Eosinophilic esophagitis (EoE) is an emerging chronic allergic inflammatory disorder that selectively affects the esophagus and is characterized clinically by symptoms of esophageal dysfunction, including vomiting and dysphagia.1 Molecularly, EoE is characterized by a TH2 immune response based on increased expression of the cytokines IL-4, IL-5, and IL-13.2 IL-13 promotes immune cell infiltration by inducing production of chemokines, such as CCL26 (eotaxin-3),3 and by impairing epithelial barrier function through reduced expression of the epithelial adhesion molecule desmoglein 14 and overexpression of the protease calpain 14,5 which is encoded by the CAPN14 gene at chromosome 2p23 and is a major susceptibility genetic locus for EoE.6,7 Patients with EoE often present with comorbid conditions, including asthma and atopic dermatitis,1 which also involve dysregulated TH2 responses and impaired barrier function. The similarity of the pathogeneses of these disorders is evidenced by the significant degree of shared genetic associations that exists among patients with these allergic disorders, including the overabundance of genetic variants in the thymic stromal lymphopoietin (TSLP)8,9 and filaggrin (FLG) loci.10
There is a critical need to elucidate the factors that initiate and propagate the TH2 responses present in patients with EoE. The disease is considered to be driven by food antigens because strict elimination diets cause complete remission of the disease11 and experimental EoE can be induced in mice through allergen exposure.12 Currently, the route of sensitization to these food antigens is not clear. It is notable that many patients with EoE are sensitized to aeroallergens13 and that there are seasonal variations in the clinical symptoms of patients with EoE.14 Additionally, experimental murine studies indicate that EoE-like disease can be induced by allergen challenge after epicutaneous15 or respiratory12 sensitization. However, after sensitization occurs, it remains unclear how the TH2 immune response is initiated and propagated upon re-exposure to food antigens, although this likely includes activation of innate immune cells, including antigen-presenting cells, by epithelium-derived, TH2-promoting innate cytokines. Notably, esophageal TSLP is expressed at increased levels in patients with EoE16,17 and activates dendritic cells18 and basophils19 to induce TH2 polarization of CD41 T cells. Additionally, Noti et al16 showed a critical role for TSLP in the pathogenesis of EoE using an experimental mouse model that induces eosinophilic infiltration and TH2 cytokine production in the esophagus after epicutaneous sensitization and repeated intragastric challenge with the food antigen ovalbumin. Genetic deficiency in TSLP or its receptor prevented induction of disease in this model, and administering TSLP-neutralizing antibodies after establishing esophageal eosinophilia reversed the features of the disease. Preliminary evidence was presented that basophils were the critical target of TSLP because depleting basophils phenocopied loss of TSLP, although the definitive experiment of adoptive transfer of wild-type and TSLP receptor–deficient basophils was not performed.
There has been much recent interest in investigating whether another epithelium-derived, TH2-promoting innate cytokine, IL-33, could serve as an additional signal to initiate and propagate the TH2 response in patients with EoE. IL-33 is an alarmin that is present during homeostasis in the nuclei of epithelial cells20,21 and is released during necrosis or cellular damage.22 Upon extracellular release, it can activate a wide variety of immune cells that express its receptor, ST2, including type 2 innate lymphoid cells,23 CD41 T cells,24 eosinophils,25 mast cells,26 and basophils.26 IL-33 and ST2 are required for induction of allergic asthma in murine models.27,28 Two recent studies have shown that esophageal biopsy specimens from patients with EoE express higher levels of IL-33 protein than those from control subjects.17,29 Additionally, there is association between genetic variants in the IL33 locus and disease risk.6 Finally, Judd et al29 showed that intraperitoneal injection of recombinant IL-33 induces esophageal eosinophilia, epithelial hyperplasia, and production of TH2-associated cytokines.
In this issue of the Journal, Venturelli et al30 provide evidence of a requirement for IL-33 in the pathogenesis of experimental murine EoE-like disease. Eosinophil infiltration and TH2-associated cytokine production were induced in the esophagus by means of repeated intranasal challenge with ovalbumin after epicutaneous sensitization by disrupting the skin epithelial barrier through tape-stripping or genetic deficiency of FLG. Disrupting the skin epithelial barrier was required because allergic sensitization did not occur in wild-type mice without tape-stripping of the skin. IL-33 was a critical mediator in this model because esophageal symptoms were not present in mice either genetically deficient in ST2 or given ST2-neutralizing antibodies. Furthermore, genetic deficiency in both FLG and ST2 prevented esophageal symptoms. Basophil depletion prevented disease induction, and esophageal symptoms were restored on adoptive transfer of wild-type, but not ST2-deficient, basophils. This observation supports that basophils were the target of IL-33, at least in part. Collectively, the data show that IL-33–ST2 interactions in basophils and impaired skin epithelial barrier integrity mediate induction of EoE-like disease induced by epicutaneous allergen sensitization (summarized in Fig 1).
FIG 1.
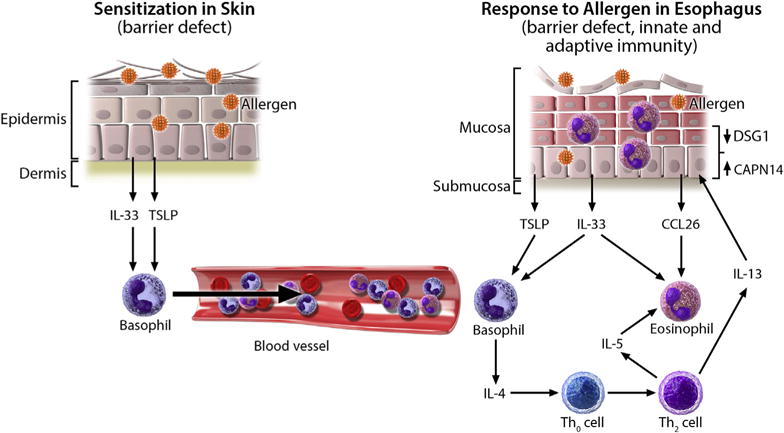
Proposed model for the development of allergic inflammation in patients with EoE. Impaired skin barrier integrity, such as that caused by injury or FLG mutations, allows for increased allergen penetration through the epithelium, causing allergic sensitization and release of IL-33 and TSLP from the epithelium. Secondary to activation by these cytokines, there is increased trafficking of basophils through the bloodstream to the esophagus. Later, direct exposure of allergens to the esophageal epithelium leads to the release of TSLP and IL-33, which induce IL-4 production from basophils and subsequent release of IL-5 and IL-13 from TH2 cells. IL-13 causes esophageal epithelial barrier impairment and increased allergen exposure by decreasing desmoglein 1 (DSG1) and increasing calpain 14 (CAPN14) expression. Additionally, IL-13 induces CCL26 production from the epithelium, which, in combination with IL-5, causes eosinophil infiltration into the esophagus.
The work presented in this article builds on the aforementioned work by Noti et al16 showing the requirement of basophils and TSLP for induction of EoE-like disease in mice, which used a different model of allergen challenge (intragastric vs intranasal). Although this work provides strong evidence for the importance of basophils in 2 different experimental mouse models, concerns remain with regard to translating these findings to human disease. Although Noti et al16 show accumulation of basophils in human biopsy specimens, this finding has not been independently confirmed by others, including Venturelli et al.30 In contrast, esophageal biopsy specimens from patients with active EoE are notable for infiltration of mast cells and eosinophils,31 which are both potently activated by IL-33. Therefore, it is likely that IL-33 activating these cells also contributes to the human disease. Additionally, the experimental antigen exposure is of supraphysiologic doses of a single antigen with one route of sensitization, whereas the human disease likely involves multiple allergens at more physiologic exposure doses and with several routes of sensitization. Finally, the mechanism through which skin sensitization leads to esophageal priming, whether involving basophils or not, remains unclear.
In summary, this study identifies that epicutaneous sensitization promotes induction of EoE-like disease in mice through a mechanism that involves IL-33/ST2–mediated activation of basophils. This study further supports the view that deficiency of FLG predisposes patients to EoE and suggests that blocking the IL-33–ST2 axis and protecting or enhancing the skin barrier would be of therapeutic value for treating EoE. It will also be of interest to apply these results directly to the esophagus because esophageal epithelial barrier function in patients with EoE is impaired, likely through IL-13–mediated dysregulation of calpain 145,6 and desmoglein 1.4
Acknowledgments
Supported by National Institutes of Health grants R37 AI045898, R01 AI124355, and U19 AI070235; the Campaign Urging Research for Eosinophilic Disease (CURED); the Buckeye Foundation; and the Sunshine Charitable Foundation and its supporters, Denise A. Bunning and David G. Bunning
We thank Shawna Hottinger for editorial assistance.
Footnotes
Disclosure of potential conflict of interest: M. E. Rothenberg receives grant support from the National Institutes of Health; consulting fees from Receptos Immune Pharmaceuticals, NKT Therapeutics, and PulmOne Therapeutics; patents with Miraca Life Science; royalties from Teva Pharmaceuticals; and stock options from PulmOne, NKT Therapeutics, and Immune Pharmaceuticals. The rest of the authors declare that they have no relevant conflicts of interest.
References
- 1.Davis BP, Rothenberg ME. Mechanisms of disease of eosinophilic esophagitis. Annu Rev Pathol. 2016;11:365–93. doi: 10.1146/annurev-pathol-012615-044241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blanchard C, Mingler MK, Vicario M, Abonia JP, Wu YY, Lu TX, et al. IL-13 involvement in eosinophilic esophagitis: transcriptome analysis and reversibility with glucocorticoids. J Allergy Clin Immunol. 2007;120:1292–300. doi: 10.1016/j.jaci.2007.10.024. [DOI] [PubMed] [Google Scholar]
- 3.Blanchard C, Wang N, Stringer KF, Mishra A, Fulkerson PC, Abonia JP, et al. Eo-taxin-3 and a uniquely conserved gene-expression profile in eosinophilic esophagi-tis. J Clin Invest. 2006;116:536–47. doi: 10.1172/JCI26679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sherrill JD, Kc K, Wu D, Djukic Z, Caldwell JM, Stucke EM, et al. Desmoglein-1 regulates esophageal epithelial barrier function and immune responses in eosinophilic esophagitis. Mucosal Immunol. 2014;7:718–29. doi: 10.1038/mi.2013.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Davis BP, Stucke EM, Khorki ME, Litosh VA, Rymer JK, Rochman M, et al. Eosinophilic esophagitis-linked calpain 14 is an IL-13-induced protease that mediates esophageal epithelial barrier impairment. JCI Insight. 2016;1:e86355. doi: 10.1172/jci.insight.86355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kottyan LC, Davis BP, Sherrill JD, Liu K, Rochman M, Kaufman K, et al. Genome-wide association analysis of eosinophilic esophagitis provides insight into the tissue specificity of this allergic disease. Nat Genet. 2014;46:895–900. doi: 10.1038/ng.3033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sleiman PM, Wang ML, Cianferoni A, Aceves S, Gonsalves N, Nadeau K, et al. GWAS identifies four novel eosinophilic esophagitis loci. Nat Commun. 2014;5:5593. doi: 10.1038/ncomms6593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rothenberg ME, Spergel JM, Sherrill JD, Annaiah K, Martin LJ, Cianferoni A, et al. Common variants at 5q22 associate with pediatric eosinophilic esophagitis. Nat Genet. 2010;42:289–91. doi: 10.1038/ng.547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sherrill JD, Gao PS, Stucke EM, Blanchard C, Collins MH, Putnam PE, et al. Variants of thymic stromal lymphopoietin and its receptor associate with eosinophilic esophagitis. J Allergy Clin Immunol. 2010;126:160–5.e3. doi: 10.1016/j.jaci.2010.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Blanchard C, Stucke EM, Burwinkel K, Caldwell JM, Collins MH, Ahrens A, et al. Coordinate interaction between IL-13 and epithelial differentiation cluster genes in eosinophilic esophagitis. J Immunol. 2010;184:4033–41. doi: 10.4049/jimmunol.0903069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Davis BP, Rothenberg ME. Emerging concepts of dietary therapy for pediatric and adult eosinophilic esophagitis. Exp Rev Clin Immunol. 2013;9:285–7. doi: 10.1586/eci.13.15. [DOI] [PubMed] [Google Scholar]
- 12.Mishra A, Schlotman J, Wang M, Rothenberg ME. Critical role for adaptive T cell immunity in experimental eosinophilic esophagitis in mice. J Leuk Biol. 2007;81:916–24. doi: 10.1189/jlb.1106653. [DOI] [PubMed] [Google Scholar]
- 13.Aceves SS. Food and aeroallergens in eosinophilic esophagitis: role of the allergist in patient management. Curr Opin Gastroenterol. 2014;30:391–5. doi: 10.1097/MOG.0000000000000088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Almansa C, Krishna M, Buchner AM, Ghabril MS, Talley N, Devault KR, et al. Seasonal distribution in newly diagnosed cases of eosinophilic esophagitis in adults. Am J Gastroenterol. 2009;104:828–33. doi: 10.1038/ajg.2008.169. [DOI] [PubMed] [Google Scholar]
- 15.Akei HS, Mishra A, Blanchard C, Rothenberg ME. Epicutaneous antigen exposure primes for experimental eosinophilic esophagitis in mice. Gastroenterology. 2005;129:985–94. doi: 10.1053/j.gastro.2005.06.027. [DOI] [PubMed] [Google Scholar]
- 16.Noti M, Wojno ED, Kim BS, Siracusa MC, Giacomin PR, Nair MG, et al. Thymic stromal lymphopoietin-elicited basophil responses promote eosinophilic esophagitis. Nat Med. 2013;19:1005–13. doi: 10.1038/nm.3281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Simon D, Radonjic-Hosli S, Straumann A, Yousefi S, Simon HU. Active eosinophilic esophagitis is characterized by epithelial barrier defects and eosinophil extracellular trap formation. Allergy. 2015;70:443–52. doi: 10.1111/all.12570. [DOI] [PubMed] [Google Scholar]
- 18.Ziegler SF, Roan F, Bell BD, Stoklasek TA, Kitajima M, Han H. The biology of thymic stromal lymphopoietin (TSLP) Adv Pharmacol. 2013;66:129–55. doi: 10.1016/B978-0-12-404717-4.00004-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Siracusa MC, Saenz SA, Hill DA, Kim BS, Headley MB, Doering TA, et al. TSLP promotes interleukin-3-independent basophil haematopoiesis and type 2 inflammation. Nature. 2011;477:229–33. doi: 10.1038/nature10329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Carriere V, Roussel L, Ortega N, Lacorre DA, Americh L, Aguilar L, et al. IL-33, the IL-1-like cytokine ligand for ST2 receptor, is a chromatin-associated nuclear factor in vivo. Proc Natl Acad Sci U S A. 2007;104:282–7. doi: 10.1073/pnas.0606854104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moussion C, Ortega N, Girard JP. The IL-1-like cytokine IL-33 is constitutively expressed in the nucleus of endothelial cells and epithelial cells in vivo: a novel ʻalarminʼ? PLoS One. 2008;3:e3331. doi: 10.1371/journal.pone.0003331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhao W, Hu Z. The enigmatic processing and secretion of interleukin-33. Cell Mol Immunol. 2010;7:260–2. doi: 10.1038/cmi.2010.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Neill DR, Wong SH, Bellosi A, Flynn RJ, Daly M, Langford TK, et al. Nuocytes represent a new innate effector leukocyte that mediates type-2 immunity. Nature. 2010;464:1367–70. doi: 10.1038/nature08900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Peine M, Marek RM, Lohning M. IL-33 in T cell differentiation, function, and immune homeostasis. Trends Immunol. 2016;37:321–33. doi: 10.1016/j.it.2016.03.007. [DOI] [PubMed] [Google Scholar]
- 25.Bouffi C, Rochman M, Zust CB, Stucke EM, Kartashov A, Fulkerson PC, et al. IL-33 markedly activates murine eosinophils by an NF-kappaB-dependent mechanism differentially dependent upon an IL-4-driven autoinflammatory loop. J Immunol. 2013;191:4317–25. doi: 10.4049/jimmunol.1301465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Saluja R, Ketelaar ME, Hawro T, Church MK, Maurer M, Nawijn MC. The role of the IL-33/IL-1RL1 axis in mast cell and basophil activation in allergic disorders. Mol Immunol. 2015;63:80–5. doi: 10.1016/j.molimm.2014.06.018. [DOI] [PubMed] [Google Scholar]
- 27.Lee HY, Rhee CK, Kang JY, Byun JH, Choi JY, Kim SJ, et al. Blockade of IL-33/ST2 ameliorates airway inflammation in a murine model of allergic asthma. Exp Lung Res. 2014;40:66–76. doi: 10.3109/01902148.2013.870261. [DOI] [PubMed] [Google Scholar]
- 28.Zoltowska AM, Lei Y, Fuchs B, Rask C, Adner M, Nilsson GP. The interleukin-33 receptor ST2 is important for the development of peripheral airway hyperresponsiveness and inflammation in a house dust mite mouse model of asthma. Clin Exp Allergy. 2016;46:479–90. doi: 10.1111/cea.12683. [DOI] [PubMed] [Google Scholar]
- 29.Judd LM, Heine RG, Menheniott TR, Buzzelli J, O’Brien-Simpson N, Pavlic D, et al. Elevated IL-33 expression is associated with pediatric eosinophilic esophagitis, and exogenous IL-33 promotes eosinophilic esophagitis development in mice. Am J Physiol Gastrointest Liver Physiol. 2016;310:G13–25. doi: 10.1152/ajpgi.00290.2015. [DOI] [PubMed] [Google Scholar]
- 30.Venturelli N, Lexmond WS, Ohsaki A, Nurko S, Karasuyama H, Fiebiger E, et al. Allergic skin sensitization promotes eosinophilic esophagitis through the IL-33-basophil axis in mice. J Allergy Clin Immunol. 2016;138:1367–80. doi: 10.1016/j.jaci.2016.02.034. [DOI] [PubMed] [Google Scholar]
- 31.Aceves SS, Chen D, Newbury RO, Dohil R, Bastian JF, Broide DH. Mast cells infiltrate the esophageal smooth muscle in patients with eosinophilic esophagitis, express TGF-b1, and increase esophageal smooth muscle contraction. J Allergy Clin Immunol. 2010;126:1198–204.e4. doi: 10.1016/j.jaci.2010.08.050. [DOI] [PubMed] [Google Scholar]


