Abstract
Few studies have evaluated the mortality or quantified the economic burden of community-onset Clostridium difficile infection (CDI). We estimated the attributable mortality and costs of community-onset CDI. We conducted a population-based matched cohort study. We identified incident subjects with community-onset CDI using health administrative data (emergency department visits and hospital admissions) in Ontario, Canada between January 1, 2003 and December 31, 2010. We propensity-score matched each infected subject to one uninfected subject and followed subjects in the cohort until December 31, 2011. We evaluated all-cause mortality and costs (unadjusted and adjusted for survival) from the healthcare payer perspective (2014 Canadian dollars). During our study period, we identified 7,950 infected subjects. The mean age was 63.5 years (standard deviation = 22.0), 62.7% were female, and 45.0% were very high users of the healthcare system. The relative risk for 30-day, 180-day, and 1-year mortality were 7.32 (95% confidence interval [CI], 5.94–9.02), 3.55 (95%CI, 3.17–3.97), and 2.59 (95%CI, 2.37–2.83), respectively. Mean attributable cumulative 30-day, 180-day, and 1-year costs (unadjusted for survival) were $7,434 (95%CI, $7,122-$7,762), $12,517 (95%CI, $11,687-$13,366), and $13,217 (95%CI, $12,062-$14,388). Mean attributable cumulative 1-, 2-, and 3-year costs (adjusted for survival) were $10,700 (95%CI, $9,811-$11,645), $13,312 (95%CI, $12,024-$14,682), and $15,812 (95%CI, $14,159-$17,571). Infected subjects had considerably higher risk of all-cause mortality and costs compared with uninfected subjects. This study provides insight on an understudied patient group. Our study findings will facilitate assessment of interventions to prevent community-onset CDI.
Introduction
Clostridium difficile infection (CDI) has been deemed “a leading cause of patient harm” by the Centers for Disease Control and Prevention with 453,000 infections, 83,000 recurrences, and 29,300 deaths estimated in the United States in 2011 [1, 2]. Approximately 29% to 58% of CDI cases have symptom onset in the community or within two or three days of a hospital admission [3–6]. These cases are classified as having community-onset CDI, regardless of whether infection originated from a healthcare facility or the community [4]. Few studies have evaluated clinical or cost outcomes in subjects with community-onset CDI [7], therefore the objective of this study was to determine the risk of all-cause mortality and healthcare costs associated with community-onset CDI.
Materials and methods
Study design, setting and subjects
We conducted a population-based propensity-score matched cohort study of individuals with community-onset CDI to examine attributable mortality and costs. The latter was determined using an incidence-based cost-of-illness approach [8] from the perspective of the Ontario Ministry of Health and Long-Term Care (healthcare payer). This approach measures long-term costs of newly diagnosed cases from an index date (e.g., diagnosis) to a defined point in time (e.g., post one year, death) in contrast to the prevalence-based approach, which evaluates the cost of disease (across varying disease stages) during a defined time period (e.g., 1 year) [8–11]. We conducted this study at the Institute for Clinical Evaluative Sciences (ICES) in Toronto, Canada, which houses provincial health administrative data on publicly funded healthcare that are linkable at the individual level using encoded unique identifiers [12]. This study was approved by the Sunnybrook Health Sciences Centre Institutional Review Board and the University of Toronto Office of Research Ethics. Written or verbal informed consent was not required from the subjects in this study, as ICES is permitted to obtain and use personal health information without patient consent for specific purposes, as outlined in Ontario’s Personal Health Information Protection Act.
We identified incident cases of community-onset CDI (symptomatic infected subjects) using the International Statistical Classification of Diseases and Related Health Problems, 10th Revision, Canada (ICD-10-CA) code A04.7 (enterocolitis due to CDI). To be classified as community-onset, this code had to be present (between January 1, 2003 and December 31, 2010) during the following healthcare encounters: a) an emergency department (ED) visit (index date: ED visit date or physician office date if CDI-related symptoms documented within two weeks prior); b) a non-elective hospital admission with CDI as the principal diagnosis and a colectomy (see S1 Table for the intervention codes for identifying colectomies) within two days of hospitalization (index date: admission date); c) a non-elective hospital admission lasting ≤2 days (index date: admission date); or d) a non-elective hospital admission with a principal diagnosis of CDI with CDI-related symptoms documented within two weeks prior to admission (index date: physician office or ED visit date). CDI-related symptoms were defined as a physician office visit coded as abdominal pain or diarrhea; or an ED visit coded as cramps, abdominal pain, diarrhea, or suspicious CDI (see S2 Table for codes used to identify these conditions). For infected subjects that had more than one healthcare encounter (i.e., a to d), the earliest encounter was used. We were unable to identify infected subjects using physician office visits alone, as there is no code specific to CDI amongst the diagnostic codes used for Ontario physician billing claims. As a result, our study population likely includes predominantly subjects with more severe infection. The entire cohort was followed until December 31, 2011.
We used propensity-score matching without replacement to match each infected subject to one randomly selected (hospitalized or community dwelling) uninfected subject (those without a record for ICD-10-CA code A04.7). The index dates for the uninfected subjects were randomly assigned between January 1, 2003 and December 31, 2010. Using a logistic regression model that regressed infection status (infected versus uninfected), the propensity score was calculated based on covariates at the index date (neighborhood income quintile, rurality), covariates within the previous 12 weeks before the index date (healthcare utilization, a record of an infection that could have led to a prescription for an antibiotic), and covariates within the two years prior to the index date (comorbidities using the John Hopkins Adjusted Clinical Groups System® which measures an individual’s health resource utilization by assigning their ICD codes into diagnosis groups, e.g., Aggregated Diagnosis Groups, Resource Utilization Bands [13–15]). See S3 Table for additional details on these covariates.
To evaluate the effect of CDI on costs prior to death, we re-matched without replacement each infected subject who died during the observation period (between 2003 and 2011) to one randomly selected uninfected subject who also died during the observation period. Using a logistic regression model that regressed infection status (infected versus uninfected), the propensity score was calculated based on neighborhood income quintile, rurality, and co-morbidities, measured three months before death.
For both matching approaches, subjects were matched on the logit of the propensity score using calipers of width equal to 0.2 of the standard deviation of the logit of the propensity score [16]. Subjects were also hard matched on sex, birth year ±3 years, and date (index date for the initial match and three months prior to death for the re-match) ±30 days. The balance of measured baseline covariates were assessed using standardized differences, with standardized differences <0.1 indicating negligible differences between the matched infected and uninfected subjects in terms of the covariates included in the propensity score matches [17].
Outcomes
We examined 30-day, 180-day, and 1-year all-cause mortality. We evaluated the costs of publicly funded healthcare services (e.g., inpatient hospitalization, ED visits, physician services) [18], expressed as total costs unadjusted for survival (30-day, 180-day, and 1-year costs) and adjusted for survival (1-, 2-, and 3-year costs).
Analysis
Using the matched sample, we estimated the difference in the probability of death (assessing its statistical significance using McNemar’s test) and the number needed to harm (NNH, reciprocal of the absolute difference in the probability of death), along with the relative risk (RR) and 95% confidence intervals (CI) for all-cause mortality [19, 20]. We also graphed the 3-year survival for the matched infected and uninfected subjects.
We calculated all cost outcomes using the matched sample, and reported costs in 2014 Canadian dollars ($1 Canadian = $0.9054 US [21]). Attributable cumulative costs unadjusted for survival were evaluated by determining the mean difference between the matched infected and uninfected subjects. To determine 95%CIs we employed bootstrapping methods [22]. Lastly, we stratified costs by sex, age group, diagnosis year, and those who died within one year of their index date.
We determined attributable cumulative costs adjusted for survival using the phase-of-care approach. This method involves organizing an individual’s observation time into phases that represent patient care from diagnosis to death and is consistent with C. difficile disease history [23–25]. We defined three phases of care: acute infection (lasting up to 6 months), continuing care (up to 110 months), and final care (lasting up to 3 months). We allocated each subject’s observation time to each phase of care in the following order: final care, acute infection, and continuing care. For example, for subjects with 12 months of observation time (index date to death date), the last 3 months were allocated to final care, the first 6 months were allocated to acute infection, and the intervening period (3 months) was allocated to continuing care. For subjects with 2 months of observation time (index date to death date), the entire time was allocated to final care.
Phase-specific costs were measured in 30-day intervals [24]. We calculated attributable phase-specific costs by evaluating the mean difference between the matched infected and uninfected subjects. We used bootstrapping methods to determine the 95%CIs [22]. Further, phase-specific costs were stratified by healthcare services (e.g., physician services, hospitalizations) to understand resource use over the course of the disease. To determine attributable cumulative mean 1-, 2-, and 3-year costs (and 95% CIs), the mean attributable phase-specific costs (acute infection, continuing care, and final care) were combined with the survival data as described by Yabroff et al. [24]. Costs calculated beyond one year were discounted at 5% annually [26]. Lastly, we stratified the 1-, 2-, and 3-year costs by sex, age group, and those who died within one year of their index date.
Sub-analysis
As a sub-analysis, among the matched cohort, we stratified the cost outcomes of those who had an ED visit as their first healthcare encounter, into those who were discharged home versus admitted to hospital.
Results
Study cohort
We identified 7,950 subjects with community-onset CDI between January 1, 2003 and December 31, 2010 (22% of all individuals with a CDI code in Ontario between 2003 and 2010, n = 36,258). Half (50.2%) had a CDI-coded ED visit (with 1,728 being admitted to hospital), 0.6% had a non-elective CDI-coded hospitalization with a colectomy conducted within two days of admission, 13.8% had a non-elective CDI-coded hospitalization lasting ≤2 days, and 35.4% had a non-elective CDI-coded hospitalization and CDI-related symptoms within two weeks prior to admission.
The estimated crude annual mean incidence of community-onset CDI was 7.8 per 100,000 population. The mean age was 63.5 years (standard deviation = 22.0), 62.7% were female, and 45.0% were very high users of the healthcare system at index date. Nearly three-quarters (72.4%) had healthcare utilization and nearly half (47.5%) had an infection prompting antibiotic therapy within the previous 12 weeks before the index date (Table 1). All subjects had between 1 and 9 years of follow-up if they did not die.
Table 1. Selected characteristics prior to matching.
| Community-onset CDI (n = 7,950) | |
|---|---|
| Agea, mean±SD, median | 63.5±22.0, 69 years |
| Female, % | 62.7 |
| Age groupa, % | |
| Children (≤18 years) | 4.1 |
| Adults (19–64 years) | 38.6 |
| Older adults (≥65years) | 57.3 |
| Crude annual incidence rateb, per 100,000 population | |
| 2003 | 4.9 |
| 2004 | 6.7 |
| 2005 | 8.2 |
| 2006 | 7.4 |
| 2007 | 8.9 |
| 2008 | 9.4 |
| 2009 | 8.7 |
| 2010 | 8.4 |
| Neighborhood income quintilea, % | |
| 1 (lowest) | 22.0 |
| 2 | 20.5 |
| 3 | 19.0 |
| 4 | 19.2 |
| 5 (highest) | 19.3 |
| Ruralitya, % | |
| Major urban | 59.9 |
| Non-major urban | 29.0 |
| Rural | 11.1 |
| Very high users of the healthcare systemc, % | 45.0 |
| Healthcare utilization within 12 weeks prior to index date, % | 72.4 |
| Possible prescription for an antibiotic within 12 weeks prior to index date, % | 47.5 |
| All-cause mortalityd, % | |
| 30 day | 11.8 |
| 180 day | 19.6 |
| 1 year | 23.0 |
| End of study period | 35.8 |
| Costs unadjusted for survivald, mean, median | |
| 30 day | $9,535, $6,324 |
| 180 day | $21,488, $9,754 |
| 1 year | $28,782, $12,705 |
aMeasured at the index date.
bNot standardized to a specific year.
cMeasure of co-morbidities (within the two years prior to the index date, see S3 Table).
dPost index date.
CDI—C.difficile infection. SD—standard deviation.
Matching results
We matched 81.0% (n = 6,437) of the infected subjects to uninfected subjects (Table 2). Thirty-four percent (n = 2,158) of the matched infected subjects died during the study period (January 2003 to December 2011). In the re-match, we matched 99.2% (n = 2,140) of these cases to similar uninfected subjects who also died (S4 Table). For both sets of matches, all standardized differences were <0.1.
Table 2. Selected characteristics of those with community-onset CDI before and after matching.
| Infected subjects | Pool of uninfected subjects | Standardized differences comparing infected subjects and pool of uninfected subjects | Unmatched infected subjects | Matched infected subjects | Matched uninfected subjects | Standardized differences comparing matched infected and uninfected subjects | |
|---|---|---|---|---|---|---|---|
| n | 7,950 | 512,184 | NA | 1,513 | 6,437 | 6,437 | NA |
| Hard match variablesa | |||||||
| Age, mean±SD | 63.5±22.0 | 57.7±21.0 | 0.27 | 62.4±24.1 | 63.8±21.4 | 64.2±21.0 | 0.02 |
| Female, % | 62.7 | 62.2 | 0.01 | 52.2 | 65.1% | 65.1% | 0.00 |
| First healthcare encounter, % | |||||||
| CDI-coded ED visit | 50.2 | NA | NA | 47.9 | 50.7 | NA | NA |
| CDI-coded non-elective hospital admission | 49.8 | NA | NA | 52.1 | 49.3 | NA | NA |
| Propensity score variables | |||||||
| Neighborhood income quintilea, % | |||||||
| 1 (lowest) | 22.0 | 20.0 | 0.05 | 22.5 | 21.9 | 20.7 | 0.03 |
| 2 | 20.5 | 20.4 | 0.00 | 21.1 | 20.4 | 22.2 | 0.04 |
| 3 | 19.0 | 19.5 | 0.01 | 18.8 | 19.1 | 19.1 | 0.00 |
| 4 | 19.2 | 19.7 | 0.01 | 18.5 | 19.3 | 19.2 | 0.00 |
| 5 (highest) | 19.3 | 20.3 | 0.03 | 19.2 | 19.3 | 18.9 | 0.01 |
| Ruralitya, % | |||||||
| Major urban | 59.9 | 70.4 | 0.22 | 56.2 | 60.8 | 60.1 | 0.01 |
| Non-major urban | 29.0 | 21.9 | 0.16 | 31.7 | 28.4 | 28.2 | 0.00 |
| Rural | 11.1 | 7.7 | 0.12 | 12.1 | 10.8 | 11.7 | 0.03 |
| Very high users of the healthcare systemb, % | 45.0 | 8.8 | 0.90 | 69.7 | 39.2 | 38.4 | 0.02 |
| Aggregated Diagnosis Groups- specific to infectionb, % | |||||||
| Time Limited: Minor- primary infections | 79.2 | 42.3 | 0.82 | 92.1 | 76.2 | 73.6 | 0.06 |
| Time Limited: Major- primary infections | 35.8 | 9.0 | 0.68 | 66.8 | 28.5 | 28.8 | 0.01 |
| Likely to Recur: Discrete- infections | 42.8 | 19.0 | 0.53 | 52.5 | 40.5 | 39.6 | 0.02 |
| Healthcare utilizationc, % | 72.4 | 11.6 | 1.56 | 99.6 | 66.0 | 65.1 | 0.02 |
| Record of an infection that may have led to an antibiotic prescriptionc, % | 47.5 | 9.6 | 0.92 | 82.2 | 39.4 | 36.0 | 0.07 |
aMeasured at the index date.
bMeasure of co-morbidities (within the two years prior to the index date, see S3 Table).
cMeasured within the previous 12 weeks before the index date.
CDI–C.difficile infection. NA—not applicable. SD—standard deviation.
Outcomes
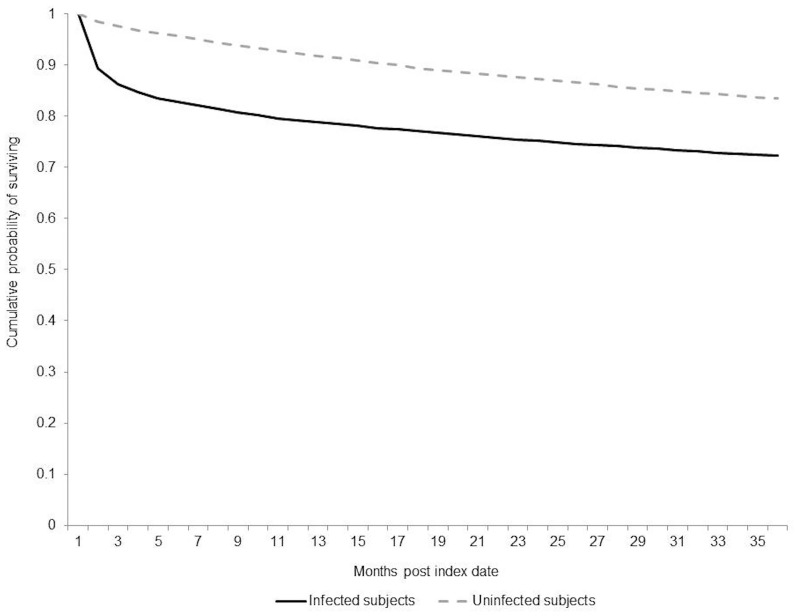
Community-onset CDI was associated with increased risk of all-cause mortality among infected subjects compared to uninfected subjects (Table 3). The absolute differences in the risk of 30-day, 180-day, and 1-year mortality were 9.2%, 12.9%, and 13.0%, respectively (p<0.0001), for a NNH for 1-year mortality of 8. The RRs for 30-day, 180-day and 1-year mortality were 7.32 (95%CI, 5.94–9.02), 3.55 (95%CI, 3.17–3.97), and 2.59 (95%CI, 2.37–2.83), respectively. Lower survival was also observed among infected subjects three years after the index date (Fig 1).
Table 3. All-cause mortality and healthcare costs attributable to community-onset CDI.
| n matched pairs | Infected subjects | Uninfected subjects | Attributable outcome (95% CI) | |
|---|---|---|---|---|
| All-cause mortalitya | ||||
| 30 days | 6,437 | 10.7% | 1.5% | 9.2%, RR = 7.32 (5.94–9.02) |
| 180 days | 6,437 | 17.9% | 5.0% | 12.9%, RR = 3.55 (3.17–3.97) |
| 1 year | 6,437 | 21.2% | 8.2% | 13.0%, RR = 2.59 (2.37–2.83) |
| Mean cost outcomes unadjusted for survivala | ||||
| 30-day cumulative costs | 6,437 | $9,234, Median = $6,130 | $1,800, Median = $240 | $7,434 ($7,122-$7,762), Median = $5,891 |
| 180-day cumulative costs | 6,437 | $20,376, Median = $9,154 | $7,859, Median = $1,760 | $12,517, ($11,687-$13,366), Median = $7,394 |
| 1-year cumulative costs | 6,437 | $27,209, Median = $11,900 | $13,992, Median = $3,817 | $13,217 ($12,062-$14,388), Median = $8,083 |
| Mean cost outcomes by phaseb | ||||
| Acute infection costs | 5,454 | $3,503 | $1,209 | $2,294 ($2,135-$2,463) |
| Continuing care costs | 5,160 | $979 | $478 | $502 ($435-$573) |
| Final care costsc | 2,140 | $13,658 | $10,560 | $3,099 ($2,364-$3,823) |
| Mean cost outcomes adjusted for survivala | ||||
| 1-year cumulative costs | 6,437 | NA | NA | $10,700 ($9,811-$11,645) |
| 2-year cumulative costs | ||||
| Undiscounted | 6,437 | NA | NA | $13,312 ($12,024-$14,682) |
| Discounted 5% | 6,437 | NA | NA | $12,646 ($11,423-$13,948) |
| 3-year cumulative costs | ||||
| Undiscounted | 6,437 | NA | NA | $15,812 ($14,159-$17,571) |
| Discounted 5% | 6,437 | NA | NA | $15,022 ($13,451-$16,692) |
aPost index date.
bCosts standardized to 30 days.
cCosts derived from the re-matched infected subjects who died (S4 Table).
CI—confidence interval. NA—not applicable. RR—relative risk.
Fig 1. Survival curve for infected subjects and their matched pairs, three years post index date.
Community-onset CDI was associated with 1.9- to 5.1-fold higher mean costs compared to uninfected subjects, up to one year post index date (Table 3). Mean attributable cumulative 30-day, 180-day, and 1-year costs per subject unadjusted for survival were $7,434 (95%CI, $7,122-$7,762), $12,517 (95%CI, $11,687-$13,366), and $13,217 (95%CI, $12,062-$14,388), respectively. Mean attributable cumulative 1-, 2-, and 3-year costs per subject adjusted for survival (undiscounted) were $10,700 (95%CI, $9,811-$11,645), $13,312 (95%CI, $12,024-$14,682), and $15,812 (95%CI, $14,159-$17,571), respectively.
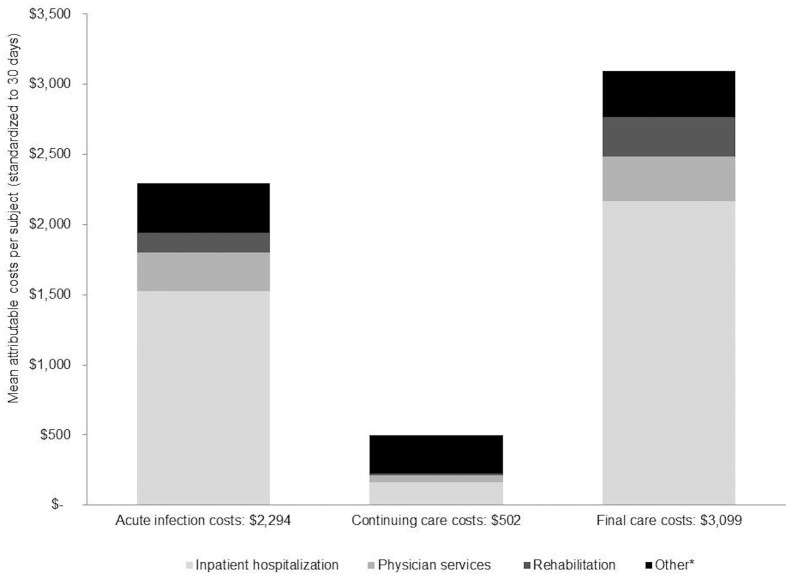
In the stratified analyses, hospitalizations and physician visits comprised the largest cost components across all phases (Fig 2). Mean attributable costs were higher among those ≥65 years of age, those infected in 2008, and those who died within one year post index date (S5 Table).
Fig 2. Stratification of phase-specific costs by healthcare services.
*Other- Includes same day surgery, ED visits, outpatient medication, non-physician services, outpatient laboratory tests, complex continuing care, home care services, long-term care, dialysis clinic visits, cancer clinic visits, and assisted devices.
Sub-analysis
Among the matched cohort (n = 6,437), 50.7% (n = 3,266) had an ED visit as their first healthcare encounter (Table 2). From this group, 58.8% (n = 1,920) were discharged home and 41.2% (n = 1,346) were admitted to hospital. Mean attributable costs were higher among those admitted to hospital versus those discharged home (Table 4).
Table 4. Stratified mean attributable costs per subject among those that had their first healthcare encounter as an ED visit.
| n matched pairs | Costs unadjusted for survival | Costs adjusted for survival | |||||
|---|---|---|---|---|---|---|---|
| Cumulative 30-day costs (95% CI) | Cumulative180-day costs (95% CI) | Cumulative1-year costs (95% CI) | Cumulative 1-year costs, undiscounted (95% CI) | Cumulative 2-year costs, undiscounted (95% CI) | Cumulative 3-year costs, undiscounted (95% CI) | ||
| Overall | 3,266 | $5,280 ($4,842-$5,733) | $9,458 ($8,387-$10,560) | $10,229 ($8,801-$11,724) | $8,517 ($7,293- $9,826) | $11,156 ($9,343- $13,071) | $13,619 ($11,267- $16,090) |
| Discharged home | 1,920 | $967 ($676-$1,239) | $2,406 ($1,495-$3,334) | $2,610 ($1,141-$4,042) | $2,839 ($1,649- $4,100) | $4,578 ($2,647- $6,718) | $6,229 ($3,595- $9,200) |
| Admitted to hospital | 1,346 | $11,433 ($10,544-$12,384) | $19,517 ($17,281-$21,872) | $21,096 ($18,013-$24,268) | $13,777 ($11,669- $16,059) | $16,842 ($13,948- $19,988) | $19,551 ($15,983- $23,438) |
Discussion
Infected subjects had 2.6- to 7.3-fold greater all-cause mortality and 1.9- to 5.1-fold greater costs compared to uninfected subjects up to one year post index date. Attributable mortality and healthcare costs were greatest close to the index date. However, differences in mortality and costs still existed three years post index date. This accumulation of costs could be due to CDI recurrences, treatment failure, need for a colectomy, or CDI increasing susceptibility for other conditions. Hospitalization was the largest cost component during the course of the disease, and mean attributable costs were higher among those aged ≥65 years, those infected in 2008 (which could be due to a virulent CDI strain in Ontario that year [27]), and those who died within one year post index date.
Our results were generally similar to published literature, where it has been reported that those with community-onset CDI have a median age of 61 years, 52% to 61% are female, and 11% die within 30 days [3, 28]. In our cohort, the median age was 69 years, 63% were female, and 12% died within 30 days. No previous studies have evaluated attributable mortality among those with community-onset CDI. However, Karas et al. reported a pooled attributable mortality for CDI within 3 months post diagnosis (mostly in hospital settings) of 6.0% overall (8.0% if only including studies published after 2000), whereas we report an attributable mortality of 9.2% at 30 days, 12.9% at 180 days, and 13.0% at 1 year [29]. One explanation for this difference could be a circulating CDI strain that caused more severe disease in Ontario during our study period. Compared to matched cohort studies focused on other conditions, attributable mortality for community-onset CDI in our cohort was comparable or lower [30, 31]. For example, Lash et al. found 180-day mortality for patients hospitalized for chronic obstructive pulmonary disease to be 16.0%, compared to 2.4% in matched uninfected patients (difference = 13.6%), whereas Su et al. found 1-year mortality for those with healthcare-associated Staphylococcus aureus infection to be 59.5%, compared to 39.3% in matched uninfected patients (difference = 20.2%) [30, 31].
In terms of economic burden, we could not compare our results to published literature. Sammons et al. did not examine attributable costs beyond a hospital stay for those with community-onset CDI [32]. Kuntz et al. did not present non-attributable costs exclusively for community-onset CDI; instead they presented costs for those identified with CDI in outpatient versus inpatient settings [33]. However, in contrast to Nanwa et al. who conducted a systematic review of the CDI costing literature, the mean attributable 30-day costs among our cohort ($7,434) were lower than their reported range of mean attributable CDI costs for hospitalized patients over a hospital stay ($10,961 to $36,960 [converted to Canadian dollars [34]]) [7]. This difference is likely because not all subjects in our cohort were hospitalized.
Our study had some limitations. We were not able to identify subjects who only had physician office visits for CDI, likely overestimating mortality among those with community-onset CDI and the economic burden of community-onset CDI per subject, as we likely captured more severe cases in our study. However, on a population level we are likely underestimating the total economic impact of community-onset CDI as not all cases are captured. We are uncertain about how many infected subjects we missed; however, Hirshon et al. found that 4% of outpatients with diarrhea tested positive for CDI between 2002 and 2007 in the US [35]. Missing subjects who only visited physician offices for CDI could explain why we captured fewer individuals with community-onset CDI than previously reported (22% versus 29% to 58% of all CDI cases [3, 4]). However, this could also be due to regional variations. Our sub-analysis of matched infected subjects who had an ED visit as their first encounter, provides some insight on the mean attributable costs of mild versus severe community-onset CDI (Table 4).
Additional uncertainly surrounds the validity of our case definition. A Canadian study found a high sensitivity (88%) and specificity (100%) associated with the ICD-9/ICD-10-CA code for CDI when compared to CDI stool toxin results in a cohort of hospitalized ulcerative colitis patients [36]. However, 50.2% of the infected subjects in our study were identified using ED visits and to the best of our knowledge, there are no published CDI validation studies among ED patients. Another study limitation was that our index dates might not represent the date of symptom onset, as this is difficult to establish with a retrospective study design and without CDI-specific laboratory data. Due to this uncertainty we did not stratify our results by where the infection may have originated (healthcare facility or community setting). However, we provide the overall costs of community-onset CDI, which is understudied in the literature and it can be assumed that the majority of subjects captured in our study were healthcare facility-associated cases, since 66.0% of the matched infected subjects had healthcare utilization within the previous 12 weeks before the index date (Table 2). Lastly, 19% of the cohort could not be matched. This proportion of infected subjects represented males and those with greater co-morbidities. Not including the latter could have led to an underestimation of mortality and costs.
The main strengths of our study were the population-based sample, allowing for our results to be generalizable and our access to individually linked datasets, which allowed us to: a) create a comprehensive algorithm to identify infected subjects with community-onset CDI; b) use and define numerous covariates in our matches; and c) incorporate a broad range of healthcare services that are publicly funded in our cost analyses.
Future studies should contrast the burden of healthcare facility-onset CDI to community-onset CDI and attempt to stratify results by where the infection may have been acquired. Also, patient out-of-pocket costs and productivity losses of those with CDI would be useful to further our understanding of CDI burden.
Conclusions
This study demonstrates that community-onset CDI is associated with increased risk of all-cause mortality and both short- and long-term economic burden. Our estimates of absolute and relative mortality and costs highlight the need for interventions to prevent community-onset CDI.
Supporting information
(DOCX)
ICD-10-CA—International Statistical Classification of Diseases and Related Health Problems, 10th Revision, Canada. OHIP—Ontario Health Insurance Plan.
(DOCX)
aFor the re-match, the index date was 3 months prior to death. b12 weeks was chosen since studies have found that the onset of CDI symptoms can occur up to 3 months after discharge from a hospital or stopping an antibiotic [6–8] (refer to the references listed with S3 Table). ICD-10- CA—International Statistical Classification of Diseases and Related Health Problems, 10th Revision, Canada. OHIP—Ontario Health Insurance Plan.
(DOCX)
aMeasured at the index date. bHighest resource utilization band measured within the previous two years before the index date (Adjusted Clinical Groups® were used to measure comorbidities- see S3 Table). CDI–C.difficile infection. NA—not applicable. SD—standard deviation.
(DOCX)
Confidence intervals not reported, however, none of the confidence intervals crossed zero. CDI—C.difficile infection. NA—not applicable.
(DOCX)
Data Availability
There are restrictions on sharing data from our study as public deposition of the individual-level data used in this study is not legally permitted due to provincial privacy legislation (Personal Health Information Protection Act) and the contractual obligations of the data sharing agreements between the Institute for Clinical Evaluative Sciences (ICES) and the data providers. Please refer to the following link for more information: http://www.ices.on.ca/Data-and-Privacy/Privacy%20at%20ICES. Please contact Dr. Jeff Kwong (jeff.kwong@utoronto.ca) with questions related to data access.
Funding Statement
This study was supported by a CIHR operating grant (grant number: MOP 130553, grant recipient: Beate Sander), the Institute for Clinical Evaluative Sciences (ICES), and Public Health Ontario (PHO). ICES and PHO are funded by annual grants from the Ontario Ministry of Health and Long-Term Care (MOHLTC). The opinions, results, and conclusions reported in this article do not necessarily represent the views of CIHR, ICES, PHO, or the MOHLTC. Parts of this material are based on data and information compiled and provided by the Canadian Institute of Health Information (CIHI). However, the analyses, conclusions, opinions and statements expressed herein are those of the authors, and not necessarily those of CIHI.
References
- 1.Centers for Disease Control and Prevention. Clostridium difficile Infection Tracking. 2015 [November 29 2015]; http://www.cdc.gov/hai/eip/Clostridium-difficile.html.
- 2.Lessa FC, Mu Y, Bamberg WM, Beldavs ZG, Dumyati GK, Dunn JR, et al. Burden of Clostridium difficile infection in the United States. N Engl J Med. 2015. February 26;372(9):825–34. 10.1056/NEJMoa1408913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Clohessy P, Merif J, Post JJ. Severity and frequency of community-onset Clostridium difficile infection on an Australian tertiary referral hospital campus. Int J Infect Dis. 2014. December;29:152–5. 10.1016/j.ijid.2014.08.009 [DOI] [PubMed] [Google Scholar]
- 4.Kutty PK, Benoit SR, Woods CW, Sena AC, Naggie S, Frederick J, et al. Assessment of Clostridium difficile-associated disease surveillance definitions, North Carolina, 2005. Infect Control Hosp Epidemiol. 2008. March;29(3):197–202. 10.1086/528813 [DOI] [PubMed] [Google Scholar]
- 5.McDonald LC, Coignard B, Dubberke E, Song X, Horan T, Kutty PK. Recommendations for surveillance of Clostridium difficile-associated disease. Infect Control Hosp Epidemiol. 2007. February;28(2):140–5. 10.1086/511798 [DOI] [PubMed] [Google Scholar]
- 6.Cohen SH, Gerding DN, Johnson S, Kelly CP, Loo VG, McDonald LC, et al. Clinical practice guidelines for Clostridium difficile infection in adults: 2010 update by the society for healthcare epidemiology of America (SHEA) and the infectious diseases society of America (IDSA). Infect Control Hosp Epidemiol. 2010. May;31(5):431–55. 10.1086/651706 [DOI] [PubMed] [Google Scholar]
- 7.Nanwa N, Kendzerska T, Krahn M, Kwong JC, Daneman N, Witteman W, et al. The Economic Impact of Clostridium difficile Infection: A Systematic Review. Am J Gastroenterol. 2015. April;110(4):511–9. 10.1038/ajg.2015.48 [DOI] [PubMed] [Google Scholar]
- 8.Larg A, Moss JR. Cost-of-illness studies: a guide to critical evaluation. PharmacoEconomics. 2011;29(8):653–71. 10.2165/11588380-000000000-00000 [DOI] [PubMed] [Google Scholar]
- 9.Lipscomb J, Yabroff KR, Brown ML, Lawrence W, Barnett PG. Health care costing: data, methods, current applications. Med Care. 2009. July;47(7 Suppl 1):S1–6. 10.1097/MLR.0b013e3181a7e401 [DOI] [PubMed] [Google Scholar]
- 10.Barlow WE. Overview of methods to estimate the medical costs of cancer. Med Care. 2009. July;47(7 Suppl 1):S33–6. 10.1097/MLR.0b013e3181a2d847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tarricone R. Cost-of-illness analysis. What room in health economics? Health Policy. [Review]. 2006;77(1):51–63. 10.1016/j.healthpol.2005.07.016 [DOI] [PubMed] [Google Scholar]
- 12.Institute for Clinical Evaluative Sciences (ICES). ICES Data. 2014 [August 29 2014]; http://www.ices.on.ca/Data-and-Privacy/ICES-data.
- 13.University of Manitoba. Concept: Adjusted Clinical Groups® (ACG®)—Overview. 2014 [August 21 2014]; http://mchp-appserv.cpe.umanitoba.ca/viewConcept.php?conceptID=1304.
- 14.The Johns Hopkins University. The Johns Hopkins ACG® System. 2013 [August 21 2014]; http://acg.jhsph.org.
- 15.Austin PC, van Walraven C, Wodchis WP, Newman A, Anderson GM. Using the Johns Hopkins Aggregated Diagnosis Groups (ADGs) to predict mortality in a general adult population cohort in Ontario, Canada. Medical Care. [Research Support, Non-U.S. Gov't]. 2011;49(10):932–9. 10.1097/MLR.0b013e318215d5e2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Austin PC. Optimal caliper widths for propensity-score matching when estimating differences in means and differences in proportions in observational studies. Pharm Stat. 2011. Mar-Apr;10(2):150–61. 10.1002/pst.433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Austin PC. An Introduction to Propensity Score Methods for Reducing the Effects of Confounding in Observational Studies. Multivariate Behav Res. 2011. May;46(3):399–424. 10.1080/00273171.2011.568786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wodchis WP BK, Nikitovic M, McKillop I,. Guidelines on Person- Level Costing Using Administrative Databases in Ontario. Working Paper Series. Toronto: Health System Performance Research Network (HSPRN); 2013.
- 19.Austin PC. A Tutorial and Case Study in Propensity Score Analysis: An Application to Estimating the Effect of In-Hospital Smoking Cessation Counseling on Mortality. Multivariate Behav Res. 2011;46(1):119–51. 10.1080/00273171.2011.540480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Agresti A, Min Y. Effects and non-effects of paired identical observations in comparing proportions with binary matched-pairs data. Stat Med. 2004. January 15;23(1):65–75. 10.1002/sim.1589 [DOI] [PubMed] [Google Scholar]
- 21.Bank of Canada. Annual Average Exchange Rates. 2014 [June 9 2015]; http://www.bankofcanada.ca/rates/exchange/annual-average-exchange-rates/.
- 22.Austin PC, Small DS. The use of bootstrapping when using propensity-score matching without replacement: a simulation study. Stat Med. 2014. October 30;33(24):4306–19. 10.1002/sim.6276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brown ML, Riley GF, Potosky AL, Etzioni RD. Obtaining long-term disease specific costs of care: application to Medicare enrollees diagnosed with colorectal cancer. Medical Care. 1999;37(12):1249–59. [DOI] [PubMed] [Google Scholar]
- 24.Yabroff KR, Lamont EB, Mariotto A, Warren JL, Topor M, Meekins A, et al. Cost of care for elderly cancer patients in the United States. Journal of the National Cancer Institute. 2008;100(9):630–41. 10.1093/jnci/djn103 [DOI] [PubMed] [Google Scholar]
- 25.Riley GF, Potosky AL, Lubitz JD, Kessler LG. Medicare payments from diagnosis to death for elderly cancer patients by stage at diagnosis. Medical Care. 1995;33(8):828–41. [DOI] [PubMed] [Google Scholar]
- 26.Canadian Agency for Drugs and Technologies in Health. Guidelines for the economic evaluation of health technologies. Ottawa.2006 [June 9 2015]; http://www.cadth.ca/media/pdf/186_EconomicGuidelines_e.pdf.
- 27.Pillai DR, Longtin J, Low DE. Surveillance data on outbreaks of Clostridium difficile infection in Ontario, Canada, in 2008–2009. Clin Infect Dis. 2010. June 15;50(12):1685–6; author reply 6. 10.1086/653007 [DOI] [PubMed] [Google Scholar]
- 28.Tan XQ, Verrall AJ, Jureen R, Riley TV, Collins DA, Lin RT, et al. The emergence of community-onset Clostridium difficile infection in a tertiary hospital in Singapore: a cause for concern. Int J Antimicrob Agents. 2014. January;43(1):47–51. 10.1016/j.ijantimicag.2013.09.011 [DOI] [PubMed] [Google Scholar]
- 29.Karas JA, Enoch DA, Aliyu SH. A review of mortality due to Clostridium difficile infection. J Infect. 2010. July;61(1):1–8. 10.1016/j.jinf.2010.03.025 [DOI] [PubMed] [Google Scholar]
- 30.Lash TL, Johansen MB, Christensen S, Baron JA, Rothman KJ, Hansen JG, et al. Hospitalization rates and survival associated with COPD: a nationwide Danish cohort study. Lung. [Research Support, Non-U.S. Gov't]. 2011;189(1):27–35. 10.1007/s00408-010-9274-z [DOI] [PubMed] [Google Scholar]
- 31.Su C-H, Chang S-C, Yan J-J, Tseng S-H, Chien L-J, Fang C-T. Excess mortality and long-term disability from healthcare-associated staphylococcus aureus infections: a population-based matched cohort study. PLoS ONE. [Research Support, Non-U.S. Gov't]. 2013;8(8):e71055 10.1371/journal.pone.0071055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sammons JS, Localio R, Xiao R, Coffin SE, Zaoutis T. Clostridium difficile infection is associated with increased risk of death and prolonged hospitalization in children. Clinical Infectious Diseases. 2013;57(1):1–8. 10.1093/cid/cit155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kuntz JL, Johnson ES, Raebel MA, Petrik AF, Yang X, Thorp ML, et al. Epidemiology and healthcare costs of incident Clostridium difficile infections identified in the outpatient healthcare setting. Infection Control & Hospital Epidemiology. 2012;33(10):1031–8. [DOI] [PubMed] [Google Scholar]
- 34.The Organisation for Economic Co-operation and Development. PPPs and exchange rates. 2014 [July 14 2014]; http://stats.oecd.org/Index.aspx?datasetcode=SNA_TABLE4.
- 35.Hirshon JM, Thompson AD, Limbago B, McDonald LC, Bonkosky M, Heimer R, et al. Clostridium difficile infection in outpatients, Maryland and Connecticut, USA, 2002–2007. Emerging Infectious Diseases. 2011. October;17(10):1946–9. 10.3201/eid1710.110069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Negron M, Barkema H, Rioux K, De Buck J, Checkley S, Beck P, et al. Accuracy of ICD9 and ICD 10 codes for clostridium difficile among ulcerative colitis patients. American Journal of Gastroenterology. 2011;106:S481. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
ICD-10-CA—International Statistical Classification of Diseases and Related Health Problems, 10th Revision, Canada. OHIP—Ontario Health Insurance Plan.
(DOCX)
aFor the re-match, the index date was 3 months prior to death. b12 weeks was chosen since studies have found that the onset of CDI symptoms can occur up to 3 months after discharge from a hospital or stopping an antibiotic [6–8] (refer to the references listed with S3 Table). ICD-10- CA—International Statistical Classification of Diseases and Related Health Problems, 10th Revision, Canada. OHIP—Ontario Health Insurance Plan.
(DOCX)
aMeasured at the index date. bHighest resource utilization band measured within the previous two years before the index date (Adjusted Clinical Groups® were used to measure comorbidities- see S3 Table). CDI–C.difficile infection. NA—not applicable. SD—standard deviation.
(DOCX)
Confidence intervals not reported, however, none of the confidence intervals crossed zero. CDI—C.difficile infection. NA—not applicable.
(DOCX)
Data Availability Statement
There are restrictions on sharing data from our study as public deposition of the individual-level data used in this study is not legally permitted due to provincial privacy legislation (Personal Health Information Protection Act) and the contractual obligations of the data sharing agreements between the Institute for Clinical Evaluative Sciences (ICES) and the data providers. Please refer to the following link for more information: http://www.ices.on.ca/Data-and-Privacy/Privacy%20at%20ICES. Please contact Dr. Jeff Kwong (jeff.kwong@utoronto.ca) with questions related to data access.