Abstract
The transport of proteins at the cell surface of Bacteroidetes depends on a secretory apparatus known as type IX secretion system (T9SS). This machine is responsible for the cell surface exposition of various proteins, such as adhesins, required for gliding motility in Flavobacterium, S-layer components in Tannerella forsythia, and tooth tissue-degrading enzymes in the oral pathogen Porphyromonas gingivalis. Although a number of subunits of the T9SS have been identified, we lack details on the architecture of this secretion apparatus. Here we provide evidence that five of the genes encoding the core complex of the T9SS are co-transcribed and that the gene products are distributed in the cell envelope. Protein-protein interaction studies then revealed that these proteins oligomerize and interact through a dense network of contacts.
Keywords: bacterial pathogenesis, membrane protein, microbiology, protein assembly, protein complex, protein purification, protein secretion, secretion
Introduction
Porphyromonas gingivalis is the causative agent of gingivitis and periodontal diseases that are responsible for teeth loss (1, 2). It causes severe lesions in periodontal tissues such as the gingiva or the alveolar bone and yields to disruption of the tooth-supporting structure (3). Periodontitis is considered a major public health concern, as it affects ∼35% of the population. Tissue alterations and damage are mainly induced by a mixture of toxin proteins secreted by bacteria, the gingipains (4). Gingipains act as adhesins or proteases that help the bacteria to adhere to periodontal tissues and to promote gingival tissue invasion by degradation of the matrix proteins fibrinogen and collagen (5, 6). The secretion of these proteins is a two-step mechanism. Gingipains carry an N-terminal signal peptide and are first addressed to the periplasm by the Sec pathway before being transported to the cell surface or to the cell exterior (7). However, the machinery responsible for the translocation of gingipains through the outer membrane remained unknown, as genes encoding a potential type II secretion system, the major two-step secretory pathway, are absent in the P. gingivalis genome (8, 9). Recently, a number of proteins responsible for the active release of these proteins at the bacterial cell surface, named Por, have been identified (10–15). Although there is little evidence that these proteins assemble a secretion machine, they were collectively grouped under the name Porphyromonas secretion system and, more recently, type IX secretion system (T9SS)4 (10, 16). In addition to the gingipains, this secretion apparatus transports the Hbp35 heme-binding protein peptidylarginine deiminase, a toxin responsible for host protein, the citrullination and rheumatoid arthritis, and Maf5, a subunit of the extracellular Maf fimbriae (17–19). Interestingly, most of the T9SS proteins share homologies with proteins encoded within genomes of species belonging to the Bacteroidetes phylum, such as Flavobacterium, Capnocytophaga, Cellulophaga, or Tannerella (8). In these strains, the T9SS is responsible for the secretion of adhesins, chitinases, or S-layer components (15, 20, 21). Although it has been proposed that the T9SS is a rotative machinery (22, 23), the overall organization and architecture of this secretion system are not known. In P. gingivalis, at least 14 genes are necessary for the function of the T9SS, including three regulators and 11 machine components (10, 24, 25). It has been reported that at least four of these components, PorK, PorL, PorM, and PorN, assemble a >1.4-MDa complex that resists blue native polyacrylamide gel electrophoresis (10). The genes encoding these four proteins are contiguous on the chromosome and located downstream of the gene encoding the PorP protein. Here we show that the five genes are co-transcribed, and we define the localization of the corresponding proteins. We further determine the topology of the PorL and PorM inner membrane proteins and provide insights into the protein-protein interactions within this complex.
Results
The porP, porK, porL, porM, and porN Genes Are Co-transcribed
Although most of the por genes required for gingipain secretion are scattered on the P. gingivalis genome, the porP (PGN_1677), porK (PGN_1676), porL (PGN_1675), porM (PGN_1674), and porN (PGN_1673) genes are contiguous on the chromosome (Fig. 1A) and are only separated by few bases (porP-porK, 56 bp; porK-porL, 40 bp; porL-porM, 3 bp; porM-porN, 8 bp) (supplemental Fig. S1). The genomic organization and the limited intergenic spaces suggest that the expression of these genes might be coordinated. To test whether the porPKLMN gene locus is transcribed as a unique polycistronic mRNA, we performed RT-PCR using oligonucleotides designed for the amplification of each gene junction (named PK, KL, LM, and MN respectively; Fig. 1A). RT-PCR experiments were performed on purified total RNA extracted from P. gingivalis cells (Fig. 1B, top panel). As controls, RT-PCR was performed on purified genome DNA (Fig. 1B, center panel) as well as on the total RNA preparation but in the absence of reverse transcriptase to test for DNA contamination (Fig. 1B, bottom panel). As shown in Fig. 1B, RT-PCR products with the expected sizes were obtained for each gene junction of the porPKLMN gene cluster from DNA or cDNA but not from RNA, suggesting that these five genes are co-transcribed.
FIGURE 1.
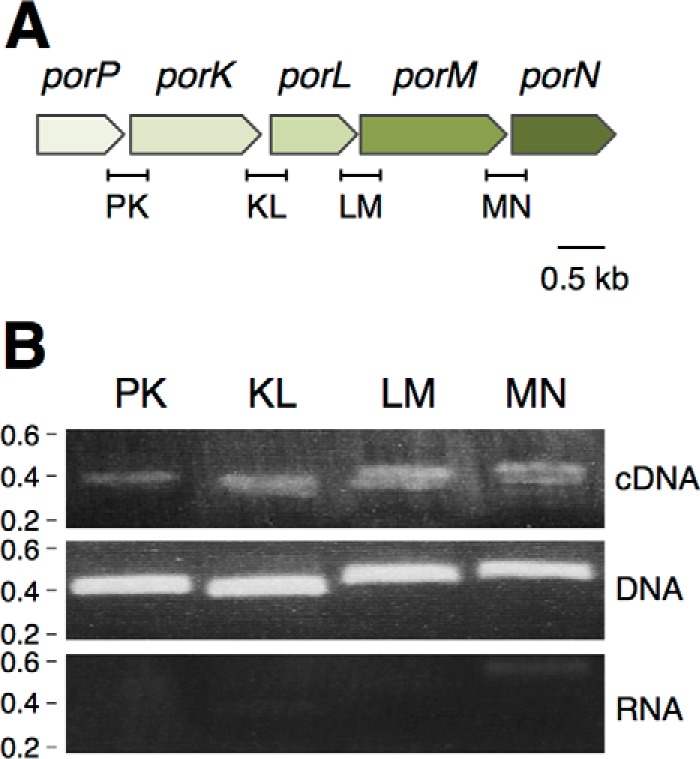
The porP, porK, porL, porM, and porN genes are co-transcribed. A, schematic representation of the P. gingivalis porP-K-L-M-N fragment (PGN_1677 to PGN_1673). The fragments corresponding to gene junctions and amplified in the RT-PCR experiments are indicated below (PK, 393 bp; KL, 377 bp; LM, 423 bp; MN, 432 bp). B, operon structure of the P. gingivalis porP-K-L-M-N fragment. Shown are agarose gel analyses of the indicated gene junctions amplified by PCR from cDNA (top panel), genomic DNA (center panel, positive control), and total RNA (bottom panel, negative control). The presence of a PCR fragment in the cDNA gel demonstrates co-transcription of the genes located 5′ and 3′ of the amplified region. Molecular weight markers (in kilobases) are indicated on the left.
The PorKLMNP Proteins Are Distributed in the Cell Envelope
Four of the five gene products of the porPKLMN operon, PorK, PorL, PorM, and PorN, have been shown to assemble a >1.4-MDa complex that resists blue native polyacrylamide gel electrophoresis (10). To gain information on the subcellular localization of these proteins, we first performed in silico analyses to identify signal peptides or trans-membrane segments.
The P. gingivalis PorK protein (gene accession no. GI:188595220) bears a signal sequence with a typical lipobox motif (supplemental Fig. S2). This motif comprises the highly conserved cysteine residue at position +1 of the mature protein, which is anticipated to be acylated. The +2 residue of the PorK protein, which defines the final localization of the lipoprotein, is a glycine. These analyses suggest that the P. gingivalis PorK protein is an outer membrane lipoprotein. Indeed, the PorK protein shares homologies with the Flavobacterium johnsoniae GldJ and GldK proteins, two components of the gliding machinery that have been experimentally demonstrated to be outer membrane lipoproteins (26).
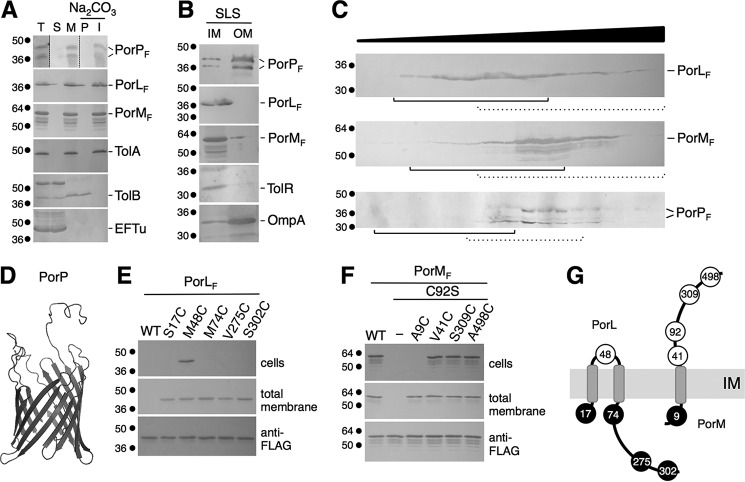
The PorN (accession no. GI:188595217) and PorP (accession no. GI:188595221) proteins bear typical signal sequences and are likely exported to the periplasm (supplemental Fig. S2). By contrast, the PorL (accession no. GI:188595219) and PorM (accession no. GI:188595218) proteins have predicted trans-membrane helices (supplemental Fig. S3). To better define the localization of the PorL, PorM, and PorP proteins, we performed fractionation of Escherichia coli cells expressing the corresponding genes fused to the FLAG epitope. Fig. 2A shows that PorL, PorM, and PorP are associated with the membrane fraction. Dissociation of peripherally associated membrane by sodium carbonate treatment of the total membrane fraction showed that these three subunits are integral membrane proteins (Fig. 2A). Finally, differential solubilization with sodium lauroyl sarcosinate (SLS), a detergent that specifically disrupts the inner membrane (Fig. 2B), and discontinuous sucrose gradient analyses (Fig. 2C and supplemental Fig. S4) demonstrated that PorP co-fractionates with outer membrane proteins, whereas PorL and, putatively, PorM are inserted into the inner membrane. Although the results were less clear for PorM, cysteine accessibility assays (see below) confirmed that PorM is anchored to the inner membrane. PorP does not bear a lipobox motif but, rather, is predicted to assemble a β barrel structure. Indeed, Phyre analyses reported that PorP is likely to be an outer membrane barrel, and a homology model could be built with 98% confidence using the structure of the Ralstonia pickettii toluene transporter TbuX protein (PDB code 3BRY) as template (Fig. 2D). Inner membrane proteins are usually embedded within the membrane via trans-membrane helices (TMHs). Computer predictions and hydrophobicity plots of the PorL and PorM sequences suggested the existence of one or several TMHs (supplemental Fig. S3). To test these predictions, we used a cysteine accessibility approach. This method is based on the accessibility of cysteine residues to 3-(N-maleimidyl-propionyl) biocytin (MPB), a sulfhydryl reagent that readily passes the OM but only inefficiently the IM of Gram-negative bacteria (27). Based on TMH predictions, cysteine substitutions were introduced into the cysteine-less PorL protein at various positions. MPB accessibility analyses in E. coli cells showed that only a cysteine positioned at residue 48 was labeled, whereas cysteine residues located at positions 17, 74, 275, and 302 remained inaccessible (Fig. 2E). These data demonstrate that PorL has cytoplasmic N and C termini and possesses two TMHs located between residues 17–48 and 48–74 (Fig. 2E). The PorM protein possesses a native cysteine residue located at position 92 that is labeled by MPB. Cysteines were introduced into the PorM C92S variant, and MPB analyses showed that, although a cysteine at position 9 was not accessible, cysteine residues at positions 41, 309, and 498 were labeled, demonstrating that PorM is a bitopic protein with in-to-out topology and a single membrane spanning the segment between residues 9 and 41 (Fig. 2F). Based on these data, we conclude that the PorK, PorL, PorM, PorN, and PorP proteins are distributed into the cell envelope and comprise two inner membrane proteins (PorL and PorM), a periplasmic protein (PorN), and an outer membrane lipoprotein (PorK) and β barrel (PorP) (Fig. 2G).
FIGURE 2.
Localization and topologies of the PorL, PorM, PorN, and PorP proteins. A, PorL, PorM, and PorP co-fractionate with integral membrane proteins. E. coli cells producing FLAG-tagged PorL, PorM, or PorP (T, total fraction) were fractionated to separate soluble (S) and membrane (M) fractions. Membranes were then treated with sodium carbonate (Na2CO3) to separate peripheral (P) and integral (I) membrane proteins. Samples from 5 × 108 cells were subjected to 12.5% SDS-PAGE and immunodetected with antibodies directed against the EFTu (soluble), TolB (soluble and peripherally associated with the membrane), and TolA (integral inner membrane) proteins and the FLAG epitope. B, total membranes from E. coli cells producing FLAG-tagged PorP, PorL, or PorM were subjected to solubilization with SLS. Solubilized IM and insolubilized OM proteins were separated. Samples from 5 × 108 cells were subjected to 12.5% SDS-PAGE and immunodetected with antibodies directed against the TolR (inner membrane) and OmpA (outer membrane) proteins and the FLAG epitope. C, total membranes from E. coli cells producing FLAG-tagged PorL, PorM, or PorP were separated on a discontinuous sedimentation sucrose gradient. The collected fractions were analyzed for content using anti-FLAG antibodies. The positions of the inner (plain lines) and outer membrane (dotted lines) fractions, based on immunodetection controls with anti-TolA (inner membrane) and anti-OmpF/anti-Pal (outer membrane) antibodies and with an NADH oxidase (inner membrane) activity test (supplemental Fig. S4), are indicated. Molecular weight markers are indicated on the left. D, homology model of the PorP protein based on the crystal structure of the R. pickettii toluene transporter TbuX protein (PDB code 3BRY), generated using HHPred/Swiss-Model. E and F, accessibility of cysteine residues. Whole cells (top panels) or total membranes (center panel) of E. coli cells producing the FLAG-tagged PorL (E) or PorM (F) WT or cysteine-substituted derivatives were treated with the MPB probe and solubilized, and the PorL and PorM proteins were immunoprecipitated using agarose beads coupled to M2 anti-FLAG antibody. Precipitated material was subjected to SDS-PAGE and Western blotting analysis using anti-FLAG antibody (to detect PorL or PorM, bottom panels) and streptavidin coupled to alkaline phosphatase (to detect biotinylated PorL or PorM derivatives). Molecular weight markers are indicated on the left. G, topology model for the PorL and PorM proteins at the inner membrane based on the cysteine accessibility experiments. The positions of the labeled and unlabeled cysteine residues are indicated by open and filled circles, respectively.
Bacterial Two-hybrid and Co-immunoprecipitation Analyses Define an Intense Interaction Network
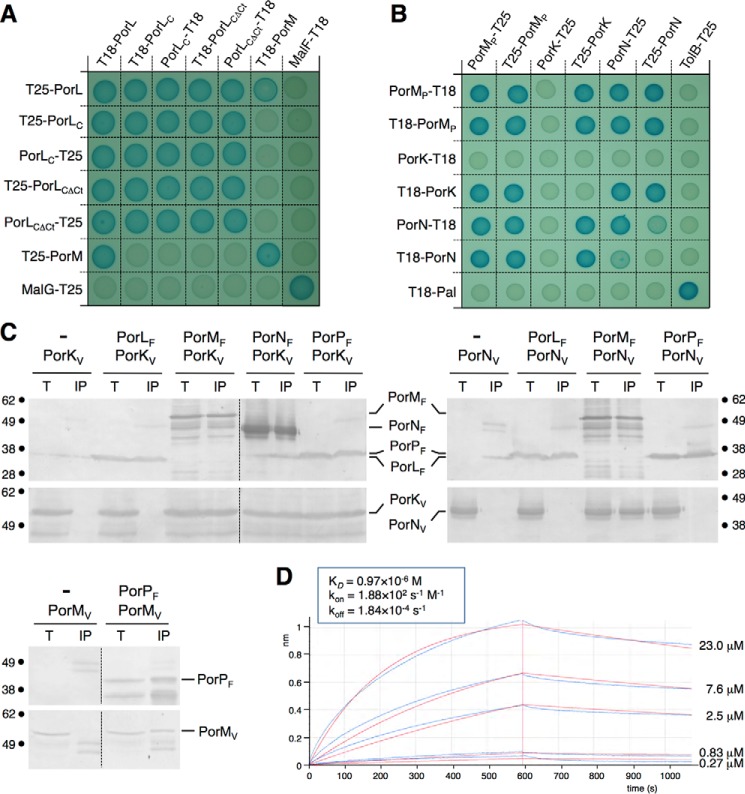
To gain insights into the architecture of the T9SS core complex, we next tested pairwise interactions among the PorK, PorL, PorM, PorN, and PorP proteins. First, interactions were assayed by bacterial two-hybrid analysis. The T18 and T25 domains of the adenylate cyclase were fused to the N termini of the PorL and PorM full-length subunits and to the N and C termini of the soluble regions of PorK, PorL, PorM, or PorN proteins, as defined by topology experiments. The PorP β barrel was not included in the assay. Fig. 3A shows that PorL and PorM oligomerize and interact with each other. The cytoplasmic segment of PorL located between the second trans-membrane segment and the C-terminal hydrophobic region (amino-acids 73–274) is sufficient to mediate oligomerization. By contrast, the PorL-PorM interaction does not involve the PorL cytoplasmic domain (PorLC), and, therefore, these data suggest that PorL and PorM interact through their trans-membrane segments. Fig. 3B reports the interactions between the PorM, PorK, and PorN periplasmic fragments: the periplasmic soluble domain of PorM (PorMP), the soluble, unacylated form of PorK, and the PorN subunit. We showed that the periplasmic domain of PorM and the PorN protein oligomerize. This approach revealed an intense network of interaction in the periplasm. In addition to the previously documented PorK-PorN interaction (28, 29), our analysis showed that PorMP contacts PorK and PorN.
FIGURE 3.
Interaction network among the PorKLMNP complex. A and B, bacterial two-hybrid assay. BTH101 reporter cells producing the indicated proteins or domains (PorLC, cytoplasmic domain of the PorL protein; PorLCΔCt, cytoplasmic domain of the PorL protein deleted from the C-terminal hydrophobic helix) fused to the T18 or T25 domain of the Bordetella adenylate cyclase were spotted on plates supplemented with IPTG and the chromogenic substrate X-gal. Interaction between the two fusion proteins is attested by the dark blue color of the colony. The MalF-MalG (A) and TolB-Pal (B) interactions served as positive controls. C, co-immunoprecipitation assays. Detergent-solubilized extracts of E. coli cells producing the indicated VSVG- and FLAG-tagged proteins were subjected to immunoprecipitation with anti-FLAG-coupled beads. The input (total soluble material, T) and the immunoprecipitated material (IP) were loaded on a 12.5% SDS-PAGE and immunodetected with anti-FLAG and anti-VSVG monoclonal antibodies. The immunodetected proteins and the molecular weight markers are indicated. D, biolayer interferometry. PorM and PorN interact with a KD of 0.97 μm. The recordings represent binding of the indicated concentration of purified PorMP to the sensor tip coupled to purified PorN (experimental and fitted curves are shown in blue and red, respectively). The response (in nanometers) is plotted versus time (in seconds). The kinetics parameters are indicated in the inset.
The interactions between these four proteins as well as PorP were then tested by co-immunoprecipitations in the heterologous host E. coli (Fig. 3C). This approach confirmed the PorM-PorK, PorM-PorN, and PorK-PorN interactions and revealed that PorP interacts with PorK and PorM (Fig. 3C). We did not detect interactions between PorL and PorK, PorL and PorN, and PorN and PorP. The strength of the interaction between the PorM periplasmic domain and PorN was measured in vitro by biolayer interferometry using the purified proteins (see below, Fig. 3D). The two proteins interact with an apparent KD of 0.97 ± 0.02 μm.
To gain further information on the oligomeric state of the Por subunits, the soluble fragments of PorK (unacylated form), PorL (cytoplasmic domain, PorLC, amino-acids 73–309), PorM (periplasmic domain, PorMP, amino-acids 36–516), and PorN (full-length mature protein) were fused to a N-terminal His6 tag and subjected to purification using metal affinity chromatography and gel filtration. Although PorK remained insoluble, we succeeded in purifying PorLC, PorMP, and PorN (Fig. 4, A–C, left panels). The three proteins were subjected to size exclusion chromatography coupled to online multiangle laser light scattering (MALS)/quasi-elastic light scattering/absorbance/refractive index analyses. Analyses of PorLC showed that this domain has a mass of 44 kDa (compared with the 29-kDa theoretical mass, Fig. 4A) suggesting that it has an elongated, non-compact conformation. PorLC elutes as two peaks (44 and 113 kDa), suggesting that it exists as a monomer and trimer (Fig. 4A). Contrarily, PorMP (107 kDa compared with the 55-kDa theoretical mass, Fig. 4B) and PorN (94 kDa compared with the 43-kDa theoretical mass, Fig. 4C) both exist as dimers.
FIGURE 4.
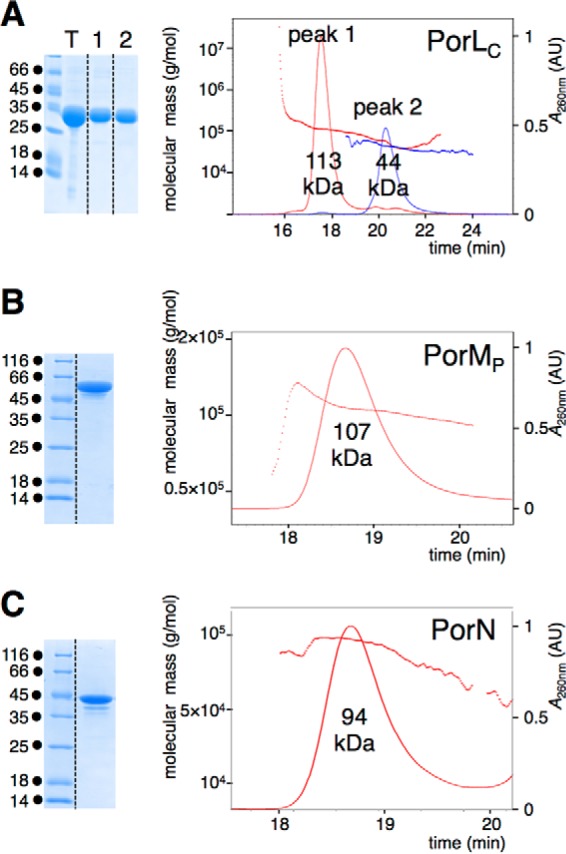
Oligomeric state of the PorL, PorM, and PorN soluble domains. A–C, the purified PorL cytoplasmic domain (PorLC, A), PorM periplasmic domain (PorMP, B), and the PorN protein (C) shown in the left panels (Coomassie Blue staining) were subjected to MALS/quasi-elastic light scattering/UV/refractive index analyses (right panels). The molecular weights of the proteins or complexes are indicated below their corresponding peaks. The absorbance at λ = 260 nm (in absorbance units, AU) and the molecular mass (in grams per milliliter) are plotted versus the volume of elution (in minutes).
Discussion
In this study, we defined the localization and topology of the subunits of the P. gingivalis T9SS PorKLMNP complex. The five proteins are distributed in the cell envelope (Fig. 5). PorL and PorM are inner membrane proteins, PorN resides in the periplasm, and PorK and PorP are an outer membrane lipoprotein and β barrel, respectively. We then defined the interaction network between these subunits, demonstrating that PorL and PorM interact, likely through their transmembrane helices. PorM has a large periplasmic domain that mediates interaction with PorK, PorN, and PorP. In addition to PorM, PorK contacts PorN and PorP (Fig. 5). Recent studies showed that PorK and PorN interact with a 1:1 stoichiometry and assemble a ring-like structure composed of 32–36 PorKN heterodimers (28, 29). Taken together, these data provide evidence that PorKLMNP assemble a cell envelope-spanning complex, with a central protein, PorM, linking inner membrane (PorLM)- and outer membrane-associated (PorKN) complexes. This situation is comparable with that of GspC, VirB10, TssM, and TolA that connect IM and OM complexes in T2SS, T4SS, T6SS, and Tol-Pal systems, respectively (30–33).
FIGURE 5.
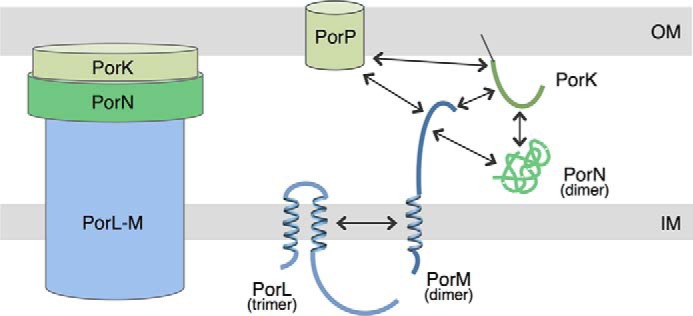
Schematic representation of the PorKLMNP T9SS core complex. Shown is a schematic of the PorKLMNP complex, highlighting the localizations and topologies of the proteins. The interactions defined by bacterial two-hybrid and co-immunoprecipitation assays are indicated by arrows. The oligomeric states of the proteins determined by gel filtration experiments are indicated in parentheses. The outer membrane-associated PorKN complex is depicted as a ring-like structure, as shown recently by negative stain electron microscopy (29).
The PorK, PorL, PorM, and PorN proteins have been shown previously to assemble a stable >1.4-MDa complex. Our results show that the porP gene is co-transcribed with the porKLMN genes and that PorP interacts with at least two components of this complex, PorM and PorK. However, Sato et al. (10) reported that PorP is not part of and does not stabilize the >1.4 MDa complex, suggesting that the interaction of PorP with the PorKLMN complex is more labile or prone to dissociation or that PorP associates with the PorKLMN complex under specific conditions. Indeed, it has been shown that, contrarily to PorK, PorL, PorM, and PorN, PorP (or its homologue in F. johnsoniae, SprF) is an accessory component of the secretion apparatus, as it is not required for the transport of all T9SS substrates (34). However, a recent cross-linking study demonstrated that the PorKN outer membrane-associated complex interacts with an OM protein, PG0189 (29).
We also showed that PorL forms trimers, whereas PorM and PorN dimerize. Although the oligomeric status of PorK was not defined in our study, the recent observation that PorK and PorN interact with a 1:1 stoichiometry (29) suggests a minimal PorK2L3M2N2 complex. This minimal complex should have a mass of ∼ 400 kDa. These data therefore suggest that either additional T9SS subunits are present in the >1.4 MDa complex or that this complex results from the multimerization of the minimal PorK2L3M2N2 complex. These two hypotheses are likely, as most secretion apparatuses form large channels to accommodate folded effectors (35), multimerization has been reported for the T6SS TssJLM membrane complex that comprises five copies of dimers of TssJLM heterotrimers (32), and additional components have been identified recently to interact with the PorKN complex (29).
It has been demonstrated previously that the T9SS is a two-step mechanism. The T9SS substrates are first exported through the inner membrane via the Sec translocon before being transported to the cell surface by the T9SS (7). However, our results stress that two proteins, PorL and PorM, are anchored to the inner membrane. Based on the localization/topology of these proteins and the recent evidence that the T9SS is a rotary machine (22, 23), we propose that PorL and PorM serve as an energy transducer complex to convert chemical energy (proton-motive force or ATP) into mechanical energy and provide the energy for T9SS assembly, dynamics, or substrate translocation through the outer membrane. Energy transducers have been evidenced, notably in the case of the T4SS, in which the VirB10 protein transduces the energy provided by the VirB11, VirB4, and VirD4 ATPases to the VirB7-VirB9 outer membrane complex (31). However, none of the T9SS subunits bear the signature of NTPases. By contrast, T9SS-dependent gliding motility has been shown to be dependent on the proton-motive force in F. johnsoniae (36). Energy transducers using the proton-motive force, such as the MotAB, TolQRA, ExbBD-TonB or AglQR complexes (37, 38), usually possess negatively charged glutamate or aspartate residues within the hydrophobic TMH. Interestingly, sequence analysis of the PorL and PorM TMH defined in our study reveals that the second TMH of PorL as well as the PorM TMH bear glutamate residues that are conserved in the F. johnsoniae GldL and GldM homologs (supplemental Fig. S5). It would therefore be interesting to determine whether PorLM constitutes a new molecular motor and how these proteins power T9SS assembly or dynamics, or substrate translocation.
Taken together, the data described in this study provide a better understanding of the P. gingivalis T9SS PorKLMNP complex and open new perspectives on the role of this complex in powering Type IX secretion. These data also pave the way for the design of specific inhibitors to prevent assembly of the PorKLMNP complex and, hence, to lessen or abolish P. gingivalis virulence.
Experimental Procedures
Bacterial Strains, Growth Conditions, Chemicals, and Antibodies
The strains used in this study are listed in supplemental Table S1. P. gingivalis ATCC33277/DSM20709 (obtained from the DSMZ collection, Germany) was used as source of DNA for cloning. P. gingivalis cells were grown anaerobically in brain heart infusion medium supplemented with menadiol (0.5 μg/ml) and hemin (5 μg/ml). E. coli K-12 DH5α, W3110, BTH101, and Rosetta(DE3)pLys were used for cloning procedures, co-immunoprecipitations, two-hybrid assays, and protein purification, respectively. Unless specified, E. coli strains were routinely grown in LB medium at 37 °C with shaking. Expression of genes from pBAD and pTet was induced for 40–45 min with l-arabinose (0.02%) and anhydrotetracyclin (0.1 μg/ml), respectively. Plasmids were maintained by addition of ampicillin (100 μg/ml), kanamycin (50 μg/ml), or chloramphenicol (40 μg/ml). Igepal CA-630, l-arabinose, and N-ethylmaleimide were purchased from Sigma-Aldrich, and 3-(N-maleimidyl-propionyl) biocytin and alkaline phosphatase-conjugated streptavidin were purchased from Pierce. The anti-TolA, anti-TolB, anti-TolR, anti-OmpA, anti-Pal, and anti-OmpF polyclonal antibodies were from our laboratory collection, and the anti-EFTu (clone mAb900, Hycult Biotech), anti-FLAG (clone M2, Sigma-Aldrich), and anti-VSVG (clone P5D4, Sigma-Aldrich) monoclonal antibodies are commercially available.
RNA Purification and RT-PCR
1010 P. gingivalis cells were resuspended in 1 ml of TE buffer (10 mm Tris-HCl (pH 8.0) and 1 mm EDTA) supplemented with lysozyme (50 mg/ml). Total RNAs were isolated using a total RNA isolation system kit (Promega) and treated with 10 units of DNase (RTS DNase kit, MoBio). The concentration and the purity of the samples were measured at A260 and by the A260/A280 ratio, respectively, using nanodrop technology (Shimadzu BioSpec-nano). cDNAs were then generated using the RT-PCR SuperScript first-strand synthesis kit (Invitrogen) using 100 ng of total RNAs and random hexamers. PCRs were performed using Q5 DNA polymerase (New England Biolabs) using 10 ng of genomic DNA (DNA), 10 ng of total RNA (RNA) or cDNA from 10 ng of total RNA (cDNA) as starting material.
Plasmid Construction
The plasmids used for this study are listed in supplemental Table S1. por genes were amplified from P. gingivalis genomic DNA extracted from 6 × 109 cells using a DNA purification kit (DNeasy Blood & Tissue, Qiagen). PCRs were performed with a Biometra thermocycler using Phusion DNA polymerase (Thermo Scientific) and custom oligonucleotides synthesized by Sigma-Aldrich (listed in supplemental Table S1). Expression plasmids were constructed by restriction-free cloning (39) as described previously (40). Briefly, the gene of interest was amplified using oligonucleotides introducing extensions annealing to the target vector. The double-stranded product of the first PCR has then been used as oligonucleotides for a second PCR using the target vector (pASK-IBA4, pASK-IBA37(+), pBAD33, pBAD24, or pLIC03) as template. PCR products were then treated with DpnI to eliminate template plasmids and transformed into DH5α-competent cells. Bacterial two-hybrid plasmids were constructed by restriction ligation. PCR products bearing 5′ XbaI and 3′ KpnI sites were digested by the corresponding restriction enzymes (New England Biolabs) and inserted into pUT18 (fusion at the N terminus of the T18 domain, X-T18), pUT18C (fusion at the C terminus of the T18 domain, T18-X), pKT25 (fusion at the C terminus of the T25 domain, T25-X) and pKTN25 (fusion at the N terminus of the T25 domain, X-T25) vectors (41) digested with the same enzymes. All constructs were verified by restriction analyses and DNA sequencing (GATC).
Fractionation
Cell fractionation assays were performed as published previously (40, 42, 43). Briefly, 2 × 109 exponentially growing cells were resuspended in 0.5 ml of Tris-HCl (10 mm, pH 8.0) and sucrose (30%) and incubated for 10 min on ice. After addition of 100 μg/ml of lysozyme and 1 mm EDTA and further incubation for 45 min on ice, 0.5 ml of Tris-HCl (10 mm, pH 8.0) supplemented with DNase (200 μg/ml) and MgCl2 (4 mm) was added, and cells were lysed by five cycles of freezing and thawing. Unbroken cells were removed by centrifugation, and soluble and membrane fractions were separated by ultracentrifugation for 40 min at 100,000 × g. Membranes were washed with 20 mm Tris-HCl (pH 8.0) and MgCl2 (2 mm), resuspended in 1 ml of Tris-HCl (20 mm, pH 8.0) supplemented with Na2CO3 (1 m), and incubated on a wheel for 1 h. The mixture was then ultracentrifuged for 40 min at 100,000 × g to separate integral membrane and peripherally membrane-associated proteins. Soluble and membrane-associated fractions were precipitated with trichloroacetic acid (15%) and resuspended in loading buffer prior to analysis by SDS-PAGE and immunoblotting. EF-Tu, TolA/TolR, TolB, and OmpF/OmpA/Pal were used as cytoplasmic, inner membrane, periplasmic, and outer membrane markers, respectively.
Differential Membrane Solubilization
Sodium lauroyl sarcosinate is an anionic detergent that selectively disrupts the inner membrane and solubilizes inner membrane proteins (44). Membranes prepared from 1010 cells using the fractionation protocol were resuspended in 1 ml of Tris-HCl (10 mm, pH 8.0) and EDTA (1 mm) supplemented with 1% SLS (Sigma-Aldrich) and incubated on a wheel for 1 h at room temperature (43). Insoluble (outer membrane) and soluble (inner membrane) fractions were collected by ultracentrifugation at 100,000 × g for 40 min prior to analysis by SDS-PAGE and immunoblotting.
Sucrose Sedimentation Gradients
Inner and outer membranes were separated using discontinuous sedimentation sucrose gradients as described previously (40, 42, 43). 4 × 1011 cells were harvested; resuspended in 3 ml of Tris-HCl (10 mm, pH 7.4), sucrose (30%), and RNase (100 μg/ml); and lysed by French press treatment (three passages at 900 p.s.i.). Total membranes were recovered by centrifugation at 100,000 × g for 40 min and resuspended in 0.5 ml of 25% sucrose containing a protease inhibitor mixture (Complete EDTA-free, Roche). The membrane fraction was then loaded on the top of a discontinuous sucrose gradient composed of the superposition of 1.5 ml of 30%, 35%, 40%, 45%, 50%, 55%, and 60% sucrose solutions (from top to bottom). Gradients were centrifuged at 90,000 × g for 90 h, and 500-μl fractions were collected from the top. The fractions were analyzed by an NADH oxidase enzymatic test and by SDS-PAGE and immunodetection. TolA and OmpF/Pal were used as inner and outer membrane markers, respectively. The NADH oxidase activity was measured in 96-well polystyrene microtitre dishes using 20 μl of each fraction diluted in 180 μl of Tris-HCl (50 mm, pH 7.5), DTT (0.2 mm), and NADH (0.5 mm). The decrease of absorbance of NADH at 340 nm, which reflects the activity of NADH oxidase, was measured every minute at 25 °C using a Tecan M200 microplate reader, and the NADH activity was calculated from the initial slope. Each fraction was tested in triplicate. NADH oxidase activities are reported as the percentage of activity in the fraction compared with the total membrane fraction.
Cysteine Accessibility Experiments (Substituted Cysteine Accessibility Method)
Cysteine accessibility experiments (27) were carried out as described previously on whole cells (40, 43, 45, 46) with modifications. Briefly, 2 × 1010 cells producing the cysteine variant were harvested, resuspended in buffer A (100 mm Hepes (pH 7.5), 250 mm sucrose, 25 mm MgCl2, and 0.1 mm KCl) to a final A600 nm of 12. MPB (Molecular Probes) was added to a final concentration of 100 μm (from a 20 mm stock freshly dissolved in DMSO), and the cells were incubated for 30 min at 25 °C. β-Mercaptoethanol (20 mm final concentration) was added to quench the biotinylation reaction, and cells were washed and resuspended in buffer A supplemented with N-ethylmaleimide (5 mm) to block all free sulfhydryl residues. After incubation for 20 min at 25 °C, cells were disrupted by sonication. Membranes recovered by ultracentrifugation for 40 min at 100,000 × g were resuspended in 1 ml of buffer B (10 mm Tris-HCl (pH 8.0), 100 mm NaCl, and 1% Triton X-100 (v/v)) supplemented with protease inhibitor mixture (Complete, Roche). After incubation on a wheel for 1 h, insoluble material was discarded by centrifugation 15 min at 20,000 × g, and solubilized proteins were subjected to immunoprecipitation using anti-FLAG-conjugated beads (M2 clone, Sigma-Aldrich). After 3 h of incubation on a wheel, beads were washed twice with 1 ml of buffer B, once with buffer B supplemented with Tween 0.1%, and once with buffer C (10 mm Tris-HCl pH 8.0, NaCl 100 mm, Triton X-100 0.1% (v/v)). Beads were air-dried, resuspended, and boiled in Laemmli buffer prior to SDS-PAGE analysis and immunodetection with anti-FLAG antibodies (to detect the proteins) or streptavidin (to detect the biotinylated proteins) coupled to alkaline phosphatase. Control experiments were performed on purified membranes instead of whole cells.
Bacterial Two-hybrid Assay
Protein-protein interactions were assessed with the adenylate cyclase-based two-hybrid technique (41) using protocols published previously (47). Briefly, the proteins to be tested were fused to the isolated T18 and T25 catalytic domains of the Bordetella adenylate cyclase. After introduction of the two plasmids producing the fusion proteins into the reporter BTH101 strain, plates were incubated at 30 °C for 48 h. Three independent colonies for each transformation were inoculated into 600 μl of LB medium supplemented with ampicillin, kanamycin, and IPTG (0.5 mm). After overnight growth at 30 °C, 10 μl of each culture was dropped onto LB plates supplemented with ampicillin, kanamycin, IPTG, and X-gal and incubated for 16 h at 30 °C. Controls included interaction assays with TolB/Pal or MalF/MalG, two protein pairs unrelated to the T9SS. The experiments were done at least in triplicate, and a representative result is shown.
Co-immunoprecipitation
1011 exponentially growing cells were harvested, resuspended in 50 mm Tris-HCl (pH 7.2), 0.5 m NaCl, and 12% PEG3350 supplemented with lysozyme (100 μg/ml) and DNase (100 μg/ml) and broken by three passages in a French press (900 p.s.i.). Unbroken cells were discarded, and lysates corresponding to 1010 cells were mixed. The mixture was sonicated (six pulses, 80% duty, Branson sonifier), incubated for 15 min at 37 °C, and sonicated (four pulses, 80% duty) before being diluted in 3 volumes of 20 mm Tris-HCl (pH 7.2), 1.35 mm EDTA, and 0.2% Igepal CA-630 supplemented with protease inhibitors (Complete, Roche). After 1 h of incubation on a wheel, insoluble material was discarded by centrifugation for 15 min at 20,000 × g, and solubilized proteins were subjected to immunoprecipitation using anti-FLAG-conjugated agarose beads (clone M2, Sigma-Aldrich) for 16 h on a wheel at 4 °C. The beads were washed twice with 1 ml of 20 mm Tris-HCl (pH 7.2), 125 mm NaCl, 1 mm EDTA, 3% PEG3350, and 0.1% Igepal CA-630 and once with the same buffer without detergent. Beads were air-dried, resuspended, and boiled in Laemmli buffer prior to SDS-PAGE analysis and immunodetection with anti-FLAG and anti-VSVG monoclonal antibodies coupled to alkaline phosphatase.
Protein Purification
Proteins were purified from Rosetta (DE3) pLysS E. coli cells (Novagen) producing the PorN protein or the PorLC or PorMP fragments cloned into the pLIC03 vector (kindly provided by BioXtal). The pLIC03 vector has been designed for ligation-independent cloning (48) and is a derivative of the pET28a+ expression vector (Novagen) in which a cassette coding for a His6 tag and a tobacco etch virus protease cleavage site followed by the suicide gene sacB flanked by BsaI restriction sites was introduced downstream of the ATG start codon. Cells were grown in ZYP-5052 autoinduction medium (49) at 37 °C for 4 h, followed by 18-h growth at 17 °C. Cells were harvested by centrifugation (4000 × g for 10 min), and the pellet was homogenized and frozen in lysis buffer (50 mm Tris-HCl (pH 8.0), 300 mm NaCl, 10 mm imidazole, 0.1 mg/ml lysozyme, and 1 mm PMSF). After thawing, DNase I (20 μg/ml) and MgSO4 (1 mm) were added, and cells were lysed by sonication. The pellet and soluble fractions were separated by centrifugation (16,000 × g for 30 min), and the proteins were purified from the soluble fraction by immobilized metal ion affinity chromatography using a 5-ml HisTrap crude (GE Healthcare) Ni2+-chelating column equilibrated in buffer A (50 mm Tris-HCl (pH 8.0), 300 mm NaCl, and 10 mm imidazole). The proteins were eluted with buffer A supplemented with 250 mm imidazole and further purified by size exclusion chromatography (HiLoad 16/60 Superdex 200 prep grade, GE Healthcare) equilibrated in 10 mm Hepes (pH 7.5) and 150 mm NaCl. For the biophysical assays, the proteins were concentrated by centrifugation using 10- or 30-kDa cutoff Amicon concentrators in the same buffer as for exclusion chromatography to 5 mg/ml. The protein concentration was determined by the absorbance of the sample at 280 nm using a NanoDrop 2000 (Thermo Scientific).
Biophysical Assays
The biophysical methods were performed as published previously (50, 51).
MALS
Size exclusion chromatography was carried out on an Alliance 2695 HPLC system (Waters) using a silica gel KW803 column (Shodex) equilibrated in 10 mm Hepes (pH 7.5) and 150 mm NaCl at a flow of 0.5 ml/min. Detection was performed using a triple-angle light-scattering detector (Mini-DAWN TREOS, Wyatt Technology), a quasi-elastic light-scattering instrument (Dynapro, Wyatt Technology), and a differential refractometer (OptilabrEX, Wyatt Technology).
Biolayer Interferometry
The purified PorN protein was first biotinylated using the EZ-Link NHS-PEG4-Biotin kit (Perbio Science). The reaction was quenched by removing the excess of biotin using a Zeba Spin desalting column (Perbio Science). Biolayer interferometry studies were performed in black 96-well plates (Greiner) at 25 °C using an OctetRed96 (ForteBio). Streptavidin biosensor tips (ForteBio) were first hydrated with 0.2 ml kinetic buffer (KB, ForteBio) for 20 min and then loaded with biotinylated PorN (10 μg/ml in KB). The association of PorN with various concentrations of PorMP (0.27, 0.83, 2.5, 7.6, and 23 μm) was monitored for 600 s, and the dissociation was followed for 1800 s in KB. Fitting of the data and constant measurements (statistical parameters: Chi2 = 2.76, R2 = 0.99) were performed with the Octet Red system software (version 7.1) using the 1:1 model. Independent experiments were run to verify that no nonspecific binding occurred, using biosensors loaded with a fragment antigen-binding (Fab) or with no protein.
Computer Analyses and Structure Modeling
Sequence signal and lipoprotein motifs were predicted using PSORTb (52), SignalP (53), and LipoP (54). Trans-membrane helix predictions were made using HMMTop (55), TMHMM (56), TMpred (57), and PHDhtm (58). Secondary structure predictions were made using the Psipred server. Structural predictions and homology modeling of the 3D structure of PorP were performed using HHpred (59) and Swiss-Model (60) using the 3.2-Å X-ray structure of the R. pickettii toluene transporter TbuX protein (PDB code 3BRY) (confidence = 98%) as template.
Miscellaneous
SDS-polyacrylamide gel electrophoresis was performed using standard protocols. For immunostaining, proteins were transferred onto nitrocellulose membranes, and immunoblots were probed with primary antibodies and goat secondary antibodies coupled to alkaline phosphatase and developed in alkaline buffer in the presence of 5-bromo-4-chloro-3-indolyl phosphate and nitro blue tetrazolium.
Author Contributions
M. S. V., M. J. C., B. I., and A. Z. performed the in vivo experiments. P. L., J. S., and C. K. purified the proteins and performed the in vitro experiments. C. C., A. R., and E. C. conceived and coordinated the study. M. S. V., P. L., A. R., and E. C. designed the experiments and prepared the figures. E. C. wrote the paper. All authors analyzed the results and approved the final version of the manuscript.
Supplementary Material
Acknowledgments
We thank Laure Journet and Eric Durand for critical reading of the manuscript; Roland Lloubès for providing antibodies against TolA, TolR, TolB, Pal, OmpA and OmpR; Romain Borne, Pascale de Philip, and Henri-Pierre Fiérobe for advice regarding growth of P. gingivalis; Haensel Fletcher and Margaret Duncan for providing protocols; Antoine Schramm and Jean-Pierre Duneau for insights regarding the PorP homology model; the members of the Cascales, Cambillau/Roussel, Lloubès, Sturgis, and Bouveret research groups for discussions; Isabelle Bringer, Annick Brun, and Olivier Uderso for technical assistance; and Kelly Diote for encouragement.
This work was supported by the CNRS, the Aix-Marseille Université, and a grant from the Agence Nationale de la Recherche (ANR-15-CE11-0019-01). The authors declare that they have no conflicts of interest with the contents of this article.

This article contains supplemental Figs. S1–S5 and Table S1.
- T9SS
- type IX secretion system
- SLS
- sodium lauroyl sarcosinate
- TMH
- trans-membrane helix
- MPB
- 3-(N-maleimidyl-propionyl) biocytin
- OM
- outer membrane
- IM
- inner membrane
- IPTG
- isopropyl 1-thio-β-d-galactopyranoside
- KB
- kinetic buffer.
References
- 1. Nakayama K. (2015) Porphyromonas gingivalis and related bacteria: from colonial pigmentation to the type IX secretion system and gliding motility. J. Periodontal. Res. 50, 1–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Sakamoto M., Umeda M., and Benno Y. (2005) Molecular analysis of human oral microbiota. J. Periodont. Res. 40, 277–285 [DOI] [PubMed] [Google Scholar]
- 3. Rôças I. N., Siqueira J. F. Jr, Santos K. R., and Coelho A. M. (2001) “Red complex” (Bacteroides forsythus, Porphyromonas gingivalis, and Treponema denticola) in endodontic infections: a molecular approach. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 91, 468–471 [DOI] [PubMed] [Google Scholar]
- 4. Potempa J., Sroka A., Imamura T., and Travis J. (2003) Gingipains, the major cysteine proteinases and virulence factors of Porphyromonas gingivalis: structure, function and assembly of multidomain protein complexes. Curr. Protein Pept. Sci. 4, 397–407 [DOI] [PubMed] [Google Scholar]
- 5. Boisvert H., Lorand L., and Duncan M. J. (2014) Transglutaminase 2 is essential for adherence of Porphyromonas gingivalis to host cells. Proc. Natl. Acad. Sci. U.S.A. 111, 5355–5360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Boisvert H., and Duncan M. J. (2010) Translocation of Porphyromonas gingivalis gingipain adhesin peptide A44 to host mitochondria prevents apoptosis. Infect. Immun. 78, 3616–3624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Veith P. D., Nor Muhammad N. A., Dashper S. G., Likić V. A., Gorasia D. G., Chen D., Byrne S. J., Catmull D. V., and Reynolds E. C. (2013) Protein substrates of a novel secretion system are numerous in the Bacteroidetes phylum and have in common a cleavable C-terminal secretion signal, extensive post-translational modification, and cell-surface attachment. J. Proteome Res. 12, 4449–4461 [DOI] [PubMed] [Google Scholar]
- 8. McBride M. J., and Zhu Y. (2013) Gliding motility and Por secretion system genes are widespread among members of the phylum Bacteroidetes. J. Bacteriol. 195, 270–278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Abby S. S., Cury J., Guglielmini J., Néron B., Touchon M., and Rocha E. P. (2016) Identification of protein secretion systems in bacterial genomes. Sci. Rep. 6, 23080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sato K., Naito M., Yukitake H., Hirakawa H., Shoji M., McBride M. J., Rhodes R. G., and Nakayama K. (2010) A protein secretion system linked to bacteroidete gliding motility and pathogenesis. Proc. Natl. Acad. Sci. U.S.A. 107, 276–281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Glew M. D., Veith P. D., Peng B., Chen Y. Y., Gorasia D. G., Yang Q., Slakeski N., Chen D., Moore C., Crawford S., and Reynolds E. C. (2012) PG0026 is the C-terminal signal peptidase of a novel secretion system of Porphyromonas gingivalis. J. Biol. Chem. 287, 24605–24617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chen Y. Y., Peng B., Yang Q., Glew M. D., Veith P. D., Cross K. J., Goldie K. N., Chen D., O'Brien-Simpson N., Dashper S. G., and Reynolds E. C. (2011) The outer membrane protein LptO is essential for the O-deacylation of LPS and the co-ordinated secretion and attachment of A-LPS and CTD proteins in Porphyromonas gingivalis. Mol. Microbiol. 79, 1380–1401 [DOI] [PubMed] [Google Scholar]
- 13. Kharade S. S., and McBride M. J. (2015) Flavobacterium johnsoniae PorV is required for secretion of a subset of proteins targeted to the type IX secretion system. J. Bacteriol. 197, 147–158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Taguchi Y., Sato K., Yukitake H., Inoue T., Nakayama M., Naito M., Kondo Y., Kano K., Hoshino T., Nakayama K., Takashiba S., and Ohara N. (2015) Involvement of an Skp-like protein, PGN_0300, in the type IX secretion system of Porphyromonas gingivalis. Infect. Immun. 84, 230–240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Shrivastava A., Johnston J. J., van Baaren J. M., and McBride M. J. (2013) Flavobacterium johnsoniae GldK, GldL, GldM, and SprA are required for secretion of the cell surface gliding motility adhesins SprB and RemA. J. Bacteriol. 195, 3201–3212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sato K., Yukitake H., Narita Y., Shoji M., Naito M., and Nakayama K. (2013) Identification of Porphyromonas gingivalis proteins secreted by the Por secretion system. FEMS Microbiol. Lett. 338, 68–76 [DOI] [PubMed] [Google Scholar]
- 17. Shoji M., Sato K., Yukitake H., Kondo Y., Narita Y., Kadowaki T., Naito M., and Nakayama K. (2011) Por secretion system-dependent secretion and glycosylation of Porphyromonas gingivalis hemin binding protein 35. PLoS ONE 6, e21372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Goulas T., Mizgalska D., Garcia-Ferrer I., Kantyka T., Guevara T., Szmigielski B., Sroka A., Millán C., Usón I., Veillard F., Potempa B., Mydel P., Solà M., Potempa J., and Gomis-Rüth F. X. (2015) Structure and mechanism of a bacterial host protein citrullinating virulence factor, Porphyromonas gingivalis peptidylarginine deiminase. Sci. Rep. 5, 11969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hasegawa Y., Iijima Y., Persson K., Nagano K., Yoshida Y., Lamont R. J., Kikuchi T., Mitani A., and Yoshimura F. (2016) Role of Mfa5 in expression of Mfa1 fimbriae in Porphyromonas gingivalis. J. Dent. Res. 95, 1291–1297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Narita Y., Sato K., Yukitake H., Shoji M., Nakane D., Nagano K., Yoshimura F., Naito M., and Nakayama K. (2014) Lack of a surface layer in Tannerella forsythia mutants deficient in the type IX secretion system. Microbiology 160, 2295–2303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Tomek M. B., Neumann L., Nimeth I., Koerdt A., Andesner P., Messner P., Mach L., Potempa J. S., and Schäffer C. (2014) The S-layer proteins of Tannerella forsythia are secreted via a type IX secretion system that is decoupled from protein O-glycosylation. Mol. Oral. Microbiol. 29, 307–320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Shrivastava A., Lele P. P., and Berg H. C. (2015) A rotary motor drives Flavobacterium gliding. Curr. Biol. 25, 338–341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Shrivastava A., and Berg H. C. (2015) Towards a model for Flavobacterium gliding. Curr. Opin. Microbiol. 28, 93–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kadowaki T., Yukitake H., Naito M., Sato K., Kikuchi Y., Kondo Y., Shoji M., and Nakayama K. (2016) A two-component system regulates gene expression of the type IX secretion component proteins via an ECF σ factor. Sci. Rep. 6, 23288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Vincent M. S., Durand E., and Cascales E. (2016) The PorX response regulator of the Porphyromonas gingivalis PorXY two-component system does not directly regulate the type IX secretion genes but binds the PorL subunit. Front. Cell Infect. Microbiol. 6, 96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Braun T. F., and McBride M. J. (2005) Flavobacterium johnsoniae GldJ is a lipoprotein that is required for gliding motility. J. Bacteriol. 187, 2628–2637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Bogdanov M., Zhang W., Xie J., and Dowhan W. (2005) Transmembrane protein topology mapping by the substituted cysteine accessibility method (SCAM(TM)): application to lipid-specific membrane protein topogenesis. Methods 36, 148–171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Glew M. D., Veith P. D., Chen D., Seers C. A., Chen Y. Y., and Reynolds E. C. (2014) Blue native-PAGE analysis of membrane protein complexes in Porphyromonas gingivalis. J. Proteomics. 110, 72–92 [DOI] [PubMed] [Google Scholar]
- 29. Gorasia D. G., Veith P. D., Hanssen E. G., Glew M. D., Sato K., Yukitake H., Nakayama K., and Reynolds E. C. (2016) Structural insights into the PorK and PorN components of the Porphyromonas gingivalis type IX secretion system. PLoS Pathog. 12, e1005820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Korotkov K. V., Johnson T. L., Jobling M. G., Pruneda J., Pardon E., Héroux A., Turley S., Steyaert J., Holmes R. K., Sandkvist M., and Hol W. G. (2011) Structural and functional studies on the interaction of GspC and GspD in the type II secretion system. PLoS Pathog. 7, e1002228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Cascales E., and Christie P. J. (2004) Agrobacterium VirB10, an ATP energy sensor required for type IV secretion. Proc. Natl. Acad. Sci. U.S.A. 101, 17228–17233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Durand E., Nguyen V. S., Zoued A., Logger L., Péhau-Arnaudet G., Aschtgen M. S., Spinelli S., Desmyter A., Bardiaux B., Dujeancourt A., Roussel A., Cambillau C., Cascales E., and Fronzes R. (2015) Biogenesis and structure of a type VI secretion membrane core complex. Nature 523, 555–560 [DOI] [PubMed] [Google Scholar]
- 33. Lloubès R., Cascales E., Walburger A., Bouveret E., Lazdunski C., Bernadac A., and Journet L. (2001) The Tol-Pal proteins of the Escherichia coli cell envelope: an energized system required for outer membrane integrity? Res. Microbiol. 152, 523–529 [DOI] [PubMed] [Google Scholar]
- 34. Rhodes R. G., Nelson S. S., Pochiraju S., and McBride M. J. (2011) Flavobacterium johnsoniae sprB is part of an operon spanning the additional gliding motility genes sprC, sprD, and sprF. J. Bacteriol. 193, 599–610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Costa T. R., Felisberto-Rodrigues C., Meir A., Prevost M. S., Redzej A., Trokter M., and Waksman G. (2015) Secretion systems in Gram-negative bacteria: structural and mechanistic insights. Nat. Rev. Microbiol. 13, 343–359 [DOI] [PubMed] [Google Scholar]
- 36. Nakane D., Sato K., Wada H., McBride M. J., and Nakayama K. (2013) Helical flow of surface protein required for bacterial gliding motility. Proc. Natl. Acad. Sci. U.S.A. 110, 11145–11150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Cascales E., Lloubès R., and Sturgis J. N. (2001) The TolQ-TolR proteins energize TolA and share homologies with the flagellar motor proteins MotA-MotB. Mol. Microbiol. 42, 795–807 [DOI] [PubMed] [Google Scholar]
- 38. Sun M., Wartel M., Cascales E., Shaevitz J. W., and Mignot T. (2011) Motor-driven intracellular transport powers bacterial gliding motility. Proc. Natl. Acad. Sci. U.S.A. 108, 7559–7564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. van den Ent F., and Löwe J. (2006) RF cloning: a restriction-free method for inserting target genes into plasmids. J. Biochem. Biophys. Methods 67, 67–74 [DOI] [PubMed] [Google Scholar]
- 40. Aschtgen M. S., Gavioli M., Dessen A., Lloubès R., and Cascales E. (2010) The SciZ protein anchors the enteroaggregative Escherichia coli type VI secretion system to the cell wall. Mol. Microbiol. 75, 886–899 [DOI] [PubMed] [Google Scholar]
- 41. Karimova G., Pidoux J., Ullmann A., and Ladant D. (1998) A bacterial two-hybrid system based on a reconstituted signal transduction pathway. Proc. Natl. Acad. Sci. U.S.A. 95, 5752–5756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Aschtgen M. S., Bernard C. S., De Bentzmann S., Lloubès R., and Cascales E. (2008) SciN is an outer membrane lipoprotein required for type VI secretion in enteroaggregative Escherichia coli. J. Bacteriol. 190, 7523–7531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Aschtgen M. S., Zoued A., Lloubès R., Journet L., and Cascales E. (2012) The C-tail anchored TssL subunit, an essential protein of the enteroaggregative Escherichia coli Sci-1 type VI secretion system, is inserted by YidC. Microbiologyopen 1, 71–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Filip C., Fletcher G., Wulff J. L., and Earhart C. F. (1973) Solubilization of the cytoplasmic membrane of Escherichia coli by the ionic detergent sodium-lauryl sarcosinate. J. Bacteriol. 115, 717–722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Jakubowski S. J., Krishnamoorthy V., Cascales E., and Christie P. J. (2004) Agrobacterium tumefaciens VirB6 domains direct the ordered export of a DNA substrate through a type IV secretion system. J. Mol. Biol. 341, 961–977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Goemaere E. L., Devert A., Lloubès R., and Cascales E. (2007) Movements of the TolR C-terminal domain depend on TolQR ionizable key residues and regulate activity of the Tol complex. J. Biol. Chem. 282, 17749–17757 [DOI] [PubMed] [Google Scholar]
- 47. Zoued A., Durand E., Brunet Y. R., Spinelli S., Douzi B., Guzzo M., Flaugnatti N., Legrand P., Journet L., Fronzes R., Mignot T., Cambillau C., and Cascales E. (2016) Priming and polymerization of a bacterial contractile tail structure. Nature 531, 59–63 [DOI] [PubMed] [Google Scholar]
- 48. Aslanidis C., and de Jong P. J. (1990) Ligation-independent cloning of PCR products (LIC-PCR). Nucleic Acids Res. 18, 6069–6074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Studier F. W. (2005) Protein production by auto-induction in high-density shaking cultures. Protein Expr. Purif. 41, 207–234 [DOI] [PubMed] [Google Scholar]
- 50. Felisberto-Rodrigues C., Durand E., Aschtgen M. S., Blangy S., Ortiz-Lombardia M., Douzi B., Cambillau C., and Cascales E. (2011) Towards a structural comprehension of bacterial type VI secretion systems: characterization of the TssJ-TssM complex of an Escherichia coli pathovar. PLoS Pathog. 7, e1002386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Flaugnatti N., Le T. T., Canaan S., Aschtgen M. S., Nguyen V. S., Blangy S., Kellenberger C., Roussel A., Cambillau C., Cascales E., and Journet L. (2016) A phospholipase A1 antibacterial type VI secretion effector interacts directly with the C-terminal domain of the VgrG spike protein for delivery. Mol. Microbiol. 99, 1099–1118 [DOI] [PubMed] [Google Scholar]
- 52. Yu N. Y., Wagner J. R., Laird M. R., Melli G., Rey S., Lo R., Dao P., Sahinalp S. C., Ester M., Foster L. J., and Brinkman F. S. (2010) PSORTb 3.0: improved protein subcellular localization prediction with refined localization subcategories and predictive capabilities for all prokaryotes. Bioinformatics 26, 1608–1615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Petersen T. N., Brunak S., von Heijne G., and Nielsen H. (2011) SignalP 4.0: discriminating signal peptides from transmembrane regions. Nat. Methods 8, 785–786 [DOI] [PubMed] [Google Scholar]
- 54. Juncker A. S., Willenbrock H., Von Heijne G., Brunak S., Nielsen H., and Krogh A. (2003) Prediction of lipoprotein signal peptides in Gram-negative bacteria. Protein Sci. 12, 1652–1662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Tusnády G. E., and Simon I. (1998) Principles governing amino acid composition of integral membrane proteins: application to topology prediction. J. Mol. Biol. 283, 489–506 [DOI] [PubMed] [Google Scholar]
- 56. Krogh A., Larsson B., von Heijne G., and Sonnhammer E. L. (2001) Predicting transmembrane protein topology with a hidden Markov model: application to complete genomes. J. Mol. Biol. 305, 567–580 [DOI] [PubMed] [Google Scholar]
- 57. Hofmann K., and Stoffel W. (1993) A database of membrane spanning protein segments. Biol. Chem. 374, 166 [Google Scholar]
- 58. Rost B., Fariselli P., and Casadio R. (1996) Topology prediction for helical transmembrane proteins at 86% accuracy. Protein Sci. 5, 1704–1718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Söding J., Biegert A., and Lupas A. N. (2005) The HHpred interactive server for protein homology detection and structure prediction. Nucleic Acids Res. 33, W244–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Biasini M., Bienert S., Waterhouse A., Arnold K., Studer G., Schmidt T., Kiefer F., Gallo Cassarino T., Bertoni M., Bordoli L., and Schwede T. (2014) SWISS-MODEL: modelling protein tertiary and quaternary structure using evolutionary information. Nucleic Acids Res. 42, W252–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.