Abstract
We explore the design of metal binding sites to modulate triple-helix stability of collagen and collagen-mimetic peptides. Globular proteins commonly utilize metals to connect tertiary structural elements that are well separated in sequence, constraining structure and enhancing stability. It is more challenging to engineer structural metals into fibrous protein scaffolds, which lack the extensive tertiary contacts seen in globular proteins. In the collagen triple helix, the structural adjacency of the carboxy-termini of the three chains makes this region an attractive target for introducing metal binding sites. We engineered His3 sites based on structural modeling constraints into a series of designed homotrimeric and heterotrimeric peptides, assessing the capacity of metal binding to improve stability and in the case of heterotrimers, affect specificity of assembly. Notable enhancements in stability for both homo and heteromeric systems were observed upon addition of zinc(II) and several other metal ions only when all three histidine ligands were present. Metal binding affinities were consistent with the expected Irving-Williams series for imidazole. Unlike other metals tested, copper(II) also bound to peptides lacking histidine ligands. Acetylation of the peptide N-termini prevented copper binding, indicating proline backbone amide metal-coordination at this site. Copper similarly stabilized animal extracted Type I collagen in a metal specific fashion, highlighting the potential importance of metal homeostasis within the extracellular matrix.
Keywords: metalloprotein design, structural metals, Type I collagen, collagen mimetic peptides, copper
De novo design of metal-protein complexes has advanced our understanding of metal control on structure and function1–3. Engineered structural metals have been successfully used to enforce the folding and oligomerization of proteins 4–9. Most work in this area has focused on globular proteins, while interactions of metals with fibrous proteins such as collagen remain poorly understood. This is despite the fact that collagens are the most abundant proteins by mass in animals, playing important functional roles in many biological processes. The fibrillar structure of collagen provides tensile strength and flexibility to tissue, cartilage, and bone. The interaction of metals with collagen is also of industrial interest. Metals such as chromium and aluminum are used as tanning agents and change both the color and structure of leather through specific interactions with collagen 10–12.
Collagen has a unique three-dimensional structure, composed of three chains that oligomerize into the triple-helix fold. Individual chains are in a poly-proline II conformation, which supercoil in the triple helix. In natural collagens, the fibrillar region extends over a thousand amino acids and contains post-translational modifications, making it challenging to express, purify and characterize its biochemical and biophysical behavior. As such, short collagen mimetic peptide (CMPs) systems have been essential tools in exploring the molecular basis for stability, specificity and higher order assembly. The most stable CMPs using biogenic amino acids consist of repeating (Gly-Pro-Hyp)n triplets 13. CMPs have been designed to probe the role of amino acid sequence on folding kinetics14, stability of the triple-helix 15–23, and triple-helical geometry15, 24.
The introduction of novel metal sites into proteins provides a way to enhance stability and use metal binding as a structural switch. There are several approaches to structure-guided design of metalloproteins. One is to construct protein folds that accommodate a metal by parameterizing backbone geometry and key ligand contacts around a metal – a de novo approach 25. Alternatively, a metal binding site can be designed into an known fold 5. In this study, we explore whether the latter approach can be used to introduce structural metals into the collagen fold in order to confer stability and specificity of triple-helix folding. Unlike globular proteins, collagen has an extended structure and a limited number of tertiary contacts, making it challenging to engineer a metal binding site. However, the structural adjacency of the three chain termini of the triple-helix make those sites potentially suited for this purpose.
Connecting the chain termini in CMPs through sidechain-backbone linkages 26–30 or other templates 31 at chain termini was shown to drive folding and enhance the stability of short Gly-Xxx-Yyy repeats, facilitating host-guest studies of amino acid substitutions in the triple-helix. Similarly, the inclusion of metal chelating ligands such as bipyridine 32–34, catechol 35 and imidazole 36 at the ends of chains increased stability of the triple helix. Metal-binding sites introduced at the ends of CMPs have been utilized to promoted higher order assembly of collagen nanostructures 34, 36–41. Here, we examine whether the inclusion of metal coordinating biogenic amino acids can enhance model peptide stability and specificity of assembly.
We are also interested in whether metal binding can be used as a tool to probe or enhance the specificity of oligomeric interactions between the three chains in the collagen triple-helix. A majority of studies of synthetic CMP systems have focused on homotrimers where the three chains have the same sequence. However, some naturally occurring collagens exist as heterotrimers; for example Type I collagen consists of both an α1 and α2 gene product which assemble as an α1:α1:α2 complex. abc-type heterotrimers are also possible, as in the case of Type V collagen which can exist as an α1:α2:α3 complex. Therefore, there is significant interest in developing CMP systems that form specific heterotrimers, providing model systems to study biophysical effects of disease causing mutations in a heteromeric context 42, 43. A number of molecular strategies have been used, including the introduction of disulfide bridges 44–46, and exploiting complementary pairing of electrostatic interactions 47–49. Analysis of natural collagen sequences shows an unusually high frequency of charged amino acids that participate in favorable interchain interactions 50–52. Brodsky and co-workers have shown these interactions increase thermal stability in CMPs 53–55. Heterotrimers incorporating extensive charge-pair networks have also been designed using automated computational approaches 56–58.
We use metal-assisted assembly of CMPs to examine whether metal binding can modulate stability and specificity of heterotrimer assembly. C-terminal histidines are introduced into a previously designed abc-type CMP heterotrimer 56. Heterospecific assembly allows us to control histidine ligand stoichiometry and study its effect on metal affinity. In the triple helix, the three chains interact in a staggered fashion with a one-residue shift in register between adjacent chains. We sought to determine whether varying linker lengths that connect histidine ligands to the triple helix could produce structurally constrained designs that would only show an enhancement in stability in the right relative register of the three chains. Although stability enhancements were clearly observed in nearly all peptide systems, metal binding sites as designed were unable to discriminate the correct register of the abc-type CMPs, indicating that structural flexibility of the binding site was a confounding factor. In the course of studying this system, a secondary histidine-independent copper(II) binding mode was found in the CMPs and in animal-extracted Type I collagen. This work highlights the challenges in structure-guided design of metal binding sites into a triple-helical scaffold.
EXPERIMENTAL PROCEDURES
Peptide Synthesis and Sequences
The peptides were synthesized using solid-phase FMOC chemistry at the Tufts University Core Facility, Boston, MA. Unless otherwise specified, N- and C-termini were uncapped. Peptides were purified to 95% purity by reverse-phase high-performance liquid chromatography (HPLC), and products were verified by mass spectrometry (Fig. S9). The sequences of the characterized peptides are listed below:
-
(POG)n and (PPG)n domain peptides
(POG)7PAH POGPOGPOGPOGPOGPOGPOGPAH (POG)7AH POGPOGPOGPOGPOGPOGPOGAH (POG)7H POGPOGPOGPOGPOGPOGPOGH (POG)10 POGPOGPOGPOGPOGPOGPOGPOGPOGPOG (PPG)10 PPGPPGPPGPPGPPGPPGPPGPPGPPGPPG -
Charged peptides
A PKGPKGPKGKOGPDGDOGDOGDOGPKGPKG APAH PKGPKGPKGKOGPDGDOGDOGDOGPKGPKGPAH AAH PKGPKGPKGKOGPDGDOGDOGDOGPKGPKGAH AH PKGPKGPKGKOGPDGDOGDOGDOGPKGPKGH B PDGDOGDOGDOGPDGKOGPDGPDGPDGDOG BPAH PDGDOGDOGDOGPDGKOGPDGPDGPDGDOGPAH BAH PDGDOGDOGDOGPDGKOGPDGPDGPDGDOGAH BH PDGDOGDOGDOGPDGKOGPDGPDGPDGDOGH C KOGPDGPDGPKGKOGPKGKOGKOGKOGKOG CPAH KOGPDGPDGPKGKOGPKGKOGKOGKOGKOGPAH CAH KOGPDGPDGPKGKOGPKGKOGKOGKOGKOGAH CH KOGPDGPDGPKGKOGPKGKOGKOGKOGKOGH
Designed metal-binding ligands and associated linkers shown in bold. O = 4R-hydroxyproline.
Peptide solutions were prepared in 10mM Tris buffer pH=7.4, with or without 150mM NaCl and metal salts. Peptide concentrations in solution were estimated by measuring absorbance at 214nm using ε214 = 2200 M−1cm−1. After preparing mixtures at room temperature, they were denatured at 80°C for 30 minutes and annealed at 4°C for at least 48 hours.
Lyophilized calf-skin collagen Type I was provided by Elastin Products Company (Cat no.: C857). Lyophilized protein was directly dissolved in either acetic acid or Tris buffer (pH 7.2) with or without the addition of metal salts.
Circular dichroism (CD)
CD measurements were conducted using the Aviv model 420SF spectrophotometer equipped with a Peltier temperature controller. For wavelength spectra, measurements were made at every 0.5nm step with an averaging time of 10 seconds at each wavelength. Scans were conducted from 190 to 260 nm at 5°C. Observed ellipticity was converted to molar ellipticity by dividing raw values by the peptide concentration, number of residues, and cell path length. For temperature induced denaturation, ellipticity was measured at 225 nm for (POG)n and (PPG)n domain peptides, or at 223 nm for charged peptides. Unless otherwise specified, total peptide concentrations used were 0.2mM. CD temperature denaturation profiles were smoothed using the Savitsky-Golay algorithm with nineteen points and a third-order polynomial 59, and melting temperatures assigned based on extrema of the first derivative.
Dynamic light scattering (DLS)
Dynamic light scattering (DLS) measurements were performed using a Zetasizer Nano ZS (Malvern Instruments, UK). Data was collected with a 3 mW He-Ne laser at a 633nm wavelength. This unit collects back scattered light at an angle of 173° and contains a built-in Peltier element temperature controller. Autocorrelation functions were determined from the average of 3 correlation functions, with an acquisition time of 2 minutes per correlation function. Reported viscosity values 60 were used for the hydrodynamic radius calculation. A detailed description of the DLS instrumental setup and data analysis methods can be found elsewhere 61, 62.
Isothermal titration calorimetry (ITC)
Isothermal titration calorimetry (ITC) measurements were carried out at 25°C using an ITC 200 calorimeter (GE Biosciences, Piscataway, NJ). All solutions were filtered through 0.2 μm syringe filters and degassed for 5 minutes prior to measurement. 35 to 40 μL aliquots of 1.67 mM ZnCl2 were injected from a 1000 rpm rotating syringe into a 0.2 mL sample cell containing 0.167 mM trimer (0.5 mM monomer) with a 180 second delay between injections. Each titration experiment was accompanied by the corresponding control experiment in which ZnCl2 was injected into a solution of buffer alone. Each injection generated a heat burst curve (μcal/s versus s), the area under which was determined by integration using Origin version 7.0 software (OriginLab Corp., Northampton, MA), to obtain a measure of the heat associated with that injection. The measure of the heat associated with each ZnCl2-buffer injection, as estimated using a linear regression analysis of the integrated data, was subtracted from that of the corresponding heat associated with each ZnCl2-peptide injection to yield the heat of ZnCl2 binding for that injection. Following removal of the point corresponding to the first low volume injection the buffer-corrected ITC profiles for the binding of each experiment were fit with a model for one set of binding sites.
RESULTS AND DISCUSSION
Metal Binding Enhances Stability
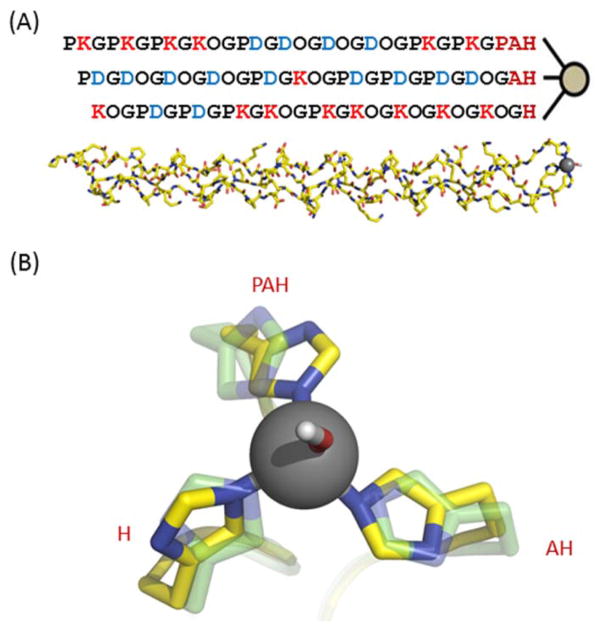
We previously designed a series of collagen peptides specifically intended to form an A:B:C heterotrimer 56. In order to design a metal binding site that targets an ABC heterotrimer, we developed a model that took advantage of the one amino acid stagger of A relative to B, and B to C. Starting with the assumption that the designed register ABC is the correct one, adding xxH, xH and H linkers to A, B and C respectively should form a blunt ended design (Fig. 1). Linkers were modeled onto the high-resolution structure of a [(POG)10]3 CMP as x-x-His, x-His and His for the leading, central and lagging strands. Backbone torsions of x and histidine, and sidechain rotamers of histidine were sampled using the modeling platform protCAD 63, 64 for those that formed a tetrahedral His3-metal site. The optimal conformation identified in the linker sampling specified all x positions in an α-helical conformation (f ≈ −60ϕ, y ≈ −45ϕ). Linker amino acids were chosen based on known backbone conformation preferences. Proline, which is favored at the beginning of α-helices 65, and alanine, which stabilizes the α-helix 66, were incorporated to optimize the target linker conformation, such that the imidazole sidechains presented a pre-organized tetrahedral metal binding site. Structures were minimized in AMBER using the ff99sb force field 67 plus parameters for a zinc pseudoatom 68. Pro-Ala-His (PAH), Ala-His (AH), and His (H) were appended to the C-terminus of A, B, and C peptides respectively to obtain APAH, BAH, and CH peptides.
Figure 1. Blunt-end design of a collagen metalloprotein.
(A) Model of peptides containing histidines at the C-terminus coordinating zinc, to stabilize the heterotrimer. (B) An initial configuration built using protCAD (green) and minimized with AMBER (yellow) to model the heterotrimeric zinc-histidine site.
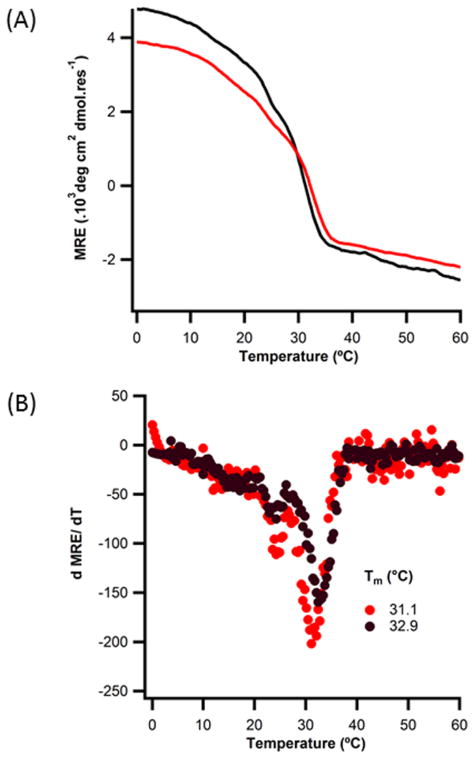
Metal binding linkers in an α-helical conformation should not extend the interchain hydrogen bonding network of the triple-helix, and were not expected to significantly perturb stability except in the presence of a coordinated metal. CD wavelength spectra and thermal denaturation profiles of the APAH:BAH:CH heterotrimer showed triple-helical structure and comparable stability to the original A:B:C (Fig. 2). Differences of ~2–3ϕC for the A:B:C system from the previously published Tm values 56 were due to the use of a Tris-buffer system instead of phosphate to allow for metal binding studies to be performed. In both peptide sets, two transitions were observed in the absence of NaCl, corresponding to potential multiple registers of association or the formation of competing species with lower thermal stability. Increased ionic strength decreased the stability of both A:B:C and APAH:BAH:CH heterotrimers and eliminated one of the transitions (Fig. S2) consistent with our previous characterization of the A:B:C heterotrimer 56.
Figure 2. Effect of linker on structure and stability of the A:B:C heterotrimer.
(A) CD temperature-melting curves monitored at 223nm of 0.2 mM ABC (red) and APAH:BAH:CH (black) collagen like peptides in phosphate buffer without salt; (B) respective first derivative plots. Effect of increasing ionic strength shown in Fig. S2.
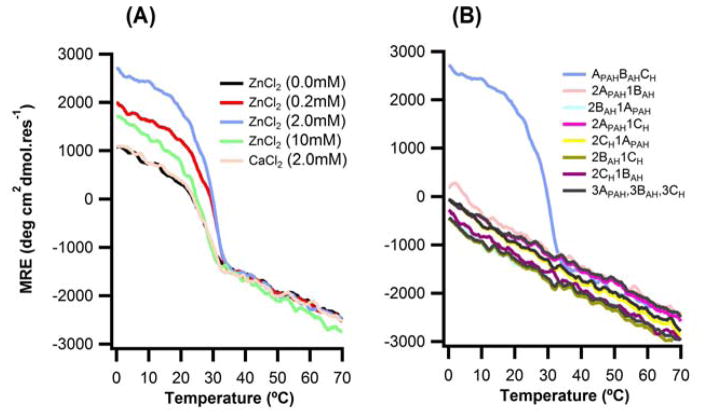
Addition of the histidine ligand and associated linkers did not perturb heterospecificity of triple-helix folding. Ten combinations of the histidine-containing peptides (APAH:BAH:CH, 2APAH:BAH, APAH:2BAH, 2APAH:CH, APAH:2CH, 2BAH:CH, BAH:2CH, 3APAH, 3BAH, 3CH) were prepared in the presence of ZnCl2, of which only APAH:BAH:CH formed a triple-helix (Fig. 3, S3A). Inclusion of ZnCl2 up to 2.0 mM stabilized the His3 containing heterotrimer. Zinc concentrations greater than 2.0 mM decreased structure and stability of APAH:BAH:CH. This is presumably due to screening of interchain electrostatic interactions upon increasing ionic strength, as was previously observed for the unmodified peptides 56. To examine whether stability enhancements could be caused by non-specific metal-salt interactions, we titrated CaCl2, which does not interact strongly with histidine, into the APAH:BAH:CH mixture. The lack of Ca(II) binding to the heterotrimer was consistent with specific coordination of imidazoles. At four-fold lower total peptide concentrations (0.05mM), the APAH:BAH:CH heterotrimer was unfolded. Under these conditions, inclusion of Zn(II) induced folding of the triple-helix and enhanced stability in a concentration dependent manner (Fig. S3B).
Figure 3. abc-type heterotrimer required for metal binding.
Circular dichroism temperature melting curves monitored at 223nm of (A) APAH:BAH:CH heterotrimer peptide in 10mm Tris buffer, pH=7.5, 150mM NaCl with varying concentration of ZnCl2 or CaCl2. (B) All ten possible combination of APAH,BAH, and CH in 10mM Tris buffer, 150mM NaCl, 2mM ZnCl2.
Effect of metal species on the stability
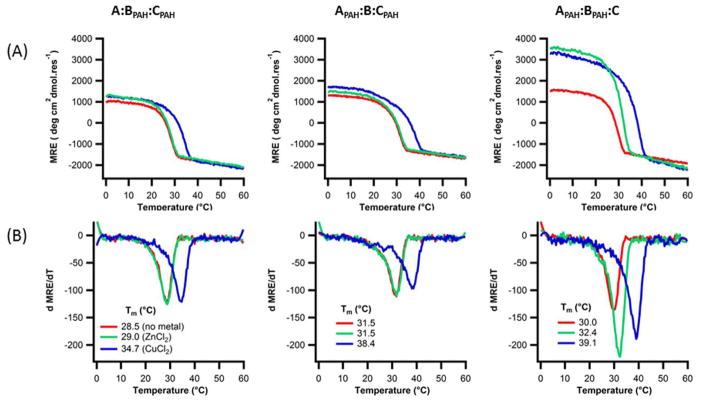
We measured the stability of APAH:BAH:CH in the presence of metal salts: ZnCl2, CaCl2, CoCl2, MnCl2, FeCl2, NiCl2, and CuCl2. No significant changes in structure or stability were seen with Mn(II), Fe(II), and Ca(II) (Fig. 4). Addition of Co(II) showed a slight increase in MRE and stability. Ni(II) and Zn(II) showed more pronounced effects, while addition of Cu(II) had the largest increase on stability, ΔTm = 12°C, consistent with previous reports of copper and zinc binding and stabilization of histidine containing peptides 36. Metal binding affinities agreed with the expected Irving-Williams series for imidazole: Ca2+ < Mn2+ < Fe2+ < Co2+ < Ni2+ < Cu2+ > Zn2+ 69.
Figure 4. Metal-dependent stabilization of the triple helix.
(A) Circular dichroism temperature melting curves monitored at 223nm of 0.18mM APAH:BAH:CH heterotrimer peptide in 10mm Tris buffer, pH=7.4, 150mM NaCl in 0.36mM concentration of different metals (B) their respective first derivative plots.
Testing the blunt-ended model
We tested the blunt-end design hypothesis using two approaches. First, a homotrimeric system was used to determine whether a blunt-end site was essential for metal binding. Homotrimers can only form staggered binding sites and might therefore be expected to poorly coordinate metal. Second, we wanted to assess whether the blunt-ended site was the optimal linker configuration. To determine this, we took advantage of the heterospecific association of A:B:C to sample linker configurations the metal binding.
A blunt-ended structure cannot form in a homotrimeric system when each chain has the same linker, instead creating a metal binding site where each histidine is staggered by one amino acid relative to the next. Thus constructing homotrimers with various linkers allows us to examine the flexibility of the metal binding site. The H, AH and PAH linkers were added to the C-terminus of a homotrimer forming (POG)7 domain. All species formed stable triple-helical structures with and without ZnCl2 (Fig. S4). Addition of ZnCl2 to (POG)7AH and (POG)7PAH increased stability by approximately 7°C (Fig. S5, Table S1). However, only a marginal effect was observed for (POG)7H stability, about 2°C. Homotrimers did not bind Ca(II), indicating that specific imidazole coordination was required to enhance stability. To model the zinc-mediated stability, relevant homotrimer structures were computed and optimized. The extent to which each homotrimer model could geometrically adopt a metal binding conformation was consistent with the observed stability enhancement (PAH, AH > H). Affinities assessed by ITC (Fig. S5) exhibit the same trend as stability measured by CD and calculated binding scores (Table S1).
Given that (POG)7H could not be modeled to adopt a metal binding site, it was suspected the small increase in stability could be due to the metal coordination by multiple triple-helices. Similar behavior was observed in related designed peptides containing a histidine at both N- and C-terminal ends of the sequence (i.e. HG(PPG)9GH) 36, where metal binding enhanced fold stability and drove higher-order assembly. Dynamic light scattering measurements (DLS) were conducted for all three homotrimer peptides in increasing concentrations of ZnCl2. DLS measures the Brownian motion of the particle and correlates it to its size. DLS confirmed the formation of higher order structures for (POG)7H upon addition of zinc, suggesting more than one peptide trimer was required to form a complete binding site. Larger species were not observed for (POG)7AH or (POG)7PAH upon addition of zinc (Fig. S6). This suggests that the increase in stability driven by intra-helical formation of a His3-metal site precludes multiple helical elements interacting with the same metal ion. In the case of (POG)7H, formation of a an intra-helical metal site requires significant conformational strain, instead promoting multi-helical metal assemblies, such as those in related systems where such behavior is intentionally designed 36, 37.
The homotrimer results clearly demonstrated that forming a blunt-ended site is not an essential requirement for metal binding. However, the blunt-end may still be the optimal configuration, requiring minimal rearrangement of the linker conformation to coordinate metal. A blunt-ended metal site can only be constructed using a heterotrimer where the leading, middle and lagging strands are augmented with PAH, AH and H linkers respectively. To systematically evaluate the relationship between binding site geometry and metal binding, we generated a library of peptide + linker variants: AH, AAH, APAH, BH, BAH, BPAH, CH, CAH, CPAH. From these nine peptides, one could potentially assemble 81 (3×3×3) linker sequence permutations on an A:B:C heterotrimer. We focused on a subset of these to determine whether a blunt-end design provided an optimal metal binding site.
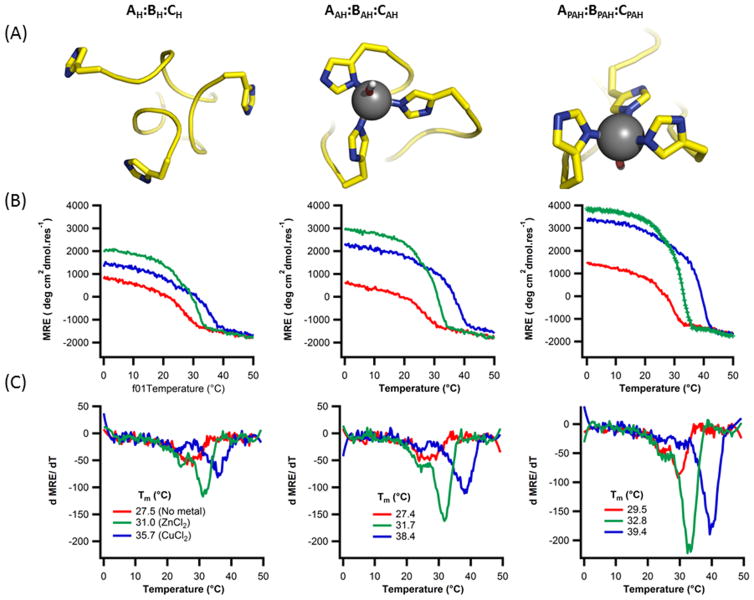
We first examined the three cases where all peptide chains A, B and C had the same linker, resulting in a staggered binding site: AH:BH:CH, AAH:BAH:CAH, and APAH:BPAH:CPAH. These linker geometries would be most comparable to the (POG)7 homotrimer experiments – i.e. they are incapable of creating a blunt-ended site. As seen in the homotrimer, the PAH linker increased the baseline stability of the heterotrimer by a few degrees Celsius relative to the AH and H linkers (Table 1, Fig. 5). Addition of Zn(II) and Cu(II) increased stability in all cases. Unlike the homotrimer (POG)7H, AH:BH:CH showed enhanced stability on par with the other two linkers. Together, these two observations suggest that heterotrimers show increased conformational flexibility at the C-terminus, presumably due to the lower content of rigid imino acids relative to (POG)7. Increased flexibility may decrease the coupling between triple-helix stability and metal binding, and reduce the effect of linker length on accommodating a metal binding site at the C-terminus of the triple helix.
Table 1.
Melting temperature (Tm) of mixture of peptides A, B, C with different combination of ligands PAH, AH, and H in the absence of metal and in the presence of 2mM ZnCl2 or CuCl2.
| Peptides | Tm(°C) | ΔTm(°C)a | ||
|---|---|---|---|---|
| No metal | + Zn(II) | + Cu(II) | computed binding scoreb | |
| same linkers | ||||
| AH:BH:CH | 27.5 | +3.5 | +8.3 | 13858 |
| AAH:BAH:CAH | 27.4 | +4.3 | +11.0 | 1007 |
| APAH:BPAH:CPAH | 29.5 | +3.3 | +9.9 | 5744 |
|
| ||||
| unique linkers | ||||
| APAH:BAH:CH c | 28.8 | +2.6 | +10.0 | 1283 |
| APAH:BH:CAH | 28.2 | +1.9 | +8.8 | 4886 |
| AAH:BPAH:CH | 28.1 | +4.7 | +9.2 | 607 |
| AAH:BH:CPAH | 28.2 | +4.2 | +10.5 | 499 |
| AH:BAH:CPAH | 28.2 | +4.5 | +9.2 | 2550 |
| AH:BPAH:CAH | 29.1 | +1.7 | +8.9 | 10380 |
ΔTm(°C) = Tm (metal) – Tm (no metal)
Linkers were modeled at the C-terminus using the structure of an idealized (PPG)10 triple-helix, assuming three peptides are listed in order of leading, middle and lagging strand. Values for the best ligand configuration are indicated here. For all ligand configurations, see Tables S2 and S3.
corresponds to the blunt end design if association state is ABC
Figure 5. Modeling and characterization of heterotrimers with staggered metal binding sites.
AH:BH:CH, AAH:BAH:CAH, and APAH:BPAH:CPAH (A) atomic models constructed in protCAD and minimized in AMBER. In the case of AH:BH:CH the mimized structure did not maintain a metal coordination competent geometry. (B) Thermal denaturation at 223nm in 10mm Tris buffer, pH=7.4, 150mM NaCl with (red) no metals (green) 0.36mM of ZnCl2 and (blue) 0.36mM CuCl2 (C) their respective first derivative plots.
Next, we examined the six states where each chain has a different linker (APAH:BAH:CH, AAH:BPAH:CH, AAH:BH:CPAH, APAH:BH:CAH, AH:BAH:CPAH, AH:BPAH:CAH), that could potentially form a blunt ended design. If A:B:C associates into one unique state and the blunt-end site is optimal for metal binding, one of theses six combinations should have a detectably higher stability than the other five. The composition of the heterotrimer is known, but the register of the three chains is not 56. As noted previously, in the absence of salt two melting transitions are observed, indicating that multiple registers may occur. Of the possible associations states: the target ABC and competing BCA, CAB are the most likely species given that these three are predicted to form the highest number of favorable charge pair interactions. CBA, BAC and ACB are unlikely to be formed as these have primarily repulsive interchain interactions with significantly lower computed interaction energies 56.
The six mixtures each formed triple-helical structures with equivalent stabilities in the absence of metal (Table 1, Fig. S7). While addition of either Zn(II) or Cu(II) stabilized the triple-helix in all cases, no one state stood out from the other five as having the highest degree of metal induced stabilization. Furthermore, the observed stability increases were within the same range as those for heterotrimers where each chain had the same linker. This indicates that linker length exerts minimal control on metal binding site affinity, and that the blunt-end design as implemented is not sufficient to discriminate the correct register of A:B:C. In natural proteins where binding sites are in loops or other disordered regions, the strong metal-ligand interactions can remodel flexible regions of the protein to adopt a metal coordination site 70. This appears to be the case at the termini of collagen as well. Another consideration is the presence of multiple transitions in many of the A:B:C-type peptide denaturation profiles, indicating that multiple species may be present. The resulting heterogeneity in binding site configurations may allow additional plasticity in metal coordination.
Modeling of all potential linker configurations Calculating a metal affinity score using a combination of geometric constraints on ligand-metal interactions and standard intramolecular forces indicates other linkers can provide good scoring coordination sites (Table S3). The blunt-end structure has the best packing energy, but other linker configurations have more ideal metal-ligand geometries. The correlation between calculated and observed stabilities for the variants tested in Table 1 are modest (R2 = 0.56 for copper and 0.42 for zinc). More sophisticated modeling approaches that adequately treat both ligand-metal and intramolecular forces could potentially improve the correlation between calculated and observed metal affinities.
Evidence for sequence-independent mode of Cu(II) binding to triple-helical peptides
It was expected that all three histidines would be required for metal binding in the other heterotrimer variants – this turned out to be the case for zinc (Fig. 6). However, the addition of Cu(II) enhanced heterotrimer stability even when a complete His3 site was absent. Furthermore, no significant correlation was observed between the degree of stabilization of heterotrimer linker combinations in the presence of Zn(II) versus Cu(II) (Table 1). This suggested that copper binds to the collagen peptides through an alternative or additional mode.
Figure 6. Metal-induced stabilization in the presence of incomplete binding sites.
(A) Thermal denaturation at 223nm in 10mm Tris buffer, pH=7.4, 150mM NaCl with (red) no metals (green) 0.36mM of ZnCl2 and (blue) 0.36mM CuCl2 (B) their respective first derivative plots.
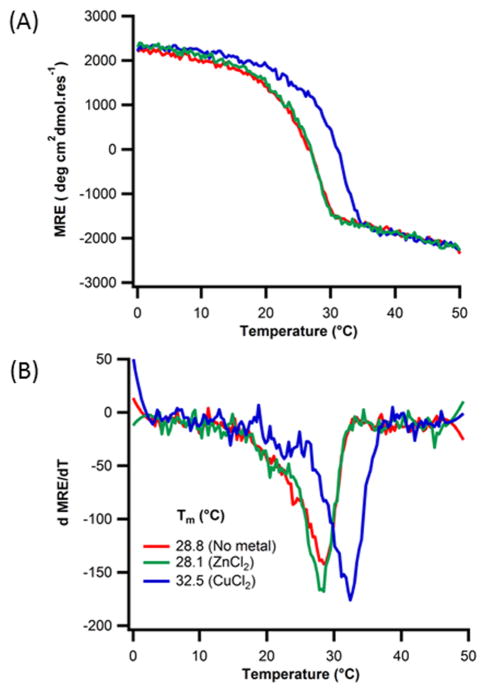
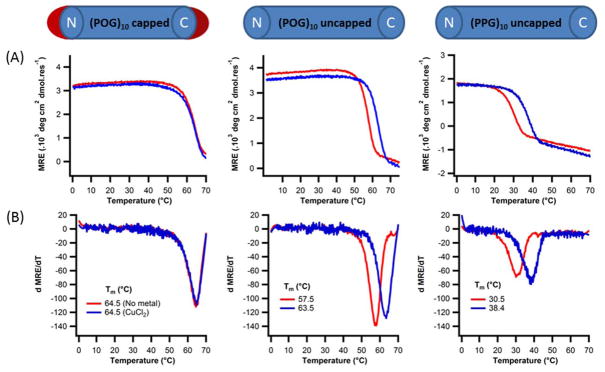
We examined metal binding to the A:B:C heterotrimer without the inclusion of C-terminal histidine. Addition of Zn(II) did not affect stability (Fig. 7), but the same concentration of Cu(II) salt raised the melting temperature by 5°C. A, B and C peptides have a high number of charged amino acids (Asp and Lys) which were designed to form networks of stabilizing charge pairs along the helix axis 56. To examine whether some of these groups may also be providing unanticipated binding sites, we examined neutral homotrimers (POG)10 and (PPG)10, also lacking C-terminal histidine groups. Again, enhancement of stability was observed in the presence of copper, but zinc had no effect (Fig. 8). The only competent metal ligands remaining in these proline-rich peptides were the backbone amides and carboxylates of peptide termini. The parallel orientation of the triple-helix chains brings together three amines or carboxylates in potentially favorable geometries for metal coordination. Capping the ends of the triple-helix by acetylation of the N-terminus and amidation of the C-terminus of (POG)10, giving Ac-(POG)10-NH2, resulted in a peptide that did not increase in stability upon addition of copper. This suggested that one of the two termini was forming a secondary copper binding site.
Figure 7. Metal binding to histidine-lacking heterotrimers.
(A) CD temperature melting curves monitored at 223nm of 0.18mM A:B:C heterotrimer peptide in 10mm Tris buffer, pH=7.4, 150mM NaCl in (red) no metal (green) 0.36mM ZnCl2 , (blue) 0.36mM CuCl2 (B) their respective first derivative plots.
Figure 8. Copper binding to neutral collagen model peptides.
(A) Circular dichroism temperature melting curves monitored at 225nm of 0.2mM (Left) capped (POG)10; (Middle) uncapped (POG)10; (Right) uncapped (PPG)10 peptide in 10mm Tris buffer, pH=7.4, 150mM NaCl with (red) no metal , (blue) 0.2mM CuCl2 (B) their respective first derivative plots.
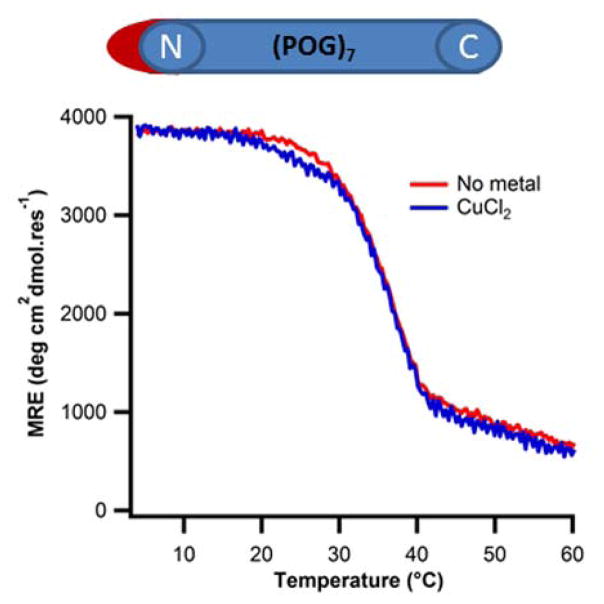
To identify on which end of the triple-helix copper binding occurs, we synthesized a singly capped variant of (POG)7 which was only acetylated on the N-terminus: Ac-(POG)7-OH. This modification prevented copper binding, indicating that the N-termini of triple-helices were responsible for coordinating copper (Fig. 9). The observed stabilization of APAH:BAH:CH by Cu(II) was due to the presence of two copper sites, one at the N-terminus and the other at the His3 site.
Figure 9. Sequence-independent binding of copper to the N-terminus of the triple helix.
(A) Circular dichroism temperature melting curves monitored at 225nm of 0.2mM N-terminus blocked (POG)7 with (blue) and without (red) CuCl2. No change in melting temperature was observed in presence of CuCl2.
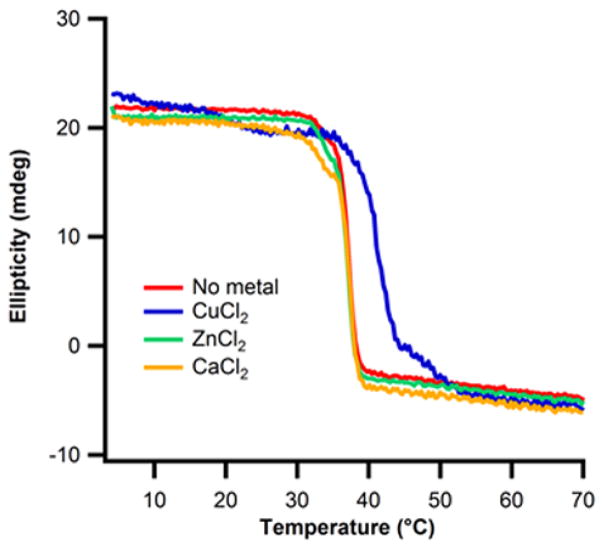
Provided copper binds to backbone amines at the N-terminus of the triple-helix in a sequence-independent manner, it was thought that similar binding would be observed to natural collagen. We measured the thermal denaturation of calf-skin Type I collagen in the absence and presence of a series of metals. Similar to the peptide result, copper raised the Tm of collagen by ~5°C (Fig. 10). No effect was seen in the presence of equivalent concentrations of zinc or calcium. Addition of copper did not perturb the oligomeric state of collagen as measured by DLS (Fig. S8), suggesting stabilization through intra-helical interactions. It should be noted that animal-extracted collagen has a short, non-triple-helical, N-terminal telopeptide, which should not present the same ligand configuration as the peptides. Therefore, the mode of copper binding may be different between natural collagen and peptide mimetics.
Figure 10. Effect of CuCl2 on natural collagen stability.
Thermal denaturation of 0.4 mg/ml Type I collagen at 222nm (A) in 10mm Tris buffer, pH=7.4, (red) no metals, (blue) 1.2mM CuCl2, (green) 1.2mM ZnCl2, (orange) 1.2mM CaCl2.
CONCLUSIONS
The poly-proline II conformation of the triple-helix is a challenging structural context in which to design metal binding sites. This is particularly evident at the termini, where chain flexibility hinders the design of a specific, structurally unique binding site. Although the protCAD software platform used here was previously successful in developing metal binding α-helix and β-sheet proteins71, 72, collagen presented a novel design challenge due to its flexibility. One of the advantages of putting the binding sites at the flexible region of a protein is that the ligands can adjust to bind the metals in their preferred geometry 70, 73. Constraining the linker geometry to promote formation of a specific heterotrimeric blunt-ended metal binding site proved to be very difficult. This work highlights the potential importance of multi-state design 74 in collagen engineering where both stability of the target and specificity against competing states are explicitly considered 75. Multi-state design protocols have been applied at the sequence modeling level to collagen. To achieve metal binding specificity, we expect similar approaches to be useful in atomistic design protocols as well.
There are now several strategies for stabilizing the collagen triple-helix using terminal augments. Natural pro-collagens contain N- and/or C-terminal globular non-collagenous domains that are responsible for directing oligomerization prior to their cleavage from the triple-helix. This has inspired the design of synthetic protein augments such as a trimeric foldon taken from bacteriophage fibritin 76, 77 or trimeric coiled coils78 which mimic the adjacent V-domain of bacterial collagens79, 80. Metal binding sites have been used to engineer self-assembling systems where assembly is gated by a metal switch 34, 37–40, 81. Metal binding combined with heterospecific inter-strand interactions provide an expanding set of tools for controlling the chemical and physical properties of collagen-derived biomaterials.
Copper binding to the N-termini of proteins has been previously observed and in some cases shown to depend on its acetylation state 82. Cu(II) has a high affinity for proline 83, and the close proximity of the three N-termini in the triple helix may provide an entactic state favoring binding. Copper is necessary for catalytic activity of lysyl-oxidase, which crosslinks animal collagen, contributing to its mechanical strength. This work suggests that copper may have additional effects on collagen matrices that may manifest in metal trafficking disorders such as Wilson’s disease. Characterizing the specific interactions between copper and collagen may further our understanding of the etiology of metal-trafficking disorders and the engineering of collagen-based biomaterials 84. Toxic metals such as chromium and aluminum affect the stability and higher-order structure of animal-extracted collagen 10–12, and are used in leather processing as tanning agents. Based on the observed broad spectrum effects of copper on many collagen-like systems, CMPs could be used as models to understand how other metals impact collagen structure in both industrial and biological contexts.
Supplementary Material
Acknowledgments
We thank Drs. Christopher Barbieri and Ann Stock, CABM, Piscataway, NJ for assistance with ITC measurements.
Footnotes
ASSOCIATED CONTENT
Supporting Information. Description of computational modeling of collagen structure, additional modeling and experimental data figures and tables, and peptide purification and characterization are including the supplemental supporting information. Supporting material may be accessed free of charge online at http://pubs.acs.org.
Funding disclosures: VN acknowledges NSF DMR-0907273, NIH DP2-OD-006478-1, and NIH R01-GM-089949-01 to carry out this work. ASP acknowledges support from Department of Science & Technology (DST-SERB) - SB/FTP/PS-073/2014, INDIA to carry out this work.
References
- 1.DeGrado WF, Summa CM, Pavone V, Nastri F, Lombardi A. De novo design and structural characterization of proteins and metalloproteins. Annu Rev Biochem. 1999;68:779–819. doi: 10.1146/annurev.biochem.68.1.779. [DOI] [PubMed] [Google Scholar]
- 2.Kennedy ML, Gibney BR. Metalloprotein and redox protein design. Curr Opin Struct Biol. 2001;11:485–490. doi: 10.1016/s0959-440x(00)00237-2. [DOI] [PubMed] [Google Scholar]
- 3.Lu Y, Yeung N, Sieracki N, Marshall NM. Design of functional metalloproteins. Nature. 2009;460:855–862. doi: 10.1038/nature08304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Clarke ND, Yuan SM. Metal search: a computer program that helps design tetrahedral metal-binding sites. Proteins. 1995;23:256–263. doi: 10.1002/prot.340230214. [DOI] [PubMed] [Google Scholar]
- 5.Klemba M, Gardner KH, Marino S, Clarke ND, Regan L. Novel metal-binding proteins by design. Nat Struct Biol. 1995;2:368–373. doi: 10.1038/nsb0595-368. [DOI] [PubMed] [Google Scholar]
- 6.Benson DE, Wisz MS, Hellinga HW. Rational design of nascent metalloenzymes. Proc Natl Acad Sci U S A. 2000;97:6292–6297. doi: 10.1073/pnas.97.12.6292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brodin JD, Ambroggio XI, Tang C, Parent KN, Baker TS, Tezcan FA. Metal-directed, chemically tunable assembly of one-, two- and three-dimensional crystalline protein arrays. Nat Chem. 2012;4:375–382. doi: 10.1038/nchem.1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dieckmann GR, McRorie DK, Lear JD, Sharp KA, DeGrado WF, Pecoraro VL. The role of protonation and metal chelation preferences in defining the properties of mercury-binding coiled coils. J Mol Biol. 1998;280:897–912. doi: 10.1006/jmbi.1998.1891. [DOI] [PubMed] [Google Scholar]
- 9.Handel TM, Williams SA, DeGrado WF. Metal ion-dependent modulation of the dynamics of a designed protein. Science. 1993;261:879–885. doi: 10.1126/science.8346440. [DOI] [PubMed] [Google Scholar]
- 10.Gayatri R, Sharma AK, Rajaram R, Ramasami T. Chromium(III)-induced structural changes and self-assembly of collagen. Biochem Bioph Res Co. 2001;283:229–235. doi: 10.1006/bbrc.2001.4713. [DOI] [PubMed] [Google Scholar]
- 11.Wu B, Mu CD, Zhang GZ, Lin W. Effects of Cr3+ on the Structure of Collagen Fiber. Langmuir. 2009;25:11905–11910. doi: 10.1021/la901577j. [DOI] [PubMed] [Google Scholar]
- 12.He LR, Cai SM, Wu B, Mu CD, Zhang GZ, Lin W. Trivalent chromium and aluminum affect the thermostability and conformation of collagen very differently. J Inorg Biochem. 2012;117:124–130. doi: 10.1016/j.jinorgbio.2012.08.017. [DOI] [PubMed] [Google Scholar]
- 13.Engel J, Chen HT, Prockop DJ, Klump H. The triple helix in equilibrium with coil conversion of collagen-like polytripeptides in aqueous and nonaqueous solvents. Comparison of the thermodynamic parameters and the binding of water to (L-Pro-L-Pro-Gly)n and (L-Pro-L-Hyp-Gly)n. Biopolymers. 1977;16:601–622. doi: 10.1002/bip.1977.360160310. [DOI] [PubMed] [Google Scholar]
- 14.Xu Y, Bhate M, Brodsky B. Characterization of the nucleation step and folding of a collagen triple-helix peptide. Biochemistry. 2002;41:8143–8151. doi: 10.1021/bi015952b. [DOI] [PubMed] [Google Scholar]
- 15.Bella J, Eaton M, Brodsky B, Berman HM. Crystal and molecular structure of a collagen-like peptide at 1.9 A resolution. Science. 1994;266:75–81. doi: 10.1126/science.7695699. [DOI] [PubMed] [Google Scholar]
- 16.Kramer RZ, Bella J, Brodsky B, Berman HM. The crystal and molecular structure of a collagen-like peptide with a biologically relevant sequence. J Mol Biol. 2001;311:131–147. doi: 10.1006/jmbi.2001.4849. [DOI] [PubMed] [Google Scholar]
- 17.Xu Y, Hyde T, Wang X, Bhate M, Brodsky B, Baum J. NMR and CD spectroscopy show that imino acid restriction of the unfolded state leads to efficient folding. Biochemistry. 2003;42:8696–8703. doi: 10.1021/bi034006n. [DOI] [PubMed] [Google Scholar]
- 18.Bretscher LE, Jenkins CL, Taylor KM, DeRider ML, Raines RT. Conformational stability of collagen relies on a stereoelectronic effect. J Am Chem Soc. 2001;123:777–778. doi: 10.1021/ja005542v. [DOI] [PubMed] [Google Scholar]
- 19.Holmgren SK, Bretscher LE, Taylor KM, Raines RT. A hyperstable collagen mimic. Chem Biol. 1999;6:63–70. doi: 10.1016/S1074-5521(99)80003-9. [DOI] [PubMed] [Google Scholar]
- 20.Holmgren SK, Taylor KM, Bretscher LE, Raines RT. Code for collagen’s stability deciphered. Nature. 1998;392:666–667. doi: 10.1038/33573. [DOI] [PubMed] [Google Scholar]
- 21.Shah NK, Ramshaw JA, Kirkpatrick A, Shah C, Brodsky B. A host-guest set of triple-helical peptides: stability of Gly-X-Y triplets containing common nonpolar residues. Biochemistry. 1996;35:10262–10268. doi: 10.1021/bi960046y. [DOI] [PubMed] [Google Scholar]
- 22.Persikov AV, Ramshaw JA, Brodsky B. Prediction of collagen stability from amino acid sequence. J Biol Chem. 2005;280:19343–19349. doi: 10.1074/jbc.M501657200. [DOI] [PubMed] [Google Scholar]
- 23.Persikov AV, Ramshaw JA, Kirkpatrick A, Brodsky B. Amino acid propensities for the collagen triple-helix. Biochemistry. 2000;39:14960–14967. doi: 10.1021/bi001560d. [DOI] [PubMed] [Google Scholar]
- 24.Okuyama K, Wu G, Jiravanichanun N, Hongo C, Noguchi K. Helical twists of collagen model peptides. Biopolymers. 2006;84:421–432. doi: 10.1002/bip.20499. [DOI] [PubMed] [Google Scholar]
- 25.Summa CM, Lombardi A, Lewis M, DeGrado WF. Tertiary templates for the design of diiron proteins. Curr Opin Struct Biol. 1999;9:500–508. doi: 10.1016/S0959-440X(99)80071-2. [DOI] [PubMed] [Google Scholar]
- 26.Roth W, Heidemann E. Triple Helix-Coil Transition of Covalently Bridged Collagen-Like Peptides. Biopolymers. 1980;19:1909–1917. [Google Scholar]
- 27.Dolz R, Heidemann E. Influence of Different Tripeptides on the Stability of the Collagen Triple Helix .1. Analysis of the Collagen Sequence and Identification of Typical Tripeptides. Biopolymers. 1986;25:1069–1080. doi: 10.1002/bip.360250607. [DOI] [PubMed] [Google Scholar]
- 28.Thakur S, Vadolas D, Germann HP, Heidemann E. Influence of Different Tripeptides on the Stability of the Collagen Triple Helix .2. An Experimental Approach with Appropriate Variations of a Trimer Model Oligotripeptide. Biopolymers. 1986;25:1081–1086. doi: 10.1002/bip.360250608. [DOI] [PubMed] [Google Scholar]
- 29.Germann HP, Heidemann E. A Synthetic Model of Collagen - an Experimental Investigation of the Triple-Helix Stability. Biopolymers. 1988;27:157–163. doi: 10.1002/bip.360270112. [DOI] [PubMed] [Google Scholar]
- 30.Fields CG, Lovdahl CM, Miles AJ, Matthias Hagen VL, Fields GB. Solid-Phase Synthesis and Stability of Triple-Helical Peptides Incorporating Native Collagen Sequences. Biopolymers. 1993;33:1695–1707. doi: 10.1002/bip.360331107. [DOI] [PubMed] [Google Scholar]
- 31.Goodman M, Feng YB, Melacini G, Taulane JP. A template-induced incipient collagen-like triple-helical structure. J Am Chem Soc. 1996;118:5156–5157. [Google Scholar]
- 32.Koide T, Yuguchi M, Kawakita M, Konno H. Metal-assisted stabilization and probing of collagenous triple helices. J Am Chem Soc. 2002;124:9388–9389. doi: 10.1021/ja026182+. [DOI] [PubMed] [Google Scholar]
- 33.Lebruin LT, Banerjee S, O’Rourke BD, Case MA. Metal ion-assembled micro-collagen heterotrimers. Biopolymers. 2011;95:792–800. doi: 10.1002/bip.21678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Przybyla DE, Chmielewski J. Metal-triggered radial self-assembly of collagen peptide fibers. J Am Chem Soc. 2008;130:12610–12611. doi: 10.1021/ja804942w. [DOI] [PubMed] [Google Scholar]
- 35.Cai WB, Kwok SW, Taulane JP, Goodman M. Metal-assisted assembly and stabilization of collagen-like triple helices. J Am Chem Soc. 2004;126:15030–15031. doi: 10.1021/ja0442062. [DOI] [PubMed] [Google Scholar]
- 36.Hsu W, Chen YL, Horng JC. Promoting self-assembly of collagen-related peptides into various higher-order structures by metal-histidine coordination. Langmuir. 2012;28:3194–3199. doi: 10.1021/la204351w. [DOI] [PubMed] [Google Scholar]
- 37.Pires MM, Chmielewski J. Self-assembly of collagen peptides into microflorettes via metal coordination. J Am Chem Soc. 2009;131:2706–2712. doi: 10.1021/ja8088845. [DOI] [PubMed] [Google Scholar]
- 38.Pires MM, Przybyla DE, Chmielewski J. A metal-collagen peptide framework for three-dimensional cell culture. Angew Chem Int Ed Engl. 2009;48:7813–7817. doi: 10.1002/anie.200902375. [DOI] [PubMed] [Google Scholar]
- 39.Przybyla DE, Chmielewski J. Metal-triggered collagen peptide disk formation. J Am Chem Soc. 2010;132:7866–7867. doi: 10.1021/ja103148t. [DOI] [PubMed] [Google Scholar]
- 40.Pires MM, Lee J, Ernenwein D, Chmielewski J. Controlling the morphology of metal-promoted higher ordered assemblies of collagen peptides with varied core lengths. Langmuir. 2012;28:1993–1997. doi: 10.1021/la203848r. [DOI] [PubMed] [Google Scholar]
- 41.Perez CM, Panitch A, Chmielewski J. A collagen peptide-based physical hydrogel for cell encapsulation. Macromol Biosci. 2011;11:1426–1431. doi: 10.1002/mabi.201100230. [DOI] [PubMed] [Google Scholar]
- 42.Gauba V, Hartgerink JD. Synthetic collagen heterotrimers: structural mimics of wild-type and mutant collagen type I. J Am Chem Soc. 2008;130:7509–7515. doi: 10.1021/ja801670v. [DOI] [PubMed] [Google Scholar]
- 43.Brodsky B, Baum J. Structural biology: modelling collagen diseases. Nature. 2008;453:998–999. doi: 10.1038/453998a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fiori S, Sacca B, Moroder L. Structural properties of a collagenous heterotrimer that mimics the collagenase cleavage site of collagen type I. J Mol Biol. 2002;319:1235–1242. doi: 10.1016/S0022-2836(02)00365-0. [DOI] [PubMed] [Google Scholar]
- 45.Ottl J, Battistuta R, Pieper M, Tschesche H, Bode W, Kuhn K, Moroder L. Design and synthesis of heterotrimeric collagen peptides with a built-in cystine-knot. Models for collagen catabolism by matrix-metalloproteases. FEBS Lett. 1996;398:31–36. doi: 10.1016/s0014-5793(96)01212-4. [DOI] [PubMed] [Google Scholar]
- 46.Kotch FW, Raines RT. Self-assembly of synthetic collagen triple helices. Proc Natl Acad Sci U S A. 2006;103:3028–3033. doi: 10.1073/pnas.0508783103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gauba V, Hartgerink JD. Self-assembled heterotrimeric collagen triple helices directed through electrostatic interactions. J Am Chem Soc. 2007;129:2683–2690. doi: 10.1021/ja0683640. [DOI] [PubMed] [Google Scholar]
- 48.Dong H, Paramonov SE, Hartgerink JD. Self-assembly of alpha-helical coiled coil nanofibers. J Am Chem Soc. 2008;130:13691–13695. doi: 10.1021/ja8037323. [DOI] [PubMed] [Google Scholar]
- 49.Gauba V, Hartgerink JD. Surprisingly high stability of collagen ABC heterotrimer: evaluation of side chain charge pairs. J Am Chem Soc. 2007;129:15034–15041. doi: 10.1021/ja075854z. [DOI] [PubMed] [Google Scholar]
- 50.Salem G, Traub W. Conformational implications of amino acid sequence regularities in collagen. FEBS Lett. 1975;51:94–99. doi: 10.1016/0014-5793(75)80861-1. [DOI] [PubMed] [Google Scholar]
- 51.Traub W, Fietzek PP. Contribution of the alpha2 chain to the molecular stability of collagen. FEBS Lett. 1976;68:245–249. doi: 10.1016/0014-5793(76)80446-2. [DOI] [PubMed] [Google Scholar]
- 52.Hulmes DJ, Miller A, Parry DA, Piez KA, Woodhead-Galloway J. Analysis of the primary structure of collagen for the origins of molecular packing. J Mol Biol. 1973;79:137–148. doi: 10.1016/0022-2836(73)90275-1. [DOI] [PubMed] [Google Scholar]
- 53.Venugopal MG, Ramshaw JA, Braswell E, Zhu D, Brodsky B. Electrostatic interactions in collagen-like triple-helical peptides. Biochemistry. 1994;33:7948–7956. doi: 10.1021/bi00191a023. [DOI] [PubMed] [Google Scholar]
- 54.Yang W, Chan VC, Kirkpatrick A, Ramshaw JA, Brodsky B. Gly-Pro-Arg confers stability similar to Gly-Pro-Hyp in the collagen triple-helix of host-guest peptides. J Biol Chem. 1997;272:28837–28840. doi: 10.1074/jbc.272.46.28837. [DOI] [PubMed] [Google Scholar]
- 55.Persikov AV, Ramshaw JA, Kirkpatrick A, Brodsky B. Electrostatic interactions involving lysine make major contributions to collagen triple-helix stability. Biochemistry. 2005;44:1414–1422. doi: 10.1021/bi048216r. [DOI] [PubMed] [Google Scholar]
- 56.Xu F, Zahid S, Silva T, Nanda V. Computational design of a collagen A:B:C-type heterotrimer. J Am Chem Soc. 2011;133:15260–15263. doi: 10.1021/ja205597g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fallas JA, Hartgerink JD. Computational design of self-assembling register-specific collagen heterotrimers. Nat Commun. 2012;3:1087. doi: 10.1038/ncomms2084. [DOI] [PubMed] [Google Scholar]
- 58.Xu F, Zhang L, Koder RL, Nanda V. De Novo Self-Assembling Collagen Heterotrimers Using Explicit Positive and Negative Design. Biochemistry. 2010;49:2307–2316. doi: 10.1021/bi902077d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Savitzky AG, MJE Smoothing and differentiation of data by simplified least squares procedures. Anal Chem. 1964;36:e13. doi: 10.1021/ac60319a045. [DOI] [PubMed] [Google Scholar]
- 60.Parmar AS, Muschol M. Lysozyme as diffusion tracer for measuring aqueous solution viscosity. J Colloid Interface Sci. 2009;339:243–248. doi: 10.1016/j.jcis.2009.07.031. [DOI] [PubMed] [Google Scholar]
- 61.Parmar AS, Gottschall PE, Muschol M. Pre-assembled clusters distort crystal nucleation kinetics in supersaturated lysozyme solutions. Biophys Chem. 2007;129:224–234. doi: 10.1016/j.bpc.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 62.Parmar AS, Muschol M. Hydration and hydrodynamic interactions of lysozyme: effects of chaotropic versus kosmotropic ions. Biophys J. 2009;97:590–598. doi: 10.1016/j.bpj.2009.04.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pike DH, Nanda V. Empirical Estimation of Local Dielectric Constants: Toward Atomistic Design of Collagen Mimetic Peptides. Biopolymers. 2015 doi: 10.1002/bip.22644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pike DH, Nanda V. Empirical estimation of local dielectric constants: Toward atomistic design of collagen mimetic peptides. Biopolymers. 2015;104:360–370. doi: 10.1002/bip.22644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Presta LG, Rose GD. Helix signals in proteins. Science. 1988;240:1632–1641. doi: 10.1126/science.2837824. [DOI] [PubMed] [Google Scholar]
- 66.O’Neil KT, DeGrado WF. A thermodynamic scale for the helix-forming tendencies of the commonly occurring amino acids. Science. 1990;250:646–651. doi: 10.1126/science.2237415. [DOI] [PubMed] [Google Scholar]
- 67.Hornak V, Abel R, Okur A, Strockbine B, Roitberg A, Simmerling C. Comparison of multiple amber force fields and development of improved protein backbone parameters. Proteins-Structure Function and Bioinformatics. 2006;65:712–725. doi: 10.1002/prot.21123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Pang YP, Xu K, El Yazal J, Prendergast FG. Successful molecular dynamics simulation of the zinc-bound farnesyltransferase using the cationic dummy atom approach (vol 9, pg 1857, 2000) Protein Science. 2000;9:2583–2583. [PMC free article] [PubMed] [Google Scholar]
- 69.Williams RJP, Frausto da Silva JJR. The Natural Selection of the Chemical Elements. Clarendon Press; 1997. [Google Scholar]
- 70.Wray JW, Baase WA, Ostheimer GJ, Zhang XJ, Matthews BW. Use of a non-rigid region in T4 lysozyme to design an adaptable metal-binding site. Protein Eng. 2000;13:313–321. doi: 10.1093/protein/13.5.313. [DOI] [PubMed] [Google Scholar]
- 71.Nanda V, Rosenblatt MM, Osyczka A, Kono H, Getahun Z, Dutton PL, Saven JG, Degrado WF. De novo design of a redox-active minimal rubredoxin mimic. J Am Chem Soc. 2005;127:5804–5805. doi: 10.1021/ja050553f. [DOI] [PubMed] [Google Scholar]
- 72.Kaplan J, DeGrado WF. De novo design of catalytic proteins. Proc Natl Acad Sci U S A. 2004;101:11566–11570. doi: 10.1073/pnas.0404387101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhang XJ, Matthews BW. Edpdb - a Multifunctional Tool for Protein-Structure Analysis. J Appl Crystallogr. 1995;28:624–630. [Google Scholar]
- 74.Fleishman SJ, Baker D. Role of the biomolecular energy gap in protein design, structure, and evolution. Cell. 2012;149:262–273. doi: 10.1016/j.cell.2012.03.016. [DOI] [PubMed] [Google Scholar]
- 75.Havranek JJ, Harbury PB. Automated design of specificity in molecular recognition. Nat Struct Biol. 2003;10:45–52. doi: 10.1038/nsb877. [DOI] [PubMed] [Google Scholar]
- 76.Frank S, Kammerer RA, Mechling D, Schulthess T, Landwehr R, Bann J, Guo Y, Lustig A, Bachinger HP, Engel J. Stabilization of short collagen-like triple helices by protein engineering. J Mol Biol. 2001;308:1081–1089. doi: 10.1006/jmbi.2001.4644. [DOI] [PubMed] [Google Scholar]
- 77.Stetefeld J, Frank S, Jenny M, Schulthess T, Kammerer RA, Boudko S, Landwehr R, Okuyama K, Engel J. Collagen stabilization at atomic level: crystal structure of designed (GlyProPro)10foldon. Structure. 2003;11:339–346. doi: 10.1016/s0969-2126(03)00025-x. [DOI] [PubMed] [Google Scholar]
- 78.Yoshizumi A, Fletcher JM, Yu Z, Persikov AV, Bartlett GJ, Boyle AL, Vincent TL, Woolfson DN, Brodsky B. Designed coiled coils promote folding of a recombinant bacterial collagen. J Biol Chem. 2011;286:17512–17520. doi: 10.1074/jbc.M110.217364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Xu Y, Keene DR, Bujnicki JM, Hook M, Lukomski S. Streptococcal Scl1 and Scl2 proteins form collagen-like triple helices. J Biol Chem. 2002;277:27312–27318. doi: 10.1074/jbc.M201163200. [DOI] [PubMed] [Google Scholar]
- 80.Yu Z, Mirochnitchenko O, Xu C, Yoshizumi A, Brodsky B, Inouye M. Noncollagenous region of the streptococcal collagen-like protein is a trimerization domain that supports refolding of adjacent homologous and heterologous collagenous domains. Protein Sci. 2010;19:775–785. doi: 10.1002/pro.356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Pires MM, Przybyla DE, Rubert Perez CM, Chmielewski J. Metal-mediated tandem coassembly of collagen peptides into banded microstructures. J Am Chem Soc. 2011;133:14469–14471. doi: 10.1021/ja2042645. [DOI] [PubMed] [Google Scholar]
- 82.Moriarty GM, Minetti CA, Remeta DP, Baum J. A revised picture of the Cu(II)-alpha-synuclein complex: the role of N-terminal acetylation. Biochemistry. 2014;53:2815–2817. doi: 10.1021/bi5003025. [DOI] [PubMed] [Google Scholar]
- 83.Stability Constants of Metal-Ion Complexes, with Solubility PRoducts of Inorganic Substances. The Chemical Society; London: 1957. Vol. Special Publications No. 6. [Google Scholar]
- 84.Dahl SLM, Rucker RB, Niklason LE. Effects of copper and cross-linking on the extracellular matrix of tissue-engineered arteries. Cell Transplant. 2005;14:367–374. doi: 10.3727/000000005783982936. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.