Abstract
Objective
This study aimed to determine the prevalence of subclinical enthesopathy in patients with psoriasis using power Doppler ultrasonography (PDUS) and its association with other disease parameters.
Material and Methods
A total of 50 patients with psoriasis (31 females) aged 19–70 years underwent a thorough clinical examination that included assessment of body mass index (BMI) and psoriasis area and severity index (PASI) score. Measurements of inflammatory markers, C-reactive protein (CRP), erythrocyte sedimentation rate (ESR), serum uric acid, and plain radiography of the heels, knees, and sacroiliac joints were performed for all patients. Patients without clinical evidence of arthritis or enthesitis underwent an ultrasonographic (US) examination. According to the US examination, patients were classified into group I (patients with enthesitis) and group II (patients without enthesitis).
Results
In group I, Achilles enthesis was the most common site of US enthesitis (33.3%), followed by distal patellar enthesis (22.2%), proximal patellar enthesis (16.7%), quadriceps enthesis (16.7%), and plantar aponeurosis enthesis (11.1%). There was a statistically significant positive correlation between the occurrence of enthesitis and the patient’s age, disease duration, PASI score, BMI, and hyperuricemia (p<0.05 for each). In contrast, there was no significant correlation between enthesitis and sex or radiographic sacroiliitis (p>0.05 for each).
Conclusion
In addition to the importance of PDUS as a complimentary tool for examining enthesis in patients with psoriasis, the presence of high PASI score, increased BMI and hyperuricemia, and a long disease duration can be considered as predictive parameters for the presence of psoriatic enthesitis.
Keywords: Psoriasis, enthesopathy, power Doppler ultrasonography
Introduction
Psoriatic arthritis, a common rheumatic disease occurring in up to one-third of patients with psoriasis, is categorized under seronegative spondyloarthropathies (1, 2). Early recognition and therapeutic interventions, particularly with new biologic treatments, are critical for preventing the destructive and debilitating changes of psoriatic arthritis (3).
Enthesitis, i.e., inflammation at the attachment of tendons and ligaments to the bones, has been suggested to be the unifying feature of psoriatic arthritis and can be considered to be enthesis-associated disorder rather than a primary synovitic arthropathy (4).
Psoriatic enthesitis, which has been postulated to be the hallmark and initial site of joint inflammation, commonly affects the lower limbs, particularly the Achilles tendon and plantar facia insertions to calcaneus, and is clinically manifested by the onset of spontaneous pain and tenderness upon pressure, causing difficulty in walking. Otherwise, it can be frequently asymptomatic (5, 6).
The diagnosis of enthesitis in clinical practice is usually based on conventional radiography, which can only demonstrate established bony erosions and spurs and gives little information regarding soft tissues, particularly during the early phases of enthesopathy (7).
Musculoskeletal ultrasonography (MSUS) has been confirmed to be a reliable tool in diagnosing enthesitis and is superior to clinical examination, conventional radiography, and even magnetic resonance imaging, particularly during the early stages of enthesopathic changes (7–9). Furthermore, subclinical enthesitis detected using MSUS, particularly with a power Doppler signal, has a predictive value for the development of structural damage in patients with psoriasis (10).
However, there is still a paucity of studies regarding the US findings of psoriatic enthesitis and their association with other clinico-radiographic abnormalities.
This study aimed to determine the prevalence of subclinical enthesopathy in patients with psoriasis using PDUS and to determine its association with other disease parameters.
Material and Methods
This study was conducted at the Departments of Rheumatology and Dermatology in our university hospital. A total of 50 (31 females) patients with psoriasis (clinically diagnosed and confirmed by skin biopsy) were recruited from the outpatient clinic of the Department of Dermatology. The age of the patients ranged from 19 to 70 years, and the duration of psoriasis ranged from 2 to 15 years.
Patients with evidence of other rheumatic or dermatological diseases, gout, malignancies, infections, previous surgical intervention, or local injections in the examined areas were excluded. Informed consent was obtained from each patient. The study was approved by our University Medical Ethics Committee.
A substantial clinical history was obtained from the patients regarding age, sex, disease duration, and previous history of arthritis/arthralgia/enthesopathy or relevant drug treatments for psoriasis. In addition, the skin lesion severity was assessed using the psoriasis area and severity index (PASI) score (11). All patients underwent clinical examination with special regard to the presence of Achilles tendinitis, plantar fasciitis, and quadriceps tendinitis or patellar ligament abnormalities. The condition was considered symptomatic if tendons were swollen, pain was spontaneous or elicited along the course of the tendon or at the tendon level or fascial insertion, or any other functional impairment. Body mass index (BMI) was calculated according to the World Health Organization classification, i.e., weight in kg/height in m2 (12).
Laboratory studies
Five milliliters of venous blood were collected from each patient; 2 mL on EDTA for erythrocyte sedimentation rate using the Westergren method, and 3 mL for the C-reactive protein using the latex agglutination test and fasting serum uric acid using a spectrophotometer.
Plain X-rays were taken of both sacroiliac joints (anteroposterior view), both feet and heels (anteroposterior and lateral views), and both knees (anteroposterior and lateral views; “standing”).
Real time MSUS using a Toshiba Xario200, Tokyo, Japan with a linear 10–12-MHz probe was performed in a dark room by two rheumatologists (A.M. and W.G.) who were completely trained in MSUS and who were blinded to the clinical data. The contact gel was applied to the skin to provide an acoustic interface.
The following five entheses were bilaterally scanned in longitudinal and axial planes: quadriceps insertion into the upper pole of the patella, patellar tendon insertion into the lower pole of the patella and that into the tibial tuberosity (patient in the supine position and extending the knees), and Achilles tendon and plantar aponeurosis insertions into the calcaneal bone (patient in the prone position and the feet hanging over the edge of the examination table at 90° flexion). The US findings were scored using the Glasgow Ultrasound Enthesitis Scoring System (GUESS) (13).
Each enthesis was scanned in grayscale to detect morphostructural changes and subsequently with the power Doppler technique to detect abnormal blood flow. The presence of at least one of the following early US findings is diagnostic for enthesitis: loss of normal fibrillar echogenicity, hypoechoic swelling of the tendon insertion, enthesophytes, effusions, and power Doppler signal (14).
Patients were classified according to the presence or absence of enthesitis into group I or group II, respectively. Group I comprised 18 patients (36%) and group II comprised 32 patients (64%).
The results were analyzed by the Statistical Package for the Social Sciences (SPSS) version 11 (SPSS Inc.; Chicago, IL, USA). Categorical data parameters were presented as frequency and percentage. Categorical data were compared using chi-square test.
A descriptive analysis was performed for all study variables, and the values were presented as absolute and relative frequencies for qualitative variables and mean and standard deviation for continuous variables. A p value of <0.05 was considered to be statistically significant.
Results
This study was included 50 patients with psoriasis (19 males and 31 females), and the age ranged from 19 to 70 years (mean, 33.8±11.2 years) and the disease duration ranged from 2 to 15 years (mean, 7.7+3.4 years). Twenty-six percent of patients had PASI score of <15, 30% had PASI score of 15–25, and 44% had PASI score of >25. Regarding BMI, 22% of patients had an average weight (BMI, 18.5–24.9), 34% were overweight (BMI, 25–29.9), and 44% were obese (BMI, >30). The demographic and clinico-radiographic characteristics of the patients are summarized in Table 1.
Table 1.
Demographic findings of the study population
| Variables | Patients |
|---|---|
| Age range (mean±SD), years | 19–70 (33.8±11.2) |
| Sex | |
| Female | 31 (62%) |
| Male | 19 (38%) |
| Disease duration range (mean±SD), years | 2–12 (7.7+3.4) |
| PASI score range (mean±SD), % | 2.7–33.2 (21.1+7.3) |
| BMI range (mean±SD) | 19.5–42 (29.9+6.4) |
| ESR | |
| Elevated (>20 mm/h) | 6 (12%) |
| Normal | 44 (88%) |
| CRP | |
| Positive (>6 mg/L) | 9 (18%) |
| Negative | 41 (82%) |
| Rheumatoid factor | |
| Positive (>1/8) | 4 (8%) |
| Negative | 46 (92%) |
| SUA | |
| Normal (<6 mg/dL) | 38 (76%) |
| Elevated | 12 (24%) |
| Radiographic sacroiliitis | 6 (12%) |
| Heel X-ray | |
| Normal | 41 (82%) |
| Abnormal (calcified Achilles tendon or calcaneal spur) | 9 (18%) |
| Ultrasonographic findings (%) | |
| Normal | 32 (64%) |
| Abnormal (enthesitis) | 18 (36%) |
PASI: Psoriasis Area and Severity Index; BMI: Body Mass Index; ESR: erythrocyte sedimentation rate; CRP: C-reactive protein; SUA: serum uric acid
Early US signs of enthesitis included loss of normal fibrillar echogenicity, hypoechoic swelling of the tendon insertion, effusions, bursitis, and increased blood flow that is detectable with a power Doppler. In group I, Achilles enthesis (33.3%) was the most common US signs of enthesitis, followed by distal patellar enthesis (22.2%), proximal patellar enthesis (16.7%), quadriceps enthesis (16.7%), and plantar aponeurosis enthesis (11.1%) with variable entheseal morphostructural abnormalities (Table 2). Figure 1 shows the different patterns of subclinical enthesitis.
Table 2.
Distribution of the morphostructural changes in group I
| No. of patients with enthesitis (%) | Entheseal thickness (n=9) | Enthesophyte (n=9) | Bone erosion (n=0) | Bursitis (n=1) | |||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
||||||
| No. | % | No. | % | No. | % | No. | % | ||
| Quadriceps enthesis | 3 (16.7%) | 1 | 11.1 | 2 | 22.2 | 0 | 0.0 | 0 | 0.0 |
| Proximal patellar enthesis | 3 (16.7%) | 2 | 22.2 | 1 | 11.1 | 0 | 0.0 | 0 | 0.0 |
| Distal patellar enthesis | 4 (22.2%) | 3 | 33.3 | 1 | 11.1 | 0 | 0.0 | 0 | 0.0 |
| Achilles enthesis | 6 (33.3%) | 1 | 11.1 | 5 | 55.5 | 0 | 0.0 | 1 | 100 |
| Plantar aponeurosis enthesis | 2 (11.1%) | 2 | 22.2 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
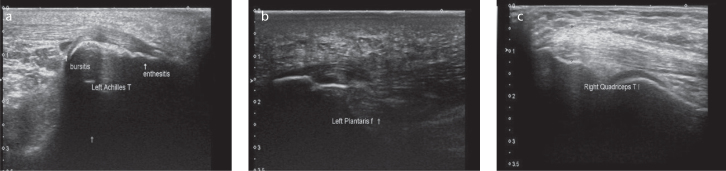
Figure 1. a–c.
Different patterns of subclinical US enthesitis. Longitudinal scan of the Achilles tendon showing enthesitis in a male patient with psoriasis aged 58 years. Note the enthesophytes and swelling of the tendon with the loss of its normal fibrillar pattern (a). Longitudinal scan of plantar aponeurosis showing abnormal entheseal insertion into the calcaneus bone with an increased hypoechogenecity of the fascia (b). Longitudinal scan of quadriceps tendon showing thickening of the tendon and enthesophytes at the patellar side in a female patient with psoriasis aged 44 years (c).
There was a statistically significant positive correlation between US enthesitis and the patient’s age, duration of psoriasis, BMI, and hyperuricemia (p<0.05 for each). According to the PASI score severity, 37 (74%) patients had a moderate–to-severe score, whereas 13 (26%) had a mild score. Patients with enthesitis had a more frequent moderate-to-severe PASI score (17/18=94.4%) than those without enthesitis (20/32=62.5%), indicating a significant positive correlation. Conversely, there was no significant correlation between enthesitis and sex distribution or radiographic sacroiliitis (p>0.05 for each) (Table 3).
Table 3.
Correlation between ultrasonographic enthesitis and clinico-radiographic characteristics
| Clinico-radiographic characteristics | Ultrasonographic findings | p | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| Normal (n=32) | Abnormal (US enthesitis) (n=18) | |||||
|
|
|
|||||
| No. | % | No. | % | |||
| Age | <25 years | 10 | 31.3 | 2 | 11.1 | 0.012 (S)* |
| 25 to <30 years | 10 | 31.3 | 3 | 16.7 | ||
| 30 to <35 years | 8 | 25.0 | 3 | 16.7 | ||
| ≥35 years | 4 | 12.4 | 10 | 55.5 | ||
| Sex dist. | Male | 12 | 37.5 | 7 | 38.9 | 0.922 (NS)* |
| Female | 20 | 62.5 | 11 | 61.1 | ||
| Disease duration | <5 years | 11 | 34.4 | 2 | 11.1 | 0.044 (S)* |
| 5 to <10 years | 11 | 34.4 | 4 | 22.2 | ||
| ≥10 years | 10 | 31.2 | 12 | 66.7 | ||
| PASI score | Mild disease (PASI, <15) | 12 | 37.5 | 1 | 5.6 | 0.035 (S)* |
| Moderate dis. (PASI, 15–25) | 7 | 21.9 | 8 | 44.4 | ||
| Severe dis. (PASI, >25) | 13 | 40.6 | 9 | 50.0 | ||
| BMI | Normal weight (18.5–24.9) | 9 | 28.1 | 2 | 11.1 | 0.011(S)* |
| Over weight (25–29.9) | 14 | 43.8 | 3 | 16.7 | ||
| Obesity (>30) | 9 | 28.1 | 13 | 72.2 | ||
| SUA | Normal | 28 | 87.5 | 10 | 55.6 | 0.011 (S)* |
| Elevated | 4 | 12.5 | 8 | 44.4 | ||
| SIJ | Normal | 30 | 93.8 | 14 | 77.8 | 0.095 (NS)* |
| Abnormal | 2 | 6.3 | 4 | 22.2 | ||
chi-square test
PASI: Psoriasis Area and Severity Index; BMI: Body Mass Index; SUA: serum uric acid; SIJ: sacroiliac joint; S: significant; NS: non-significant
Discussion
Common sites of enthesitis in psoriatic arthritis include the insertion of the Achilles tendon to the calcaneus bone, insertion of the plantar fascia to the calcaneus bone, insertion of the patellar tendon to the tibial tuberosity, and insertion of tendons to the humerus (15). Many studies have recently documented the validity of MSUS for detecting subclinical enthesitis in patients with psoriasis and psoriatic arthritis (7–9, 14, 16–18).
In their long-term study, McGonagle et al. (4) suggested that psoriatic arthritis is an enthesitis-related disease rather than synovitis-related disease. Salvarani et al. (18) also agreed that patients with isolated peripheral enthesitis can be considered as a subset of patients with psoriatic arthritis. Asymptomatic US enthesopathy was present in 36% of patients in our study, while it was present in 56% (8), 33.3% (14), 11.6% (19), and 32.9% (20) in previous studies.
Achilles enthesitis was the most common US sign in this study. A similar observation was reported by Ozcakar et al. (8), De Simone et al. (17), and Gutierrez et al. (21).
We found a significant correlation between the patient’s age and US enthesitis, as previously reported (19, 21, 22). Conversely, we found no significant difference between the sex distribution and US abnormalities, which was also previously reported (19).
In this study, we found a significant correlation between the disease duration and US enthesitis, which is consistent with that reported by Ozcakar et al. (8) in their study on US of the Achilles tendon in patients with psoriasis and Ash et al. (23) who examined the association between nail changes and subclinical enthesitis. This was contrary to that reported by Naredo et al. (19) and Gutierrez et al. (21). We found a statistically significant correlation between the PASI score and entheseal US findings; the same was reported by Sakkas et al. (24) in patients with psoriatic arthritis and Husic et al. (25) in their study using US for diagnosis and follow-up of psoriatic arthritis.
However, contrary to our study, De Simone et al. (17) in their US study of Achilles tendon enthesitis in 59 patients with psoriasis did not find a correlation between the PASI score and entheseal US findings, which was same as that reported by Bandinelli et al. (26) in their study on patients with early psoriatic arthritis.
While Eder et al. (20) and Gisondi et al. (22) reported a significant correlation of US enthesitis and BMI, which is the same as our study result, Gutierrez et al. (21) did not find this correlation depending on the mean GUESS score.
We found a statistically significant correlation between hyperuricemia and entheseal US findings. This is in agreement with the study of Pineda et al. (27) who found that hyperuricemia (>6 mg/dL in males and 5.5 mg/dL in females) was clearly correlated with entheseal US findings.
According to the presence of sacroiliitis, we found no considerable difference with US enthesitis, which is in agreement with that reported by Ozcakar et al. (8) in their aforementioned study.
This study has some limitations. First, absence of the analysis of intrinsic enthesopathic findings and independently correlating them to the clinico-radiographic findings of psoriasis. Second, the dichotomous manner of reporting the US findings as normal and abnormal led to considering minute enthesopathic changes as large changes. Third, although the examined entheses were 250, the dichotomous interpretation minimized the size of the study population. Furthermore, the lack of a control group is a prominent limitation because of the presence of age-related and mechanical-induced enthesitis-like lesions. Further large-scale studies with long-term follow-up are required to support our results and to help in the prediction and early diagnosis of psoriatic arthritis.
In conclusion, PDUS is an important tool for examining entheses in patients with psoriasis, and the presence of a high PASI score, an increased BMI, hyperuricemia, and a long disease duration could be considered as predictive parameters for the presence of psoriatic enthesitis.
Footnotes
Ethics Committee Approval: Ethics committee approval was received for this study from the ethics committee of Al Azhar University School of Medicine.
Informed Consent: Written informed consent was obtained from patients who participated in this study.
Peer-review: Externally peer-reviewed.
Author Contributions: Design - A.M., M.D.; Supervision - M.D., E.M.; Materials - W.G., E.M.; Data Collection and/or Processing - W.G., A.M.; Analysis and/or Interpretation - E.M., A.M., W.G.; Writing Manuscript - A.M.; Critical Review - E.M., M.D.
Conflict of Interest: No conflict of interest was declared by the authors.
Financial Disclosure: The authors declared that this study has received no financial support.
References
- 1.Menter A, Gottlieb A, Feldman SR, Van Voorhees A, Leonardi C, Gordon K, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis, section 1. Overview of psoriasis and guide lines of care for the treatment of psoriasis with biologics. J Am Acad Dermatol. 2008;58:826–50. doi: 10.1016/j.jaad.2008.02.039. https://doi.org/10.1016/j.jaad.2008.02.039. [DOI] [PubMed] [Google Scholar]
- 2.Dimitrios TB, Ioannis OT. Psoriatic arthritis. In: Klippel JH, Stone JH, Crofford LeJ, White PH, editors. Primer on the Rheumatic Diseases. Atlanta, GA: Arthritis Foundation; 1997. pp. 175–179. [Google Scholar]
- 3.Rozenblit M, Lebwohl M. New biologics for psoriasis and psoriatic arthritis. Dermatol Ther. 2009;22:56–60. doi: 10.1111/j.1529-8019.2008.01216.x. https://doi.org/10.1111/j.1529-8019.2008.01216.x. [DOI] [PubMed] [Google Scholar]
- 4.McGonagle D, Conaghan PG, Emery P. Psoriatic arthritis: a unified concept twenty years on. Arthritis Rheum. 1999;42:1080–7. doi: 10.1002/1529-0131(199906)42:6<1080::AID-ANR2>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 5.Frediani B, Falsetti P, Storri L, Allegri A, Bisogno S, Baldi F, et al. Quadricipital tendon enthesitisin psoriatic arthritis and rheumatoid arthritis: ultrasound examinations and clinical correlations. J Rheumatol. 2001;28:2566–8. [PubMed] [Google Scholar]
- 6.Scarpa R, Cuocolo A, Peluso R, Atteno M, Gisonni P, Iervolino S, et al. Early psoriatic arthritis: the clinical spectrum. J Rheumatol. 2008;35:137–41. [PubMed] [Google Scholar]
- 7.Galluzzo E, Lischi DM, Taglione E, Lombardini F, Pasero G, Perri G, et al. Sonographic analysis of the ankle in patients with psoriatic arthritis. Scand J Rheumatol. 2000;29:52–5. doi: 10.1080/030097400750001806. https://doi.org/10.1080/030097400750001806. [DOI] [PubMed] [Google Scholar]
- 8.Ozçakar L, Cetin A, Inanici F, Kaymak B, Gurer CK, Kölemen F. Ultrasonographical evaluation of the Achilles tendon in psoriasis patients. Int J Dermatol. 2005;44:930–2. doi: 10.1111/j.1365-4632.2004.02235.x. https://doi.org/10.1111/j.1365-4632.2004.02235.x. [DOI] [PubMed] [Google Scholar]
- 9.Kamel M, Eid H, Mansour R. Ultrasound detection of heel enthesitis: A comparison with magnetic resonance imaging. J Rheumatol. 2003;30:774–8. [PubMed] [Google Scholar]
- 10.El Miedany Y, El Gaafary M, Youssef S, Ahmed I, Nasr A. Tailored approach to early psoriaticarthritis patients: clinical and ultrasonographic predictors for structural joint damage. Clin Rheumatol. 2015;34:307–13. doi: 10.1007/s10067-014-2630-2. https://doi.org/10.1007/s10067-014-2630-2. [DOI] [PubMed] [Google Scholar]
- 11.Feldman SR, Krueger GG. Psoriasis assessment tools in clinical trials. Ann Rheum Dis. 2005;46:65–8. doi: 10.1136/ard.2004.031237. https://doi.org/10.1136/ard.2004.031237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.WHO Expert Consultation. Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet. 2004;363:157–63. doi: 10.1016/S0140-6736(03)15268-3. https://doi.org/10.1016/S0140-6736(03)15268-3. [DOI] [PubMed] [Google Scholar]
- 13.Balint PV1, Kane D, Wilson H, McInnes IB, Sturrock RD. Ultrasonography of entheseal insertions in the lower limb in spondyloarthropathy. Ann Rheum Dis. 2002;61:905–10. doi: 10.1136/ard.61.10.905. https://doi.org/10.1136/ard.61.10.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.De Filippis LG, Caliri A, Lo Gullo R, Bartolone S, Miceli G, Cannavò SP, et al. Ultrasonography in the early diagnosis of psoriasis-associated enthesopathy. Int J Tissue React. 2005;27:159–62. [PubMed] [Google Scholar]
- 15.Heuft-Dorenbosch L, Spoorenberg A, van Tubergen A, Landewé R, van ver Tempel H, Mielants H, et al. Assessment of enthesitis in ankylosing spondylitis. Ann Rheum Dis. 2003;62:127–32. doi: 10.1136/ard.62.2.127. https://doi.org/10.1136/ard.62.2.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Filippucci E, De Angelis R, Salaffi F, Grassi W. Ultrasound, skin and joints in psoriatic arthritis. J Rheumatol. 2009;36(Suppl I):83-35-38. doi: 10.3899/jrheum.090220. [DOI] [PubMed] [Google Scholar]
- 17.De Simone C, Guerriero C, Giampietruzzi AR, Costantini M, Di-Gregorio F, Amerio P. Achilles tendinitis in psoriasis: clinical and sonographic findings. J Am Acad Dermatol. 2003;49:217–22. doi: 10.1067/s0190-9622(03)00904-6. https://doi.org/10.1067/S0190-9622(03)00904-6. [DOI] [PubMed] [Google Scholar]
- 18.Salvarani C, Cantini F, Olivieri I, Macchioni P, Niccoli L, Padula A, et al. Isolated peripheralenthesitis and/or dactylitis: a subset of psoriatic arthritis. J Rheumatol. 1997;24:1106–10. [PubMed] [Google Scholar]
- 19.Naredo E, Möller I, de Miguel E, Batlle-Gualda E, Acebes C, Brito E, et al. High prevalence of ultrasonographic synovitis and enthesopathy in patients with psoriasis without psoriatic arthritis: a prospective case-control study. Rheumatology. 2011;50:1838–48. doi: 10.1093/rheumatology/ker078. https://doi.org/10.1093/rheumatology/ker078. [DOI] [PubMed] [Google Scholar]
- 20.Eder L, Barzilai M, Peled N, Gladman DD, Zisman D. The use of ultrasound for the assessment of enthesitis in patients with spondyloarthritis. Clin Radiol. 2013;68:219–23. doi: 10.1016/j.crad.2012.07.018. https://doi.org/10.1016/j.crad.2012.07.018. [DOI] [PubMed] [Google Scholar]
- 21.Gutierrez M, Filippucci E, De Angelis R, Salaffi F, Filosa G, Ruta S, et al. Subclinical entheseal involvement in patients with psoriasis: an ultrasound study. Semin Arthritis Rheum. 2011;40:407–12. doi: 10.1016/j.semarthrit.2010.05.009. https://doi.org/10.1016/j.semarthrit.2010.05.009. [DOI] [PubMed] [Google Scholar]
- 22.Gisondi P, Tinazzi I, El-Dalati G, Gallo M, Biasi D, Barbara LM, et al. Lower limb enthesopathy inpatients with psoriasis without clinical signs of arthropathy: a hospital-based case-control study. Ann Rheum Dis. 2008;67:26–30. doi: 10.1136/ard.2007.075101. https://doi.org/10.1136/ard.2007.075101. [DOI] [PubMed] [Google Scholar]
- 23.Ash ZR, Tinazzi I, Gallego CC, Kwok C, Wilson C, Goodfield M, et al. Psoriasis patients with naildisease have a greater magnitude of underlying systemic subclinical enthesopathy than those with normal nails. Ann Rheum Dis. 2012;71:553–6. doi: 10.1136/annrheumdis-2011-200478. https://doi.org/10.1136/annrheumdis-2011-200478. [DOI] [PubMed] [Google Scholar]
- 24.Sakkas LI, Alexia I, Simopoulou T, Vlychou M. Enthesitis in psoriatic arthritis. Semin Arthritis Rheum. 2013;43:325–34. doi: 10.1016/j.semarthrit.2013.04.005. https://doi.org/10.1016/j.semarthrit.2013.04.005. [DOI] [PubMed] [Google Scholar]
- 25.Husic R, Anja Ficjan A, Christina Duftner C, Dejaco C. Use of ultrasound for diagnosis and follow-up of psoriatic arthritis. EMJ Rheumatol. 2014:65–72. [Google Scholar]
- 26.Bandinelli F, Prignano F, Bonciani D, Bartoli F, Collaku L, Candelieri A, et al. Ultrasound detects occult entheseal involvement in early psoriaticarthritis independently of clinical features and psoriasis severity. Clin Exp Rheumatol. 2013;31:219–24. [PubMed] [Google Scholar]
- 27.Pineda C, Amezcua-Guerra LM, Solano C, Rodriguez-Henríquez P, Hernández-Díaz C, Vargas A, et al. Joint and tendon subclinical involvement suggestive of gouty arthritis in asymptomatic hyperuricemia: an ultrasound controlled study. Arthritis Res Ther. 2011;13:R4. doi: 10.1186/ar3223. https://doi.org/10.1186/ar3223. [DOI] [PMC free article] [PubMed] [Google Scholar]