Abstract
Hypophosphatemia causes rickets by impairing hypertrophic chondrocyte apoptosis. Phosphate induction of MEK1/2-ERK1/2 phosphorylation in hypertrophic chondrocytes is required for phosphate-mediated apoptosis and growth plate maturation. MEK1/2 can be activated by numerous molecules including Raf isoforms. A- and B-Raf ablation in chondrocytes does not alter skeletal development, whereas ablation of C-Raf decreases hypertrophic chondrocyte apoptosis and impairs vascularization of the growth plate. However, ablation of C-Raf does not impair phosphate-induced ERK1/2 phosphorylation in vitro, but leads to rickets by decreasing VEGF protein stability. To determine whether Raf isoforms are required for phosphate-induced hypertrophic chondrocyte apoptosis, mice lacking all three Raf isoforms in chondrocytes were generated. Raf deletion caused neonatal death and a significant expansion of the hypertrophic chondrocyte layer of the growth plate, accompanied by decreased cleaved caspase-9. This was associated with decreased phospho-ERK1/2 immunoreactivity in the hypertrophic chondrocyte layer and impaired vascular invasion. These data further demonstrated that Raf kinases are required for phosphate-induced ERK1/2 phosphorylation in cultured hypertrophic chondrocytes and perform essential, but partially redundant roles in growth plate maturation.
Keywords: apoptosis, caspase, chondrocyte, growth plate, Raf kinase, phosphate, rickets
Introduction
Longitudinal bone growth is dependent upon the tightly regulated proliferation and differentiation of growth plate chondrocytes. Resting chondrocytes differentiate into proliferating chondrocytes that further differentiate into pre-hypertrophic and hypertrophic chondrocytes (1). Although recent investigations demonstrate that 60% of osteocalcin-expressing osteoblasts in growing bone originate from Col10a1-Cre-expressing cells (2), it is not currently known what percentage of hypertrophic chondrocytes gives rise to osteoblasts versus undergo apoptosis. Hypertrophic chondrocytes secrete angiogenic factors that promote vascular invasion, undergo apoptosis, and are replaced by mineralized bone (3, 4). Studies in numerous mouse models demonstrate that hypophosphatemia impairs apoptosis of terminally differentiated hypertrophic chondrocytes, which results in rickets (5–7). These studies establish a critical role for serum phosphate in normal growth plate maturation.
Exposure of cultured hypertrophic chondrocytes to phosphate leads to ERK1/2 phosphorylation and activation of the mitochondrial apoptotic pathway. Blocking phosphate induction of ERK1/2 phosphorylation with the MEK1/2 inhibitor U0126 impairs hypertrophic chondrocyte apoptosis in vivo and in vitro, leading to expansion of the hypertrophic chondrocyte layer of the growth plate (8). MEK1/2 can be activated by numerous signaling pathways, including Raf kinases. In the growth plate, A-Raf and B-Raf are primarily expressed in proliferative chondrocytes. Ablation of either or both of these Raf isoforms does not significantly affect normal growth plate development (9). In contrast, C-Raf is predominantly expressed in the hypertrophic chondrocyte layer of the growth plate (10). Chondrocyte-specific ablation of C-Raf leads to expansion of the hypertrophic layer of the growth plate, associated with decreased hypertrophic chondrocyte apoptosis. However, C-Raf is not required for phosphate-induced ERK1/2 phosphorylation in cultured hypertrophic chondrocytes, but rather causes rickets by increasing ubiquitin-dependent VEGF protein degradation and impaired vascular invasion at the chondro-osseous junction (11). To define a role for Raf isoforms in normal growth plate maturation and phosphate-induced hypertrophic chondrocyte apoptosis, mice with chondrocyte-specific ablation of all three Raf isoforms were generated.
Results
Raf Isoforms Have Non-redundant Actions in Normal Growth Plate Maturation
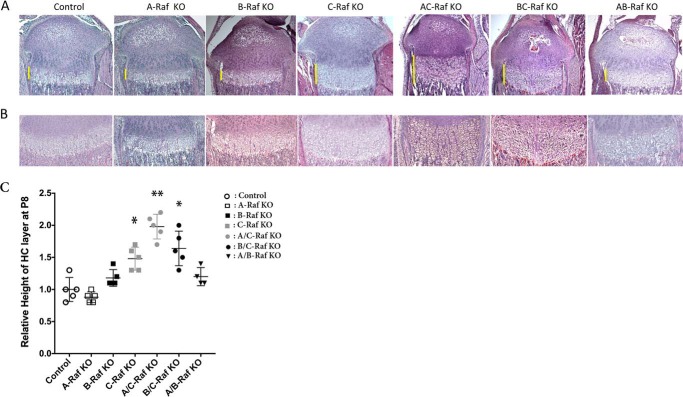
To generate mice with chondrocyte-specific ablation of B- and C-Raf, mice expressing the Col2a1-Cre transgene (12) were bred to mice with floxed B-Raf and C-Raf (B-Raffl/fl or C-Raffl/fl) alleles (13, 14). Offspring were bred to A-Raf−/y (A-Raf KO mice) (15) to obtain mice lacking all three Raf isoforms in chondrocytes. A-Raf−/y mice exhibit growth retardation and neurologic abnormalities after the second week of life; thus, postnatal phenotypes were analyzed on day 8 (P8).2 As reported previously, A-Raf−/y, B-Raff/f;Col2Cre, and C-Raff/f;Col2Cre mice were phenotypically normal at P8 (9, 11), as were mice lacking two of the three Raf isoforms (A/B-Raf, A/C-Raf, or B/C-Raf) in chondrocytes. Histological analyses revealed a normal tibial growth plate in A-Raf−/y mice, in B-Raff/f;Col2Cre mice, and in A-Raf−/y; B-Raff/f;Col2Cre mice (Fig. 1). As reported previously, C-Raff/f;Col2Cre mice exhibited an expansion of the hypertrophic chondrocyte layer of the growth plate (11) (Fig. 1). Chondrocyte-specific ablation of B-Raf did not alter the growth plate of the C-Raff/f;Col2Cre mice (Fig. 1); however, A-Raf−/y;C-Raff/f;Col2Cre mice exhibited an increase in the number of hypertrophic chondrocytes per column relative to that observed in the C-Raff/f;Col2Cre mice, suggesting non-redundant functions of A- and C-Raf in normal growth plate maturation (Fig. 1).
FIGURE 1.
Growth plate histology of single and compound Raf mutants. A and B, H&E staining was performed on tibiae of P8 mice: control, A-Raf KO (A-Raf−/y), B-Raf KO (B-Raff/f;Col2Cre), C-Raf KO (C-Raff/f;Col2Cre), A/C-Raf KO (A-Raf−/y;C-Raff/f;Col2Cre), B/C-Raf KO (B-Raff/f;Col2Cre; C-Raff/f;Col2Cre), and A/B-Raf KO (A-Raf−/y;B-Raff/f;Col2Cre). Data are representative of 4–5 mice per genotype. Bars indicate the extent of the hypertrophic chondrocyte layer. A, ×4 magnification. B, ×10 magnification. C, relative height of the hypertrophic chondrocyte layer of the growth plate in Raf KO versus control. Data are representative of 4–5 mice per genotype. Error bars indicate ± S.E. *, p < 0.02 versus control, **, p = 0.0023 versus C-Raf KO. HC, hypertrophic chondrocytes.
To determine whether the modest phenotype observed in the growth plate of mice lacking two of the three Raf isoforms was due to compensation by the remaining Raf isoform, mice lacking all three isoforms in chondrocytes were generated. Consistent with a critical role for Raf signaling in the growth plate, ablation of all three Raf isoforms led to a neonatal lethal phenotype, as did the presence of a single A-Raf allele in female mice. Thus, analyses of the growth plate were performed on embryonic day 18.5 (E18.5). Mice lacking all three Raf isoforms in chondrocytes (A-Raf−/Y; B-Raffl/fl; C-Raffl/fl;Col2Cre triple KO mice) exhibited significant limb abnormalities (Fig. 2A) relative to A-Raf+/Y; B-Raffl/fl; C-Raffl/fl Cre-negative littermates, as did females with a single A-Raf allele. Triple knock-out mice exhibited ribcage abnormalities and expansion of the metaphysis of the long bones (Fig. 2B) due to a marked expansion of the hypertrophic layer of the growth plate (Fig. 2C). Consistent with this, expansion of the ColX mRNA expressing the hypertrophic chondrocyte domain was observed in mice lacking all three Raf isoforms in chondrocytes (Fig. 2D). The femurs of the triple KO were 24% longer than those of control littermates (p = 0.02, n = 5), whereas tibiae, humeri, and skull parameters were not significantly different.
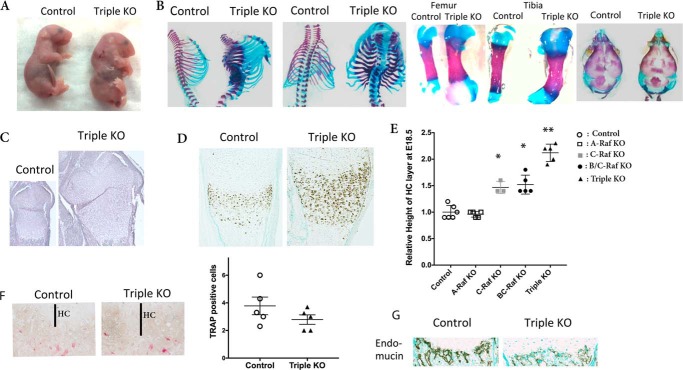
FIGURE 2.
Ablation of Raf isoforms in chondrocytes leads to expansion of the hypertrophic chondrocyte layer and impaired vascular invasion. A, E18.5 control and triple KO embryos (A-Raf−/y;B-Raff/f;Col2Cre;C-Raff/f;Col2Cre). B, skeletal preparations of the axial skeleton, femur, tibia, and skull of control and triple KO embryos. C, H&E staining of the growth plate of control and triple KO tibiae at E18.5. D, type X collagen in situ hybridization of control and triple KO tibiae at E18.5. E, relative height of the growth plate hypertrophic chondrocyte layer of Raf KO and control mice at E18.5. Error bars indicate ± S.E. *, p < 0.001 versus control, **, p = 0.0001 versus C-Raf KO. HC, hypertrophic chondrocytes. F, tartrate-resistant acid phosphatase (TRAP) staining of control and triple KO tibias and quantitation of number of osteoclasts at the chondro-osseous junction. Error bars indicate ± S.E. G, endomucin immunostaining on tibiae of E18.5 control and triple KO embryos. Data are representative of five mice per genotype.
As reported previously, C-Raff/f;Col2Cre mice exhibit an expansion of the hypertrophic chondrocyte layer at E18.5 relative to Cre-negative littermates (11). A-Raf−/y growth plates were similar to those of control mice, whereas the growth plates of mice lacking B/C-Raf exhibited a significant expansion of the hypertrophic layer (Fig. 2E) similar to that observed in C-Raf KO mice at E18.5.
Vascular invasion is required for normal growth plate maturation and hypertrophic chondrocyte apoptosis (16). Although ablation of all three Raf isoforms did not significantly alter osteoclast number at the chondro-osseous junction (Fig. 2F), E18.5 embryos lacking all three Raf isoforms in chondrocytes exhibited a marked reduction in vascular invasion based on the dramatic decrease in immunoreactivity for the endothelial marker, endomucin (Fig. 2G), relative to wild type littermates.
Raf Signaling in Chondrocytes Is Required for Hypertrophic Chondrocyte Apoptosis
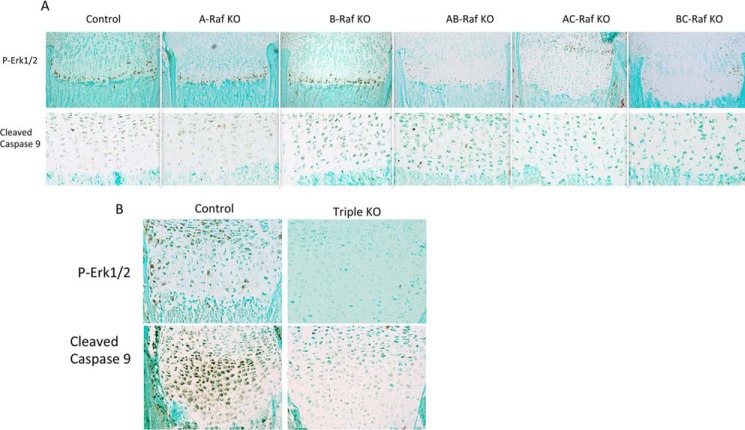
Raf kinases are known to induce phosphorylation of MEK1/2-ERK1/2, which is required for normal growth plate maturation. At P8, pERK1/2 and cleaved caspase-9 immunoreactivity were preserved in A-Raf−/y, B-Raff/f;Col2Cre, and A-Raf−/y; B-Raff/f;Col2Cre mice, correlating with lack of expansion of the hypertrophic chondrocyte layer of the growth plate (Fig. 3A). Consistent with the known effects of C-Raf ablation, pERK1/2 and cleaved caspase-9 immunoreactivity were decreased in growth plates of mice lacking C-Raf alleles (11). A similar decrease in pERK1/2 and caspase-9 immunoreactivity is seen in A-Raf−/y; C-Raff/f;Col2Cre and B-Raffl/fl; C-Raffl/fl;Col2Cre mice, correlating with the expansion of the hypertrophic chondrocyte layer observed relative to A-Raf+/+ and A-Raf+/y Cre-negative littermates (Fig. 3A). The growth plates of E18.5 mice lacking all three Raf isoforms in chondrocytes exhibited a dramatic decrease in pERK1/2 and cleaved caspase-9 immunoreactivity, demonstrating that impaired hypertrophic chondrocyte apoptosis underlies the marked expansion of the hypertrophic chondrocyte layer observed in the growth plates of these mice (Fig. 3B).
FIGURE 3.
Ablation of Raf isoforms in chondrocytes decreases pERK1/2 and impairs hypertrophic chondrocyte apoptosis. A and B, immunostaining for pERK1/2 and cleaved caspase-9 in the tibial metaphyses of P8 (A) and E18.5 (B) mice. Data are representative of five mice per genotype.
Raf Isoforms Regulate Proliferation of Growth Plate Chondrocytes
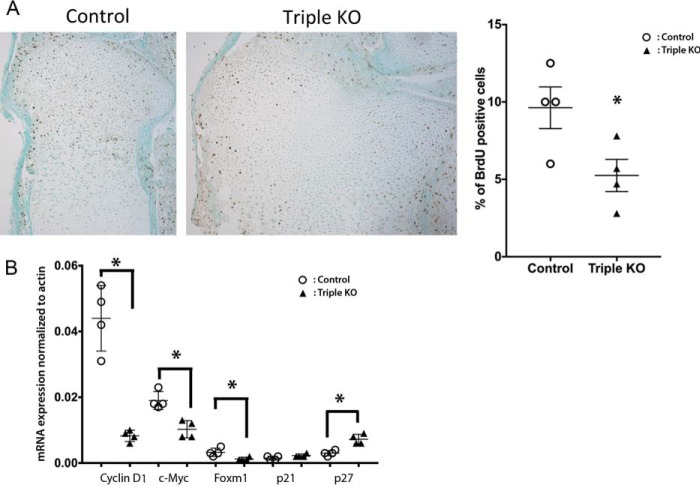
Constitutive activation of MEK1 in chondrocytes impairs proliferation (17). Thus, to determine whether enhanced proliferation contributes to growth plate expansion in mice lacking all three Raf isoforms in chondrocytes, chondrocyte proliferation was evaluated by BrdU incorporation. Mice lacking all three Raf isoforms in chondrocytes exhibited a 61% reduction in the percentage of BrdU-positive proliferative chondrocytes (Fig. 4A), in the setting of an 80 and 97% decrease in expression of B-Raf and C-Raf, respectively, evaluated by quantitative RT-PCR (A-Raf KO mice are a global knock-out). Thus, ablation of Raf isoforms decreases chondrocyte proliferation in the developing growth plate. Consistent with this, cyclin D1, c-Myc, and Foxm1 expression was decreased in primary proliferating chondrocytes from triple KO mice, whereas p27 was increased (Fig. 4B).
FIGURE 4.
Deletion of all three Raf isoforms impairs chondrocyte proliferation. A, BrdU immunostaining of growth plates of E18.5 control and triple KO embryos. Graph indicates the percentage of BrdU-positive chondrocytes in the proliferating layer of tibial growth plates (*, p = 0.04). Data are representative of four mice per genotype. B, evaluation of gene expression by quantitative RT-PCR. Data are representative of four mice per genotype. Error bars indicate ± S.E. *, p < 0.025.
Raf Isoforms Are Essential for Phosphate-induced ERK1/2 Phosphorylation in Hypertrophic Chondrocytes
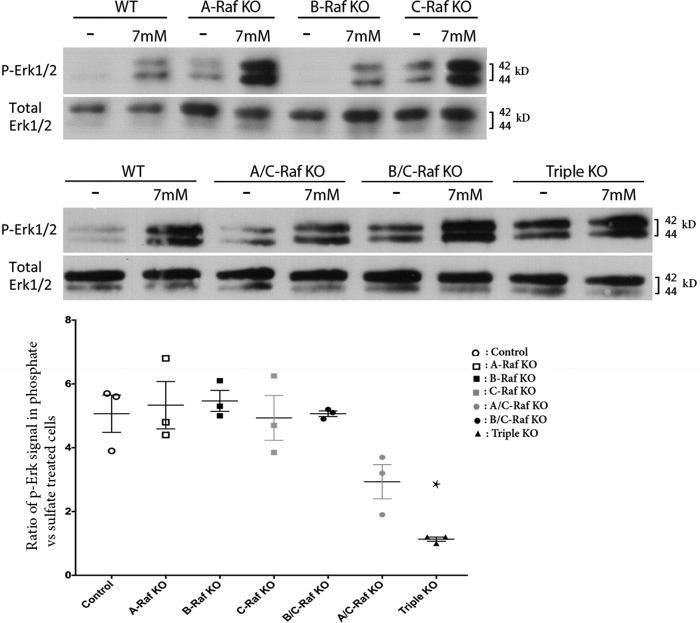
Because MEK1/2 activation of ERK1/2 phosphorylation is required for phosphate-mediated hypertrophic chondrocyte apoptosis (8), investigations were performed to determine whether phosphate activation of this signaling pathway requires Raf isoforms. Mice lacking C-Raf in chondrocytes exhibit a decrease in growth plate pERK1/2 immunoreactivity. However, ablation of C-Raf in chondrocytes does not abolish phosphate-mediated ERK1/2 phosphorylation in vitro, suggesting that C-Raf is not required for this process or that other Raf isoforms can compensate for the absence of C-Raf. Notable in this respect, the expression of A-Raf and B-Raf in hypertrophic chondrocytes is 7.6 and 42.7% that of C-Raf (11). To determine the contributions of Raf isoforms to phosphate-induced ERK1/2 phosphorylation, primary hypertrophic chondrocytes from mice lacking one or all three Raf isoforms, or from females with one A-Raf allele, were treated with sodium phosphate or sodium sulfate for 30 min. The absence of A-Raf, B-Raf, or C-Raf did not impair phosphate-induced ERK1/2 phosphorylation (Fig. 5). However, studies in chondrocytes isolated from mice lacking all three Raf isoforms demonstrated dramatic impairment in phosphate-induced ERK1/2 phosphorylation. Similar findings were observed in chondrocytes from females with a single A-Raf allele (supplemental Fig. 1), the latter of which exhibit a phenotype similar to mice lacking all Raf isoforms in chondrocytes (supplemental Fig. 1). These data define a critical role for Raf kinases in phosphate-induced ERK1/2 phosphorylation and normal growth plate maturation.
FIGURE 5.
Raf isoforms are essential for phosphate-induced ERK1/2 phosphorylation. Hypertrophic chondrocytes were incubated with 7 mm sodium sulfate (−) or sodium phosphate (+) for 30 min prior to Western analysis of whole cell extracts for phosphorylated (P) and total ERK1/2. Data are representative of three independent chondrocyte preparations per genotype. The graph shows the ratio of pERK1/2 signal in the Western blots of phosphate- versus sulfate-treated chondrocytes, normalized for total ERK1/2 in the same sample, as quantitated by ImageJ64 software. *, p = 0.0012 versus control.
Discussion
Phosphate and the MEK1/2-ERK1/2 signaling pathway have been shown to play a critical role in normal growth plate maturation (8). The current studies demonstrate an essential role for Raf kinases in phosphate-induced ERK1/2 phosphorylation, hypertrophic chondrocyte apoptosis, and normal plate maturation. Global ablation of B-Raf or C-Raf isoforms causes embryonic lethality in mice (18, 19); therefore, mice with chondrocyte-specific B-Raf and C-Raf ablation were used to investigate the role of B-Raf and C-Raf in phosphate-mediated growth plate maturation. Despite 20% residual mRNA expression of B-Raf and 3% residual expression of C-Raf in chondrocytes, these studies demonstrate a critical role for Raf isoforms in growth plate maturation, phosphate-induced ERK1/2 phosphorylation, and hypertrophic chondrocyte apoptosis.
Previous investigations have shown an important role for C-Raf in stabilizing VEGF protein in chondrocytes (11). Although mice with global ablation of A-Raf do not exhibit a growth plate phenotype, mice lacking both A- and C-Raf in chondrocytes exhibit a more significant expansion of the hypertrophic chondrocyte layer of the growth plate than do mice with chondrocyte-specific C-Raf ablation, suggesting that A- and C-Raf exert non-redundant functions in chondrocytes. In contrast, the absence of B-Raf in chondrocytes does not alter the growth plate phenotype of mice lacking either A-Raf or C-Raf. However, a non-redundant function for B-Raf is evident from the severe phenotype observed in mice lacking all three Raf isoforms in chondrocytes. Consistent with the essential role of Raf isoforms in normal growth plate maturation, males lacking all three Raf isoforms in chondrocytes and females with only one A-Raf allele exhibit neonatal lethality, in the setting of significant, but incomplete ablation of B- and C-Raf. The inability of a single A-Raf allele to prevent neonatal lethality in female mice suggests that X-inactivation may decrease the expression of A-Raf from the remaining allele. Consistent with this, mRNA levels of A-Raf in the chondrocytes of these females is <30% of that seen in control.
The phenotype of mice lacking all three Raf isoforms in chondrocytes is analogous to that seen in mice lacking both Erk1 and Erk2 in chondrocytes (20, 21). Col2a1-Cre-mediated ablation of Erk2 in Erk1 knock-out mice results in expansion of the terminally differentiated hypertrophic chondrocyte layer of the growth plate and neonatal lethality. Neither cleaved caspase-9 nor TUNEL staining was evaluated in these mice. However, analogous to the current studies, an increase in length of the long bones was observed, primarily due to expansion of the epiphysis (21). Similar to mice lacking all three Raf isoforms in chondrocytes, this was associated with a decrease in chondrocyte proliferation (20). Analogous to our findings with Raf isoform ablation, mice expressing a single Erk allele had a significantly milder phenotype than those with ablation of all four alleles. Osx-Cre-mediated Erk2 ablation in the hypertrophic chondrocytes and osteoblasts of Erk1 null mice also leads to a marked expansion of the hypertrophic chondrocyte layer of the growth plate and is accompanied by impaired terminal chondrocyte differentiation (22). Paradoxically, diffuse TUNEL staining in the hypertrophic chondrocyte layer of the growth plate is observed, in contrast to the TUNEL staining present in the most differentiated hypertrophic chondrocytes in normal mice. Of note, in these studies, mediated recombination resulted in a 30% reduction of Erk2, and did not lead to neonatal lethality.
The epidermal growth factor receptor (EGFR) also plays a critical role in growth plate development. Blocking EGFR signaling leads to expansion of the hypertrophic chondrocyte layer of the growth plate without altering chondrocyte proliferation, differentiation, or vascular invasion. Rather, a dramatic decrease in chondrocyte MMPs and osteoclast recruitment at the chondro-osseous junction is observed (23). A similar impairment in MMP expression and osteoclast recruitment is observed in mice lacking TGFα (24). However, ablation of Raf isoforms does not impair osteoclast recruitment (Fig. 2F) or chondrocyte MMP13 expression (data not shown).
VEGF signaling is also required for normal growth plate maturation and hypertrophic chondrocyte apoptosis (16). C-Raff/f;Col2Cre mice exhibit expansion of the hypertrophic chondrocyte layer associated with impaired vascular invasion due to increased ubiquitin-dependent degradation of VEGF protein (11). Impaired vascular invasion was also observed in mice lacking all three Raf isoforms in chondrocytes and in Erk1 null mice with chondrocyte-specific Erk2 ablation (21). However, in contrast to mice with chondrocyte-specific C-Raf ablation, phosphate induction of ERK1/2 phosphorylation was prevented in mice lacking all three Raf isoforms in chondrocytes as well as in chondrocytes of females with a single A-Raf allele, demonstrating that Raf isoforms contribute to normal growth plate maturation by stabilizing VEGF protein levels as well as promoting phosphate-mediated ERK1/2 phosphorylation. Interestingly, basal ERK1/2 phosphorylation is increased in the Raf triple knock-out mice. An increase in Ras activity is known to occur with Raf inhibition, and leads to an increase in pERK1/2. However, this requires the presence of active Raf isoforms (25). Thus, the 20% residual expression of B-Raf and/or 3% residual expression of C-Raf could contribute to the increase in basal pERK1/2 by Ras-dependent C-Raf/B-Raf signaling (26). The molecular basis for the increase in Ras activity observed with Raf inhibition is not established, but has been shown to be associated with activation of receptor tyrosine kinases (RTKs), including the EGFR and FGFR3 (27, 28).
Expression of conditionally active Raf isoforms in vitro increases proliferation of several cell types (29), and constitutive activation of B-Raf in vivo leads to tumors including melanomas and lung adenomas (30). Although B-Raf ablation in skin tumors reduces cell proliferation (31), the absence of a growth plate phenotype in mice with chondrocyte-specific B-Raf ablation suggests a redundant role for Raf isoforms in the regulation of chondrocyte proliferation. However, similar to mice lacking ERK1/2 in chondrocytes (20), chondrocyte proliferation was reduced in the growth plates of mice lacking all three Raf isoforms in chondrocytes. Cyclin D1, which is induced upon activation of C-Raf in a fibroblast cell line (32), was decreased in the triple KO mice, as was c-Myc, which is increased in the setting of increased B-Raf signaling (33). Foxm1, which mediates Raf-induced proliferation in fibroblast cell lines, (34) was also decreased in the chondrocytes from triple KO mice. P27, which negatively regulates proliferation of multiple cell types including chondrocytes (35), and is induced upon Raf inhibition (36), was increased in the triple KO chondrocytes, whereas p21, which mediates C-Raf regulation of chondrocyte proliferation (37), was unchanged.
The current investigations define an essential role for Raf isoforms in phosphate-induced ERK1/2 phosphorylation and normal growth plate maturation in vivo. Ablating Raf signaling leads to expansion of the hypertrophic chondrocyte layer of the growth plate, impaired vascular invasion, impaired hypertrophic chondrocyte apoptosis, and neonatal lethality. Thus, these studies support a critical role for Raf-MEK1/2-ERK1/2 in phosphate signaling and growth plate maturation.
Experimental Procedures
Animal Studies
Animal studies were approved by the Massachusetts General Hospital Institutional Animal Care Committee. All mice were on a mixed C57BL/6J, 129T2/SvEmsJ, 129/OLA background, maintained in a virus- and parasite-free barrier facility, and exposed to a 12-h light/dark cycle. Mice with chondrocyte-specific ablation of C-Raf or B-Raf were generated by crossing mice expressing Cre recombinase driven by the collagen type II (Col2a1) promoter with mice bearing C-Raf alleles floxed at exon 3 (C-Raff/f;Col2Cre) (13) or B-Raf alleles floxed at exon 12 (B-Raff/f;Col2Cre) (14). Germline A-Raf knock-out mice (A-Raf XKOY) have been characterized previously (15). These mice were bred to create mice with deletion of A-/C-Raf, B-/C-Raf, or A-/B-Raf, or deletion of all three Raf kinases in chondrocytes. Growth plates were analyzed at P8 for postnatal studies in single Raf mutant mice. Mice lacking three Raf isoforms in chondrocytes, or females with a single A-Raf allele, were analyzed at E18.5 because of their neonatal lethal phenotype. Both female and male mice were studied. The phenotype of mice with chondrocyte-specific Raf ablation (homozygous for the floxed B- or/and C-Raf allele and heterozygous for the Col2-Cre transgene (B- or C-Raff/f;Col2Cre)) was compared with that of Cre-negative littermates homozygous for the Raf floxed allele (B- or C-Raff/f).
Histology and Skeletal Preparations
Bones were fixed in 4% paraformaldehyde in PBS (pH 7.4) and demineralized in 20% EDTA (pH 8.0) prior to being processed for paraffin sectioning. Morphology of the growth plate was assessed by H&E staining of 5-μm paraffin sections. pERK1/2 (Cell Signaling, 9101, 1/500), endomucin (Abcam, ab106100, 1/200), and cleaved caspase-9 (Abcam, ab52298, 1/100) immunohistochemistry and tartrate-resistant acid phosphatase (TRAP) staining was performed as described previously (8, 11, 38). In situ hybridization was performed on paraffin sections using digoxigenin-labeled probes as reported previously (39). To evaluate gross long bone morphology, skeletal preparations were stained with Alizarin red and Alcian blue as reported previously (40).
Evaluation of Chondrocyte Proliferation
Pregnant females were injected at 18.5 days post-coitum with 0.25 mg/g BrdU with 30 μg/g of 5-fluoro-2′-deoxyuridine (FdU) (to decrease background) 2.5 h before harvesting embryos. BrdU was detected on paraffin sections using the BrdU staining kit (Invitrogen). Total and BrdU-positive cells in the proliferating chondrocyte layer were counted.
Cell Culture
Primary chondrocytes were isolated from the rib cages of P2 pups or E18.5 embryos by sequential collagenase II digestions and then plated at a density of 3 × 105/cm2 (5, 41). Cells were cultured in DMEM supplemented with 10% FBS, 1% penicillin/streptomycin, and 25 μg/ml ascorbic acid at 37 °C, 5% CO2 for 7–10 days. To evaluate phosphate induction of ERK1/2 phosphorylation, chondrocytes were serum-restricted (0.5% FBS) overnight prior to treatment. Cells were treated with sodium sulfate or sodium phosphate at a concentration of 7 mm for 30 min.
Evaluation of Gene Expression
Total RNA was isolated from proliferating primary chondrocytes using the RNeasy Mini Kit (Qiagen) and reverse-transcribed with SuperScript II (Roche Applied Science). Quantitative real-time PCR was performed using the QuantiTect SYBR Green RT-PCR Kit (Qiagen) on an Opticon DNA Engine (MJ Research). Gene expression was normalized to that of actin (Actb) for each sample (42).
Western Analysis
Whole-cell lysates of primary chondrocytes were prepared as described previously (8). Protein concentration was calculated using the BCA protein assay (Pierce), and 7 μg of protein was subjected to Western analysis. Membranes were blocked with 5% non-fat dried milk prior to incubation with rabbit antibodies against pERK1/2 (1:1000; Cell Signaling, 9101) or ERK1/2 (1:1000; Cell Signaling, 9102). Following incubation with horseradish peroxidase-conjugated secondary antibodies (goat anti-rabbit IgG HRP antibody; 1:2000; Santa Cruz Biotechnology, sc-2004), signals were detected using ECL Plus (Amersham Biosciences). Quantitation of band intensity was performed with ImageJ64 software.
Statistical Analysis
Student's t test was used to analyze significance between two groups. p < 0.05 was considered significant.
Author Contributions
G. P. designed and performed studies and wrote the manuscript. E. T. P. performed studies. E. S. L. designed and performed studies and reviewed the manuscript. M. B. provided reagents and reviewed the manuscript. C. P. provided reagents and reviewed the manuscript. M. B. D. designed studies and wrote the manuscript.
Supplementary Material
This work was supported by National Institutes of Health Grants P30 AR061313 and R01 AR061376 (to M. B. D.). The authors declare that they have no conflicts of interest with the contents of this article. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.

This article contains supplemental Fig. 1.
- P8
- postnatal day 8
- E18.5
- embryonic day 18.5
- pERK1/2
- phospho-ERK1/2
- EGFR
- epidermal growth factor receptor
- MMP
- matrix metalloproteinase.
References
- 1. Kronenberg H. M. (2003) Developmental regulation of the growth plate. Nature 423, 332–336 [DOI] [PubMed] [Google Scholar]
- 2. Zhou X., von der Mark K., Henry S., Norton W., Adams H., and de Crombrugghe B. (2014) Chondrocytes transdifferentiate into osteoblasts in endochondral bone during development, postnatal growth and fracture healing in mice. PLoS Genet. 10, e1004820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Maes C., Kobayashi T., Selig M. K., Torrekens S., Roth S. I., Mackem S., Carmeliet G., and Kronenberg H. M. (2010) Osteoblast precursors, but not mature osteoblasts, move into developing and fractured bones along with invading blood vessels. Dev. Cell 19, 329–344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Carlevaro M. F., Cermelli S., Cancedda R., and Descalzi Cancedda F. (2000) Vascular endothelial growth factor (VEGF) in cartilage neovascularization and chondrocyte differentiation: auto-paracrine role during endochondral bone formation. J. Cell Sci. 113, 59–69 [DOI] [PubMed] [Google Scholar]
- 5. Sabbagh Y., Carpenter T. O., and Demay M. B. (2005) Hypophosphatemia leads to rickets by impairing caspase-mediated apoptosis of hypertrophic chondrocytes. Proc. Natl. Acad. Sci. U.S.A. 102, 9637–9642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Donohue M. M., and Demay M. B. (2002) Rickets in VDR null mice is secondary to decreased apoptosis of hypertrophic chondrocytes. Endocrinology 143, 3691–3694 [DOI] [PubMed] [Google Scholar]
- 7. Bergwitz C., Roslin N. M., Tieder M., Loredo-Osti J. C., Bastepe M., Abu-Zahra H., Frappier D., Burkett K., Carpenter T. O., Anderson D., Garabedian M., Sermet I., Fujiwara T. M., Morgan K., Tenenhouse H. S., and Juppner H. (2006) SLC34A3 mutations in patients with hereditary hypophosphatemic rickets with hypercalciuria predict a key role for the sodium-phosphate cotransporter NaPi-IIc in maintaining phosphate homeostasis. Am. J. Hum. Genet. 78, 179–192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Miedlich S. U., Zalutskaya A., Zhu E. D., and Demay M. B. (2010) Phosphate-induced apoptosis of hypertrophic chondrocytes is associated with a decrease in mitochondrial membrane potential and is dependent upon Erk1/2 phosphorylation. J. Biol. Chem. 285, 18270–18275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Provot S., Nachtrab G., Paruch J., Chen A. P., Silva A., and Kronenberg H. M. (2008) A-Raf and B-Raf are dispensable for normal endochondral bone development, and parathyroid hormone-related peptide suppresses extracellular signal-regulated kinase activation in hypertrophic chondrocytes. Mol. Cell. Biol. 28, 344–357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kaneko Y., Tanzawa H., and Sato K. (1994) The proto-oncogene C-raf-1 is highly expressed only in the hypertrophic zone of the growth plate. Calcif. Tissue Int. 54, 426–430 [DOI] [PubMed] [Google Scholar]
- 11. Liu E. S., Raimann A., Chae B. T., Martins J. S., Baccarini M., and Demay M. B. (2016) c-Raf promotes angiogenesis during normal growth plate maturation. Development 143, 348–355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ovchinnikov D. A., Deng J. M., Ogunrinu G., and Behringer R. R. (2000) Col2a1-directed expression of Cre recombinase in differentiating chondrocytes in transgenic mice. Genesis 26, 145–146 [PubMed] [Google Scholar]
- 13. Jesenberger V., Procyk K. J., Rüth J., Schreiber M., Theussl H. C., Wagner E. F., and Baccarini M. (2001) Protective role of Raf-1 in Salmonella-induced macrophage apoptosis. J. Exp. Med. 193, 353–364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Chen A. P., Ohno M., Giese K. P., Kühn R., Chen R. L., and Silva A. J. (2006) Forebrain-specific knockout of B-raf kinase leads to deficits in hippocampal long-term potentiation, learning, and memory. J. Neurosci. Res. 83, 28–38 [DOI] [PubMed] [Google Scholar]
- 15. Pritchard C. A., Bolin L., Slattery R., Murray R., and McMahon M. (1996) Post-natal lethality and neurological and gastrointestinal defects in mice with targeted disruption of the A-Raf protein kinase gene. Curr. Biol. 6, 614–617 [DOI] [PubMed] [Google Scholar]
- 16. Gerber H. P., Vu T. H., Ryan A. M., Kowalski J., Werb Z., and Ferrara N. (1999) VEGF couples hypertrophic cartilage remodeling, ossification and angiogenesis during endochondral bone formation. Nat. Med. 5, 623–628 [DOI] [PubMed] [Google Scholar]
- 17. Murakami S., Balmes G., McKinney S., Zhang Z., Givol D., and de Crombrugghe B. (2004) Constitutive activation of MEK1 in chondrocytes causes Stat1-independent achondroplasia-like dwarfism and rescues the Fgfr3-deficient mouse phenotype. Genes Dev. 18, 290–305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wojnowski L., Zimmer A. M., Beck T. W., Hahn H., Bernal R., Rapp U. R., and Zimmer A. (1997) Endothelial apoptosis in Braf-deficient mice. Nat. Genet. 16, 293–297 [DOI] [PubMed] [Google Scholar]
- 19. Wojnowski L., Stancato L. F., Zimmer A. M., Hahn H., Beck T. W., Larner A. C., Rapp U. R., and Zimmer A. (1998) Craf-1 protein kinase is essential for mouse development. Mech. Dev. 76, 141–149 [DOI] [PubMed] [Google Scholar]
- 20. Matsushita T., Chan Y. Y., Kawanami A., Balmes G., Landreth G. E., and Murakami S. (2009) Extracellular signal-regulated kinase 1 (ERK1) and ERK2 play essential roles in osteoblast differentiation and in supporting osteoclastogenesis. Mol. Cell. Biol. 29, 5843–5857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sebastian A., Matsushita T., Kawanami A., Mackem S., Landreth G. E., and Murakami S. (2011) Genetic inactivation of ERK1 and ERK2 in chondrocytes promotes bone growth and enlarges the spinal canal. J. Orthop. Res. 29, 375–379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Chen Z., Yue S. X., Zhou G., Greenfield E. M., and Murakami S. (2015) ERK1 and ERK2 regulate chondrocyte terminal differentiation during endochondral bone formation. J. Bone Miner. Res. 30, 765–774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zhang X., Siclari V. A., Lan S., Zhu J., Koyama E., Dupuis H. L., Enomoto-Iwamoto M., Beier F., and Qin L. (2011) The critical role of the epidermal growth factor receptor in endochondral ossification. J. Bone Miner. Res. 26, 2622–2633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Usmani S. E., Pest M. A., Kim G., Ohora S. N., Qin L., and Beier F. (2012) Transforming growth factor α controls the transition from hypertrophic cartilage to bone during endochondral bone growth. Bone 51, 131–141 [DOI] [PubMed] [Google Scholar]
- 25. Lito P., Pratilas C. A., Joseph E. W., Tadi M., Halilovic E., Zubrowski M., Huang A., Wong W. L., Callahan M. K., Merghoub T., Wolchok J. D., de Stanchina E., Chandarlapaty S., Poulikakos P. I., Fagin J. A., and Rosen N. (2012) Relief of profound feedback inhibition of mitogenic signaling by RAF inhibitors attenuates their activity in BRAFV600E melanomas. Cancer Cell 22, 668–682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Spirli C., Morell C. M., Locatelli L., Okolicsanyi S., Ferrero C., Kim A. K., Fabris L., Fiorotto R., and Strazzabosco M. (2012) Cyclic AMP/PKA-dependent paradoxical activation of Raf/MEK/ERK signaling in polycystin-2 defective mice treated with sorafenib. Hepatology 56, 2363–2374 [DOI] [PMC free article] [PubMed] [Google Scholar] [Research Misconduct Found]
- 27. Vakana E., Pratt S., Blosser W., Dowless M., Simpson N., Yuan X. J., Jaken S., Manro J., Stephens J., Zhang Y., Huber L., Peng S. B., and Stancato L. F. (2016) LY3009120, a panRAF inhibitor, has significant anti-tumor activity in BRAF and KRAS mutant preclinical models of colorectal cancer. Oncotarget, 10.18632/oncotarget.14002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Yadav V., Zhang X., Liu J., Estrem S., Li S., Gong X. Q., Buchanan S., Henry J. R., Starling J. J., and Peng S. B. (2012) Reactivation of mitogen-activated protein kinase (MAPK) pathway by FGF receptor 3 (FGFR3)/Ras mediates resistance to vemurafenib in human B-RAF V600E mutant melanoma. J. Biol. Chem. 287, 28087–28098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Thiel G., Ekici M., and Rössler O. G. (2009) Regulation of cellular proliferation, differentiation and cell death by activated Raf. Cell Commun. Signal. 7, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Urosevic J., Sauzeau V., Soto-Montenegro M. L., Reig S., Desco M., Wright E. M., Cañamero M., Mulero F., Ortega S., Bustelo X. R., and Barbacid M. (2011) Constitutive activation of B-Raf in the mouse germ line provides a model for human cardio-facio-cutaneous syndrome. Proc. Natl. Acad. Sci. U.S.A. 108, 5015–5020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kern F., Doma E., Rupp C., Niault T., and Baccarini M. (2013) Essential, non-redundant roles of B-Raf and Raf-1 in Ras-driven skin tumorigenesis. Oncogene 32, 2483–2492 [DOI] [PubMed] [Google Scholar]
- 32. Kerkhoff E., and Rapp U. R. (1997) Induction of cell proliferation in quiescent NIH 3T3 cells by oncogenic c-Raf-1. Mol. Cell. Biol. 17, 2576–2586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Magudia K., Lahoz A., and Hall A. (2012) K-Ras and B-Raf oncogenes inhibit colon epithelial polarity establishment through up-regulation of c-myc. J. Cell Biol. 198, 185–194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ma R. Y., Tong T. H., Cheung A. M., Tsang A. C., Leung W. Y., and Yao K. M. (2005) Raf/MEK/MAPK signaling stimulates the nuclear translocation and transactivating activity of FOXM1c. J. Cell Sci. 118, 795–806 [DOI] [PubMed] [Google Scholar]
- 35. Emons J. A., Marino R., Nilsson O., Barnes K. M., Even-Zohar N., Andrade A. C., Chatterjee N. A., Wit J. M., Karperien M., and Baron J. (2006) The role of p27Kip1 in the regulation of growth plate chondrocyte proliferation in mice. Pediatr. Res. 60, 288–293 [DOI] [PubMed] [Google Scholar]
- 36. Joseph E. W., Pratilas C. A., Poulikakos P. I., Tadi M., Wang W., Taylor B. S., Halilovic E., Persaud Y., Xing F., Viale A., Tsai J., Chapman P. B., Bollag G., Solit D. B., and Rosen N. (2010) The RAF inhibitor PLX4032 inhibits ERK signaling and tumor cell proliferation in a V600E BRAF-selective manner. Proc. Natl. Acad. Sci. U.S.A. 107, 14903–14908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Beier F., Taylor A. C., and LuValle P. (1999) The Raf-1/MEK/ERK pathway regulates the expression of the p21Cip1/Waf1 gene in chondrocytes. J. Biol. Chem. 274, 30273–30279 [DOI] [PubMed] [Google Scholar]
- 38. Ko F. C., Martins J. S., Reddy P., Bragdon B., Hussein A. I., Gerstenfeld L. C., and Demay M. B. (2016) Acute phosphate restriction impairs bone formation and increases marrow adipose tissue in growing mice. J. Bone Miner. Res. 31, 2204–2214 [DOI] [PubMed] [Google Scholar]
- 39. Zalutskaya A. A., Cox M. K., and Demay M. B. (2009) Phosphate regulates embryonic endochondral bone development. J. Cell. Biochem. 108, 668–674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Papaioannou G., Inloes J. B., Nakamura Y., Paltrinieri E., and Kobayashi T. (2013) let-7 and miR-140 microRNAs coordinately regulate skeletal development. Proc. Natl. Acad. Sci. U.S.A. 110, E3291–E3300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Lefebvre V., Garofalo S., Zhou G., Metsäranta M., Vuorio E., and De Crombrugghe B. (1994) Characterization of primary cultures of chondrocytes from type II collagen/β-galactosidase transgenic mice. Matrix Biol. 14, 329–335 [DOI] [PubMed] [Google Scholar]
- 42. Livak K. J., and Schmittgen T. D. (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 25, 402–408 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.