Abstract
Objective:
Ankaferd hemostat is the first topical hemostatic agent about the red blood cell–fibrinogen relations tested in the clinical trials. Ankaferd hemostat consists of standardized plant extracts including Alpinia officinarum, Glycyrrhiza glabra, Thymus vulgaris, Urtica dioica, and Vitis vinifera. The aim of this study was to determine the effect of Ankaferd hemostat on viability of melanoma cell lines.
Methods:
Dissimilar melanoma cell lines and primary cells were used in this study. These cells were treated with different concentrations of Ankaferd hemostat to assess the impact of different dosages of the drug. All cells treated with different concentrations were incubated for different time intervals. After the data had been obtained, one-tailed T-test was used to determine whether the Ankaferd hemostat would have any significant inhibitory impact on cell growth.
Results:
We demonstrated in this study that cells treated with Ankaferd hemostat showed a significant decrease in cell viability compared to control groups. The cells showed different resistances against Ankaferd hemostat which depended on the dosage applied and the time treated cells had been incubated. We also demonstrated an inverse relationship between the concentration of the drug and the incubation time on one hand and the viability of the cells on the other hand, that is, increasing the concentration of the drug and the incubation time had a negative impact on cell viability.
Conclusion:
The findings in our study contribute to our knowledge about the anticancer impact of Ankaferd hemostat on different melanoma cells.
Keywords: Ankaferd hemostat, anticancer, melanoma
Introduction
Ankaferd hemostat (ABS) is the first topical hemostatic agent about the red blood cell (RBC)–fibrinogen relations tested in the clinical trials.1 ABS consists of standardized plant extracts including Alpinia officinarum, Glycyrrhiza glabra, Thymus vulgaris, Urtica dioica, and Vitis vinifera.2 ABS-stimulated pharmacological modulation of essential erythroid proteins (ankyrin, spectrin, and actin) can lead to vital eythroid aggregation via acting on fibrinogen gamma.3 The pleiotropic effects of ABS on vascular endothelium, blood cells, angiogenesis, cellular proliferation, vascular dynamics, and cellular mediators have been investigated.4–8 The use of ABS in the gastrointestinal (GI) system hemorrhages to control bleeding and/or infected GI wounds is also evident.9 Controlled clinical trials indicated the safety and efficacy of ABS for the control of clinical bleedings in an extensive variety of settings.10–17 Since the survival rates of metastatic melanoma 5 years had remained below 25%, there is a continued need for new therapeutic and/or complementary approaches in this field.18 For some tumors, plant extracts may have a beneficial anti-tumor effect and may work synergistically with the standard chemotherapeutics.
Melanocytes are the cells that produce “melanin” pigment giving the skin its color. They are present in the basal layer of the epidermis and protect the underlying layers of the skin from sun ray and other environmental factors. Melanocytes can turn into melanoma if their DNA undergoes any damage.19 Melanomas can be seen everywhere in the body and mainly appear as moles. Benign moles have the potential to turn into melanomas.20 There are other types of skin cancer: basal cell and squamous cell cancers (often called non-melanoma skin cancers) which are more responsive to medical treatment than melanoma. Melanomas can also metastasize through lymph nodes to internal organs.21 The number of patients diagnosed with melanoma has been increasing recently and approximately 53,000 people die annually of melanoma worldwide.21 The aim of this study was to determine the effect of ABS on viability of melanoma cells.
Materials and methods
Cells and cell lines
The primary cells were from Hadassah Medical Center in Jerusalem. Cell lines were from İstanbul University. M7, M24, M307, and M133 were used as primary cells. The following cell lines were used for this study: SK-MEL-10 (CVCL_6020), SK-MEL-9 (CVCL_U934), A2058 (ATCC® CRL-11147™), and MeWo (ATCC HTB-65™). All of the ethical considerations were strictly handled in accordance with the Helsinki Declaration.
Cell culture assays
The cells were developed in Roswell Park Memorial Institute (RPMI) 1640 medium containing 10% fetal bovine serum, 1% penicilium/streptomycin, and 1% l-glutamine. They were incubated at 37°C with 5% level of CO2 in cell culture until they reached 70% confluency.
In vitro cytotoxicity assays
ABS, a combination of different plants as described in the introduction, was used to treat the cells. (100 mL product includes 6 mg dried root extract of Urtica dioica, 8 mg dried leaf extract of Vitis vinifera, 9 mg dried leaf extract of Glycyrrhiza glabra, 7 mg dried leaf extract of Alpinia officinarum, and 5 mg dried grass extract of Thymus vulgaris.) Cells and media were cultured to the plates. Each well contained 5000 cells and 100 µL final volume. Three plates were prepared with A2058 cell line. ABS concentrations were prepared by diluting with phosphate-buffered saline (PBS; 100%, 87.5%, 75%, 62.5%, 50%, 37.5%, 25%, and 12.5% of ABS). They were incubated for 2, 5, and 8 h, respectively. After different time intervals of incubation, the plates were treated with CellTiter-Glo® (Promega) reagent. The viability of the cells was measured by Biotech Microplate Reader 15 min of applying the reagent. According to the results, the same experiment design was modified and applied to all of the cells. Cell suspension and medium mixture were cultured to the plates. Each well contained 1000 cells and 100 µL volume in total. Five plates for each cell (40 plates in total) were prepared. Different concentrations of ABS were prepared (25%, 12.5%, 6.25%, 3.13%, 1.56%, 0.78%, 0.39%, and 0.19%). Control group consisted of 100% ABS, 100% PBS, and 100% RPMI 1640 medium. Three wells were used for each concentration. After 24 h, the media in the wells were replaced with prepared concentrations (100 µL in total). First plate of each cell was not placed into the incubator after treatment. The other plates were incubated for 30, 60, 90, and 120 min, respectively. The plates which were not incubated were treated with CellTiter-Glo reagent following 15 min after treatment with the ABS-PBS mixtures. The other plates were treated with the reagent following their incubation intervals. All plates were read by Biotech Microplate Reader following 15 min of applying the reagent.
Statistical analyses
One-tailed T-test was used to analyze the data using GraphPad Prism Version 6.01 (GraphPad Software, Inc., CA, USA). T-test was used to compare the optical density (OD) values of the treated cells and untreated cells. Statistical significance was declared at p < 0.05. All the values are reported as means ± standard deviation (SD), and they are calculated using Microsoft Excel Ver.3.2013.
Results
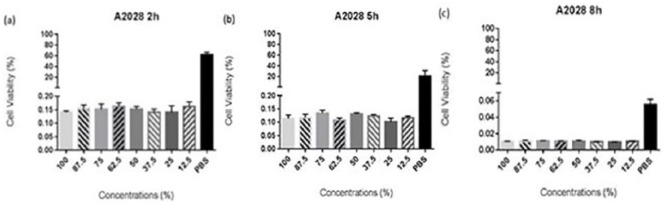
ABS concentrations affected the cells by decreasing their viability for all three time periods (Figure 1). The first experimental model was used to determine the relation between time and effectiveness of different ABS concentrations. For this purpose, different concentrations of ABS were prepared (including controls as 100% ABS, 100% medium, and 100% PBS). The cells were treated with different concentrations of ABS and incubated for 2, 5, and 8 h to evaluate the cell viabilities in PBS. After results are obtained from the first experiment model, it was seen that when time increases, the viability of the cells which were treated with PBS decreases. It means that the experiment conducted for 5 and 8 h was not reliable in the sense that the cell viability decreased not just because of ABS but also because of PBS. Considering these findings, the maximum time period was limited to 2 h for each experiment.
Figure 1.
The survival of the cells within PBS ABS mixture. Using A2058 cell line, three plates were prepared by diluting ABS with PBS in different concentrations (100%, 87.5%, 75%, 62.5%, 50%, 37.5%, 25%, and 12.5% of ABS). Following the 2, 5, and 8 h of incubation, the cell viabilities were measured using CellTiter-Glo (Promega) reagent and the Biotech Microplate Reader.
In the light of the first experimental findings, ABS ratio in the ABS mixtures was minimized so that the least amount of ABS that has significant effect on the melanoma cells can be observed. The new concentrations were limited to a maximum amount of 25% and the time for incubation was limited to 30, 60, 90, and 120 min and then measured by CellTiter-Glo Luminescent Cell Viability Assay (Figures 2–4). Moreover, cell viability for one plate of each cell type was measured after 15 min of incubation. The second experimental model which was applied to cells confirmed the anticancer effects of ABS. As it is seen, different incubation times have different effects on the primary cells (Figure 4). All primary cells were affected by ABS concentrations as evidenced by a decrease in cell viabilities. 1.56% was the lowest statistically significant ABS concentration which was effective on all the cells in all incubation intervals. We believe that this effect was not caused by PBS in the mixture since treating with only PBS did not have a significant effect on the cell viability. It can be concluded that ABS created statistically significant differences between the control group and the cells treated with the mixture.
Figure 2.
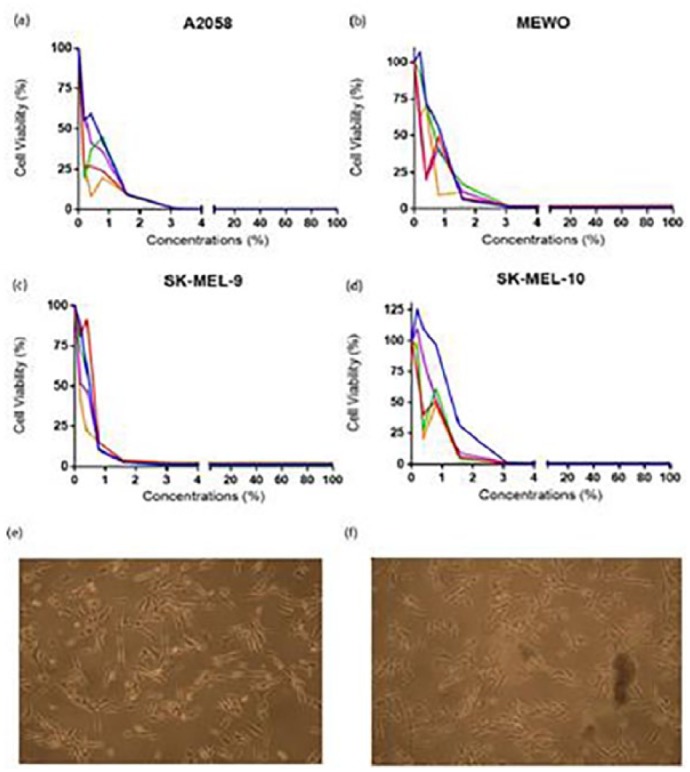
(a) A2058, (b) MeWo, (c) SK-MEL-9, and (d) SK-MEL-10 show cell viability versus concentrations applied. The cell viability for the cell lines have been decreased as the applied concentration has increased. The microscopic images (bright field, 10× magnification) taken (e) before the concentrations applied to M307 cell line and (f) after are shown.
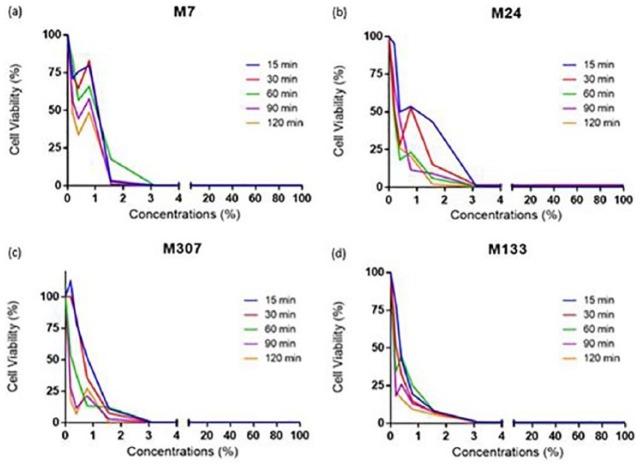
Figure 3.
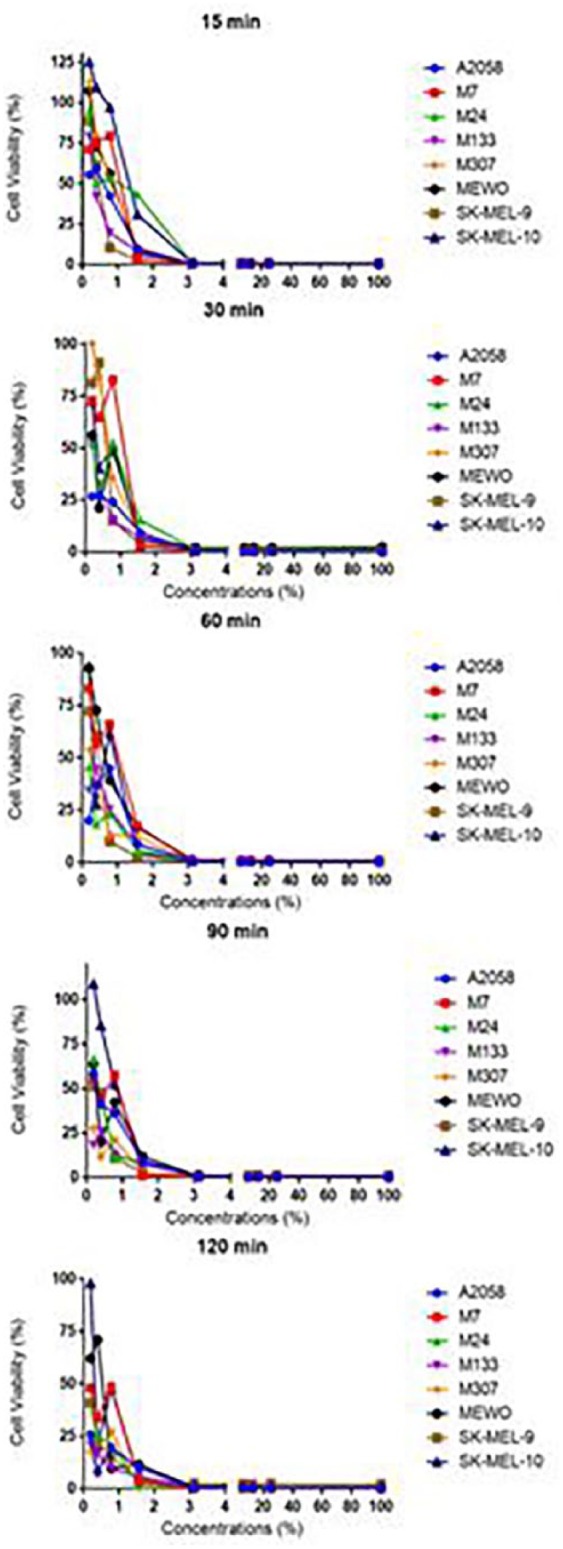
The cell viability versus applied concentrations for different incubation times; five plates were prepared for each cell line primary cells. After treating with different concentrations (25%, 12.5%, 6.25%, 3.13%, 1.56%, 0.78%, 0.39%, and 0.19% of ABS), and incubating for 15, 30, 60, 90, and 120 min, the cell viability was measured using CellTiter-Glo (Promega) reagent and the Biotech Microplate Reader.
Figure 4.
The cell viability versus concentrations applied for primary cells M7, M24, M307, and M133. 25%, 12.5%, 6.25%, 3.13%, 1.56%, 0.78%, 0.39%, and 0.19% of ABS have been applied and the cell viability was measured using CellTiter-Glo (Promega) reagent and the Biotech Microplate Reader after 15, 30, 60, 90, and 120 min of incubation.
It was seen that primary cells have different resistances against the drug. Effectiveness of ABS is different in cell lines compared to primary cells which can be seen from figures and the p-values calculated for both cell types (Figures 2 and 4). The incubation time did not make any difference at ABS concentrations above 3.125%. At these concentrations regardless of incubation time, ABS showed a similar effect on cell viabilities. This indicates that at high ABS concentrations, 15 min of incubation is enough to decrease the cell viability to a significant level, which is supported by the corresponding p-values. However, the incubation time is an important factor for ABS concentrations below 3.125%. When concentration of ABS is below 1%, incubating A2058 cell line for 120 min was more effective than incubating for 15 min. The p-values calculated for these cell lines demonstrate that even though 120 min of incubation had a higher impact on the cells, all of the incubation times were significantly effective on the cells for all of the concentrations used. These data show that A2058 cells are not resistant to the drug at any concentration during any incubation time, while SK-MEL-10 was resistant to the mixtures below 1.56% for all the incubation times. It can be concluded that different cell lines have different resistance against the drug used in this study. The data demonstrate that 0.19% is the lowest concentration of ABS to affect A2058 cell line in 15, 30, 60, 90, and 120 min. Therefore, it is concluded that applying 0.19% ABS for 15 min is enough for the drug to exert its maximum effect on A2058 cell line. ABS concentration of 0.19% affected different primary cells and cell lines in different time periods. Those periods were determined as 120 min for SK-MEL-9, 90 min for MeWo, 90 min for M7, 30 min for M24, 90 min for M133, and 120 min for M307. However, in 120 min, the concentrations (0.19%, 0.39%, and 0.78%) were not effective on SK-MEL-10 cell line, whereas ABS exerted an effect in 30 min at 1.56% concentration.
In conclusion, it has been observed that the most resistant cell type was SK-MEL-10, and the least resistant cell type was A2058 since the effect of 0.19% ABS could be seen in just 15 min. The anticancer effect of ABS was also apparent on the light microscopic images of untreated M307 primary cell. The images were obtained by bright field microscope with 10× magnification after 2 h of 3.125% ABS treatment (Figure 2). In Figure 2, untreated cells are piled and detached from the surface, demonstrating the difference caused by ABS.
Discussion
The antineoplastic effects of ABS were first investigated on Saos-2 osteosarcoma cell existence and development.1 Saos-2, an osteosarcoma cell line often employed in drug resistance studies, was developed in RPMI media containing 10% fetal calf serum (FCS), 1% pen/strep, and 1% Na-pyruvate. After the development, cells were transported into 12-well tissue plates, in which 2, 4, 6, 8, and 10 µL/mL concentrations of ABS solution was mixed with the growth medium. Control group was cultured in growth media in the absence of ABS. Growth of Saos-2 cells was followed-up for 17 days throughout which yellow and opaque-looking aggregates were reported in cultures developing in the presence of ABS. There was a dose-dependent suppression in cell proliferation and a notable decrease was detected in the survival of Saos-2 cells. Aggregate formation amplified with higher doses of ABS and dose-dependent suppression was detected in cell invasion. ABS-treated Saos-2 osteosarcoma cells lose adhesion in vitro.1 Following this initial study,1 effective antineoplastic role of ABS on colon cancer was further verified. Two types of cells were placed independently in 12-well tissue culture containers, and ABS solutions with 2, 4, 6, 8, and 10 µL/mL concentrations were mixed with the culture medium. The cells developed in culture medium in the absence of ABS were used as control. The growth of CaCo-2 and Saos-2 cells was followed-up for 16 days. ABS application to culture medium leads to yellow and cloudy aggregates, increasing in parallel with ABS concentration. Consequently, it was realized that the suppression of cellular reproduction and reduction in viability of human colon CaCo-2 cells were correlated with applied ABS concentration in vitro. The data have proven that the invasion of cells was also suppressed in a dose-dependent manner. The suppressor effect of ABS on CaCo-2 cells was detected at 2 µL/mL level and becomes more prominent at 10 µL/mL concentration. It was also realized that CaCo-2 cells that were exposed to ABS lost their adhesive features in vitro and significant viability loss was detected.1
In a recent study, it was shown that intravesical administration of ABS is at least as efficacious as epinephrine in terms of congestion, edema, necrosis, and ulceration.22 Moreover, ABS can be considered as a better option in inducing regenerating epithelium than epinephrine.22 ABS can induce apoptosis, regulate cellular proliferation, and decrease tumor vascularization.23–27 Experimental antineoplastic activities of ABS have been shown in leukemic and lymphoid neoplastic cell lines.28 ABS could alter proteinase-activated receptor 1 (PAR1) and endothelial protein C receptor (EPCR) expression in K562 and Jurkat cells in a time- and dose-dependent manner. Additionally, ABS treatment had induced apoptosis in leukemia cells. Possible involvement of PAR1 and p21 in the apoptotic process was observed in Jurkat cells.29 Antineoplastic effects of ABS at higher doses (>0.5 mu g/mL) and induction of cellular differentiation at lower ABS doses (<0.5 mu g/mL) in leukemic cells28 could highlight the importance of dose schedule for the administration in experimental and future clinical trials. In a study by Odabas et al.,30 the cytotoxicity of ABS was evaluated on human pulp fibroblasts in vitro. ABS was eluted with fresh Dulbecco’s Modified Eagle’s Medium (DMEM) without serum for 72 h at 37°C. The results of Odabas’ study had shown that ABS is cytotoxic to human pulp fibroblasts by modified 3-(4,5-dimethyl-thiazol-2-yl)-2,5-diphenyl-tetrazolium bromide assay, which depended on the concentration of ABS applied. More dilutions exhibited less cytotoxic characteristics compared to the more concentrated forms on human pulp fibroblasts.30 ABS can cause vital erythroid aggregation that takes place in conjunction with the spectrin and ankyrin receptors on RBC membranes. Essential erythroid proteins are spectrin alpha, actin-depolymerization factor, nicotinamide adenine dinucleotide-hydrogen (NADH) dehydrogenase [ubiquinone] 1 alpha subcomplex, and mitochondrial nicotinamide adenosine dinucleotide phosphate (NADP) [+] dependent malic enzyme.31 ABS affects the levels of various critical proteins and factors including protein 2 (AP2), androgen receptor (AR), cyclic adenosine monophosphate (AMP) response element or activating transcription factor 1 (CRE-ATF1), cyclic AMP response element binding protein (CREB), E2F1-5, E2F6, interferon (IFN)-stimulated response element (ISRE), Myc-Max, nuclear factor-1 (NF-1), protein 53 or tumor protein 53 (p53), peroxisome proliferator-activated receptor (PPAR), and Yin Yang 1 (YY1) transcription factors.32 These regulator molecules affect distinct steps of cellular proliferation such as cell vascular hemostasis, angiogenesis, signal transduction, apoptosis, inflammation, acute phase reaction, immunity, and several metabolic molecular pathways. Therefore, the protein content and their effects on the transcriptomics may be active in the cellular action of ABS.1,32
Natural products are commonly preferred for the production of anticancer drugs as the desired property of an anticancer drug is to have the ability to kill the cancer cells and cause no harm to normal human cells. ABS can decrease the viability of the cells significantly and is reported to have no cytotoxic properties.33 Those quantities make ABS a potential drug for the management of melanoma. The experiments presented in this study were undertaken on human cells. We aimed to investigate whether the drug had any cytotoxic impact on normal cells in our study. The results demonstrate that ABS is not cytotoxic on normal cells at any prescribed dosage or any incubation interval. The study aimed to assess the growth inhibitory activity of ABS on primary melanoma cells (M7, M24, M133, and M307) and cell lines (SK-MEL-9, SK-MEL-10, MeWo, and A2058). To achieve this, melanoma cell lines and primary cells were treated with different concentrations of ABS and incubated for different time intervals. Wells treated with ABS had lower OD values, whereas the untreated ones had higher OD values, indicating that ABS caused a significant decrease in the viability of the cells. Different cells have different resistance levels against ABS. Although in some previous studies the effect of ABS was observed after more than 10 days, in this study, ABS was applied to the cancer cells for a maximum of 2 h.28,29,34,35 Therefore, it can be said that this model is more efficient. There is limited number of studies on anticancer effect of ABS. Göker et al.35 investigated the anticancer impact of ABS on the viability and growth of osteosarcoma cells (Saos-2). The authors reported an inhibition in cell growth on Day 17, following treatment of the cells with 2 µL/mL ABS.35 They also showed that when the concentration of the drug increased from 2 to 10 µL, the inhibitor effect of the drug was observed significantly. The authors concluded that the inhibitory effect of the drug was correlated with its concentration; however, the team did not manage to explain the exact mechanism behind it.35 It is known that ABS has several effects on cell cycle regulation, apoptosis, angiogenesis, signal transduction, inflammation, immunologic processes, and metabolic pathways. Therefore, this study focuses on the impact of ABS on melanoma cells (primary cells and cell lines).29 Goker et al.34 also investigated the impact of ABS on colon cancer cells (CaCo-2) and reported a decrease in cell viability following 16 days of being treated with ABS. The effect of the drug began to appear at 2 µL and to become significant at 10 µL; however, the authors did not report on the anticancer mechanism of the drug. In Akalin et al.,28 the impact of ABS on the mesenchymal stem cells has been investigated. The authors observed that depending on the applied dosage of ABS, the cells stuck to each other and their growth was inhibited. Yet, another study by Mumcuoglu et al.29 on the anticancer effects of ABS on leukemia cells reported that the expression of PAR1 and EPCR depended on the dosage of ABS applied on K562 and Jurkat cells. In addition, it has been discovered that ABS triggers the apoptosis of the leukemia cells.29 Akalin et al.28 reported that B-cell chronic lymphocytic leukemia (B-CLL)cells treated with ABS (dosage: 0.5, 1, and 2 µg/mL) prevented the inflammation and, compared to 0.1 and 0.25 µg/mL, managed to kill more than 50% of the cells. The authors also observed that ABS, when added to the medium, prevented the transformation of the BCLLs into aggressive blastic lymphocytes. The team examined the antineoplastic effects at high doses and the promotion of cell differentiation at low doses. The main limitation of this study is the lack of in vivo models for the validation of the in vitro data obtained from myeloma cell lines. Nevertheless, our promising preliminary data shall be considered for the design of melanoma mice models subjected to the systemic, oral, and/or topical intra-tumoral application of ABS to demonstrate the impact of the drug on the progression of the living body melanoma disease. To conclude, the findings in our study contribute to our knowledge about the anticancer impact of ABS on different melanoma cells. In future, more studies should be conducted to clarify the anticancer impact of ABS.
Acknowledgments
The authors would like to thank İstanbul University for providing the cells.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical approval: Ethical approval was not sought for this study because the research was performed in cell lines in vitro that are not subjected to the ethical committee approval.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by Research Fund of the Hacettepe University (project no. THD-2015-6479).
References
- 1. Haznedaroglu BZ, Beyazit Y, Walker SL, et al. Pleiotropic cellular, hemostatic, and biological actions of Ankaferd hemostat. Crit Rev Oncol Hematol 2012; 83(1): 21–34. [DOI] [PubMed] [Google Scholar]
- 2. Beyazit Y, Kurt M, Kekilli M, et al. Evaluation of hemostatic effects of Ankaferd as an alternative medicine. Altern Med Rev 2010; 15(4): 329–336. [PubMed] [Google Scholar]
- 3. Ozel-Demiralp D, Igci N, Ayhan B, et al. Prohemostatic and antithrombin activities of Ankaferd hemostat are linked to fibrinogen gamma chain and prothrombin by functional proteomic analyses. Clin Appl Thromb Hemost 2012; 18(6): 604–610. [DOI] [PubMed] [Google Scholar]
- 4. Deveci A, Coban AY, Tanriverdi Cayci Y, et al. In vitro effect of Ankaferd Blood Stopper®: a plant extract against mycobacterium tuberculosis isolates. Mikrobiyoloji Bulteni 2013; 47(1): 71–78 (in Turkish). [DOI] [PubMed] [Google Scholar]
- 5. Akkoc N, Akcelik M, Haznedaroglu IC, et al. In vitro anti-bacterial activities of Ankaferd medicinal plant extract. Turkiye Klinikleri Tip Bilimleri Dergisi 2009; 29(2): 410–415. [Google Scholar]
- 6. Ciftci S, Keskin F, Ozcan SK, et al. In vitro antifungal activity of Ankaferd Blood Stopper against candida albicans. Curr Ther Res Clin Exp 2011; 72(3): 120–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Fisgin NT, Cayci YT, Coban AY, et al. Antimicrobial activity of plant extract Ankaferd Blood Stopper®. Fitoterapia 2009; 80(1): 48–50. [DOI] [PubMed] [Google Scholar]
- 8. Saribas Z, Sener B, Haznedaroglu IC, et al. Antimicrobial activity of Ankaferd Blood Stopper® against nosocomial bacterial pathogens. Cent Eur J Med 2010; 5(2): 198–202. [Google Scholar]
- 9. Beyazit Y, Kekilli M, Haznedaroglu IC, et al. Ankaferd hemostat in the management of gastrointestinal hemorrhages. World J Gastroenterol 2011; 17(35): 3962–3970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Akpinar MB, Atalay A, Atalay H, et al. Ankaferd blood stopper decreases postoperative bleeding and number of transfusions in patients treated with clopidogrel: a double-blind, placebo-controlled, randomized clinical trial. Heart Surg Forum 2015; 18(3): E118–E123. [DOI] [PubMed] [Google Scholar]
- 11. Beyazit Y, Kart T, Kuscu A, et al. Successful management of bleeding after dental procedures with application of blood stopper: a single center prospective trial. J Contemp Dent Pract 2011; 12(5): 379–384. [DOI] [PubMed] [Google Scholar]
- 12. Iynen I, Bozkus F, San I, et al. The hemostatic efficacy of Ankaferd Blood Stopper in adenoidectomy. Int J Pediatr Otorhinolaryngol 2011; 75(10): 1292–1295. [DOI] [PubMed] [Google Scholar]
- 13. Keceli HG, Aylikci BU, Koseoglu S, et al. Evaluation of palatal donor site haemostasis and wound healing after free gingival graft surgery. J Clin Periodontol 2015; 42(6): 582–589. [DOI] [PubMed] [Google Scholar]
- 14. Odabas ME, Cinar C, Tulunoglu O, et al. A new haemostatic agent’s effect on the success of calcium hydroxide pulpotomy in primary molars. Pediatr Dent 2011; 33(7): 529–534. [PubMed] [Google Scholar]
- 15. Teker AM, Korkut AY, Gedikli O, et al. Prospective, controlled clinical trial of Ankaferd Blood Stopper in children undergoing tonsillectomy. Int J Pediatr Otorhinolaryngol 2009; 73(12): 1742–1745. [DOI] [PubMed] [Google Scholar]
- 16. Teker AM, Korkut AY, Kahya V, et al. Prospective, randomized, controlled clinical trial of Ankaferd Blood Stopper in patients with acute anterior epistaxis. Eur Arch Otorhinolaryngol 2010; 267(9): 1377–1381. [DOI] [PubMed] [Google Scholar]
- 17. Yaman E, Gorken F, Pinar Erdem A, et al. Effects of folk medicinal plant extract Ankaferd Blood Stopper® in vital primary molar pulpotomy. Eur Arch Paediatr Dent 2012; 13(4): 197–202. [DOI] [PubMed] [Google Scholar]
- 18. Serrano OK, Parrow NL, Violet PC, et al. Antitumor effect of pharmacologic ascorbate in the B16 murine melanoma model. Free Radic Biol Med 2015; 87: 193–203. [DOI] [PubMed] [Google Scholar]
- 19. Pfeifer GP, Besaratinia A. UV wavelength-dependent DNA damage and human non-melanoma and melanoma skin cancer. Photochem Photobiol Sci 2012; 11: 90–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Tsao H, Bevona C, Goggins W, et al. The transformation rate of moles (melanocytic nevi) into cutaneous melanoma: a population-based estimate. Arch Dermatol 2003; 139: 282–288. [DOI] [PubMed] [Google Scholar]
- 21. Fellner C. Ipilimumab (Yervoy) prolongs survival in advanced melanoma: serious side effects and a hefty price tag may limit its use. P T 2012; 7: 503–530. [PMC free article] [PubMed] [Google Scholar]
- 22. Kilic O, Akand M, Karabagli P, et al. Hemostatic efficacy and histopathological effects of Ankaferd Blood Stopper in an experimental rat model of cyclophosphamide-induced hemorrhagic cystitis. Urology 2016; 94: 313. [DOI] [PubMed] [Google Scholar]
- 23. Aktas B, Basar O, Yilmaz B, et al. Serum M30 and M65 levels and effects of Ankaferd blood stopper in cerulein induced experimental acute pancreatitis model in rats. Int J Clin Exp Med 2014; 7(7): 1676–1683. [PMC free article] [PubMed] [Google Scholar]
- 24. Eren E, Basoglu MS, Kulduk E, et al. Mucosal trauma induced apoptosis in guinea pig middle ear: comparison of hemostatic agents. Int J Pediatr Otorhinolaryngol 2014; 78(12): 2222–2228. [DOI] [PubMed] [Google Scholar]
- 25. Huri E, Akgul T, Astarci M, et al. The effect of a novel hemostatic agent, Ankaferd Bloodstopper® (Abs), on renal tubular apoptosis in rat partial nephrectomy model. J Endourol 2009; 23: A2.19891543 [Google Scholar]
- 26. Huri E, Haznedaroglu IC, Akgul T, et al. Biphasic effects of Ankaferd blood stopper on renal tubular apoptosis in the rat partial nephrectomy model representing distinct levels of hemorrhage. Saudi Med J 2010; 30(8): 864–868. [PubMed] [Google Scholar]
- 27. Karabiyik A, Gulec S, Yilmaz E, et al. Reversible protease-activated receptor 1 downregulation mediated by Ankaferd blood stopper inducible with lipopolysaccharides inside the human umbilical vein endothelial cells. Clin Appl Thromb Hemost 2011; 17(6): E165–E170. [DOI] [PubMed] [Google Scholar]
- 28. Akalin I, Okur FV, Haznedaroglu IC, et al. Acute in vitro effects of ABS (Ankaferd hemostat) on the lymphoid neoplastic cells (B-CLL and RAJI tumor cell lines). UHOD 2014; 24(4): 253–259. [Google Scholar]
- 29. Mumcuoglu M, Akin DF, Ezer U, et al. Ankaferd Blood Stopper induces apoptosis and regulates PAR1 and EPCR expression in human leukemia cells. Egypt J Med Human Gen 2015; 16(1): 19–27. [Google Scholar]
- 30. Odabas ME, Erturk M, Cinar C, et al. Cytotoxicity of a new hemostatic agent on human pulp fibroblasts in vitro. Med Oral Patol Oral Cir Bucal 2011; 16(4): e584–e587. [PubMed] [Google Scholar]
- 31. Demiralp DO, Haznedaroglu IC, Akar N. Functional proteomic analysis of Ankaferd® Blood Stopper. Turk J Hematol 2010; 27(2): 70–77. [DOI] [PubMed] [Google Scholar]
- 32. Yilmaz E, Gulec S, Torun D, et al. The effects of Ankaferd® Blood Stopper on transcription factors in HUVEC and the erythrocyte protein profile. Turk J Hematol 2011; 28(4): 276–285. [DOI] [PubMed] [Google Scholar]
- 33. Isler SC, Demircan S, Cakarer S, et al. Effects of folk medicinal plant extract Ankaferd Blood Stopper on early bone healing. J Appl Oral Sci 2010; 18: 409–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Goker H, Kilic E, Cetinkaya D. Anti-cancer activity of Ankaferd on human colon cancer (CaCo-2) in vitro. In: Haznedaroglu IC, Goker H, Ozdemir O, et al. (eds) Ankaferd: scientific perspectives and basic-clinical data. Istanbul, Turkey: Naviga Publications, 2008, p. 108. [Google Scholar]
- 35. Goker H, Cetinkaya D, Kilic E. Anti-cancer activity of Ankaferd Blood Stopper on osteosarcom (SAOS-2) cell lines in vitro. In: Haznedaroglu IC, Goker H, Ozdemir O, et al. (eds) Ankaferd: scientific perspectives and basic-clinical data. Istanbul, Turkey: Naviga Publications, 2008, p. 109. [Google Scholar]