Abstract
Objective
Benign joint hypermobility syndrome refers to hypermobile individuals with musculoskeletal symptoms in the absence of any systemic rheumatic disease; its prevalence is approximately 0.5%. In animal studies, bilirubin has been shown to reduce fibrosis induced by bleomycin. It has been suggested that bilirubin leads to hypermobility that affects the structure or function of collagen. In addition, our observation is that hypermobility occurs more often in patients with indirect hyperbilirubinemia. In this study, we aimed to evaluate hypermobility in patients with indirect hyperbilirubinemia.
Material and Methods
We recruited 120 consecutive patients with indirect hyperbilirubinemia from a tertiary gastroenterology outpatient clinic and examined them for hypermobility. Hypermobility was evaluated using the Beighton criteria, and other relevant clinical findings were recorded. In addition, a group of healthy individuals (n=107) without hyperbilirubinemia were included as controls.
Results
The mean ages of the patients and controls were 33.4±12.9 and 36.2±11.2 years, respectively (p=0.09). In total, 100 (83%) patients and 78 (73%) controls were male (p=0.075). The mean indirect bilirubin levels were 1.44±0.66 mg/dL in the patient group and 0.37±0.18 mg/dL in the control group. Based on the Beighton score, 23 patients (19.2%) in the patient group and 3 (2.8%) individuals in the control group had joint hypermobility. The difference between the groups was statistically significant (p<0.001).
Conclusion
According to the results of our study, findings of joint hypermobility are more frequent in patients with indirect hyperbilirubinemia than in controls.
Keywords: Hypermobility, hyperbilirubinemia, Beighton scoring
Introduction
The term joint hypermobility (JH) refers to the increased passive or active movements of a joint beyond its normal range. The 9-point Beighton scoring system has been recognized as the gold standard for making a diagnosis of JH (Table 1) after a modification to the criteria was suggested by Carter et al in 1964 (1, 2). This scoring system, giving a maximum of 9 points, measures the flexibility of five body areas (spine/hips, paired elbows, fifth metacarpophalangeal, thumb/wrists, and knees), is denoted in Table 1. Higher scores do not show greater degrees of JH, but rather the number of joints affected in a limited selection. A score of 4 or more out of 9 is generally considered to represent the presence of generalized JH.
Table 1.
The Beighton scoring system for detecting hypermobility
| Movement | Points | |
|---|---|---|
| The ability to do the following: | Right | Left |
| 1 Passive dorsiflexion of the fifth finger beyond 90° | 1 | 1 |
| 2 Passive dorsiflexion of the thumbs to the flexor aspects of the forearms | 1 | 1 |
| 3 Hyperextension of the elbows beyond 10° | 1 | 1 |
| 4 Hyperextension of the knees beyond 10° | 1 | 1 |
| 5 While forward flexion of the trunk with knees full extended, palms of the hands can rest flat on the floor | 1 | |
This system gives a participant a score of 0 to 9, and 9 points mean maximal joint hypermobility (constructed from Reference 1)
Individuals with benign joint hypermobility syndrome (JHS) are defined as hypermobile individuals with musculoskeletal symptoms in the absence of any systemic rheumatic disease (3). The prevalence of JHS in the general population is approximately 0.5%, although there are reports indicating higher frequencies (4). The Beighton criteria have been introduced for the diagnosis and epidemiological studies of JHS (Table 2) (5). Although a phenotypic overlap with inherited connective tissue disorders, such as Ehlers-Danlos syndrome or Marfan syndrome, has been suggested before, it has lately been revealed to be more than a simple clinical situation because gynecologic abnormalities arising from pelvic floor weakness, such as uterine prolapse, chronic pain, functional disorders of the gastrointestinal tract, and dysautonomia, have also started being recognized as complications associated with JHS (6–10).
Table 2.
Beighton criteria
| Major criteria |
|
Minor criteria
|
Joint hypermobility syndrome is diagnosed as the presence two major criteria, one major and two minor criteria, or four minor criteria. Two minor criteria suffice if there is an unequivocally affected first-degree relative (constructed from reference 5)
Biochemical or structural disorders affecting collagen synthesis are usually seen in those with JHS. Beyond genetic abnormalities affecting collagen synthesis in hereditary connective tissue disorders, there may be other mechanisms leading to a defective collagen structure. One of the main concerns is bilirubin. In an animal study, bilirubin has been shown to reduce fibrosis induced by bleomycin through anti-oxidative action by attenuating pulmonary fibrosis, partly by inhibiting lung inflammation and transforming growth factor (TGF) beta 1 (TGF-β1) production (11). In an in vitro study, scanning electron micrographs showed the ordering of collagen fibrils that was found to be more uniform with increases in the concentration of bilirubin, substantiating the role of bilirubin involvement in collagen fibrillogenesis (12). These studies suggest that bilirubin leads to hypermobility by affecting the structure or function of collagen.
Based on our observation in clinical practice that hypermobility is more often seen in hyperbilirubinemic patients, we aimed to evaluate JH in patients with indirect hyperbilirubinemia in this study.
Material and Methods
Patients
This case-control study was conducted in a rheumatology outpatient clinic of a tertiary referral center between December 2014 and July 2015. In total, 120 consecutive patients (20 females, 17%) with indirect hyperbilirubinemia, due to Gilbert’s syndrome, who were referred from a tertiary gastroenterology outpatient clinic, were recruited. In addition, a group of normobilirubinemic healthy individuals (n=107, 29 females, 27%) were included as the control group. All participants were examined in terms of the presence of JH. Demographic parameters, family history, anthropometric measurements, systolic and diastolic blood pressures, medications, and laboratory results of the participants were directly recorded in a Microsoft Excel 2010 file. Patients with evidence of any target organ damage from high blood pressure (BP), cardiac disease, thyroid dysfunction, liver diseases other than Gilbert’s syndrome, liver parenchymal and cholestatic causes of bilirubin elevation, renal failure, malignancy, and other chronic, infectious or inflammatory diseases were excluded. The patients were asked to sit down for 10 min before their BP was recorded using a manual mercury sphygmomanometer. The mean of two BPs was calculated. The mean arterial BP was calculated according to the following formula: MAP=DBP+(SBP−DBP)/3). The complete blood count, erythrocyte sedimentation rate (ESR), renal and hepatic function test results, and serum C-reactive protein, electrolyte, serum lipid, and thyroid hormone levels in the patient and control groups were recorded. All participants provided written informed consent. The study protocol was approved by the Research and Ethics Committees of Gülhane Training and Research Hospital.
Anthropometric Measurements
Weight (kg) and height (cm) were recorded, and the BMI was calculated as body weight/height2 (kg/m2). Measurements of upper and lower segments and overarm length were performed.
Assessment of Hypermobility
JH was evaluated using the Beighton criteria (Table 1) (1). The Beighton criteria were used to assess JHS (Table 2) (5). Other relevant clinical findings were recorded. The patients and controls were questioned and examined in detail in terms of anthropometric measurements, the presence of arachnodactyly, high palate, myopia, arthralgia, joint dislocation, soft tissue lesions, marfanoid habitus, skin striae, hyperextensibility, back pain, skin elasticity, trophic changes, varicose veins, hernia, and uterine/rectal prolapse. The Beighton score was calculated, and the participants were evaluated in terms of the Beighton criteria.
Laboratory studies
The participants’ laboratory results, in last visit, consisting routine biochemistry tests (including bilirubin levels), calcium levels, whole blood count, and acute phase reactants were recorded.
Statistical analyses
Statistical Packages for the Social Sciences (SPSS) 22.0 (IBM Corp.; Armonk, NY, USA) statistical package was used for statistical analysis. The independent samples t-test, chi-square test, and correlation tests were used. Quantitative variables were expressed as mean±standard deviation. The Kolmogorov-Smirnov and Shapiro-Wilk tests were used to determine the distribution characteristics of the variables. Levene’s test was used to determine the equality of variance. Differences between the groups were studied for significance using the independent samples t-test as appropriate. Categorical variables were compared by the chi-square test. Pearson and Spearman correlation analyses were used to evaluate relationships between variables. Results of the analysis were expressed as percentage for qualitative variables and mean±standard deviation for continuous variables. A two-sided p<0.05 was considered significant.
Results
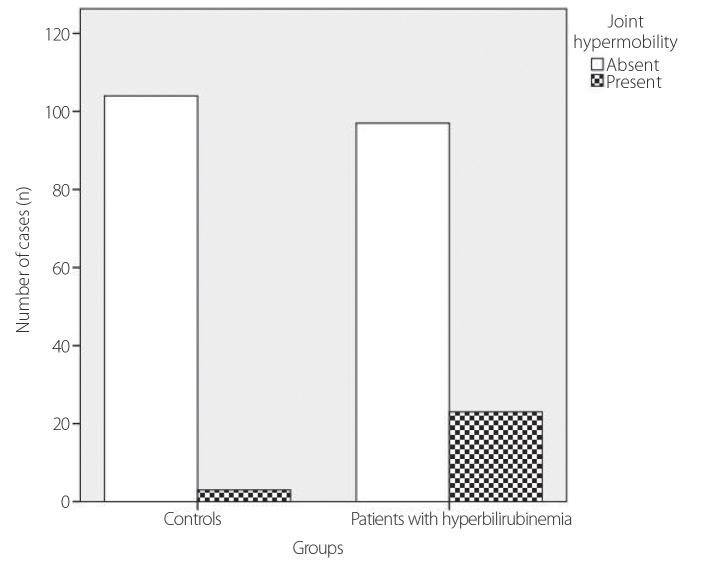
The mean ages of the patients and controls enrolled in the study were 33.4±12.9 and 36.2±11.2 years, respectively (p=0.09). In total, 100 (83%) patients and 78 (73%) controls were males (p=0.075). The demographic and laboratory characteristics and anthropometric measurements of the patient and control groups are shown in Table 3. The distributions of age and sex and means of BMI between the two groups were similar (p>0.05). The mean indirect bilirubin levels were 1.44±0.66 mg/dL in the patient group and 0.37±0.18 mg/dL in the control group. Based on the Beighton score, 23 patients (19.2%) in the patient group and 3 (2.8%) individuals in the control group had JH, and the difference between the groups was statistically significant (p<0.001) (Figure 1). According to the Beighton criteria, 1 participant (1%) in the control group and 4 patients (3%) in the patient group had JHS. The difference between the groups was not statistically significant (p=0.220).
Table 3.
The demographic, anthropometric, and laboratory comparisons of the patient and control groups.
| Patients (n=120) | Controls (n=107) | p | |
|---|---|---|---|
| Age | 33.42±12.91 | 36.15±11.28 | 0.09 |
| Sex (F) (n, %) | 20 (16.7) | 29 (27.1) | 0.075* |
| Overarm length (cm) | 174.5±10.13 | 171.07±10.78 | 0.014 |
| Height (cm) | 174.89±8.98 | 171.25±9.21 | 0.003 |
| Serum indirect bilirubin | 1.44±0.66 | 0.37±0.18 | <0.001 |
| Joint hypermobility n (%) | 23 (19.2) | 3 (2.8) | <0.001* |
| Joint hypermobility syndrome n (%) | 4 (3.3) | 1 (0.9) | 0.220* |
Chi-square test, otherwise independent samples t-test was used.
Figure 1.
Chart representing the difference in joint hypermobility between hyperbilirubinemic patients and controls. The difference was statistically significant (p<0.001)
Discussion
In this study, we found that indirect hyperbilirubinemic patients tended to have more hypermobile joints. To our knowledge, this observation has not yet been documented in the literature. This may lead to some clinical implications, such as noting the bilirubin levels of patients with JH or some kind of treatments lowering bilirubin levels to resolve hypermobility of the joints of such patients.
Collagen is a major component of the extracellular matrix. Of the total collagen synthesized, collagen types I and III constitute 85–90% and 8–11%, respectively. Under physiological circumstances, for the formation of the triple helix, hydroxylation of proline is essential and an un-hydroxylated collagen is rather susceptible to non-specific proteolysis (13). So, the balance in the hydroxylation of collagen is very important in terms of producing healthy collagen. Also, many soluble mediators and cytokines can also affect fibroblast functions. Ascorbic acid is an important molecule effecting collagen synthesis. Some cytokines, like transforming growth factor-α (TGF-α) and interferon-γ (IFN-γ) are important modulators of collagen synthesis. There is much evidence on the regulation of these functions by the addition of various cytokines to cell cultures. TGF-β, TNF-α, and IFN-γ may inhibit or stimulate production of dermal extracellular matrix proteins and collagenase enzyme (14–17).
When the balance between the hydroxylation and oxidation in collagen synthesis is taken into account, the anti-oxidant effect of bilirubin may, at least, impair collagen synthesis. In a previous study, it has been documented that the anti-oxidative action of bilirubin can attenuate bleomycin-induced pulmonary fibrosis, partly by inhibiting lung inflammation and production of TGF-β1 (11). This leads to the idea that parenterally given bilirubin inhibits inflammation-associated fibrosis, i.e., collagen synthesis in these rats. In fact, synthesis of the inflammatory state-associated collagen and structural collagen may not mean the same things; but this finding is worth discussing.
Increased bilirubin levels may affect collagen at the molecular level. According to the current literature, some molecular interactions between collagen and bilirubin molecules have been noted. In an in vitro study, the morphology and quantity of bilirubin binding to collagen varied in a concentration-dependent manner, and the adsorption rate in protein solutions changed. These microscopic studies on collagen–bilirubin interactions confirm that bilirubin affects fibrillogenesis and changes the rate of organization of collagen molecules depending on the bilirubin concentration (12). In another study investigating the molecular affinity between these two molecules, bilirubin showed a higher affinity to collagen at a concentration of approximately 25 nM/mg (18). In this study, the association rate, which shows the increased affinity of bilirubin to collagen, was calculated, and the affinity of bilirubin to collagen was found to be 8.89×10−3 s−1. The predominant binding of bilirubin to collagen was found to be electrostatic in nature. This study may help understand the affinity of the collagen–bilirubin complex during jaundice-diseased tissues and the bilirubin–collagen interaction in hypermobile patients. There may also be some enzymatically effected mechanisms in bilirubin–collagen interactions. Heme oxygenase-1 (HO-1) regulates collagen synthesis and might be affected from the imbalance in the oxidative–anti-oxidative status due to increased bilirubin levels. HO-1 plays a critical part in fibrogenic gene expression by antagonizing enhanced myocardial oxidative stress, angiogenesis, and wound healing (19, 20).
Another point of discussion on hyperbilirubinemia in hypermobile individuals is the high turnover of articular tissues. It has previously been documented in a study by Bird et al. (21) that JH leads to osteoarthrosis and chondrocalcinosis over time. Articular and periarticular tissue proteins, especially collagen, exposed to high friction forces may lead to high protein degradation end products. These possible mechanisms need further studies to reveal the true associations.
Our study has some limitations. First, our case-control study contains some observational data, and we cannot neglect the random effect on our observations. For this reason, increasing the patient number could be reasonable. Second, there may be inter- or intra-observer differences in the Beighton scoring of patients with hypermobility. This may cause some disturbances in the interpretation of our results. A recheck of the Beighton scores of participants by more blind clinicians could be beneficial; however, this is time consuming. Finally, bilirubin levels might fluctuate. This can be overcome by measuring bilirubin levels at multiple time points. Although bilirubin levels were measured by a standardized method, i.e., after 8–12 h of fasting, it might be considered as a limitation of the study. Further studies are needed on this subject.
These preliminary results suggest that findings of JH are more frequent in patients with indirect hyperbilirubinemia compared to healthy controls. These results necessitate further prospective studies to comment on the effect of bilirubin on collagen and the resulting clinical importance.
Acknowledgements
The authors would like to thank clinical members of the Division of Rheumatology, and clinical members of the Division of Gastroenterelogy, Gülhane Training and Research Hospital, for assistance with the conduct of the study.
Footnotes
Ethics Committee Approval: Ethics committee approval was received for this study from the ethics committee of Gülhane Training and Research Hospital, 07.04.2015-198.
Informed Consent: Written informed consent was obtained from patients who participated in this study.
Peer-review: Externally peer-reviewed.
Author Contributions: Concept - M.Ç., A.D.; Design - M.Ç., S.Y.; Supervision - S.Y., A.D.; Resources - M.Ç., K.Ö.; Materials - M.Ç., M.Çakar; Data Collection and/or Processing - M.Ç., M.Çakar; Analysis and/or Interpretation - M.Ç., M.Çakar; Literature Search - M.Ç., M.Çakar; Writing Manuscript - M.Ç., M.Çakar; Critical Review - A.D., S.Y.; Other - K.O., İ.Ç.
Conflict of Interest: No conflict of interest was declared by the authors.
Financial Disclosure: The authors declared that this study has received no financial support.
References
- 1.Beighton P, Solomon L, Soskolne CL. Articular mobility in an African population. Ann Rheum Dis. 1973;32:413–8. doi: 10.1136/ard.32.5.413. https://doi.org/10.1136/ard.32.5.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Carter C, Wilkinson J. Persistent joint laxity and congenital dislocation of the hip. J Bone Jt Surg. 1964;46:40–5. [PubMed] [Google Scholar]
- 3.Kirk JA, Ansell BM, Bywaters EG. The hypermobility syndrome. Musculoskeletal complaints associated with generalized joint hypermobility. Ann Rheum Dis. 1967;26:419–25. doi: 10.1136/ard.26.5.419. https://doi.org/10.1136/ard.26.5.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grahame R, Hakim AJ. Joint hypermobility syndrome is highly prevalent in general rheumatology clinics, its occurrence and clinical presentation being gender, age and race-related. Ann Rheum Dis. 2006;65(Suppl II):263. [Google Scholar]
- 5.Grahame R, Bird HA, Child A. The revised (Brighton 1998) criteria for the diagnosis of benign joint hypermobility syndrome (BJHS) J Rheumatol. 2000;27:1777–9. [PubMed] [Google Scholar]
- 6.AL-Rawi ZS, Al-Rawi ZT. Joint hypermobility in women with genital prolapse. Lancet. 1982;1:1439–41. doi: 10.1016/s0140-6736(82)92453-9. https://doi.org/10.1016/S0140-6736(82)92453-9. [DOI] [PubMed] [Google Scholar]
- 7.Gazit Y, Nahir AM, Grahame R, Jacob G. Dysautonomia in the joint hypermobility syndrome. Am J Med. 2003;115:33–40. doi: 10.1016/s0002-9343(03)00235-3. https://doi.org/10.1016/S0002-9343(03)00235-3. [DOI] [PubMed] [Google Scholar]
- 8.Sacheti A, Szemere J, Bernstein B, Tafas T, Schechter N, Tsipouras P. Chronic pain is a manifestation of the Ehlers-Danlos syndrome. J Pain Symptom Manage. 1997;14:88–93. doi: 10.1016/s0885-3924(97)00007-9. https://doi.org/10.1016/S0885-3924(97)00007-9. [DOI] [PubMed] [Google Scholar]
- 9.Grahame R. Pain, distress and joint hyperlaxity. Joint Bone Spine. 2000;67:157–63. [PubMed] [Google Scholar]
- 10.Fikree A, Aziz Q, Grahame R. Joint hypermobility syndrome. Rheum Dis Clin North Am. 2013;39:419–30. doi: 10.1016/j.rdc.2013.03.003. https://doi.org/10.1016/j.rdc.2013.03.003. [DOI] [PubMed] [Google Scholar]
- 11.Wang H, Yamaya M, Okinaga S, Jia Y, Kamanaka M, Takahashi H. Bilirubin ameliorates bleomycin-induced pulmonary fibrosis in rats. 2002;165:406–11. doi: 10.1164/ajrccm.165.3.2003149. [DOI] [PubMed] [Google Scholar]
- 12.Usharani N, Jayakumar GC, Rao JR, Chandrasekaran B, Nair BU. A microscopic evaluation of collagen-bilirubin interactions: In vitro surface phenomenon. J Microsc. 2014;253:109–18. doi: 10.1111/jmi.12101. https://doi.org/10.1111/jmi.12101. [DOI] [PubMed] [Google Scholar]
- 13.Gelse K, Pöschl E, Aigner T. Collagens--structure, function, and biosynthesis. Adv Drug Deliv Rev. 2003;55:1531–46. doi: 10.1016/j.addr.2003.08.002. https://doi.org/10.1016/j.addr.2003.08.002. [DOI] [PubMed] [Google Scholar]
- 14.Phillips CL, Combs SB, Pinnell SR. Effects of ascorbic acid on proliferation and collagen synthesis in relation to the donor age of human dermal fibroblasts. J Invest Dermatol. 1994;103:228–32. doi: 10.1111/1523-1747.ep12393187. https://doi.org/10.1111/1523-1747.ep12393187. [DOI] [PubMed] [Google Scholar]
- 15.Roberts AB, Sporn MB, Assoian RK, Smith JM, Roche NS, Wakefield LM, et al. Transforming growth factor type beta: rapid induction of fibrosis and angiogenesis in vivo and stimulation of collagen formation in vitro. Proc Natl Acad Sci. 1986;83:4167–71. doi: 10.1073/pnas.83.12.4167. https://doi.org/10.1073/pnas.83.12.4167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mauviel A, Heino J, Kähäri V-M, Hartmann DJ, Loyau G, Pujol JP, et al. comparative effects of interleukin-1 and tumor necrosis factor-alpha on collagen production and corresponding procollagen mRNA levels in human dermal fibroblasts. J Invest Dermatol. 1991;96:243–9. doi: 10.1111/1523-1747.ep12462185. https://doi.org/10.1111/1523-1747.ep12462185. [DOI] [PubMed] [Google Scholar]
- 17.Chung JH, Youn SH, Kwon OS, Cho KH, Youn J, Il Eun HC. Regulations of collagen synthesis by ascorbic acid, transforming growth factor-β and interferon-γ in human dermal fibroblasts cultured in three-dimensional collagen gel are photoaging- and aging-independent. J Dermatol Sci. 1997;15:188–200. doi: 10.1016/s0923-1811(97)00607-5. https://doi.org/10.1016/S0923-1811(97)00607-5. [DOI] [PubMed] [Google Scholar]
- 18.Nagarajan U, Gladstone Christopher J, Chandrasekaran B, Jonnalagadda RR, Balachandran UN, Kohsaku K. Studies on the molecular significance in the interaction of bilirubin with collagen. Int J Biol Macromol. 2013;33:4965–71. doi: 10.1016/j.ijbiomac.2013.08.001. https://doi.org/10.1016/j.ijbiomac.2013.08.001. [DOI] [PubMed] [Google Scholar]
- 19.Hou C, Shen L, Huang Q, Mi J, Wu Y, Yang M, et al. The effect of heme oxygenase-1 complexed with collagen on MSC performance in the treatment of diabetic ischemic ulcer. Biomaterials. 2013;34:112–20. doi: 10.1016/j.biomaterials.2012.09.022. https://doi.org/10.1016/j.biomaterials.2012.09.022. [DOI] [PubMed] [Google Scholar]
- 20.Yu H, Huang J, Wang S, Zhao G, Jiao X, Zhu L. Overexpression of Smad7 suppressed ROS/MMP9-dependent collagen synthesis through regulation of heme oxygenase-1. Mol Biol Rep. 2013;40:5307–14. doi: 10.1007/s11033-013-2631-2. https://doi.org/10.1007/s11033-013-2631-2. [DOI] [PubMed] [Google Scholar]
- 21.Bird HA, Tribe CR, Bacon PA. Joint hypermobility leading to osteoarthrosis and chondrocalcinosis. Ann Rheum Dis. 1978;37:203–11. doi: 10.1136/ard.37.3.203. https://doi.org/10.1136/ard.37.3.203. [DOI] [PMC free article] [PubMed] [Google Scholar]