Abstract
Objective
This study aimed to investigate the relations between calreticulin (CRT) serum level and both disease activity and severity parameters in juvenile idiopathic arthritis (JIA).
Material and Methods
In this study, 60 children with JIA and 50 age-and-sex-matched healthy subjects were enrolled. The assessment of the disease activity was done using juvenile arthritis disease activity score 27 (JADAS-27). The assessment of disease severity was done via gray-scale ultrasonography (US) and power Doppler US (PDUS). Enzyme-linked immunosorbent assay (ELISA) was used to assay the serum level of human CRT.
Results
The mean serum CRT levels in JIA patients was 8.6±1.2 ng/mL and showed a highly significant increase (p=0.001) as compared to the mean serum levels in the controls (5.02±0.77 ng/mL). There were statistically significant positive correlations between the serum CRT levels and disease duration, tender joint count, swollen joint count, visual analog scale, erythrocyte sedimentation rate, JADAS-27, C-reactive protein, rheumatoid factor titer, and ultrasonographic grading for synovitis and neovascularization.
Conclusion
Elevated serum CRT levels in JIA patients and its correlations with JIA disease activity and severity parameters signified that CRT might be used as a novel biomarker for disease activity and severity in JIA.
Keywords: Juvenile idiopathic arthritis, calreticulin, activity score, synovitis
Introduction
Juvenile idiopathic arthritis (JIA) denotes a group of chronic arthropathies, constituting the most common rheumatic condition in children. It is also an inflammatory joint disease characterized by chronic synovitis and associated with extra-articular manifestations, such as fever, anorexia, weight loss, anemia, lymphadenopathy, pericarditis, or eye affection (1).
Juvenile idiopathic arthritis International League of Associations for Rheumatology (ILAR) classification includes systemic onset arthritis, oligoarthritis, seropositive polyarthritis, seronegative polyarthritis, psoriatic arthritis, enthesitis-related arthritis (ERA), and undifferentiated arthritis on the basis of clinical and laboratory data during the first 6 months of the disease and family history features (2). Nowadays, musculoskeletal ultrasound (MSUS) plays an important role in detecting the presence of synovitis, joint damage, and diagnosing acute disease flare (3).
Calreticulin (CRT) is a 46-kDa Ca2+-binding chaperone comprising structurally and functionally peculiar domains with certain functions; it renders calcium to become inactive that is enrolled in the regulation of intracellular Ca2+ homeostasis and endoplasmic reticulum Ca2+ storage capacity (4).
Cell surface CRT is considered to be a signal-transducing receptor for the members of the collectin family, including C1q- and mannose-binding lectin; therefore, it is an important innate immune system receptor. CRT participates in the clearance of apoptotic cells and is involved in the link between tolerance and autoimmune diseases. Furthermore, due to its lectin activity, CRT can maintain misfolded glycoproteins, folding intermediates, or partially assembled oligomers, opposing their aggregation and assisting their conformational maturation (5).
This study aimed to investigate the relations between serum CRT levels and both disease activity and severity parameters in JIA.
Material and Methods
This study was conducted on 60 JIA cases, fulfilling the ILAR classification criteria of JIA (2), admitted to the Rheumatology, Rehabilitation, and Physical Medicine Department of the Benha University Hospitals. Here 50 age-and-sex-matched apparently healthy children represented the control group. The cases were selected in the period between August 2014 and February 2015. Prior written consents were taken from the parents of each patient and control included in this study. This study obtained the approval of the Ethical Committee of the School of Medicine, Benha University.
Patients having associated neurological diseases, those with diabetes mellitus, acute or chronic infections, any other causes for arthritis, and those with malignancies were not enrolled in this study.
The following were conducted on the patients: taking full history, thorough clinical examination including locomotor system examination, slit-lamp examination, and plain radiography on the affected joint. Juvenile arthritis disease activity score 27 (JADAS-27) was calculated using 4 measured variables: active joint count (AJC), physician global assessment (PGA) of the disease activity using a visual analog scale (VAS), patient global evaluation (PGE) of the child’s well-being determined by VAS, count of joints with active disease (evaluating 27 joints), and erythrocyte sedimentation rate (ESR). The 27 joints included the cervical spine, elbows, wrists, 1st to 3rd metacarpophalangeal, proximal interphalangeal, hips, knees, and ankles. There is no weighting of joints. To avoid excessive weight in the overall index, ESR is normalized to a score ranging from 0 to 10 by using the formula (ESR-20)/10 (6). JADAS-27 is calculated as a simple linear sum of the scores of its 4 components, which yields a total score of 0–57, with higher scores associated with worse disease activity
Assessment of disease severity
A commercially available Logiq E real-time scanner (General Electric Medical Systems; Milwaukee, Wisconsin, USA) was used for the US examination using high-frequency (8–13 MHz) linear transducer gray-scale ultrasonography (US) and power Doppler US (PDUS) assessment was done in a minimum of 2 planes (longitudinal and transverse) including the affected joints. Synovitis detected by the gray-scale US was graded as mild, moderate, or severe We used 6.7 MHz for power Doppler signal standardized with a lower pulse repetition frequency of 750 MHz and low wall filters). The color gain was increased to the highest values not creating PD signals under the bony cortex (7). The PDUS signal was scored on a semi-quantitative four-grade scale: 0 means no signs of vascularization, 1 denotes mild (presence of single/vessel dots), 2 equals moderate (presence of confluent vessel dots in less than half of the synovial area), and 3 refers marked (presence of confluent vessel dots in more than half of the synovial area) (8). PD was also graded semi-quantitatively from 0 to 3: 0 denotes none; 1, minor; 2, moderate; and 3, major presence. The total US score was calculated as the sum of the synovitis and PD scores of each joint (9). The US examination was performed by a single observer who was blinded to the condition of the patients.
Laboratory investigations included the following: complete blood count (CBC) was determined by using a Sysmex 5000 counter; ESR, Westergren method; C-reactive protein (CRP) and rheumatoid factor (RF) titer, latex agglutination slide tests; and anti-nuclear antibody (ANA) testing, indirect immunofluorescence technique (IMMCO Diagnostics; New York, USA).
CRT measurement
Serum samples were collected from each patient and control subject and stored at −20 °C. The serum CRT level was detected by double-antibody sandwich ELISA (Xin Yue; Shanghai, China). All the reagents, samples, standards, and washing solution were prepared according to the manufacturer’s instructions. Briefly, 40 μL of serum sample, 10 μL of rabbit anti-human CRT, and 50 μL of horseradish-peroxidase-labeled streptavidin were added to the test wells. After that, the plate was incubated at 37°C for 1 h with gentle shaking. After washing, 50 μL of chromogenic solution A followed by 50 μL of chromogenic solution B were applied to each well with proper mixing and the reaction was incubated for 10 min at 37°C in the dark. After adding the stop solution, the wells were assessed by ELISA plate reader within 15 min at 450 nm.
Statistical analyses
Statistical analyses were performed using the SPSS software (version 19.0, IBM Corp.; Armonk, NY, USA). According to the type of data, the following tests were used to test the differences for significance: differences between frequencies (qualitative variables) and percentages in groups were compared by means of the chi-square test (χ2), and differences between the means (quantitative variables) in 2 parametric groups were compared by the Student’s t-test (t). Non-parametric data were analyzed by means of the Mann–Whitney (MW) U-test. Pearson correlation was used. The p-value was assumed to be significant when p≤0.05 and highly significant when p≤0.001. Differences among the groups were analyzed by means of 1-way ANOVA tests.
Results
The patients included 40 (66.6%) girls and 20 (33.3%) boys whose ages ranged between 4 and 12 years (mean: 8.10±2.67 years). Further, 50 age-and-sex-matched apparently healthy children represented the controls. The disease duration ranged between 1 and 8 years with a mean duration of 4.05±2.04 years. The number of tender joints ranged between 1 and 9 joints with a mean of 4.50±2.69 joints. ESR ranged between 4 and 85 mm/h with a mean of 28.55±21.92 mm/h. The mean of CRP in JIA patients was 22.4±10.4 mg/dL. Further, 6 cases (10%) were RF-positive and the other 54 cases (90%) were RF-negative: the mean of the RF titer was 33.4±17.99 IU. There were 15 cases (25%) of the polyarticular subtype, 30 (50%) cases of the oligoarticular subtype, 10 cases (16.66%) of the ERA subtype, and 5 cases (8.33) of the systemic-onset subtype. JADAS-27 scores ranged between 1.70 and 14.50 with a mean of 7.24±3.3. Moreover, the hemoglobin (Hb) concentration ranged between 9.11 and 14.22 gm/dL with a mean of 11.23±1.28 gm/dL. Furthermore, red blood cells count ranged between 3.71×106 and 5.82×106 cells/mm3 with a mean of 4.86±0.53×106 cells/mm3; however, white blood cells (WBCs) count ranged between 4.32×103 and 13.83×103 cells/mm3 with a mean of 7.94±2.67×103 cells/mm3 and platelet count ranged between 205×103 and 292.7×103 cells/mm3 with a mean of 233±76.02 cells/mm3. Only 24 cases (40%) out of 30 were positive for ANA. Further, 5 cases (8.33%) had a history of uveitis.
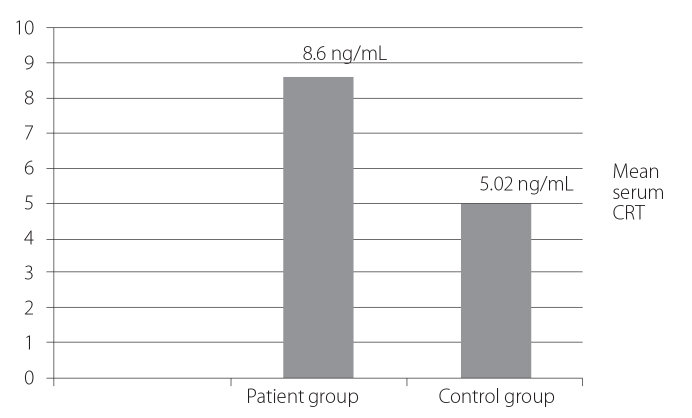
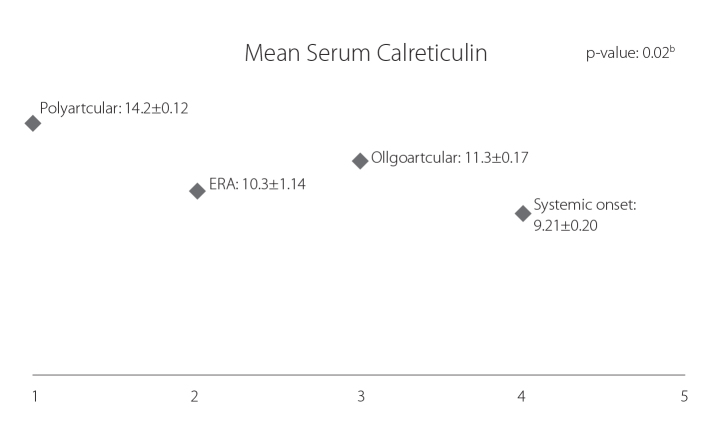
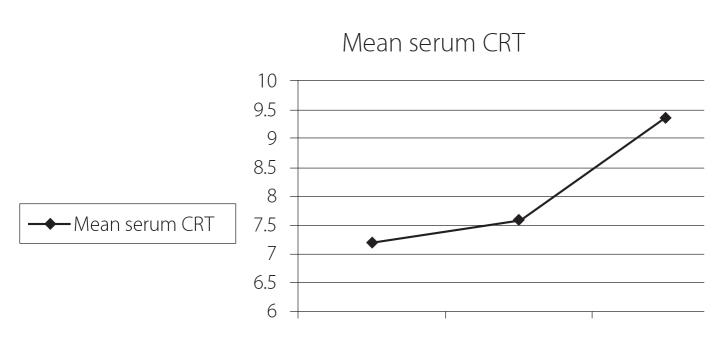
Figure (1) shows that the mean serum CRT level was significantly higher in the patient group (8.6±1.2 ng/mL) as compared to the control group (5.02±0.77 ng/mL) (p=0.001). Figure (2) shows that the mean serum CRT level was the highest in the polyarticular subtype (14.2±12 ng/mL) as compared to other JIA subtypes with statistically significant differences (p=0.02). Figure (3) shows that the serum CRT levels are highly statistically significant associated with the disease activity score (JADAS-27) in patients with JIA.
Figure 1.
Comparison between serum CRT levels in JIA patients (8.6±1.2 ng/mL) and controls (5.02±0.77 ng/mL)
CRT: calreticulin; JIA: juvenile idiopathic arthritis
Figure 2.
Comparison between different subtypes of JIA according to serum CRT levels: serum CRT level was significantly higher (p=0.02) in polyarticular subtype (14.2 ng/mL) when compared to the other JIA subtypes
CRT: calreticulin; JIA: juvenile idiopathic arthritis
Figure 3.
Relations between serum CRT levels in patients with JIA and JADAS-27 (p=0.001)
CRT: calreticulin; JIA: juvenile idiopathic arthritis; JADAS-27: juvenile arthritis disease activity score 27
In Table 1, there were statistically significant positive correlations between the serum CRT levels and disease duration (p=0.011), TJC (p=0.001), SJC (p=0.01), VAS (p=0.001), ESR (p=0.002), JADAS-27 (p=0.001), CRP (p=0.001), RF titer (p=0.007), US synovitis grading (p=0.005), and Doppler score for synovial vascularization (p=0.04). There were no significant correlations between the serum CRT level and age (p=0.31), Hb% (p=0.3), WBCs (p=0.17), platelet count (p=0.97), and ANA titer (p=0.19).
Table 1.
Correlations between serum CRT level and the studied variables in JIA patients
| Variables | Patient group (n=60) | |
|---|---|---|
|
| ||
| r | p | |
| Age | 0.192 | 0.31 |
| Disease duration (ys) | 0.459 | 0.011* |
| TJC | 0.925 | 0.001* |
| SJC | 0.461 | 0.01* |
| VAS | 0.914 | 0.001* |
| ESR | 0.536 | 0.0002* |
| JDAS 27 | 0.998 | 0.001* |
| RF titer | 0.528 | 0.007* |
| ANA | 0.345 | 0.19 |
| CRP titer | 0.258 | 0.0001* |
| Hb | 0.193 | 0.31 |
| WBCs | 0.254 | 0.17 |
| PLTs | 0.031 | 0.87 |
| Total US score | 0.370 | 0.04* |
TJC: total joint count; SJC: swollen joint count; VAS: visual analog scale; ESR: erythrocytes sedimentation rate; JADAS: juvenile arthritis disease activity score; RF: rheumatoid factor, ANA: antinuclear antibodies; CRP: C-reactive protein; Hb: hemoglobin; PLT: platelet; WBC: white blood cells; US: ultrasound
p>0.05: insignificant; p<0.05*: significant
Discussion
Several disease activity indices in JIA depending on different clinical, laboratory, and physical measures have been introduced. Further objective information about the underlying disease processes may augment clinical assessments (10). Laboratory tests represent one approach to such additional information, and markers such as ESR and CRP levels have been incorporated into disease activity assessment in patients with JIA. However, ESR and CRP can be affected by anemia and the presence of immunoglobulins, including rheumatoid factor (RF). Therefore, these markers may not be trustable in all JIA patients (11).
Calreticulin is a ubiquitous multifunctional Ca2+-binding protein. It is originally characterized as an endoplasmic reticulum molecular chaperone. Extracellular CRT attaches to the surface of many cells and is involved in signal transduction events associated with innate immunity, cell adhesion, and apoptosis (12).
Recently, CRT was used in a number of autoimmune processes such as molecular mimicry, epitope spreading, complement inactivation, and stimulation of inflammatory mediators (e.g., nitric oxide (NO) production). These findings made us depict that CRT is not just an auto-antigen in susceptible individuals but also plays an active role in the pathology of various autoimmune diseases (13).
Liu et al. (14) demonstrated elevated serum CRT levels in cancer patients. Additionally, recombinant CRT fragments, namely, r CRT/39–272 and r CRT/18–424, are potent macrophage activators that are capable of inducing tumor necrosis factor-α (TNF-α) and interleukin (IL)-6 production. This may explain the correlation between serum CRT levels and autoimmune disorders, since TNF-α and IL-6 represent predominant inflammatory cytokines released by activated macrophages (15).
In this study, the serum CRT level was significantly higher (p=0.001) in JIA patients in comparison with controls, which was in consistence with the results of Ni et al. (16) who demonstrated that the serum CRT levels were higher in patients with rheumatoid arthritis (RA) when compared with healthy controls in their study on 70 RA patients and 35 healthy controls. Furthermore, our result agreed with that of Hong et al. (17) who documented that the serum CRT level was significantly higher in their RA patients than the control group. Moreover, the present study revealed statistically significant correlations between the serum CRT levels and CRP (p<0.001), ESR (p=0.002), swollen joints count (p=0.01), tender joints count (p=0.001), and JADAS-27 (p=0.001): these results were consistent with the results of Tarr et al. (18) who found that CRT levels were positively correlated with ESR, CRP, SJC, and DAS-28 in RA patients.
It is noteworthy that there were statistically significant relations between the serum CRT levels and US grading of synovitis (p=0.001), and this was in accordance with Heba et al. (19) who concluded that the serum CRT levels are related to the degree of activity and severity of the disease detected by US. These findings emphasized that serum CRT levels may be considered as a potential marker for the assessment of disease severity in JIA patients. Holoshitz et al. (20) demonstrated that CRT increased the secretion of IL-6 and IL-23 and induced the generation of T-helper lymphocyte (Th17) cells. We found that there was a statistically significant correlation between the serum CRT levels and total US score for synovitis and synovial neovascularization (p=0.04). This was similar to Ding et al. (21) who measured the CRT levels in the synovial fluid and sera of RA patients and suggested that CRT may be involved in angiogenesis events in RA through an NO signaling pathway. Not only did we find more statistically significant differences (p=0.001) regarding the serum CRT levels in the polyarticular subtype within the JIA subtypes but also a high statistically significant correlation between the RF titer and serum CRT level (p=0.007). We concluded that elevated serum CRT levels in JIA patients and its correlations with JIA disease activity and severity parameters signify that CRT might be used as a novel biomarker for disease activity and severity in JIA.
Key messages.
Serum CRT levels were significantly higher in JIA patients than healthy controls.
CRT might be used as a potential biomarker for disease activity in JIA.
The possible role of CRT in the pathological process of JIA should be investigated.
CRT might be the potential target for RA therapy.
Acknowledgements
The authors would like to thank all children and their parents who participated in this study.
Footnotes
Ethics Committee Approval: Ethics committee approval was received for this study from the ethics committee of Benha University School of Medicine, Benha, Egypt.
Informed Consent: Written informed consent was obtained from patients’ parents who participated in this study.
Peer-review: Externally peer-reviewed.
Author Contributions: Concept - N.I.H., R.M.F.; Design - N.I.H., R.M.F., A.A.A, M.I.Y; Supervision - N.I.H., R.M.F.; Resources - N.I.H., R.M.F., A.A.A, M.I.Y.; Materials - N.I.H., R.M.F., A.A.A, M.I.Y.; Data Collection and/or Processing - N.I.H., R.M.F.; Analysis and/or Interpretation - N.I.H. R.M.F., A.A.A, M.I.Y.; Literature Search - N.I.H., R.M.F.; Writing Manuscript - N.I.H., R.M.F., A.A.A, M.I.Y.; Critical Review - N.I.H.
Conflict of Interest: No conflict of interest was declared by the authors.
Financial Disclosure: The authors declared that this study has received no financial support.
References
- 1.Haines KA. Juvenile idiopathic arthritis: therapies in the 21st century. Bull NYU Hosp Jt Dis. 2007;65:205–11. [PubMed] [Google Scholar]
- 2.Petty RE, Southwood TR, Manners P, Baum J, Glass DN, Goldenberg J, et al. International League of Associations for Rheumatology classification of juvenile idiopathic arthritis: second revision, Edmonton, 2001. J Rheumatol. 2004;31:390–24. [PubMed] [Google Scholar]
- 3.Chao MP, Jaiswal S, Weissman-Tsukamoto R, Alizadeh AA, Gentles AJ, Volkmer J, et al. Calreticulin is the dominant pro-phagocytic signal on multiple human cancers and is counterbalanced by CD47. Sci Transl Med. 2010;2:63–94. doi: 10.1126/scitranslmed.3001375. https://doi.org/10.1126/scitranslmed.3001375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Torp-Pedersen ST, Terslev L. Settings and artefacts relevant in colour/power Doppler ultrasound in rheumatology. Ann Rheum Dis. 2008;67:143–9. doi: 10.1136/ard.2007.078451. https://doi.org/10.1136/ard.2007.078451. [DOI] [PubMed] [Google Scholar]
- 5.Duo CC, Gong FY, He XY, Li YM, Wang J, Zhang JP, et al. Soluble calreticulin induces tumor necrosis factor-α (TNF-α) and interleukin (IL)-6 production by macrophages through mitogen-activated protein kinase (MAPK) and NFκB signaling pathways. Int J Mol Sci. 2014;15:2916–28. doi: 10.3390/ijms15022916. https://doi.org/10.3390/ijms15022916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Consolaro A, Ruperto N, Bazso A, Pistorio A, Magni-Manzoni S, Filocamo G, et al. Development and validation of a composite disease activity score for juvenile idiopathic arthritis. Arthritis Rheum. 2009;61:658–66. doi: 10.1002/art.24516. https://doi.org/10.1002/art.24516. [DOI] [PubMed] [Google Scholar]
- 7.Möller B, Bonel H, Rotzetter M, Villiger PM, Ziswiler HR. Measuring finger joint cartilage by ultrasound as a promising alternative to conventional radiograph imaging. Arthritis Rheum. 2009;61:435–41. doi: 10.1002/art.24424. https://doi.org/10.1002/art.24424. [DOI] [PubMed] [Google Scholar]
- 8.Torp-Pedersen ST, Terslev L. Settings and artefacts relevant in colour/power Doppler ultrasound in rheumatology. Annals of the Rheumatic Diseases. 2008;67:143–9. doi: 10.1136/ard.2007.078451. https://doi.org/10.1136/ard.2007.078451. [DOI] [PubMed] [Google Scholar]
- 9.Naredo E, Rodríguez M, Campos C, Rodríguez-Heredia JM, Medina JA, Giner E, et al. Validity, reproducibility, and responsiveness of a twelve-joint simplified power Doppler ultrasonographic assessment of joint inflammation in rheumatoid arthritis. Arthritis Rheum. 2008;59:515–22. doi: 10.1002/art.23529. https://doi.org/10.1002/art.23529. [DOI] [PubMed] [Google Scholar]
- 10.Farheen K, Agarwal SK. Assessment of disease activity and treatment outcomes in rheumatoid arthritis. J Manag Care Pharm. 2011;17(Suppl B):9–13. doi: 10.18553/jmcp.2011.17.s9-b.S09. https://doi.org/10.18553/jmcp.2011.17.s9-b.S09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lin Q, Cao YG, Gao J. Serum calreticulin is a negative biomarker in patients with Alzheimer's disease. Int J Mol Sci. 2014;15:21740–53. doi: 10.3390/ijms151221740. https://doi.org/10.3390/ijms151221740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Duus K, Pagh RT, Holmskov U, Hojrup P, Skov S, Houen G. Interaction of calreticulin with CD40 ligand, TRAIL and Fas ligand. Scand J Immunol. 2007;66:501–7. doi: 10.1111/j.1365-3083.2007.01999.x. https://doi.org/10.1111/j.1365-3083.2007.01999.x. [DOI] [PubMed] [Google Scholar]
- 13.Kuroki Y, Takahashi M, Nishitani C. Pulmonary collections in innate immunity of the lung. Cell Microbiol. 2007;9:1871–9. doi: 10.1111/j.1462-5822.2007.00953.x. https://doi.org/10.1111/j.1462-5822.2007.00953.x. [DOI] [PubMed] [Google Scholar]
- 14.Liu R, Gong J, Chen J, Li Q, Song C, Zhang J, et al. Calreticulin as a potential diagnostic biomarker for lung cancer. Cancer Immunol Immunother. 2012;61:855–64. doi: 10.1007/s00262-011-1146-8. https://doi.org/10.1007/s00262-011-1146-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huang SH, Zhao LX, Hong C, Duo CC, Guo BN, Zhang LJ, et al. Self-oligomerization is essential for enhanced immunological activities of soluble recombinant calreticulin. PLoS One. 2013;8:e64951. doi: 10.1371/journal.pone.0064951. https://doi.org/10.1371/journal.pone.0064951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ni M, Wei W, Wang Y, Zhang N, Ding H, Shen C, et al. Serum levels of calreticulin in correlation with disease activity in patients with rheumatoid arthritis. J Clin Immunol. 2013;33:947–53. doi: 10.1007/s10875-013-9885-2. https://doi.org/10.1007/s10875-013-9885-2. [DOI] [PubMed] [Google Scholar]
- 17.Hong C, Qiu X, Li Y, Huang Q, Zhong Z, Zhang Y, et al. Functional analysis of recombinant calreticulin fragment 39–272: implications for immunobiological activities of calreticulin in health and disease. J Immunol. 2010;185:4561–9. doi: 10.4049/jimmunol.1000536. https://doi.org/10.4049/jimmunol.1000536. [DOI] [PubMed] [Google Scholar]
- 18.Tarr JM, Winyard PG, Ryan B, Harries LW, Haigh R, Viner N, et al. Extracellular calreticulin is present in the joints of patients with rheumatoid arthritis and inhibits FasL (CD95L)-mediated apoptosis of T cells. Arthritis Rheum. 2010;62:2919–29. doi: 10.1002/art.27602. https://doi.org/10.1002/art.27602. [DOI] [PubMed] [Google Scholar]
- 19.Heba AS, Marwa AH, Hanaa HE, Asmaa MA. Serum calreticulin in rheumatoid arthritis and its relation with disease activity and severity. Int J of Adv Res. 2015;3:102–9. [Google Scholar]
- 20.Holoshitz J, DeAlmeida DE, Ling S. A role for calreticulin in the pathogenesis of rheumatoid arthritis. Ann N Y Acad Sci. 2010;1209:91–8. doi: 10.1111/j.1749-6632.2010.05745.x. https://doi.org/10.1111/j.1749-6632.2010.05745.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ding H, Hong C, Wang Y, Liu J, Zhang N, Shen C, et al. Calreticulin promotes angiogenesis via activating nitric oxide signaling pathway in rheumatoid arthritis. Clin Exp Immunol. 2014;178:236–44. doi: 10.1111/cei.12411. https://doi.org/10.1111/cei.12411. [DOI] [PMC free article] [PubMed] [Google Scholar]