Abstract
Objective
The interleukin 17 (IL-17) cytokine family is involved in a number of chronic inflammatory diseases. In spite of contradictory findings and a lack of causality in clinical studies, IL-17 inhibition for systemic lupus erythematosus (SLE) has regained attention as a potential therapeutic pathway, after demonstrating disease-modifying capabilities in ankylosing spondylitis. We investigated the clinical associations of interleukin 17 A (IL-17A) in patients with SLE.
Material and Methods
A cross-sectional study was performed involving SLE patients (n=102; age: 49 years; 86% female) recruited from a regional registry. IL-17A levels were determined by immunoassay, disease activity by Systemic Lupus Erythematosus Disease Activity Index-2K (SLEDAI-2K), and cumulative damage by Systemic Lupus International Collaborative Clinics Damage Index (SDI) scores. Non-parametric techniques were used to examine the association between IL-17A and disease activity and autoantibody profiles were compared with healthy controls (n=31): principal component analysis (PCA) was used to determine the interplay of immune cells across disease states and damage development in SLE patients.
Results
SLE patients had higher IgG levels, lower T-cell and B-cell counts, but median IL-17A levels did not differ from the controls (28.4 vs. 28.4 pg/mL, p=0.9). In SLE patients, IL-17A did not correlate with SLEDAI-2K or SDI, but was inversely related with age (correlation coefficients, Rs.=–0.29, p<0.05), systolic blood pressure (Rs.=–0.31, p<0.05), years of smoking (Rs.=–0.43, p<0.05), cumulative heart (Rs.=–0.22, p<0.05), and malignancy damage (Rs.=–0.18, p<0.05). Serological correlations for IL-17A existed with immunoglobulin G (IgG) levels (Rs.=0.21, p<0.05), high sensitivity C-reactive protein (hs-CRP) levels (Rs.=0.28, p<0.05), proteinuria (Rs.=0.64, p<0.05), and pre-albumin (Rs.=–0.22, p<0.05). Longitudinal data showed only modest fluctuation in IL-17A levels, independent of SLEDAI-2K.
Conclusion
These results suggest that IL-17A, while participating in inflammation, may also serve a protective purpose in SLE patients.
Keywords: IL-17A, Systemic Lupus Erythematosus, SLEDAI, organ damage
Introduction
Interleukin 17A (IL-17A) is the predominant cytokine of the interleukin 17 (IL-17) family (1, 2). In humans, IL-17A is primarily expressed by the T-helper-17 (Th17) subset of CD4+ T cells (2), but is also produced by neutrophils, natural killer (NK) cells, and CD8+ and double-negative (DN) T cells (3). Studies involving systemic lupus erythematosus (SLE) patients have reported increased numbers of Th17 cells in sera and in the biopsied tissue of individuals with kidney damage and lupus nephritis (LN) (4, 5). Increased IL-17A levels have also been reported in the sera of SLE patient subsets, particularly those with LN (4–6). Furthermore, in lupus-prone mice, the over-expression of IL-17A and subsequent expansion of DN T cells in the tubulointerstitial space has been linked to the development of nephritis (4, 7–11).
Interleukin 17A is a multifunctional cytokine that impacts neutrophil recruitment, mediating both T-helper-1 (Th1) and T-helper-2 (Th2) cytokine production, and it possesses angiogenic properties through apoptosis modulation (3, 12, 13). IL-17A production, in vivo and in vitro, is primarily controlled by transforming growth factor beta 1 (TGF-β1) and interleukin 6 (IL-6) via the activation of signal transducer and activator of transcription 3 (STAT-3) in mouse and human models, respectively (14–16). Under normal circumstances, IL-17A has the ability to recruit neutrophils to arrest tumor cells (2), whereas excessive IL-17A can contribute toward tumor growth by overcoming interferon gamma (IFN-γ) tumor surveillance properties (14, 17, 18). Similarly, excess interleukin 17F (IL-17F) can support tumor proliferation by increasing the local vessel growth (17, 19). Given IL-17’s pleiotropic nature, its role in the pathogenesis of human and experimental SLE may involve more than participating in site-specific inflammation alone (20). With the recent reports of IL-17 inhibition in achieving clinical benefit in ankylosing spondylitis, questions will be raised regarding the potential to exploit IL-17 inhibition in SLE patients or certain symptomatic groups (21, 22). Therefore, the aim of this research was to investigate IL-17A levels, clinical and serological associations between SLE patients and healthy controls, as well as to determine the association of IL-17A levels with disease activity and organ damage within SLE patients.
Material and Methods
In a cross-sectional study design, we recruited 102 SLE patients, who fulfilled the relevant American College of Rheumatology’s classification criteria for SLE and had detailed clinical information and blood samples collected during an extended outpatient visit. A group of 31 healthy volunteers served as controls for serological measures. Disease activity was recorded using the Systemic Lupus Erythematosus Disease Activity Index-2K (SLEDAI-2K) and the Physician Global Disease Activity and Patient Global Disease Activity visual analogue scales (VAS). Active disease is defined as having SLEDAI-2K scores of ≥3 (23). The Systemic Lupus International Collaborative Clinics (SLICC) Damage Index (SDI) was used to quantify overall and organ-specific damage (24). Overall, 69% patients used corticosteroids (prednisone) at a median dose of 3.75 mg per day (Table 1). Cardiovascular events were defined as a confirmed occurrence of myocardial infarction, thrombosis, or stroke. We included patients in our malignancy category when a solid or hematological cancer had developed. This included cancer(s) of the thyroid, vulva, cervical, lung, skin, and bladder, as well as leukemia or lymphoma.
Table 1.
Descriptors of SLE cohort and association with IL-17A levels
| SLE | IL-17 (Rs) | |
|---|---|---|
| Female | 87 (87%) | −0.09 |
| Age (mean) | 49 (16) 19–89 | −0.29** |
| Disease duration (months) | 125.5 (IQR 62–212) | 0.07 |
| Systolic Blood Pressure | 131.62 (22) 79–186 | −0.31** |
| Weight | 71.81 (16) 47–156 | 0.18 |
| Years of daily smoking | 20 (IQR 13–28) | −0.43** |
| Cardiovascular events ever | 24 (25.3%) | −0.07 |
| Malignancy ever | 12 (12.6%) | −0.24* |
| SLEDAI-2K | 6 (IQR 2–11) | 0.05 |
| Physician Global Disease Activity (VAS) | 2.7 (2.08) | 0.05 |
| Patient Global Disease Activity (VAS) | 3.40 (2.52) | −0.06 |
| SLICC-Damage Index | 1 (IQR 0–2) | −0.06 |
| Current Medication Usage | ||
| Prednisone daily (mg) | 3.75 (IQR 0–6.25) | −0.04 |
| OH-chloroquine | 58 (67.4%) | 0.01 |
| Immunosuppressive medication | 25 (34.2%) | −0.03 |
| Anticoagulants (Warfarin & Aspirin) | 33 (45.2%) | 0.04 |
| NSAIDs | 15 (20%) | 0.07 |
NSAIDs: non-steroidal anti-inflammatory drugs; SLEDAI-2K: Systemic Lupus Erythematosus Disease Activity Index-2K Figures reflect numbers (%), median (interquartile range), or Spearman rank correlation coefficient (Rs.)
Correlation is statistically significant at p<0.05
Correlation is strongly statistically significant at p<0.01
Serology
Anti-double-stranded DNA (anti-dsDNA) and other autoantibody assays were performed at the clinical immunology laboratory by a validated ELISA (EliATM and VarelisA®; Phadia GmbH, Freiburg, Germany). IL-17A, B-cell-activating factor (BAFF), interleukin 1 beta (IL-1β), interleukin 4 (IL-4), IL-6, interleukin 10 (IL-10), interleukin 12 (IL-12), IFN-γ, macrophage inflammatory protein 1-alpha (MIP-1α), macrophage inflammatory protein 1-beta (MIP-1β), monocyte chemoattractant protein 1 (MCP-1), tumor necrosis factor-alpha (TNF-α), and transforming growth factor beta 1 (TGF-β1) were measured by a quantitative sandwich immunoassay (Single Analyte ELISArray™ kit; SuperArray Bioscience Corp., Frederick, MD, USA) with all the assays run in duplicate and the results, averaged. The manufacturer’s recommendations were followed throughout, and the same lot was used for each cytokine. For statistical purposes, values below the limit of detection (LOD) were replaced by the LOD value. The normal upper limit for the IL-17A cytokine was defined as the upper 95% percentile.
Statistical analysis
Results were presented with a measure of central tendency, i.e., median with inter-quartile range or mean with standard deviation or a count and percentage. Differences between groups assessed with the t-test, non-parametric Mann-Whitney U test, and chi-square test, wherever appropriate. The Rs. values were derived by using the Spearman’s rho correlation test.
The longitudinal course of IL-17 levels was determined retrospectively for 18 SLE patients (n=18) with repeated measures of IL-17 prior to the baseline. This sub-cohort was similar in age, female predominance (86%), follow-up time, SLEDAI-2K, and both patient and physician Global Assessment of Disease Activity VAS values. However, the longitudinal cohort was more likely to have sustained organ damage (SDI), p=0.02. The comparison of the distribution of serial IL-17A results was analyzed non-parametrically by Friedman two-way analysis of variance by ranks. Statistical significance was set at α=0.05 or 5% level. The statistical analysis was performed on Statistical Package for the Social Sciences (SPSS) Version 22.0 (IBM Corp.; Armonk, NY, USA).
A principal component analysis (PCA) was performed using SPSS (Factor Analysis package) to determine the interplay of IL-17A amongst 33 serological parameters of the immune system (described in Table 2, 3) across various disease states of the 102 participants. Seven serological covariates were excluded due to missing data causing an inability of the PCA analysis to achieve convergence during an Oblimin rotation with Kaiser normalization. The final PCA iteration included 26 serological variables, as presented in Figure 1. It was found that the two first principal components, i.e., PC1 and PC2, accounted cumulatively for 49.74%, 46.51%, 50.12%, and 45.28% of SLICC-DI<1, SLICC-DI≥1, SLEDAI<3, and SLEDAI≥3, respectively.
Table 2.
Laboratory findings in SLE
| Serological Parameter | Controls | SLE patients | Independent Kruskall-Wallis Test (p) | Correlation with IL-17 (SLE serology only) | Correlation with high-IL 17 (n=5) |
|---|---|---|---|---|---|
| IL-17A (pg/mL) | 28.4 (IQR 28.4, 88.33) | 28.4 (IQR 28.4, 63.5) | 0.948 | - | - |
| ESR (mm) | 11 (IQR 4, 22) | 20.0 (IQR 10.5, 33.5) | 0.002 | 0.08 | 0.14 |
| hs-CRP | 1.18 (IQR 0.65, 2.29) | 2.1 (IQR 0.6, 5.0) | 0.32 | 0.28** | 0.22* |
| Hemoglobin (g/L) | 13.7 (IQR 12.75, 14.25) | 13.1 (IQR 12.0, 14.1) | 0.103 | −0.15 | −0.04 |
| WBC | 6.60 (IQR 4.90, 7.80) | 5.8 (IQR 4.2, 7.1) | 0.061 | −0.01 | 0.05 |
| Platelets | 265.5 (IQR 212.5, 330.0) | 255.5 (IQR 212.0, 296.0) | 0.338 | −0.21* | −0.12 |
| Albumin | 45.5 (IQR 44.5, 46.5) | 43.0 (IQR 41.0, 45.0) | <0.001 | −0.08 | 0.01 |
| Pre-albumin | 0.26 (IQR 0.23, 0.29) | 0.3 (IQR 0.2, 0.3) | 0.566 | −0.22* | −0.19 |
| Creatinine (umol/L) | 62.0 (IQR 57.0, 67.0) | 61.0 (IQR 52.0, 70.0) | 0.748 | 0.02 | 0.08 |
| IgG | 12.3 (IQR 10.5, 13.0) | 13.3 (IQR 10.9, 16.3) | 0.041 | 0.21* | 0.19 |
| IgM | 0.91 (IQR 0.71, 1.49) | 0.95 (IQR 0.73, 1.46) | 0.682 | 0.21* | 0.19 |
| Anti-dsDNA(IU) | 0 (IQR 0, 0) | 15.0 (IQR 0.0, 90.2) | - | −0.01 | 0.22* |
| C3 (mg/L) | 1.13 (IQR 0.93, 1.34) | 0.95 (IQR 0.80, 1.11) | 0.009 | 0.11 | 0.04 |
| Positive Coombs | 0 (0%) | 18 (20%) | - | 0.26* | 0.16 |
| Lymphocytes | 2.0 (IQR 2.0, 2.0) | 1.3 (IQR 0.9, 1.8) | <0.001 | 0.01 | 0.13 |
| CD4-cells | 0.99 (IQR 0.71, 1.26) | 0.6 (IQR 0.4, 0.8) | <0.001 | −0.03 | 0.02 |
| B-cells | 0.24 (IQR 0.19, 0.32) | 0.10 (IQR 0.04, 0.22) | 0.001 | 0.06 | 0.08 |
| NK-cells | 0.25 (IQR 0.21, 0.28) | 0.10 (IQR 0.07, 0.17) | <0.001 | 0.03 | 0.09 |
Figures represent median values with interquartile range.
IL-17A: Interleukin 17 A; ESR: erythrocyte sedimentation rate; hs-CRP: high sensitivity C-reactive protein; WBC: white blood cells; IgG: immunoglobulin G; IgM: immunoglobulin M; Anti-dsDNA: anti-double-stranded DNA; C3: complement component 3; natural killer (NK) cells.
Numbers in the last two columns show Rs.
Significantly associated with IL-17, p<0.05
Significantly associated with IL-17, p<0.001
Table 3.
Cytokine interactions in SLE
| Cytokine | Controls | SLE patients | Independent Kruskall-Wallis Test (p) | Correlation with IL-17 (SLE serology only) | Correlation with high-IL 17 (n=5) |
|---|---|---|---|---|---|
| IL-17A (pg/mL) | 28.40 (28.40, 88.33) | 28.4 (IQR 28.4, 63.5) | 0.948 | - | 0.43** |
| BAFF (pg/mL) | 1.62 (IQR 1.13, 2.36) | 1.7 (IQR 1.3, 2.3) | <0.001 | 0.11 | −0.08 |
| IFN-γ (pg/mL) | 43.79 (IQR 19.6, 119.34) | 62.5 (IQR 19.6,134.1) | 0.075 | 0.35** | 0.38** |
| IL-1β (pg/mL) | 17.90 (IQR 17.90, 348.08) | 17.9 (IQR 17.9, 17.9) | <0.001 | 0.54** | 0.42** |
| IL-4 (pg/mL) | 7 (IQR 7.0, 7.0) | 7.0 (IQR 7.0, 7.0) | 0.517 | 0.45** | 0.39** |
| IL-6 (pg/mL) | 14 (IQR 14, 18.2) | 14.0 (IQR 14.0, 19.5) | 0.857 | 0.51** | 0.29** |
| IL-10 (pg/mL) | 5.90 (IQR 5.90, 23.64) | 5.9 (IQR 5.9, 22.0) | 0.598 | 0.49** | 0.30** |
| IL-12 (pg/mL) | 26.15 (IQR 12.6, 62.8) | 24.6 (IQR 12.6, 61.7) | 0.197 | 0.32** | 0.32** |
| MCP-1 (pg/mL) | 144.84 (IQR 82.4, 239.34) | 133.7 (IQR 78.7, 219.8) | <0.001 | 0.092 | 0.24* |
| MIP-1α (pg/mL) | - | 15.0 (IQR 15.0, 103.5) | - | 0.35** | 0.25* |
| MIP-1β (pg/mL) | - | 204.3 (IQR 161.2, 292.5) | - | 0.30** | 0.27** |
| TNF-α (pg/mL) | - | 34.3 (IQR 21.4, 87.4) | - | 0.41** | 0.34** |
| TGF-β1 (pg/mL) | - | 592.3 (IQR 347.1, 859.7) | - | −0.01 | 0.04 |
Figures represent median values with interquartile range.
Interleukins 4, 6, 10, 12, and 17A (IL-4). Interleukin-1β, B-cell-activating factor (BAFF), interferon gamma (IFN-γ), monocyte chemoattractant protein 1 (MCP-1), macrophage inflammatory protein 1 alpha (MIP-1α), macrophage inflammatory protein 1 beta (MIP-1β), tumor necrosis factor alpha (TNF-α), and transforming growth factor beta 1 (TGF-β1).
Numbers in the last two columns show Rs.
Significantly associated with IL-17, p<0.05,
Significantly associated with IL-17, p<0.001
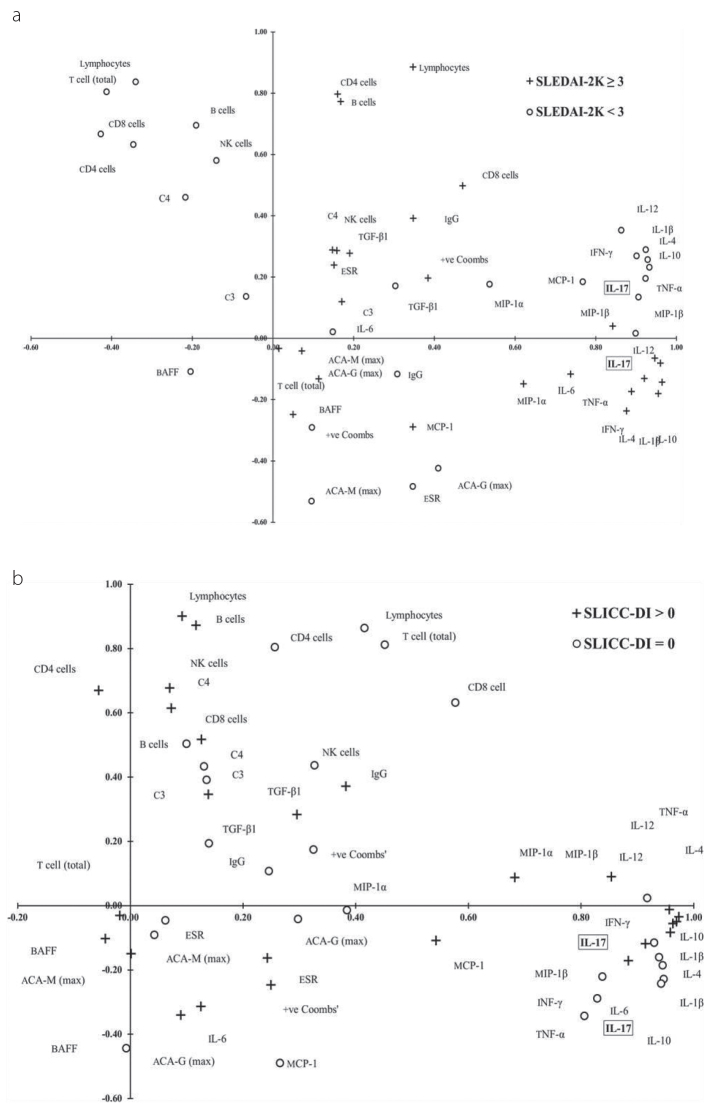
Figure 1. a, b.
Factor matrix of the PCA for SLEDAI-2K (a) and SDI (b). Projection of 102 SLE patients, described by 26 variables (listed in Tables 2 and 3) for the first two principal components, PC1 (x-axis) and PC2 (y-axis) as determined by PCA for disease activity [SLEDAI <3 = O, SLEDAI ≥3 = +] (a) and organ damage [SLICC-DI=0 = O, SLICC-DO > 0 = +] (b). The graphs provide quantitative information on the similarity of variables as determined by the PCA: the distance of the factor to the null represents the correlation of that factor to the system, i.e., SLEDAI-2K or SLICC-DI status. The shorter the distance between two or a cluster of factors, more similar their effects are on SLEDAI-2K or SLICC-DI (see Methods for a more detailed description)
Principal component analysis can illustrate the interplay of many parameters by optimizing the variance between the selected variables. In Figure 1 (SLEDAI-2K) and Figure 2 (SLICC-DI), the correlation amongst the variables is represented by the proximity of the vector points, i.e., the shorter the distance between two vector points, more similar is the influence of these two variables, or a cluster of variables, on the variance of the other variables in the PCA; further, the strength of the variables’ ability to influence the variance on other variables in the PCA is determined by the distance of the vector point from the origin.
Figure 2.
Serial course of IL-17A levels over the pre-study (retrospective) disease course in 18 patients with SLE
Ethics
All the participants provided informed and written consents for the use of their anonymized data and samples collected as part of a protocol approved by the regional ethics committee (REC North 2015/1400).
Results
Descriptors
Systemic lupus erythematosus patients and controls were effectively matched for age (49 vs. 50 years, p>0.05) and gender (87% female vs. 77% male, p=0.09). SLE patients showed modest Global Disease Activity with a SLEDAI-2K score of 6 (IQR 2, 11), an average Physician VAS of 2.7±2.1, and average Patient VAS of 3.4±2.5 (Table 1). The disease activity was mostly related to migraines, arthritis, low complement levels, positive anti-dsDNA, rash, alopecia, and Raynaud’s phenomenon. The median SDI score was 1 (IQR 0, 2; range: 0–9) with the most frequent organ damage cited as being either musculoskeletal (21%), neurological (19%), heart (15%), or malignancy (13%). At the time of this study, 57% patients were taking hydroxychloroquine; 37%, immunosuppressive (IS) medication, i.e., azathioprine, mycophenolate, methotrexate, or cyclophosphamide; and 41%, some form of anticoagulant therapy.
Clinical associations for IL-17A
Median IL-17A levels were similar for SLE patients and controls (28.4 vs. 28.4 pg/mL, p=0.90). The use or dose of prednisolone was unrelated to IL-17A levels, nor were IL-17A levels influenced by the use of anti-malarial, IS, or anticoagulant therapies. IL-17A did not correlate with SLEDAI-2K, but was inversely associated with age (Rs.=–0.29, p<0.004), systolic blood pressure (Rs.=–0.31, p=0.002), and years of smoking (Rs.=–0.43, p=0.001). The IL-17A level did not correlate with the overall SDI, but was inversely related with cumulative heart damage (Rs.=–0.22, p=0.025) and a history of cancer (Rs.=–0.24, p=0.019).
Serological associations for IL-17A
IL-17A was correlated with high sensitivity C-reactive protein (hs-CRP) (Rs.=0.28, p=0.008), immunoglobulin G (IgG) (Rs.=0.21, p=0.049) and immunoglobulin M (IgM) (Rs.=0.21, p=0.066), and positive Coombs’ test (Rs.=0.26, p=0.015). IL-17A was inversely correlated with platelet count (Rs.=–0.21, p=0.034) and pre-albumin levels (Rs.=–0.22, p=0.03) (Table 2). IL-17A correlated with a range of pro-inflammatory cytokines including IL-6 (Rs.=0.51, p<0.001) (Table 2), but not with regulatory lupus cytokines, such as BAFF (Rs.=0.101), MCP-1 (Rs.=0.092), or TGF-1β (Rs.=–0.098) (p>0.10 for all of them).
PCA of cytokine levels including IL-17A
In the PCA of a low disease activity state (SLEDAI-2k<3), the 1st principal component (PC1) included the cytokines IL-10, IL-4, IL-1β, TNF-α, IL-17, IFN-γ, MIP-1β, IL-12, MCP-1, and MIP-1α (Figure 1a). Notably, IL-6 did not participate in this cytokine group. The PC1 for patients with SLEDAI-2k≥3 included IL-10, IL-4, IL1-β, IL-12, IL-17, TNFα, IFN-γ MIP-1β, MIP-1α, and IL-6. Regulatory cytokines TGF-β1, BAFF, and MCP-1 were strongly correlated with this cytokine group.
In the PCA for SLE patients free of organ damage (i.e., SLICC-DI=0), PC1 was a group of cytokines including IL-1β, IL-4, IL-10, IL-17, IL-6, IL-1β, IL-12, MIP-1β, IFN-γ, and TNF-α (Figure 1b). PC2 included lymphocytes, T cells (total), CD4 cells, CD8 cells, B cells, NK cells, complement component 4 (C4), and complement component 3 (C3). The PCA analysis of SLE patients with organ damage, i.e., SLICC-DI≥1, produced a PC1 predominated by cytokines including IL-4, TNFα, IL-10, IL-1β, IL-12, IFN-γ, IL-17, MIP-1β, MIP-1α, and MCP-1. TGF-β1 was not correlated with the other PC1 cytokines. MCP-1 had little influence on the other variables in the PCA for SLICC-DI<1, but became a component of PC1 in the PCA for SLICC≥1. Additionally, BAFF was close to the origin for SLICC-DI<1, moving away from the origin for SLICC-DI≥3. Despite BAFF not being correlated with the other variables in PC1 or PC2 for SLICC≥3, it did exhibit an increased effect on the variation in the model by moving beyond the –0.30 to 0.30 threshold on the x- and y-axes in Figure 2.
Longitudinal course of IL-17A
Serial measures of IL-17A were performed in randomly collected sera prior to the research visit in 18 SLE patients (Figure 2). The Friedman two-way analysis of variance by ranks determined that there was no statistically significant difference in the distribution of IL-17A levels within patients across visits (p=0.24).
Discussion
In this well-managed SLE cohort, the overall IL-17A levels were not significantly higher than those expressed by age-and-gender-matched healthy controls. Additionally, IL-17A levels were relatively stable over time and were unrelated to SLE disease activity (SLEDAI-2K) or cumulative damage (SLICC-DI). IL-17A levels strongly correlated with a number of serological markers of inflammation, and they were also modestly associated with lower damage scores for malignancy and heart conditions. These results suggest that in SLE, IL-17A could maintain its pleiotropic characteristics, undertaking a more complex role than simply orchestrating inflammation.
There is experimental evidence regarding the involvement of IL-17 in lupus-like inflammation from studies in knockout mouse, where IL-17A can contribute toward the development of renal immune deposits (25). The evidence for a role in the pathophysiology of human SLE is less striking (11). A number of studies, mainly from Asian cohorts, showed a correlation of IL-17A levels or the number of Th17 cells with disease activity or organ damage (26–29). However, one of the seminal studies has since been retracted (30). Our results, which include a PCA, indicate that IL-17A does not exhibit discernibly different characteristics across disease activity and organ damage states, which was a similar finding to that of Zhao et al. (31), Cheng et al. (32), and more recently, Vincent et al. (33). Furthermore, genetic studies have also been unable to establish a direct link between the IL-23/IL-17 axes and SLE (34). Several studies have suggested that IL-17 acts in a site-specific manner in SLE and, therefore, may be a biomarker of specific disease activity, as observed in LN (5, 33, 35). However, we were unable to confirm this finding in our cohort. Our demonstration of IL-17A levels correlating with acute-phase reactants and immunoglobulin levels supports the possible involvement of IL-17A in the inflammatory pathway in SLE, although the main source and driver of IL-17A levels remain unclear. The PCA results regarding the interplay of cytokines and immune cells across states of disease activity indicate that other than IL-6 and TGF-β1, there were few distinguishing biomarkers between patients with active and inactive disease. The interaction between IL-17A and other pro- and anti-inflammatory cytokines needs further study, but IL-17A seems to be a mostly non-specific inflammatory marker in humans with SLE (35).
Interestingly, we found an inverse correlation or protective effect for IL-17 on cumulative heart damage and malignancy frequency in this SLE cohort. Although we did not conform to the sample size required to conduct the appropriate regression modeling to adjust for age and other risk factors, we did find that IL-17A was inversely correlated with the age of SLE patients, too, which could point toward an age effect in IL-17A levels and its effects. A study by Simon et al. (36) demonstrated that lower IL-17A levels were associated with a higher risk of cardiovascular problems. Additionally, the inhibition of IL-17A by the monoclonal antibody Secukinumab caused an unexpected increase in the incidence of stroke(s) and myocardial infarction(s) as compared to a placebo (37). Notwithstanding the sample size limitations, our findings of lesser heart damage with higher levels of IL-17A support the view that IL-17A may exert a protective effect on the cardiovascular system: a plausible mechanism of action for this could be due to the down-regulation of vascular cell adhesion molecule 1 (VCAM-1) activity in the development of atherosclerosis (38).
Furthermore, IL-17A demonstrated an inverse correlation on cancer frequency in this SLE cohort. This protective effect was robust with a significant difference in the mean IL-17A level for those without and with a history of cancer (103 vs. 31 pg/mL). IL-17A has been described in various in vivo and in vitro models to possess both pro- and anti-tumor properties (2, 39). IL-17A, secreted by Th17 cells, can promote tumor growth through inducing vascular endothelial growth factor (VEGF) and increasing proangiogenic activity (17). The increased vasculature about the tumor provides more oxygen and nutrients, enabling its proliferation. On the other hand, IL-17A induces DC maturation, activation of macrophages, neutrophil recruitment, and NK cell and T-cell-induced cytolysis; all of them contribute toward the destruction of tumor cells (2). Our study, therefore, adds an important clinical perspective, demonstrating that at levels similar to healthy controls, IL-17A possesses net anti-tumor functionality in SLE patients. However, further investigation is required to determine the circumstances and levels of IL-17A that would contribute toward such a state (2, 14, 18, 39).
There is a dearth of evidence regarding the long-term course of cytokines, including IL-17A, in SLE patients (40). Zickert et al. (5) showed that IL-17A levels were reduced 7 months after cyclophosphamide-based induction treatment for LN. In a random subset of patients in our cohort who did not receive induction treatment, longitudinal IL-17A levels did not fluctuate significantly over several years of observation. While an increase in cytokine levels over time in the general population has been described and considered to represent unhealthy aging due to underlying inflammatory processes (41), in a cohort of early arthritis patients, there was no significant change in IL-17A over a series of developmental stages (40).
The limitations of this study lie in the fact that our patients were all of Northern European descent and were mostly in a state of low disease activity, such that results cannot be extrapolated to cohorts with a different genetic or clinical makeup. Our results are based on clinical and serological findings and, therefore, cannot confirm the cellular source or causation of effects by IL-17A, for which further experimental studies will be needed. The strength of this study is the availability of a large range of disease characteristics in all the patients, the inclusion of longitudinal organ damage data, and the introduction of PCA to delineate the complexity of cytokine involvement in SLE.
In conclusion, IL-17A levels in this SLE cohort were similar to controls. IL-17A levels correlated with markers of inflammation including a range of other cytokines in SLE patients. While not clearly related to disease activity, IL-17A levels were inversely related to blood pressure, heart damage, and malignancy development. This dual role of IL-17A suggests that inhibiting pro-inflammatory IL-17 effects in SLE patients could have wider and significant clinical implications.
Footnotes
Ethics Committee Approval: Ethics committee approval was received for this study from the ethics committee of North Norway (REC North 2015/1400).
Informed Consent: Written informed consent was obtained from patients who participated in this study.
Peer-review: Externally peer-reviewed.
Author Contributions: Concept - J.C.; Design - W.R., J.C.; Supervision - J.C., G.OE.; Resources - J.C, G.OE.; Materials - J.C, G.OE.; Data Collection and/or Processing - G.OE, J.C.; Analysis and/or Interpretation - WR., S.G., J.C.; Literature Search - S.SG., W.R., J.C; Writing Manuscript - W.R., S.., G.OE., J.C; Critical Review - W.R., S.G., G.OE., J.C.
Conflict of Interest: No conflict of interest was declared by the authors.
Financial Disclosure: This study was supported by an unrestricted grant from The Arthritis Foundation of Western Australia.
Refrences
- 1.Yao Z, Painter SL, Fanslow WC, Ulrich D, Macduff BM, Spriggs MK, et al. Human IL-17: a novel cytokine derived from T cells. J Immunol. 1995;155:5483–6. [PubMed] [Google Scholar]
- 2.Murugaiyan G, Saha B. Protumor vs antitumor functions of IL-17. J Immunol. 2009;183:4169–75. doi: 10.4049/jimmunol.0901017. https://doi.org/10.4049/jimmunol.0901017. [DOI] [PubMed] [Google Scholar]
- 3.Ohl K, Tenbrock K. Inflammatory cytokines in systemic lupus erythmatosus. J Biomed Biotechnol. 2011;2011:432595. doi: 10.1155/2011/432595. https://doi.org/10.1155/2011/432595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yap D, Lai K. Cytokines and their roles in the pathogenesis of systemic lupus erythematosus: from basics to recent advances. J Biomed Biotechnol. 2010;2010:365083. doi: 10.1155/2010/365083. https://doi.org/10.1155/2010/365083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zickert A, Amoudruz P, Sundström Y, Rönnelid J, Malmström V, Gunnarsson I. IL-17 and IL-23 in lupus nephritis - association to histopathology and response to treatment. BMC Immunol. 2015;16:1–10. doi: 10.1186/s12865-015-0070-7. https://doi.org/10.1186/s12865-015-0070-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Postal M, Peliçari KO, Sinicato NA, Marini R, Costallat LT, Appenzeller S. Th1/Th2 cytokine profile in childhood-onset systemic lupus erythematosus. Cytokine. 2013;61:785–91. doi: 10.1016/j.cyto.2012.11.023. https://doi.org/10.1016/j.cyto.2012.11.023. [DOI] [PubMed] [Google Scholar]
- 7.Gottschalk TA, Tsantikos E, Hibbs ML. Pathogenic inflammation and its therapeutic targeting in systemic lupus erythematosus. Front Immunol. 2015;6:550. doi: 10.3389/fimmu.2015.00550. https://doi.org/10.3389/fimmu.2015.00550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhu M, Mo H, Li D, Luo X, Zhang L. Th17/Treg imbalance induced by increased incidence of atherosclerosis in patients with systemic lupus erythematosus (SLE) Clin Rheumatol. 2013;32:1045–52. doi: 10.1007/s10067-013-2237-z. https://doi.org/10.1007/s10067-013-2237-z. [DOI] [PubMed] [Google Scholar]
- 9.Kay SD, Poulsen MK, Diederichsen AC, Voss A. Coronary, carotid, and lower-extremity atherosclerosis and their interrelationship in Danish patients with systemic lupus erythematosus. J Rheumatol. 2016;43:315–22. doi: 10.3899/jrheum.150488. https://doi.org/10.3899/jrheum.150488. [DOI] [PubMed] [Google Scholar]
- 10.Lopez-Pedrera C, Aguirre MA, Barbarroja N, Cuadrado MJ. Accelerated atherosclerosis in systemic lupus erythematosus: role of proinflammatory cytokines and therapeutic approaches. J Biomed Biotechnol. 2010;2010 doi: 10.1155/2010/607084. pii: 607084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Martin JC, Baeten DL, Josien R. Emerging role of IL-17 and Th17 cells in systemic lupus erythmatosus. Clin Immunol. 2014;154:1–12. doi: 10.1016/j.clim.2014.05.004. https://doi.org/10.1016/j.clim.2014.05.004. [DOI] [PubMed] [Google Scholar]
- 12.Su DL, Lu ZM, Shen MN, Li X, Sun LY. Roles of pro- and anti-inflammatory cytokines in the pathogenesis of SLE. J Biomed Biotechnol. 2012;2012:347141. doi: 10.1155/2012/347141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu Y, Liao J, Zhao M, Wu H, Yung S, Chan TM, et al. Increased expression of TLR2 in CD4+ T cells from SLE patients enhances immune reactivity and promotes IL-17 expression through histone modifications. Eur J Immunol. 2015;45:2683–93. doi: 10.1002/eji.201445219. https://doi.org/10.1002/eji.201445219. [DOI] [PubMed] [Google Scholar]
- 14.Nam JS, Terabe M, Kang MJ, Chae H, Voong N, Yang YA, et al. Transforming growth factor beta subverts the immune system into directly promoting tumor growth through interleukin-17. Cancer Res. 2008;68:3915–23. doi: 10.1158/0008-5472.CAN-08-0206. https://doi.org/10.1158/0008-5472.CAN-08-0206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chizzolini C, Chicheportiche R, Alvarez M, de Rham C, Roux-Lombard P, Ferrari-Lacraz S, et al. Prostaglandin E2 synergistically with interleukin-23 favors human Th17 expansion. Blood. 2008;112:3696–703. doi: 10.1182/blood-2008-05-155408. https://doi.org/10.1182/blood-2008-05-155408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu SJ, Tsai JP, Shen CR, Sher YP, Hsieh CL, Yeh YC, et al. Induction of a distinct CD8 Tnc17 subset by transforming growth factor-beta and interleukin-6. J Leukoc Biol. 2007;82:354–60. doi: 10.1189/jlb.0207111. https://doi.org/10.1189/jlb.0207111. [DOI] [PubMed] [Google Scholar]
- 17.Robak E, Kulczycka-Siennicka L, Gerlicz Z, Kierstan M, Korycka-Wolowiec A, Sysa-Jedrzejowska A. Correlations between concentrations of interleukin (IL)-17A, IL-17B and IL-17F, and endothelial cells and proangiogenic cytokinesin systemic lupus erythmatosus patients. Eur Cytokine Netw. 2013;24:60–8. doi: 10.1684/ecn.2013.0330. [DOI] [PubMed] [Google Scholar]
- 18.Blankenstein T, Qin Z. The role of IFN-gamma in tumor transplantation immunity and inhibition of chemical carcinogenesis. Curr Opin Immunol. 2003;15:148–54. doi: 10.1016/s0952-7915(03)00007-4. https://doi.org/10.1016/S0952-7915(03)00007-4. [DOI] [PubMed] [Google Scholar]
- 19.Robak E, Kulczycka-Siennicka L, Gerlicz Z, Kierstan M, Korycka-Wolowiec A, Sysa-Jedrzejowska A. Correlations between concentrations of interleukin (IL)-17A, IL-17B and IL-17F, and endothelial cells and proangiogenic cytokines in systemic lupus erythematosus patients. Eur Cytokine Netw. 2013;24:60–8. doi: 10.1684/ecn.2013.0330. [DOI] [PubMed] [Google Scholar]
- 20.Nalbandian A, Crispín JC, Tsokos GC. Interleukin-17 and systemic lupus erythematosus: current concepts. Clin Exp Immunol. 2009;157:209–15. doi: 10.1111/j.1365-2249.2009.03944.x. https://doi.org/10.1111/j.1365-2249.2009.03944.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mease PJ, McInnes IB, Kirkham B, Kavanaugh A, Rahman P, van der Heijde D, et al. Secukinumab inhibition of interleukin-17A in patients with psoriatic arthritis. N Eng J Med. 2015;373:1329–39. doi: 10.1056/NEJMoa1412679. https://doi.org/10.1056/NEJMoa1412679. [DOI] [PubMed] [Google Scholar]
- 22.Hueber W, Sands BE, Lewitzky S, Vandemeulebroecke M, Reinisch W, Higgins PD, et al. Secukinumab, a human anti-IL-17A monoclonal antibody, for moderate to severe Crohn's disease: unexpected results of a randomised, double-blind placebo-controlled trial. Gut. 2012;61:1693–700. doi: 10.1136/gutjnl-2011-301668. https://doi.org/10.1136/gutjnl-2011-301668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yee CS, Farewell VT, Isenberg DA, Griffiths B, Teh LS, Bruce IN, et al. The use of Systemic Lupus Erythematosus Disease Activity Index-2000 to define active disease and minimal clinically meaningful change based on data from a large cohort of systemic lupus erythematosus patients. Rheumatology (Oxford) 2011;50:982–8. doi: 10.1093/rheumatology/keq376. https://doi.org/10.1093/rheumatology/keq376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gladman D, Goldsmith C, Urowitz M, Bacon P, Fortin P, Ginzler E, et al. The Systemic Lupus International Collaborating Clinics/American College of Rheumatology (SLICC/ACR) Damage Index for Systemic Lupus Erythematosus International Comparison. J Rheumatol. 2000;27:373–6. [PubMed] [Google Scholar]
- 25.Wong CK, Lit LC, Tam LS, Li EK, Wong PT, Lam CW. Hyperproduction of IL-23 and IL-17 in patients with systemic lupus erythematosus: implications for Th17-mediated inflammation in auto-immunity. Clin Immunol. 2008;127:385–93. doi: 10.1016/j.clim.2008.01.019. https://doi.org/10.1016/j.clim.2008.01.019. [DOI] [PubMed] [Google Scholar]
- 26.Wong CK, Ho CY, Li EK, Lam CW. Elevation of proinflammatory cytokine (IL-18, IL-17, IL-12) and Th2 cytokine (IL-4) concentrations in patients with systemic lupus erythematosus. Lupus. 2000;9:589–93. doi: 10.1191/096120300678828703. https://doi.org/10.1191/096120300678828703. [DOI] [PubMed] [Google Scholar]
- 27.Yang J, Chu Y, Yang X, Gao D, Zhu L, Yang X, et al. Th17 and natural Treg cell population dynamics in systemic lupus erythematosus. Arthritis Rheum. 2009;60:1472–83. doi: 10.1002/art.24499. https://doi.org/10.1002/art.24499. [DOI] [PubMed] [Google Scholar]
- 28.Ballantine L, Midgley A, Watson L, Beresford M. THU0292 Stimulation of juvenile systemic lupus erythematosus blood cells induces IL-17 production. Ann Rheum Dis. 2013;71(Suppl 3):254. https://doi.org/10.1136/annrheumdis-2012-eular.2257. [Google Scholar]
- 29.Yang XY, Wang HY, Zhao XY, Wang LJ, Lv QH, Wang QQ. Th22, but not Th17 might be a good index to predict the tissue involvement of systemic lupus erythematosus. J Clin Immunol. 2013;33:767–74. doi: 10.1007/s10875-013-9878-1. https://doi.org/10.1007/s10875-013-9878-1. [DOI] [PubMed] [Google Scholar]
- 30.Doreau A, Belot A, Bastid J, Riche B, Trescol-Biemont M-C, Ranchin B, et al. Retraction: Interleukin 17 acts in synergy with B cell-activating factor to influence B cell biology and the pathophysiology of systemic lupus erythematosus. Nat Immunol. 2014;15:894. doi: 10.1038/ni.1741. https://doi.org/10.1038/ni0914-894a. [DOI] [PubMed] [Google Scholar]
- 31.Zhao XF, Pan HF, Yuan H, Zhang WH, Li XP, Wang GH, et al. Increased serum interleukin 17 in patients with systemic lupus erythematosus. Mol Biol Rep. 2010;37:81–5. doi: 10.1007/s11033-009-9533-3. https://doi.org/10.1007/s11033-009-9533-3. [DOI] [PubMed] [Google Scholar]
- 32.Cheng F, Guo Z, Xu H, Yan D, Li Q. Decreased plasma IL22 levels, but not increased IL17 and IL23 levels, correlate with disease activity in patients with systemic lupus erythematosus. Ann Rheum Dis. 2009;68:604–6. doi: 10.1136/ard.2008.097089. https://doi.org/10.1136/ard.2008.097089. [DOI] [PubMed] [Google Scholar]
- 33.Vincent FB, Northcott M, Hoi A, Mackay F, Morand EF. Clinical associations of serum interleukin-17 in systemic lupus erythematosus. Arthritis Res Ther. 2013;15:R97. doi: 10.1186/ar4277. https://doi.org/10.1186/ar4277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sestak AL, Fürnrohr BG, Harley JB, Merrill JT, Namjou B. The genetics of systemic lupus erythematosus and implications for targeted therapy. Ann Rheum Dis. 2011;70(Suppl 1):i37–i43. doi: 10.1136/ard.2010.138057. https://doi.org/10.1136/ard.2010.138057. [DOI] [PubMed] [Google Scholar]
- 35.Martin JC, Baeten DL, Josien R. Emerging role of IL-17 and Th17 cells in systemic lupus erythematosus. Clin Immunol. 2014;154:1–12. doi: 10.1016/j.clim.2014.05.004. https://doi.org/10.1016/j.clim.2014.05.004. [DOI] [PubMed] [Google Scholar]
- 36.Simon T, Taleb S, Danchin N, Laurans L, Rousseau B, Cattan S, et al. Circulating levels of interleukin-17 and cardiovascular outcomes in patients with acute myocardial infarction. Eur Heart J. 2013;34:570–7. doi: 10.1093/eurheartj/ehs263. https://doi.org/10.1093/eurheartj/ehs263. [DOI] [PubMed] [Google Scholar]
- 37.McInnes IB, Mease PJ, Kirkham B, Kavanaugh A, Ritchlin CT, Rahman P, et al. Secukinumab, a human anti-interleukin-17A monoclonal antibody, in patients with psoriatic arthritis (FUTURE 2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2015;386:1137–46. doi: 10.1016/S0140-6736(15)61134-5. https://doi.org/10.1016/S0140-6736(15)61134-5. [DOI] [PubMed] [Google Scholar]
- 38.Taleb S, Tedgui A, Mallat Z. IL-17 and Th17 cells in atherosclerosis: subtle and contextual roles. Arterioscler Thromb Vasc Biol. 2015;35:258–64. doi: 10.1161/ATVBAHA.114.303567. https://doi.org/10.1161/ATVBAHA.114.303567. [DOI] [PubMed] [Google Scholar]
- 39.Guery L, Hugues S. Th17 cell plasticity and functions in cancer immunity. BioMed Res Int. 2015;2015:314620. doi: 10.1155/2015/314620. https://doi.org/10.1155/2015/314620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gullick NJ, Abozaid HS, Jayaraj DM, Evans HG, Scott DL, Choy EH, et al. Enhanced and persistent levels of interleukin (IL)-17(+) CD4(+) T cells and serum IL-17 in patients with early inflammatory arthritis. Clin Exp Immunol. 2013;174:292–301. doi: 10.1111/cei.12167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stowe RP, Peek MK, Cutchin MP, Goodwin JS. Plasma cytokine levels in a population-based study: relation to age and ethnicity. J Gerontol A Biol Sci Med Sci. 2010;65:429–33. doi: 10.1093/gerona/glp198. https://doi.org/10.1093/gerona/glp198. [DOI] [PMC free article] [PubMed] [Google Scholar]