 10.1186/1744-8069-9-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.McCoy ES, Zylka MJ. Enhanced behavioral responses to cold stimuli following CGRPalpha sensory neuron ablation are dependent on TRPM8. Mol Pain. 2014;10:69. doi: 10.1186/1744-8069-10-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Klein AH, Sawyer CM, Carstens MI, Tsagareli MG, Tsiklauri N, Carstens E. Topical application of l-menthol induces heat analgesia, mechanical allodynia, and a biphasic effect on cold sensitivity in rats. Behav Brain Res. 2010;212:179–186. doi: 10.1016/j.bbr.2010.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Klein AH, Sawyer CM, Takechi K, et al. Topical hindpaw application of l-menthol decreases responsiveness to heat with biphasic effects on cold sensitivity of rat lumbar dorsal horn neurons. Neuroscience. 2012;219:234–242. doi: 10.1016/j.neuroscience.2012.05.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Patel R, Goncalves L, Newman R, et al. Novel TRPM8 antagonist attenuates cold hypersensitivity after peripheral nerve injury in rats. J Pharmacol Exp Ther. 2014;349:47–55. doi: 10.1124/jpet.113.211243. [DOI] [PubMed] [Google Scholar]
- 61.Proudfoot CJ, Garry EM, Cottrell DF, et al. Analgesia mediated by the TRPM8 cold receptor in chronic neuropathic pain. Curr Biol. 2006;16:1591–1605. doi: 10.1016/j.cub.2006.07.061. [DOI] [PubMed] [Google Scholar]
- 62.Vinuela-Fernandez I, Sun L, Jerina H, et al. The TRPM8 channel forms a complex with the 5-HT(1B) receptor and phospholipase D that amplifies its reversal of pain hypersensitivity. Neuro-pharmacology. 2014;79:136–151. doi: 10.1016/j.neuropharm.2013.11.006. [DOI] [PubMed] [Google Scholar]
- 63.Anderson EM, Jenkins AC, Caudle RM, Neubert JK. The effects of a co-application of menthol and capsaicin on nociceptive behaviors of the rat on the operant orofacial pain assessment device. PLoS One. 2014;9:e89137. doi: 10.1371/journal.pone.0089137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lashinger ES, Steiginga MS, Hieble JP, et al. AMTB, a TRPM8 channel blocker: Evidence in rats for activity in overactive bladder and painful bladder syndrome. Am J Physiol Renal Physiol. 2008;295:F803–F810. doi: 10.1152/ajprenal.90269.2008. [DOI] [PubMed] [Google Scholar]
- 65.Ramachandran R, Hyun E, Zhao L, et al. TRPM8 activation attenuates inflammatory responses in mouse models of colitis. Proc Natl Acad Sci U S A. 2013;110:7476–7481. doi: 10.1073/pnas.1217431110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kim YS, Park JH, Choi SJ, et al. Central connectivity of transient receptor potential melastatin 8-expressing axons in the brain stem and spinal dorsal horn. PLoS One. 2014;9:e94080. doi: 10.1371/journal.pone.0094080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Burgos-Vega CC, Ahn DD, Bischoff C, et al. Meningeal transient receptor potential channel M8 activation causes cutaneous facial and hindpaw allodynia in a preclinical rodent model of headache. Cephalalgia. 2016;36:185–193. doi: 10.1177/0333102415584313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Brederson JD, Kym PR, Szallasi A. Targeting TRP channels for pain relief. Eur J Pharmacol. 2013;716:61–76. doi: 10.1016/j.ejphar.2013.03.003. [DOI] [PubMed] [Google Scholar]
- 69.Fallon MT, Storey DJ, Krishan A, et al. Cancer treatment-related neuropathic pain: Proof of concept study with menthol—A TRPM8 agonist. Support Care Cancer. 2015;23:2769–2777. doi: 10.1007/s00520-015-2642-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lehto SG, Weyer AD, Zhang M, et al. AMG2850, a potent and selective TRPM8 antagonist, is not effective in rat models of inflammatory mechanical hypersensitivity and neuropathic tactile allodynia. Naunyn Schmiedebergs Arch Pharmacol. 2015;388:465–476. doi: 10.1007/s00210-015-1090-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Andersson KE. Potential future pharmacological treatment of bladder dysfunction. Basic Clin Pharmacol Toxicol. 2016 Mar 17; doi: 10.1111/bcpt.12577. doi: 10.1111. [DOI] [PubMed] [Google Scholar]
- 72.Franken J, Uvin P, De Ridder D, Voets T. TRP channels in lower urinary tract dysfunction. Br J Pharmacol. 2014;171:2537–2551. doi: 10.1111/bph.12502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mistretta FA, Russo A, Castiglione F, et al. DFL23448, a novel transient receptor potential melastin 8-selective ion channel antagonist, modifies bladder function and reduces bladder overactivity in awake rats. J Pharmacol Exp Ther. 2016;356:200–211. doi: 10.1124/jpet.115.228684. [DOI] [PubMed] [Google Scholar]
- 74.Ito H, Aizawa N, Sugiyama R, et al. Functional role of the transient receptor potential melastatin 8 (TRPM8) ion channel in the urinary bladder assessed by conscious cystometry and ex vivo measurements of single-unit mechanosensitive bladder afferent activities in the rat. BJU Int. 2016;117:484–494. doi: 10.1111/bju.13225. [DOI] [PubMed] [Google Scholar]
- 75.Knowlton WM, McKemy DD. TRPM8: From cold to cancer, peppermint to pain. Curr Pharm Biotechnol. 2011;12:68–77. doi: 10.2174/138920111793937961. [DOI] [PubMed] [Google Scholar]
- 76.Liu Z, Wu H, Wei Z, et al. TRPM8: A potential target for cancer treatment. J Cancer Res Clin Oncol. 2016;142:1871–1881. doi: 10.1007/s00432-015-2112-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Benemei S, Patacchini R, Trevisani M, Geppetti P. TRP channels. Curr Opin Pharmacol. 2015;22:18–23. doi: 10.1016/j.coph.2015.02.006. [DOI] [PubMed] [Google Scholar]
- 78.Bertamino A, Ostacolo C, Ambrosino P, et al. Tryptamine-based derivatives as transient receptor potential melastatin type 8 (TRPM8) channel modulators. J Med Chem. 2016;59:2179–2191. doi: 10.1021/acs.jmedchem.5b01914. [DOI] [PubMed] [Google Scholar]
- 79.Andrews MD, Af Forselles K, Beaumont K, et al. Discovery of a selective TRPM8 antagonist with clinical efficacy in cold-related pain. ACS Med Chem Lett. 2015;6:419–424. doi: 10.1021/ml500479v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Horne DB, Tamayo NA, Bartberger MD, et al. Optimization of potency and pharmacokinetic properties of tetrahydroisoquinoline transient receptor potential melastatin 8 (TRPM8) antagonists. J Med Chem. 2014;57:2989–3004. doi: 10.1021/jm401955h. [DOI] [PubMed] [Google Scholar]
- 81.Ohmi M, Shishido Y, Inoue T, et al. Identification of a novel 2-pyridyl-benzensulfonamide derivative, RQ-00203078, as a selective and orally active TRPM8 antagonist. Bioorg Med Chem Lett. 2014;24:5364–5368. doi: 10.1016/j.bmcl.2014.10.074. [DOI] [PubMed] [Google Scholar]
- 82.Zhu B, Xia M, Xu X, et al. Arylglycine derivatives as potent transient receptor potential melastatin 8 (TRPM8) antagonists. Bioorg Med Chem Lett. 2013;23:2234–2237. doi: 10.1016/j.bmcl.2013.01.062. [DOI] [PubMed] [Google Scholar]
- 83.Miller S, Rao S, Wang W, Liu H, Wang J, Gavva NR. Antibodies to the extracellular pore loop of TRPM8 act as antagonists of channel activation. PLoS One. 2014;9:e107151. doi: 10.1371/journal.pone.0107151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Almeida MC, Hew-Butler T, Soriano RN, et al. Pharmacological blockade of the cold receptor TRPM8 attenuates autonomic and behavioral cold defenses and decreases deep body temperature. J Neurosci. 2012;32:2086–2099. doi: 10.1523/JNEUROSCI.5606-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Gavva NR, Davis C, Lehto SG, Rao S, Wang W, Zhu DX. Transient receptor potential melastatin 8 (TRPM8) channels are involved in body temperature regulation. Mol Pain. 2012;8:36. doi: 10.1186/1744-8069-8-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Winchester WJ, Gore K, Glatt S, et al. Inhibition of TRPM8 channels reduces pain in the cold pressor test in humans. J Pharmacol Exp Ther. 2014;351:259–269. doi: 10.1124/jpet.114.216010. [DOI] [PubMed] [Google Scholar]
- 87.Victor TW, Hu X, Campbell JC, Buse DC, Lipton RB. Migraine prevalence by age and sex in the United States: A life-span study. Cephalalgia. 2010;30:1065–1072. doi: 10.1177/0333102409355601. [DOI] [PubMed] [Google Scholar]
- 88.Kosaras B, Jakubowski M, Kainz V, Burstein R. Sensory innervation of the calvarial bones of the mouse. J Comp Neurol. 2009;515:331–348. doi: 10.1002/cne.22049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Schueler M, Neuhuber WL, De Col R, Messlinger K. Innervation of rat and human dura mater and pericranial tissues in the parieto-temporal region by meningeal afferents. Headache. 2014;54:996–1009. doi: 10.1111/head.12371. [DOI] [PubMed] [Google Scholar]
- 90.Pradhan AA, Smith ML, McGuire B, Tarash I, Evans CJ, Charles A. Characterization of a novel model of chronic migraine. Pain. 2014;155:269–274. doi: 10.1016/j.pain.2013.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Oshinsky ML. Gomonchareonsiri S. Episodic dural stimulation in awake rats: A model for recurrent headache. Headache. 2007;47:1026–1036. doi: 10.1111/j.1526-4610.2007.00871.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


