Abstract
The mitochondrial Elongation Factor Tu (EF-Tu), encoded by the TUFM gene, is a highly conserved GTPase, which is part of the mitochondrial protein translation machinery. In its activated form it delivers the aminoacyl-tRNAs to the A site of the mitochondrial ribosome. We report here on a baby girl with severe infantile macrocystic leukodystrophy with micropolygyria and a combined defect of complexes I and IV in muscle biopsy, caused by a novel mutation identified in TUFM. Using human mutant cells and the yeast model, we demonstrate the pathological role of the novel variant. Moreover, results of a molecular modeling study suggest that the mutant is inactive in mitochondrial polypeptide chain elongation, probably as a consequence of its reduced ability to bind mitochondrial aa-tRNAs. Four patients have so far been described with mutations in TUFM, and, following the first description of the disease in a single patient, we describe similar clinical and neuroradiological features in an additional patient.
Keywords: TUFM, Mitochondrial translation, Leukodystrophy, OXPHOS defects
Highlights
-
•
Using a custom-targeted panel, a novel TUFM mutation was found.
-
•
The patient shows a severe infantile macrocystic leukodystrophy with micropolygyria.
-
•
In Saccharomyces cerevisiae the mutation impairs mitochondrial respiration.
-
•
The 3D model supports that the mutation destabilizes the protein structure.
1. Introduction
In mammalian cells, oxidative phosphorylation (OXPHOS) exploits the proton gradient across the inner membrane of the mitochondria, generated by respiration, to carry out the condensation of ADP and Pi into ATP [1]. The mitochondrial OXPHOS system is composed of five multiheteromeric complexes, constituted by approximately 80 different protein subunits, of which only 13 are encoded by mitochondrial DNA (mtDNA). Importantly, mtDNA also encodes the 12S and 16S ribosomal RNA (mt rRNA) and 22 transfer RNAs (mt tRNA), necessary for the in situ translation of the 13 mtDNA-encoded proteins [2], [3], [4]. The nuclear-encoded subunits of the mitochondrial respiratory chain (MRC) complexes, as well as a huge number of proteins necessary for normal mitochondrial biogenesis (such as assembly of the mitochondrial respiratory chain complexes, mtDNA replication, transcription and translation, biosynthesis of prosthetic groups), are all encoded by the nuclear genome (nDNA) and synthesized in the cytosol before being transported into the organelle [5].
The mitochondrial genes are translated within the organelles by their own protein synthesis machinery, composed of both RNAs and proteins. The mitochondrial translation factors are different from the cytosolic ones, but the mechanism of translation is essentially the same, including four main steps: initiation, elongation, termination and recycling of the ribosome [6]. Impaired mitochondrial translation causes a subgroup of mitochondrial diseases usually manifesting as severe combined MRC dysfunctions due to defective activities of the mtDNA-encoded subunits which are part of the oxidative phosphorylation complexes I, III, IV and V [4], [7]. The clinical presentations of these disorders include a wide spectrum of diseases and phenotypes, which are often disabling, progressive or fatal, affecting the brain, liver, skeletal muscle, heart and other organs [8].
The mitochondrial Elongation Factor Tu (EF-Tu), encoded by the TUFM gene, is a highly conserved GTPase, which, in its activated form (GTP:EF-Tu), delivers the aminoacyl-tRNAs to the A site of the mitochondrial ribosome through the formation of a ternary complex. As this process requires energy, GTP:EF-Tu is converted into an EF-Tu:GDP inactive complex. The latter is released from the ribosome, and serves as a substrate for the Elongation Factor Ts (EF-Ts), which promotes the exchange of GDP with GTP, thus reactivating it [9], [10]. Several, mainly regulatory, functions of EF-Tu have been described, including aminoacyl-tRNA surveillance in mammalian mitochondria [11]. A chaperone function, preventing thermal aggregation of proteins and enhancing protein refolding in vitro, has also been reported for EF-Tu [12]. So far, few mutations in TUFM have been reported, with only the first patient having a detailed phenotypic description, while the other patients have barely no phenotypic and biochemical functional assessment [13], [14], [15].
Here we report a novel c.964G>A mutation in the TUFM gene, in a patient suffering with lactic acidosis, encephalopathy associated with leukodystrophy with micropolygyria, and mild liver dysfunction. Biochemically, a combined defect of complexes I and IV was found in muscle. We present results from clinical and functional analyses plus in vivo characterization of the equivalent mutation in yeast. We also present results of a molecular modeling study we performed to investigate the effect of the mutation on the protein structure.
2. Materials and methods
2.1. Standard protocol approvals, registrations, and patient consent
The study was approved by the Ethical Committees of the Bambino Gesù Children's Hospital, Rome, Italy, in agreement with the Declaration of Helsinki. Informed consent was signed by the parents of the patient.
2.2. Mutational analysis
Genomic DNA was extracted by standard methods from leukocytes. The coding exons and exon–intron boundaries of EFG2 and EARS2 as well the entire mtDNA were amplified by PCR using appropriated primer oligonucleotides. The patient was included in a targeted resequencing performed at the BGI-Shenzhen (BGI-Shenzhen, Shenzhen, China). A custom probe library was used for targeted enrichment (Agilent SureSelectXT Custom Kit), designed to capture coding exons and flanking intronic stretches (20 nt) of 1381 genes known to be functionally related to mitochondrial disorders (“Mitoexome”) [16], followed by deep sequencing using Illumina Hiseq technology (median reads depth = 255X). Sanger sequencing was used to validate the parallel sequencing data, as well as to check variant segregation in the family.
2.3. Functional studies
2.3.1. Human samples
MRC complex activities were measured in muscle biopsy as described [17]. Human fibroblasts, obtained from skin biopsy, were grown in DMEM supplemented with 10% fetal bovine serum, 4.5 g/l glucose and 50 μg/ml uridine. Complex V activity (in the direction of ATP synthesis) was measured in fibroblast mitochondria of patient and age-matched controls, using reported spectrophotometric methods [18]. For electrophoresis in SDS polyacrylamide gels (SDS-PAGE), 40 μg of fibroblasts mitochondria were loaded in a 12% denaturating gel. For immunoblot analysis, PVDF membranes were probed with monoclonal antibodies and reactive bands were detected using Lite Ablot Extend Long Lasting Chemiluminescent Substrate (Euroclone, Pero (Mi), Italy). Densitometry analysis was performed using Quantity One software (BioRad, Hercules, CA, USA).
The following antibodies from MitoSciences (Eugene, OR, USA) were used: complex II – 70 kDa (SDHA); complex III – UQCRC2 (Core protein 2); complex IV – subunit II (COXII), subunit IV (COXIV) and subunit Va (COXVa); complex V – subunit alpha (ATP5A1), and porin (VDAC). NDUFB11 antibody was purchased from Proteintech (San Diego, CA, USA), and EF-TuM antibody was purchased from Abcam (Cambridge, MA, USA). For the densitometric analysis, the levels of EF-Tu and the different subunits of the mitochondrial complexes were normalized against VDAC and were obtained from at least three independent experiments.
2.3.2. Mitochondrial protein synthesis
Analysis of mitochondrial protein synthesis was performed as previously described [19], [20]. Immortalized fibroblasts at 70% confluence were labeled for 1 h with [35S]-L-methionine in the presence of 100 μg/ml emetine, an inhibitor of cytosolic protein synthesis. Samples were kept at − 80 °C until use. 20 μg of total cellular protein were loaded on a Novex™ 18% Tris-Glycine precast SDS polyacrylamide gel (Invitrogen). The gel was then fixed and dried, and the mitochondrial translation products were visualized using a Storage Phosphor Screen and a Typhoon 9410 Variable Mode Imager (GE Healthcare).
For all experiments, age-matched controls were used.
2.3.3. Yeast experiments
Yeast strains used to perform experiments described in this paper were the WT MCC123 (Mat a, ade2-1, ura3-52, leu2, kar1‐1) rho + [21]. The TUF1 null strain (ΔTUF1) was obtained by transformation of MCC123 strain (Mat a, ade2-1, ura3-52, kar1-1) with the Kan- MX4 cassette as already described [10]. Experimental procedures as well as plasmids construction are exhaustively described in the Supplementary materials.
2.4. Model building and analysis
Human and yeast mitochondrial Elongation Factor Tu (EF-Tu) and Elongation Factor Ts (EF-Ts) sequences were collected from the Entrez-NCBI database (IDs: NP_003312.3, NP_014830.1 and AAH93068.1, respectively). Models of human EF-Tu in the GDP-bound, Ts-bound and tRNA-bound forms and of yeast EF-Tu in the GDP-bound and tRNA-bound were obtained by Modeller v9.12 [22], based on templates at 51 to 60% sequence identity. Details on the modeling procedure are reported in the Supplementary Materials.
Inter-domain contact maps were plotted by the COCOMAPS server [23] with the default 8 Å cut-off distance. In such maps, a dot is present at the cross-over of two residues giving a contact, i.e. having any pair of atoms closer than the cut-off distance. Related information on the interface area and inter-domain H-bonds was also obtained by COCOMAPS.
2.5. Statistical analysis
Data are presented as mean ± SD. The Student's t-test was used for the analysis of statistical significance. A p value < 0.05 was considered significant (*: p < 0.05; **: p < 0.005; ***: p < 0.0005).
3. Results
3.1. Case report
The patient was the second daughter of Italian healthy non-consanguineous parents. She was born pre-term at 34 weeks of gestation. At birth body weight was 1730 g, length was 45 cm, head circumference was 29 cm. Soon after birth she presented metabolic acidosis (pH 7.32; EB -16 mmol/L; NaHCO3 14 mEq/L) with hyperlactacidemia (16 mmol/L), which was partially corrected by intravenous bicarbonate. She also had ketonuria. Neurological examination revealed choreo-athetotic movements and cranial ultra-sound showed increased signal in the right fronto-temporal and thalamic regions, foci of grey matter heterotopia in the right ventricle, subependimal cysts in the basal ganglia, a thin corpus callosum and polymicrogyria. Somatic growth proceeded normally in the following months. At 3 months of age, she was admitted to our hospital for the persistence of lactic acidosis and transient episodes of hypoglycemia (34–40 mg/dl, nv 55–110). Physical examination showed microcephaly and an arched palate. At neurological examination she presented convergent strabismus, abnormal fixation and pursuit, axial hypotonia, poor spontaneous motility and partially acquired head control. Brain MRI disclosed a right cerebral atrophy with polymicrogyria, demyelination of supratentorial white matter and multiple cystic lesions in the fronto-temporal regions and basal ganglia (Fig. 1A). EEG showed an alternating paroxysmal pattern without clinically evident seizures; a second EEG displayed a marked improvement of epileptic abnormalities. She underwent a quadriceps muscle biopsy that revealed diffuse decrease of the histochemical reaction to cytochrome c oxidase (COX). The MRC complex activities in muscle showed a combined defect of CI and CIV after normalization to citrate synthase (27% and 60% reduction to the controls mean, respectively). Accordingly, biochemical findings on fibroblasts mitochondria documented a defect of complex V activity using either substrate, as expected for multiple MRC defects (Fig. S1). Cardiac examination and fundus oculi were normal. A fasting test showed hypoglycemia (33 mg/dl) without ketonuria, and concomitant raise of serum transaminases (GOT 119, GPT 51 UI/L, nv 5–40). She had persistent lactic acidosis (5.7 mmol/L, nv 0.6–2.3), and the urine organic acids profile showed increased levels of Krebs cycle intermediates. Plasma and urine acylcarnitine levels were in the normal range, plasmatic alanine was increased (595 micromol/l, nv 150–400). At 6 months, neurological examination showed persistent axial hypotonia and limb spasticity became evident. The patient died at the age of 10 months.
Fig. 1.
Brain MRI and genetic features. (A) Brain MRI performed at age 3 months. T2 weighted axial sections (a, c); T2 weighted coronal sections (b, d). Notice hyperintensity of the cerebellar white matter (delayed myelination for age) and an asymmetric and global polymicrogyria with malformed basal ganglia and multiple cysts that seem to be prevalent in the thalami. (B) Electropherograms of the genomic region of both parents and the patient showing the c.964G>A variant in exon 8 of TUFM. (C) Protein sequence alignment (ClustalW) highlight the high homology of the corresponding amino acid variation G322R.
Fig. S1.
Spectrofotometric determination of complex V activity. ATP synthesis in fibroblasts mitochondria was reduced with either substrates used (succinate (S): − 32%; malate (M): − 55%; pyruvate + malate (P + M): − 47%). Data are expressed as mean ± SD of three independent experiments performed in three different batches of mitochondria. All reported differences are highly significant (p < 0.0005).
3.2. Mutational analysis
mtDNA pathogenic mutations and mutations in a few other candidate genes, such as EFG2 and EARS2, were ruled out. Next, parallel sequencing of a panel including 1381 genes, known to be associated with mitochondrial function, was performed. After excluding annotated frequent SNPs (> 1%), we prioritized changes according to the presence of homozygous or compound heterozygous variants with functional impact (i.e., non-synonymous variants and changes affecting splice sites), according to a recessive inherited trait. We identified a single gene entry, TUFM (NC_000016.10, NM_003321), harboring a homozygous mutation (c.964G > A; p.G322R), never reported in public (dbSNP, ExAC, Exome Variant Server) and in-house databases. Sanger sequencing confirmed the mutation in homozygous state in the patient and its segregation in the parents (Fig. 1B). The G322 residue is highly conserved from humans to yeast (Fig. 1C).
3.3. Functional studies
3.3.1. Human
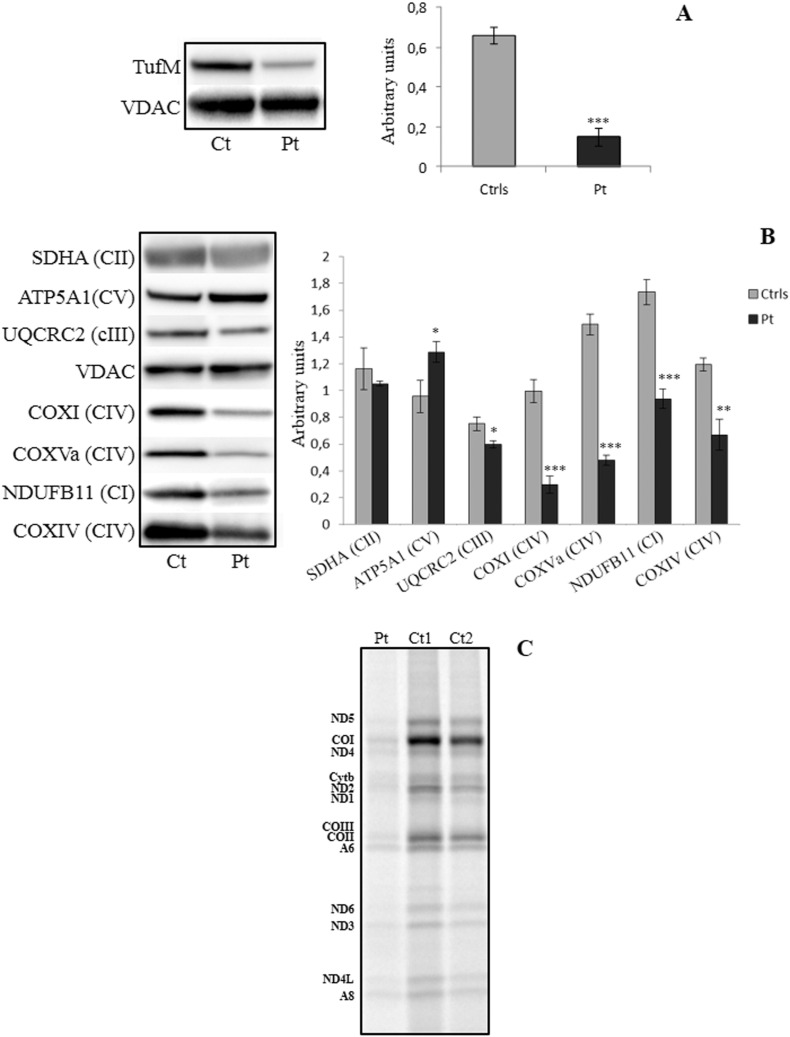
Drastic reduction of EF-Tu amount was detected in patient's fibroblast mitochondria (Fig. 2A). In keeping with reduced MRC activities in the muscle biopsy, we found severe reduction in the amount of complex I and complex IV subunits by immunoblot analysis (Fig. 2B). Moreover, markedly reduced synthesis of all the mtDNA-encoded subunits was evident in mutant immortalized fibroblasts (Fig. 2C).
Fig. 2.
Functional studies in human tissue. (A) Fibroblasts mitochondria from control and patients cells were separated on a 12% SDS-PAGE and reactive bands were probed with a specific antibody against EF-Tu protein. (B) Same samples were tested for the expression of the single subunits of the mitochondrial CI (NDUFB11), CII (SDHA), CIII (UQCRC2), CIV (COXI, COXIV, COXVa) and CV (ATP5A1). The levels of EF-Tu and the different subunits of the mitochondrial complexes reported in A and B histograms were normalized against VDAC. Data are presented as a mean ± SD of at least 3 independent experiments. (C) Mitochondrial translation study in control and mutant immortalized fibroblasts. The experiment has been repeated twice in two different batches of cells and both showed same result. *: p < 0.05; **: p < 0.005; ***: p < 0.0005.
3.3.2. Yeast
To validate the pathogenic role of the TUFM mutation in vivo we performed a gene complementation experiment in the budding yeast Saccharomyces cerevisiae, a facultative aerobic organism. The yeast mitochondrial EF-Tu factor shows 60% identity to the human one, which has a longer carboxy-terminal sequence and differs in the mitochondrial targeting sequence. Yeast EF-Tu carries out also the reactivation of the GTP-EF-Tu complex, which is operated in humans by a distinct factor, EF-Ts. These differences can explain why overexpression of human EF-Tu does not rescue the oxidative growth defect of ΔTUF1 yeast strain [10]. Therefore, we constructed a mutated yeast gene encoding the base substitution G311R, equivalent to the human G322R, inserted in a centromeric plasmid (pC-) or a multicopy plasmid (pE-). Serial dilutions of cells were plated on complete medium containing glucose (Fig. 3A) or glycerol (Fig. 3B) as carbon sources, and incubated at two different temperatures (28 °C and 37 °C). Whilst glycolytic growth was comparable in all strains (glucose medium), oxidative growth of the ΔTUF1 strain in glycerol containing media was impaired and rescued by high-copy expression of both wild type and mutated EF-Tu version. Conversely, low-copy expression of the mutated EF-Tu did not rescue the ΔTUF1 oxidative growth defect. The oxygen consumption capability of the analyzed strains is in agreement with their oxidative growth (Fig. 3C). All together, these results show that the G322R mutation impairs mitochondrial respiration in yeast and support the idea that the deleterious effect of the mutation consists in a reduction of the protein functionality.
Fig. 3.
Functional studies in yeast. (A) Growth and respiration capability of WT and transformant cells. Serial dilutions of WT, WT deleted of TUF1 gene (ΔTUF1) and transformant cells with empty, with multicopy (pE-) or centromeric (pC-) plasmids, bearing the WT TUF1 gene or its mutated version G311R, were spotted on YP plates containing 2% glucose or (B) 3% glycerol as carbon source and incubated at 28 and 37 °C. Pictures were acquired after three days of growth. (C) Comparison of oxygen consumption curves of the same cells as above, previously grown in selective minimal medium and refreshed four hours in 0.25% glucose containing media. In this figure has been reported one of at least three experiments.
3.4. Model building and analysis
To investigate the structural effect of the G322R mutation, we built 3D models for human and yeast mt EF-Tu in the GDP-bound, and GTP/tRNA-bound forms and for the human EF-Tu/EF-Ts complex, based on available structures of high sequence identity orthologs from B. taurus (mitochondrial), E. coli, T. thermophilus and T. aquaticus.
In the EF-Tu structure, an α/β domain containing the GTP/GDP binding site, domain 1, is followed by two shorter β-barrel domains, 2 and 3, participating in the tRNA binding. Mutation G322R is located in domain 2, on a loop region connecting two strands. In the GDP-bound (inactive) form, the mutated residue is exposed to the solvent and far in space from both domains 1 and 3 (Fig. 4A). In this form, a bulkier R322 can substitute G322 with no dramatic structural strain. However, R322 would be in close proximity to R255, located on the linker connecting domains 1 and 2, determining a possible electrostatic destabilization. Although in the EF-Ts-bound form, domain 1 gets closer to domains 2 and 3, there seems to still be room to accommodate the mutant R322 (not shown). Conversely, in the GTP/tRNA-bound (active) form, in which domain 1 rotates by about 90° relative to domains 2 and 3 [24], residue 322 is found at the interface with domain 1 (Fig. 4B) and in close proximity to residues H60, N62, D148 and R56. Overall, the interaction between domain 1 and domains 2–3 becomes much tighter in the GTP/tRNA bound form, compared to the GDP-bound one. This can easily be seen from Fig. 4C-D, where the inter-domain contact maps are reported for the active and inactive forms of human mt EF-Tu. In these maps, each dot represents a contact (spatial proximity) between two residues on different protein regions, specifically domain 1 and domains 2–3. The higher dots density in the map of the protein active form (Fig. 4D) clearly indicates a more extended inter-domain interface, as compared to the inactive one. The interface area between domain 1 and domains 2–3 indeed dramatically increases from 650 Å2 (inactive) to 2976 Å2 (active). G322, whose position in the maps is highlighted by a red vertical line, is clearly part of the extended inter-domain interface of the EF-Tu active form (see also Fig. 4B). The number of H-bonds between the domain 1 and domains 2–3 also significantly increases in the active form, with 14 inter-domain H-bonds (vs. the 2 of the inactive form), including 3 salt-bridges. Two salt bridges, R56-D323 and D148-R255, are close to the mutation site, and are important for stabilizing the interaction between domains 1 and 2 in the protein active structure. Furthermore, G322 itself participates with its backbone in a H-bond with the side-chain of N62. A three-dimensional representation of the H-bonds network around the mutation site in the GTP/tRNA-bound form is given in the inset of Fig. 4B. Notably, the side chain of R255 is within 4 Å from the α-carbon of G322, thus preventing its substitution by any bulkier amino acid. The multiple alignment reported in Fig. 4E shows that all residues mentioned above are widely conserved in EF-Tu across different organisms, thus underling their importance. Taken together, results of the modeling study support the experimental evidence that the G322R mutation destabilizes the protein structure both in its inactive and active configurations, particularly disfavoring the GTP/tRNA-bound (active) configuration.
Fig. 4.
Model building. (A, C) Comparison between the inactive GDP-bound and (B, D) active GTP/tRNA-bound structure of human mt EF-Tu. (A–B) 3D representation, with domain 1 in gold, domain 2 in blue, the mutation site in red, and domain 3 in silver. (Inset) Blow up of the EF-Tu GTP/tRNA 3D model around the mutation site. G322 and residues having their side-chain in close proximity to it are shown in a stick representation and labelled. (C–D) Contact maps (i.e. maps where each dot represents a spatial contact between two residues) as obtained by COCOMAPS between domain 1 (residues 1–253) and domains 2–3. G322 position in the maps is indicated by a dashed red line. (E) Sequence alignment between human and yeast mt EF-Tu and mt B. taurus (mitochondrial), E. coli, T. thermophilus and T. aquaticus EF-Tu, whose structures were used in the comparative modelling procedure. The reported numbering corresponds to human mt EF-Tu. Position of mutated G322 is shadowed in red; residues within 4 Å from the mutation site and/or participating in inter-domain salt-bridges next to it are shadowed in yellow (if belonging to domain 1) and blue (domain 2).
4. Discussion
Mitochondrial homeostasis is carried out by thousands of gene products derived from cytoplasmic protein synthesis and by 13 proteins translated from mtDNA genes. Mitochondrial–specific translation includes at least 27 nucleus-encoded factors that have been related to different mitochondrial diseases [25]. In 2007, the first mutation in TUFM, a p.R339Q substitution, was identified in a patient affected by severe infantile macrocystic leukodystrophy with micropolygyria [13]. Two additional cases were later reported [14], [15], for which no detailed clinical and neuroradiological features, as well no functional and biochemical analysis were included.
Here we report on a new pathological variant in TUFM, identified in a baby girl with an overlapping phenotype to the first patient reported [13]. Both of these patients showed metabolic acidosis at birth and hyperlactacidemia. Neurological examination revealed severe encephalopathy and leukodystrophy with micropolygyria. Moreover, these two children died around one year of age. We used targeted MitoExome analysis to characterize the genetic defect of our patient and identified a novel c.964G>A mutation in the TUFM gene, changing the evolutionary conserved mt EF-Tu G322 amino acid residue to R322. This mutation produces a drastic reduction of the protein (around 80%) and, as expected for a mitochondrial translation defect, we observed a multiple reduction of MRC activities, associated to markedly decreased mtDNA translation and reduced amount of several subunits of mtDNA-dependent MRC complexes.
We also studied the pathological role of this mutation in vivo by complementation experiments using Saccharomyces cerevisiae. In yeast, the presence of TUF1-G311R, which is equivalent to the human TUFM-G322R mutation, was shown to affect the EF-Tu activity, by impairing mitochondrial functionality when expressed by a centromeric (low copy number) plasmid. However, high amount of the mutated protein (produced by high copy number plasmid) was shown to rescue the defective phenotype, probably due to a higher amount of protein with a residual functional activity.
Analysis of 3D models we built for human mt EF-Tu suggests that the G322R mutation can be sterically accommodated in the GDP-bound form of the protein, although with a possible electrostatic destabilization consequent to the introduction of the positive charge. However, the mutation clearly destabilizes the GTP/tRNA bound, active form. In this ‘compact’ form it is indeed impossible to accommodate a bulky arginine at position 322 without affecting the physiological interaction between domains 1 and 2, thus interfering with the network of H-bonds and salt-bridges, which maintain the required inter-domain orientation. Thus, the G322R mutation could prevent or hamper the binding of mutant EF-Tu to the tRNAs and their delivery to the ribosome for the protein synthesis. Remarkably, the mutation (R339Q) previously reported in TUFM [13] was also located in domain 2 of the protein and was proven to hamper the formation of the GTP:EF-Tu:aminoacyl-tRNA ternary complex [26]. In vitro experiments indeed showed the R339Q mutant to be inactive in mitochondrial polypeptide chain elongation, as a result of its inability to bind mitochondrial aa-tRNAs. Whereas in vivo characterization of the equivalent mutation in yeast has shown that the activity of EF-Tu was not impaired [27].
Taken together, experimental data reported in this manuscript and obtained by investigation of human mutant cells and the yeast model, assisted by in silico predictions from 3D structural modeling, indicate that the G322R mutation leads to combined destabilization and partial loss of mtEF-Tu function, which explains the severe neurological and metabolic features found in our patient.
5. Conclusions
Of the four patients who have been associated to mutations in TUFM, only the first one described by Valente [13] and the current patient, have detailed clinical, neuroimaging and biochemical functional reports. Clinical and neuroimaging findings are particularly important because allow us to increase awareness favoring a candidate gene approach to address and validate molecular genetic assessment for variants in TUFM. Future work is warranted to explain the exquisite sensitivity of the brain to impaired EF-Tu and the consistent clinical and neuropathological features found in EF-Tu mutant patients.
The following are the supplementary data related to this article.
Supplementary material
Disclosure statement
The authors declare that there is no conflict of interest.
Funding sources
This work was supported by the Telethon Grant [GGP11011]; the Italian Ministry of Health [GR2010–2316392]; and A. M. is currently funded by Pasteur Institute - Cenci Bolognetti Foundation (Project Under 40, 2015-2017).
Transparency Document
Transparency document.
Acknowledgments
The authors acknowledge the Italian Association of Mitochondrial Disease Patients and Families (Mitocon).
Footnotes
The Transparency document associated with this article can be found, in online version.
References
- 1.Greaves L.C., Reeve A.K., Taylor R.W., Turnbull D.M. Mitochondrial DNA and disease. J. Pathol. 2012;226:274–286. doi: 10.1002/path.3028. [DOI] [PubMed] [Google Scholar]
- 2.Smits P., Smeitink J., van den Heuvel L. Mitochondrial translation and beyond: processes implicated in combined oxidative phosphorylation deficiencies. J. Biomed. Biotechnol. 2010;2010:737385. doi: 10.1155/2010/737385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chrzanowska-Lightowlers Z.M., Horvath R., Lightowlers R.N. 175th ENMC International Workshop: mitochondrial protein synthesis in health and disease. Neuromuscul. Disord. 2011;21:142–147. doi: 10.1016/j.nmd.2010.10.005. [DOI] [PubMed] [Google Scholar]
- 4.Rötig A. Human diseases with impaired mitochondrial protein synthesis. Biochim. Biophys. Acta. 1807;2011:1198–1205. doi: 10.1016/j.bbabio.2011.06.010. [DOI] [PubMed] [Google Scholar]
- 5.Vafai S.B., Mootha V.K. Mitochondrial disorders as windows into an ancient organelle. Nature. 2012;491:374–383. doi: 10.1038/nature11707. [DOI] [PubMed] [Google Scholar]
- 6.Christian B.E., Spremulli L.L. Mechanism of protein biosynthesis in mammalian mitochondria. Biochim. Biophys. Acta. 1819;2012:1035–1054. doi: 10.1016/j.bbagrm.2011.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boczonadi V., Horvath R. Mitochondria: impaired mitochondrial translation in human disease. Int. J. Biochem. Cell Biol. 2014;48:77–84. doi: 10.1016/j.biocel.2013.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Diodato D., Ghezzi D., Tiranti V. The mitochondrial aminoacyl tRNA synthetases: genes and syndromes. Int. J. Cell Biol. 2014;2014:787956. doi: 10.1155/2014/787956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bosch L., Kraal B., Van der Meide P.H., Duisterwinkel F.J., Van Noort J.M. The elongation factor EF-Tu and its two encoding genes. Prog. Nucleic Acid Res. Mol. Biol. 1983;30:91–126. doi: 10.1016/s0079-6603(08)60684-4. [DOI] [PubMed] [Google Scholar]
- 10.Montanari A., Zhou Y.F., D'Orsi M.F., Bolotin-Fukuhara M., Frontali L., Francisci S. Analyzing the suppression of respiratory defects in the yeast model of human mitochondrial tRNA diseases. Gene. 2013;527:1–9. doi: 10.1016/j.gene.2013.05.042. [DOI] [PubMed] [Google Scholar]
- 11.Nagao A., Suzuki T., Suzuki T. Aminoacyl-tRNA surveillance by EF-Tu in mammalian mitochondria. Nucleic Acids Symp. Ser. (Oxf.) 2007;51:41–42. doi: 10.1093/nass/nrm021. [DOI] [PubMed] [Google Scholar]
- 12.Suzuki H., Ueda T., Taguchi H., Takeuchi N. Chaperone properties of mammalian mitochondrial translation elongation factor Tu. J. Biol. Chem. 2007;282:4076–4084. doi: 10.1074/jbc.M608187200. [DOI] [PubMed] [Google Scholar]
- 13.Valente L., Tiranti V., Marsano R.M. Infantile encephalopathy and defective mitochondrial DNA translation in patients with mutations of mitochondrial elongation factors EFG1 and EF-Tu. Am. J. Hum. Genet. 2007;80:44–58. doi: 10.1086/510559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wortmann S.B., Koolen D.A., Smeitink J.A., van den Heuvel L., Rodenburg R.J. Whole exome sequencing of suspected mitochondrial patients in clinical practice. J. Inherit. Metab. Dis. 2015;38:437–443. doi: 10.1007/s10545-015-9823-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kohda M., Tokuzawa Y., Kishita Y. A comprehensive genomic analysis reveals the genetic landscape of mitochondrial respiratory chain complex deficiencies. PLoS Genet. 2016;12 doi: 10.1371/journal.pgen.1005679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Calvo S.E., Compton A.G., Hershman S.G. Molecular diagnosis of infantile mitochondrial disease with targeted next-generation sequencing. Sci. Transl. Med. 2012;4 doi: 10.1126/scitranslmed.3003310. (118ra10) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bugiani M., Invernizzi F., Alberio S. Clinical and molecular findings in children with complex I deficiency. Biochim. Biophys. Acta. 1659;2004:136–147. doi: 10.1016/j.bbabio.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 18.Rizza T., Vazquez-Memije M.E., Meschini M.C. Assaying ATP synthesis in cultured cells: a valuable tool for the diagnosis of patients with mitochondrial disorders. Biochem. Biophys. Res. Commun. 2009;383:58–62. doi: 10.1016/j.bbrc.2009.03.121. [DOI] [PubMed] [Google Scholar]
- 19.Chomyn A. In vivo labeling and analysis of human mitochondrial translation product. Methods Enzymol. 1996;264:197–211. doi: 10.1016/s0076-6879(96)64020-8. [DOI] [PubMed] [Google Scholar]
- 20.Fernández-Silva P., Acín-Pérez R., Fernández-Vizarra E., Pérez-Martos A., Enriquez J.A. In vivo and in organello analyses of mitochondrial translation. Methods Cell Biol. 2007;80:571–588. doi: 10.1016/S0091-679X(06)80028-2. [DOI] [PubMed] [Google Scholar]
- 21.Mulero J.J., Fox T.D. Alteration of the Saccharomyces cerevisiae COX2 mRNA 5′-untranslated leader by mitochondrial gene replacement and functional interaction with the translational activator protein PET111. Mol. Biol. Cell. 1993;4:1327–1335. doi: 10.1091/mbc.4.12.1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sali A., Blundell T.L. Comparative protein modelling by satisfaction of spatial restraints. J. Mol. Biol. 1993;234:779–815. doi: 10.1006/jmbi.1993.1626. [DOI] [PubMed] [Google Scholar]
- 23.Vangone A., Spinelli R., Scarano V., Cavallo L., Oliva R. COCOMAPS: a web application to analyze and visualize contacts at the interface of biomolecular complexes. Bioinformatics. 2011;27:2915–2926. doi: 10.1093/bioinformatics/btr484. [DOI] [PubMed] [Google Scholar]
- 24.Kjeldgaard M., Nissen P., Thirup S., Nyborg J. The crystal structure of elongation factor EF-Tu from Thermus aquaticus in the GTP conformation. Structure. 1993;1:35–50. doi: 10.1016/0969-2126(93)90007-4. [DOI] [PubMed] [Google Scholar]
- 25.Hällberg B.M., Larsson N.G. Making proteins in the powerhouse. Cell Metab. 2014;20:226–240. doi: 10.1016/j.cmet.2014.07.001. [DOI] [PubMed] [Google Scholar]
- 26.Valente L., Shigi N., Suzuki T., Zeviani M. The R336Q mutation in human mitochondrial EFTu prevents the formation of an active mt-EFTu.GTP.aa-tRNA ternary complex. Biochim. Biophys. Acta. 2009;1792:791–795. doi: 10.1016/j.bbadis.2009.06.002. [DOI] [PubMed] [Google Scholar]
- 27.Akama K., Christian B.E., Jones C.N., Ueda T., Takeuchi N., Spremulli L.L. Analysis of the functional consequences of lethal mutations in mitochondrial translational elongation factors. Biochim. Biophys. Acta. 1802;2010:692–708. doi: 10.1016/j.bbadis.2010.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material
Transparency document.