Abstract
Systemic lupus erythematosus (SLE) is a chronic autoimmune disease characterized with immune complex formation and renal involvement of lupus and may include several kinds of pathological conditions, but mostly, it is associated with immune complex-induced glomerular disease. Pauci-immune lupus nephritis is a very rare condition. We describe a 45-year-old female patient with pauci-immune crescentic necrotizing lupus nephritis and briefly discuss the possible mechanism and pathogenesis.
Keywords: Lupus, glomerulonephritis, pauci-immune
Introduction
Rapidly progressive glomerulonephritis (RPGN) is characterized by a rapid loss of kidney functions. Because of the microscopic features, RPGN is also called crescentic glomerulonephritis (GN). Crescentic GN is categorized by immunohistology into three groups: 1. anti-glomerular basement membrane (anti-GBM) cresentic GN with linear GBM staining for immunoglobulin G, 2. immune complex crescentic GN with granular staining of glomeruli for immunoglobulins or complement, and 3. crescentic GN with little or no glomerular staining for immunoglobulins or complements (pauci-immune crescentic GN). The most common reason of pauci-immune GNs is anti-neutrophil cytoplasmic antibody (ANCA)-associated vasculitis, including granulomatosis with polyangiitis, microscopic polyangiitis, and eosinophilic granulomatosis with polyangiitis, and renal limited vasculitis (1). There are several types of renal diseases in systemic lupus erythematosus (SLE), with the most common being immune complex-mediated glomerular disease, which is usually differentiated with a renal biopsy. In addition, renal diseases as pauci-immune GN may be rarely seen in lupus patients (2–4).
We report a rare case with ANCA-negative pauci-immune crescentic necrotizing lupus nephritis and briefly discuss the possible mechanism and pathogenesis.
Case Presentation
Our case was a 45-year-old female patient diagnosed with SLE 2 months previously with the initial presentation of polyarthritis, fever, and oral ulcer. Her laboratory test showed a high erythrocyte sedimentation rate (67 mm/h) and C-reactive protein level (8.6 mg/dL). Rheumatoid factor and anti-citrullinated peptide antibody were negative, anti-nuclear antibodies (ANA) and double-stranded DNA antibody (anti-dsDNA) were positive [anti-dsDNA: 538 (<200)]. She was treated with 10 mg prednisolone and hydroxychloroquine. She was referred to our hospital because of new appearance of proteinuria and hematuria. She had no history of Raynaud’s phenomenon, upper respiratory tract symptoms, cough, neuropathy, asthma, skin lesion, diarrhea, abdominal pain, diabetes mellitus, hypertension, or use of nephrotoxic agent. Her physical examination was remarkable for anemia and arthralgia. She had no clinical infection. Laboratory findings were as follows: white blood cell count 7000/mm3, hemoglobin level 10.6 g/dL, platelet count 274.000 /mm3, erythrocyte sedimentation rate 39 mm/h, C-reactive protein level 0.319 mg/dL, fibrinogen level 302 mg/dL, ANA positivity (a titer of 1/1000–1/3200 in a homogenous pattern by the indirect immunofluorescence method), anti-ds-DNA level 1.14 (n: 0–1.1), extractable nuclear antigen antibody negativity, complement (C) 3 level 62 mg/dL (n: 90–180), C4 level 11.4 mg/dL (n: 10–40), and serum creatinine level 1.85 mg/dL. Urinalysis revealed 3+ protein with 15 red blood cells. The 24-h urine collection revealed that the protein level was 1.72 g/day. The lupus anticoagulant test was negative. The anti-cardiolipin IgG antibody level was 4.02 GPLU/L (normal, 0–19 GPLU/L) and anti-cardiolipin IgM antibody level was 2.21 MPLU/L (normal, 0–19 MPLU/L). Coombs’ test was positive. Anti-neutrophil cytoplasmic antibody (ANCA) was negative by the indirect immunofluorescence method. Enzyme-Linked Immunosorbent Assay (ELISA) test for ANCA could not be performed in our hospital; hence, we could not evaluate it. A kidney biopsy was performed, and pulse methylprednisolone and cyclophosphamide therapy was given without waiting for biopsy results. Three days after the initiation of the treatment, the serum creatinine level began to decrease.
Renal biopsy findings
The biopsy contained 45 glomeruli. Three of them were globally sclerotic and two were segmentally sclerotic. Twelve of the glomeruli had fibrous crescents, 13 had fibrocellular crescents, and three had cellular crescents. Segmental fibrinoid necrosis of capillary tufts was seen in three of the glomeruli. There was a mild increase in mesangial cellularity in two glomeruli. Other glomeruli were almost completely normal. There was chronic inflammatory cell infiltration and mild fibrosis in the interstitium. There were few foci of tubular atrophy. Congo red and crystal violet staining were negative. No feature of thrombotic microangiopathy was identified. There was no finding for vasculitis in arterial walls, and there was no thrombus in glomerular capillary loops. Immunofluorescence microscopy showed no staining for IgG, IgA, IgM, fibrinogen, C1q, and C3 (Figure 1, 2).
Figure 1.
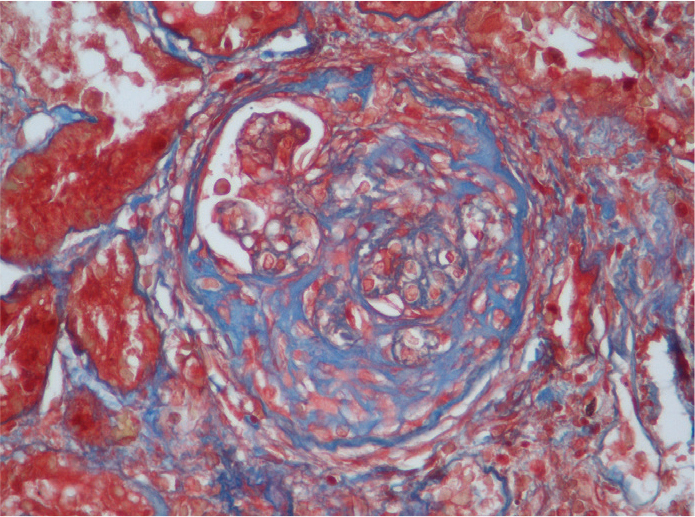
Glomerulus stained with Masson trichrome showing a large fibrocellular crescent (×400).
Figure 2.
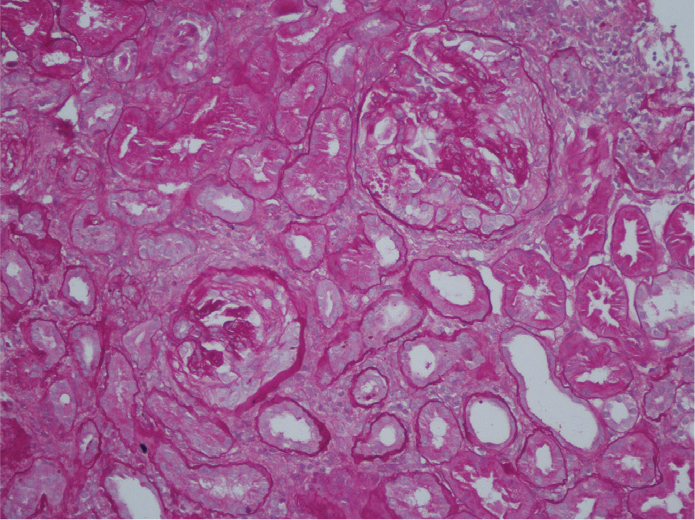
Two Periodic acid–Schiff (PAS)-stained glomeruli showing a well-formed cellular crescent (×200).
Discussion
Renal involvement of lupus may include several kinds of pathological conditions, but mostly, it is associated with immune complex-mediated glomerular disease. Pauci-immune lupus nephritis is a very rare condition. Most pauci-immune GNs have been found to be ANCA positive, but 10–30% of the cases could be ANCA negative (1). ANCAs are detected by indirect immunofluorescence in approximately 20% of patients with SLE (5). Some cases of lupus nephritis with histological features, including prominent necrosis and crescent formation with minimal or no endocapillary proliferation or subendothelial deposits, have been reported and have been interpreted as having superimposed ANCA-associated GN (6). In addition, ANCA negative pauci-immune lupus nephritis is defined in the literature (2–4).
The degree of subendothelial deposit formation roughly correlates with the degree of endocapillary proliferation in lupus nephritis (7). In most cases defined as having pauci-immune lupus nephritis in the literature, there was endocapillary proliferation, which is indicative of lupus nephritis (2–4). In our case, there was diffuse crescentic necrotising GN without endocapillary proliferation and immune complex deposition. Therefore, it was a pure pauci-immune GN. In this case, we can ask ourselves the following question: Is this a new subgroup or an incidental case? Hill et al. (7) suggested that although lupus nephritis is generally regarded as classic immune complex GN, the subgroups of lupus nephritis class IV-segmental and lupus nephritis class IV-global may have a different pathogenesis. They concluded that class IV-global lesions behave as an immune complex disease, having positive correlations with the extent of immune deposits and negative correlations with serum complement levels—the model traditionally assumed for lupus nephritis as a whole. Fibrinoid necroses without associated endocapillary proliferation occurred with some frequency in segmental proliferative cases but never in global proliferative cases. According to these results, Hill et al. (7) suggested that the mechanism underlying the pathogenesis of pure segmental class IV lupus nephritis without endocapillary proliferation and subendothelial deposition can be explained by a pauci-immune system. In the study by Hill et al. (7), ANCA was proposed as a potential pathomechanism, but the prevalence of ANCA seropositivity was not studied. In addition, research has claimed the importance of ANCA in the pathogenesis of lupus nephritis without endocapillary proliferation and subendothelial deposition but with crescents and necrosis (6). There have been some necrotizing lupus nephritis cases in the literature, which were ANCA negative, like our case (2–4). If so, what is the basic mechanism underlying ANCA-negative pauci-immune GN and ANCA-negative pauci-immune lupus nephritis? The pathogenesis of ANCA-negative pauci-immune GN is not clear. In a study by Eisenberger et al. (8), neutrophil infiltration could be found in glomeruler lesions in the absence of ANCA. Wang et al. (9) found that neutrophil gelatinase-associated lipocalin and lactroferrin were significantly higher in ANCA-negative patients than in ANCA-positive ones. These studies revealed that neutrophils play a key role in ANCA-negative pauci-immune GN.
Neutrophils also play an important role in lupus pathogenesis and the development of organ damage in SLE (10). There are some studies on lipocalin 2, which is produced by neutrophils. Lipocalin 2 also has a predictive value in terms of disease activation. A study by Elewa et al. (11) showed that there was a highly significant positive correlation between anti-dsDNA and urinary neutrophil gelatinase-associated lipocalin level with active lupus nephritis.
Patients with SLE may develop other kidney disorders that may be related or unrelated to SLE and SLE management (12). Our patient had no history of infections, drug-associated renal disease, hypertension, and diabetes mellitus. However, in our case, immunofluorescence examination revealed no immune complex deposition, but due to the lack of electron microscopic examination, we were unable to assess whether the immune complex deposition was minimal. Except for few glomeruli that have minimal mesangial proliferation, most glomeruli were normal, and this supports the pauci-immune GN. Hence, we thought that pure pauci-immune GN without endocapillary proliferation and immune complex deposition may be a rare variant of lupus nephritis rather than an incidental case.
Renal involvement in lupus is widely known as an immune complex disorder and is associated with a full-house pattern in immunofluorescence, but it must be known that without endocapillary proliferation, crescentic necrotizing pauci-immune GN may be a very rare variant of lupus nephritis. Other causes of ANCA negative pauci-immune GN should be kept in mind during differential diagnosis.
Footnotes
Ethics Committee Approval: N/A.
Informed Consent: Written informed consent was obtained from patient who participated in this study.
Peer-review: Externally peer-reviewed.
Author Contributions: Concept - D.U.C., C.K.; Design - D.U.C., C.K.; Supervision - D.U.C., C.K.; Resources - D.U.C., G.T, M.F.A.; Materials - D.U.C., G.T.; Data Collection and/or Processing - D.U.C.; Analysis and/or Interpretation - D.U.C, C.K.; Literature Search - D.U.C.; Writing Manuscript - D.U.C.; Critical Review - D.U.C, C.K, M.F.A.; Other - D.U.C, G.T.
Conflict of Interest: No conflict of interest was declared by the authors.
Financial Disclosure: The author declared that this study has received no financial support.
References
- 1.Chen M, Kallenberg CG, Zhao MH. ANCA-negative pauci-immune crescentic glomerulonephritis. Nat Rev Nephrol. 2009;5:313–8. doi: 10.1038/nrneph.2009.67. https://doi.org/10.1038/nrneph.2009.67. [DOI] [PubMed] [Google Scholar]
- 2.Akhtar M, al-Dalaan A, el-Ramahi KM. Pauci-immune necrotizing lupus nephritis: report of two cases. Am J Kidney Dis. 1994;23:320–5. doi: 10.1016/s0272-6386(12)80991-7. https://doi.org/10.1016/S0272-6386(12)80991-7. [DOI] [PubMed] [Google Scholar]
- 3.Charney DA, Nassar G, Truong L, Nadasdy T. “Pauci-Immune” proliferative and necrotizing glomerulonephritis with thrombotic microangiopathy in patients with systemic lupus erythematosus and lupus-like syndrome. Am J Kidney Dis. 2000;35:1193–206. doi: 10.1016/s0272-6386(00)70058-8. https://doi.org/10.1016/S0272-6386(00)70058-8. [DOI] [PubMed] [Google Scholar]
- 4.Ferrario F, Napodano P, Giordano A, Gandini E, Boeri R, D’Amico G. Peculiar type of focal and segmental lupus glomerulitis: glomerulonephritis or vasculitis? Contrib Nephrol. 1992;99:86–93. doi: 10.1159/000421694. https://doi.org/10.1159/000421694. [DOI] [PubMed] [Google Scholar]
- 5.Sen D, Isenberg DA. Antineutrophil cytoplasmic autoantibodies in systemic lupus erythematosus. Lupus. 2003;12:651–8. doi: 10.1191/0961203303lu456rr. https://doi.org/10.1191/0961203303lu456rr. [DOI] [PubMed] [Google Scholar]
- 6.Nasr SH, D’Agati VD, Park HR, Sterman PL, Goyzueta JD, Dressler RM, et al. Necrotizing and crescentic lupus nephritis with antineutrophil cytoplasmic antibody seropositivity. Clin J Am Soc Nephrol. 2008;3:682–90. doi: 10.2215/CJN.04391007. https://doi.org/10.2215/CJN.04391007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hill GS, Delahousse M, Nochy D, Bariety J. Class IV-S versus class IV-G lupus nephritis: clinical and morphologic differences suggesting different pathogenesis. Kidney Int. 2005;68:2288–97. doi: 10.1111/j.1523-1755.2005.00688.x. https://doi.org/10.1111/j.1523-1755.2005.00688.x. [DOI] [PubMed] [Google Scholar]
- 8.Eisenberger U, Fakhouri F, Vanhille P, Beaufils H, Mahr A, Guillevin L, et al. ANCA-negative pauci-immune renal vasculitis: histology and outcome. Nephrol Dial Transplant. 2005;20:1392–9. doi: 10.1093/ndt/gfh830. https://doi.org/10.1093/ndt/gfh830. [DOI] [PubMed] [Google Scholar]
- 9.Wang F, Chen M, Zhao MH. Neutrophil degranulation in antineutrophil cytoplasmic antibody-negative pauci-immune crescentic glomerulonephritis. J Nephrol. 2009;22:491–6. [PubMed] [Google Scholar]
- 10.Smith CK, Kaplan MJ. The role of neutrophils in the pathogenesis of systemic lupus erythematosus. Curr Opin Rheumatol. 2015;27:448–53. doi: 10.1097/BOR.0000000000000197. https://doi.org/10.1097/BOR.0000000000000197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Elewa EA, El Tokhy MA, Fathy SE, Talaat AM. Predictive role of urinary neutrophil gelatinase-associated lipocalin in lupus nephritis. Lupus. 2015;24:138–46. doi: 10.1177/0961203314550225. https://doi.org/10.1177/0961203314550225. [DOI] [PubMed] [Google Scholar]
- 12.Anders HJ, Weening JJ. Kidney disease in lupus is not always ‘lupus nephritis’. Arthritis Res Ther. 2013;15:108. doi: 10.1186/ar4166. [DOI] [PMC free article] [PubMed] [Google Scholar]


