Abstract
We assessed the prospective association between baseline levels of physical activity (PA) and the incidence of newly developed diabetic retinopathy (DR) in patients with type 2 diabetes. Data from 1,814 patients with type 2 diabetes without DR were obtained from a Japanese diabetes registry at Tenri Hospital, Nara, Japan. To assess the independent correlations between baseline PA levels and newly developed DR, the participants were divided into five categories based on their PA levels. A Cox proportional hazards model with time-varying exposure information was used and adjusted for potential confounders to assess the independent correlations. At baseline, the mean age, BMI, and hemoglobin A1c levels of the patients were 65.5 years, 24.5 kg/m2, and 7.2% (54 mmol/mol), respectively. After 2 years, newly developed DR was confirmed in 184 patients (10.1%). Patients with newly developed DR had longer duration of type 2 diabetes (14.7 versus 11.0 years, p < 0.0001), higher systolic blood pressure (139.2 versus 135.1 mmHg, p = 0.0012), lower estimated glomerular filtration rate (74.0 versus 77.1 mL/min/1.73 m2, p = 0.0382), greater urinary albumin–creatinine ratio (4.00 versus 2.45 mg/mmol, p < 0.0039), and higher HbA1c levels (7.5 versus 7.2%, p = 0.0006) than those without newly developed DR. The multivariable-adjusted hazard ratios for DR development were 0.87 (95% CI, 0.53–1.40; p = 0.557), 0.83 (95% CI, 0.52–1.31; p = 0.421), 0.58 (95% CI, 0.35–0.94; p = 0.027), and 0.63 (95% CI, 0.42–0.94; p = 0.025)for the second, third, fourth, and fifth PA categories, respectively, compared with the reference category of patients with a mean PA of 0 metabolic equivalent of task-hours/week). Higher PA levels are independently associated with a lower incidence of DR in Japanese patients with type 2 diabetes.
Introduction
The prevalence of type 2 diabetes mellitus has significantly increased worldwide, which has in turn increased the burdens on individuals and health-care systems [1]. One such burden is the increased prevalence of chronic complications, such as diabetic retinopathy (DR). DR has an insidious onset and is a major cause of vision loss in patients aged 20–64 years [2]. Thus, effective diagnostic methods and therapeutic tools are needed to prevent DR in patients with diabetes. Identification of clinical features in patients with diabetes that can predict the development and progression of DR is crucial; suggested risk factors include a long history of diabetes, elevated blood glucose levels, increased blood pressure, and impaired kidney function [3–7].
Studies have shown that increased physical activity (PA) is associated with a substantially reduced risk of cardiovascular events [8,9] and that a low PA level is an independent predictor of all-cause mortality in patients with type 2 diabetes [10,11]. However, whether physical inactivity is a risk factor for DR or higher PA is associated with a reduced risk of developing DR remains unclear. Chronic running exercise was found to alleviate the early progression of nephropathy in rats [12]. In humans, studies in patients with type 1 diabetes have shown no significant association between PA and DR [13–15]. Conversely, the Wisconsin Epidemiologic Study of Diabetic Retinopathy (WESDR) showed that only female patients who engaged in team sports had a reduced risk of having proliferative DR [16]. Moreover, the Finnish Diabetic Nephropathy (FinnDiane) Study demonstrated a significant association between low PA levels and proliferative DR in patients with type 1 diabetes [17]. However, few large-scale prospective studies have evaluated the association between PA and newly developed DR in patients with type 2 diabetes.
We studied data from a large-scale single-center registry of Japanese patients with type 2 diabetes to elucidate the prospective relationship between baseline PA levels and the development of DR over a 2-year follow-up period.
Materials and methods
Patients
Patient data were derived from the second-year survey of the Diabetes Distress and Care Registry at Tenri [18–26], a study conducted at the Tenri Hospital, a regional tertiary-care teaching hospital in Nara, Japan. In brief, this cohort study was aimed at evaluating the cross-sectional and prospective associations between the psycho-socioeconomic factors behind, biomarkers of, therapeutic modalities for, and incidences of chronic complications in patients with diabetes. The registry recruited patients with diabetes who visited the outpatient clinic at the Tenri Hospital between October 2009 and December 2010. We conducted our survey from January to December in 2011, 2012, and 2013. We excluded patients diagnosed with prediabetes (by an oral glucose tolerance test), gestational diabetes, type 1 diabetes, or diabetes induced by steroid use or other endocrine diseases. These exclusions enabled us to obtain data from patients with type 2 diabetes. At registration, the attending physician confirmed the diagnosis according to the Classification and Diagnostic Criteria of Diabetes Mellitus by the Japan Diabetes Society. For this analysis, we included only patients with available baseline PA data who did not have DR. The ethics committee of the Tenri Hospital approved this study, and written informed consent was obtained from every participant before its initiation. Patient records were de-identified and analyzed anonymously.
Inclusion and exclusion criteria
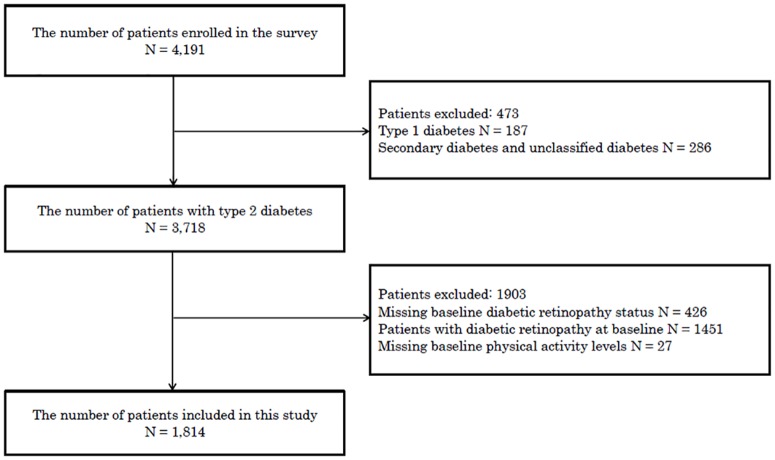
For the analysis, we included only patients whose baseline PA data were available and who did not have DR in 2011. We began collecting data on DR in 2011; therefore, we used the 2011 data as the baseline data in this study. In 2011, of the 4,330 eligible patients with diabetes (mean age, 65.6 ± 12.1 years; 40.5% were female; 4.6% had type 1 diabetes; and 92.3% had type 2 diabetes), 4,191 provided consent to participate in this study. In total, 3,718 were confirmed to have type 2 diabetes. Twenty-seven patients were excluded because of missing baseline PA data, and 426 patients were excluded because of missing baseline DR status data. Furthermore, we excluded 1,451 patients who already had DR at baseline. The remaining 1,814 patients were included in this study (Fig 1).
Fig 1. Inclusion and exclusion criteria of patients in this study.
Fig 1 shows the inclusion and exclusion criteria for patients in this study.
Data collection
In the survey, patients underwent routine inquiries about their medical history, a physical examination, and laboratory tests. The following demographic data were collected: age, sex, height, body weight, systolic blood pressure (sBP), diastolic blood pressure (dBP), smoking status, and alcohol consumption. Medical history, including micro- and macrovascular complications, and treatment modalities, including medication for hypertension, were also recorded. Laboratory tests included the evaluation of hemoglobin A1c (HbA1c) levels, lipid profiles, serum creatinine and uric acid levels, high-sensitivity C-reactive protein (hs-CRP) levels, and the urinary Albumin:creatinine ratio from a spot urine sample. HbA1c levels were expressed in accordance with the National Glycohemoglobin Standardization Program (NGSP) as recommended by the Japanese Diabetes Society [27] and in NGSP and International Federation of Clinical Chemistry units. The estimated glomerular filtration rate (eGFR) was calculated using the following equation proposed by the Japanese Society of Nephrology [28]:
Outcomes
DR status was evaluated at 1, 2, and 3 years after registration. The relationship between PA and DR development was the primary concern. We requested all the patients to receive an ophthalmological consultation every year. DR was assessed using a dilated fundus examination conducted by ophthalmologists blinded to the systemic parameters of this study. During the follow-up period, patients who did not receive a fundus examination were not excluded. Patients with good glycemic control are less likely to consult ophthalmologists; therefore, we believed that overestimation of the incidence of DR may result from the exclusion of these patients. In this study, DR grading was based on published literature and performed using the Fukuda scale [29]. We assessed whether DR had developed but did not evaluate detailed DR stages or whether it was non-proliferative or proliferative. The change in DR status and time point at which it occurred were recorded based on the results of a fundus examination by the end of 2013. In patients with an asymmetric DR manifestation, the most severe DR was selected for grading.
Physical activity
PA was measured at baseline using a Japanese short version of the International Physical Activity Questionnaire (IPAQ) [30]. IPAQ assessed the duration and frequency of walking, moderate PA (MPA), and vigorous PA (VPA) lasting for at least 10 min in a typical week. We estimated PA by multiplying the reported duration (h) per week of walking, MPA, and VPA by their respective metabolic equivalent of tasks (METs; walking = 3.3 METs; MPA = 4 METs; and VPA = 8 METs) to obtain the estimated energy expenditure in MET-hours/week [31]. Using these values, total moderate-to-vigorous PA (MVPA) was defined as follows [32]:
Statistical analysis
Continuous variables were expressed as means and standard deviation or medians and interquartile ranges (IQR) for variables with non-normal distribution. Intergroup differences between five PA categories were evaluated using one-way ANOVA or the Wilcoxon rank-sum test. An unpaired two sample Student’s t-test was used to compare continuous variables between patients with and without newly developed DR. Fisher’s exact test or the chi-squared test was used to examine categorical variables.
Total MVPA was categorically evaluated. To define the categories, we chose an MVPA cutoff of 8.25 MET-hours/week, corresponding to the American Diabetes Association recommendation of 150 min/week of MVPA (brisk walking in this case) [33]. For those with ≥8.25 MET-hours/week, we used tertiles within this sufficiently active group to determine further cutoffs (23.1, 55.1). Finally, the participants were divided into five categories: mean PA levels (in MET-hours/week) of 0, 4.8 (IQR, 3.3–6.6), 13.2 (IQR, 11–16.5), 26.4 (IQR, 23.1–34.6), and 77 (IQR, 55.1–128.3).
The associations between PA categories and DR development were analyzed using the Cox proportional hazards model with time-varying exposure information, considering clustering within attending physicians. The relationship between baseline PA and DR development was estimated in a cohort of patients without DR at baseline. The person-time was calculated as the period between the registration date until the day the outcome was confirmed or the end of follow-up (whichever occurred first). The hazard ratio (HR; 95% CIs) was estimated for the outcome in comparison with a reference category of patients with a mean PA of 0 MET-hour/week. In the analysis, the following three statistical models were used: a crude model; an age- and sex-adjusted model; and a model adjusted for age, sex, BMI, sBP, dBP, heart rate, high-density lipoprotein (HDL) levels, low-density lipoprotein levels, triglyceride levels, eGFR, HbA1c levels, duration of diabetes, diabetes therapy, and history of cardiovascular disease (CVD). We selected these covariates because they are known to be associated with PA and DR. In this multivariable-adjusted Cox proportional hazards model, sBP, dBP, BMI, and HbA1c were evaluated as time-varying exposure information. We adjusted sBP, dBP, BMI, and HbA1c levels as time-varying variables using data from the second and third year observation periods of this study. We selected these covariates as time-varying variables because they are known to vary over time and are associated with DR. Conversely, we did not evaluate PA as a time-varying variable because PA was not evaluated every year in this investigation. All p values were two-sided, and p values <0.05 were considered to be statistically significant. All analyses were performed using Stata/SE version 12.1 (StataCorp LP, College Station, TX, USA).
Results
Patient characteristics
Table 1 displays the demographic characteristics and laboratory data of the patients according to their PA categories. At baseline, their mean age was 65.5 years, mean BMI was 24.5 kg/m2, and mean HbA1c level was 7.2% (54 mmol/mol). Patients with higher PA tended to be younger (p = 0.0001), predominantly male (p < 0.0001), and have a lower BMI (p = 0.0118), lower resting heart rate (p = 0.0003), higher HDL levels (p = 0.0056), and lower hs-CRP levels (p = 0.0415). A lower percentage of them had never smoked, a higher percentage of them had a history of smoking (p < 0.0001), and a lower percentage of them had a history of CVD (p < 0.0001).
Table 1. Baseline participant characteristics for each of the physical activity categoriesa.
| Physical activity level categories | |||||||
|---|---|---|---|---|---|---|---|
| All subjects | Category 1 | Category 2 | Category 3 | Category 4 | Category 5 | ||
| n = 1814 | n = 245 | n = 340 | n = 431 | n = 388 | n = 410 | p value | |
| Physical activity levels, MET-hours/week | |||||||
| Median (interquartile range) | 16.5 (5.5–37.1) | 0 | 4.8 (3.3–6.6) | 13.2 (11–16.5) | 26.4 (23.1–34.6) | 77 (55.1–128.3) | |
| Range, MET-hours/week | 0 | 0.3–8.24 | 8.25–19.82 | 19.83–41.99 | 42– | ||
| Age, years | 65.5 (11.5) | 68.4 (11.7) | 65.2 (13.2) | 64 (11.5) | 65.4 (10.9) | 65.7 (10.2) | 0.0001 |
| Female, % | 37.2 | 46.9 | 42.9 | 40.8 | 30.4 | 29.3 | < 0.0001 |
| Duration of diabetes mellitus, years | 11.4 (8.6) | 11.8 (9.5) | 11.7 (9.1) | 11.4 (8.1) | 11.1 (8.2) | 11.2 (8.3) | 0.763 |
| BMI, kg/m2 | 24.5 (4.3) | 24.5 (4.3) | 25.1 (4.5) | 24.4 (4.3) | 24.3 (4.3) | 24 (3.9) | 0.0118 |
| Systolic blood pressure, mm Hg | 135.6 (17.5) | 134.1 (18) | 135.4 (17.9) | 136.2 (16.7) | 136.2 (16.8) | 135.3 (18.5) | 0.5982 |
| Diastolic blood pressure, mm Hg | 73.6 (11.9) | 72.4 (11.8) | 73.5 (12) | 74.3 (11.7) | 74.7 (11.7) | 72.8 (12.1) | 0.0508 |
| Heart rate, beats/min | 73.6 (12.5) | 74.2 (12.4) | 75.3 (13.1) | 73.9 (12.1) | 73.8 (13) | 71.3 (12) | 0.0003 |
| HDL, mmol/L | 1.44 (0.4) | 1.38 (0.39) | 1.41 (0.38) | 1.44 (0.4) | 1.49 (0.42) | 1.46 (0.38) | 0.0056 |
| LDL, mmol/L | 2.69 (0.73) | 2.69 (0.69) | 2.74 (0.72) | 2.7 (0.71) | 2.67 (0.77) | 2.65 (0.73) | 0.4827 |
| bTriglyceride, mmol/L | 1.52 (1.04–2.12) | 1.60 (1.09–2.11) | 1.56 (1.09–1.56) | 1.51 (0.06–2.13) | 1.48 (1.03–2.12) | 1.45 (0.98–2.14) | 0.8481 |
| Creatinine, μmol/L | 69.6 (40.1) | 69.5 (31.4) | 73.8 (66.3) | 67.5 (39.5) | 69.6 (27) | 68.5 (23.3) | 0.2631 |
| eGFR, mL/min/1.73m2 | 76.7 (22.2) | 74.6 (24.4) | 75.8 (24.3) | 78.3 (21) | 76.2 (20.8) | 77.6 (21.2) | 0.1952 |
| bUrinary Albumin:creatinine ratio, mg/mmolCr | 2.57 (1.42–6.52) | 3.85 (1.83–9.27) | 2.43 (1.30–6.78) | 2.37 (1.33–5.67) | 2.36 (1.39–5.53) | 2.55 (1.47–6.79) | 0.5121 |
| ALT, IU/L | 25.7 (19.7) | 26 (18) | 26.8 (22.3) | 25.5 (19.1) | 25.4 (18) | 25.1 (20.4) | 0.8052 |
| AST, IU/L | 26.9 (14.8) | 27.5 (14.7) | 26.8 (15.6) | 26.6 (14.3) | 26.6 (12.8) | 27.4 (16.5) | 0.8872 |
| Gamma GTP, IU/L | 45.7 (67.9) | 45.8 (54) | 43.5 (45.5) | 44.6 (83.5) | 50.1 (84.3) | 44.4 (54) | 0.6699 |
| bHighly sensitive C-reactive protein, μg/L | 800 (100–1600) | 900 (500–2000) | 900 (100–2000) | 600 (100–1500) | 700 (100–1500) | 700 (100–1400) | 0.0415 |
| HbA1c | |||||||
| NGSP, % | 7.2 (1.1) | 7.2 (1.1) | 7.4 (1.1) | 7.3 (1.1) | 7.2 (1) | 7.2 (1) | 0.0204 |
| IFCC, mmol/mol | 54 (11) | 53.3 (11.2) | 55.7 (11.6) | 54.1 (11.4) | 53.1 (10.3) | 53.6 (10.5) | 0.0204 |
| Smoking, % | < 0.0001 | ||||||
| Never | 41 | 50.2 | 44 | 43 | 36.3 | 35.1 | |
| Past | 41.8 | 29.4 | 38.1 | 40.7 | 48.7 | 47.1 | |
| Current | 17.2 | 20.4 | 18 | 16.3 | 15 | 17.8 | |
| ACE inhibitor use, % | 5 | 3.7 | 7.4 | 4.4 | 3.9 | 5.6 | 0.162 |
| ARB use, % | 31.6 | 34.3 | 31.2 | 30.2 | 30.4 | 33.2 | 0.740 |
| Diabetes therapy, % | 0.071 | ||||||
| Diet only | 19.4 | 17.1 | 15.7 | 19.0 | 22.3 | 21.2 | |
| Oral medication only | 54.5 | 53.5 | 52.5 | 55.0 | 55.1 | 55.9 | |
| Insulin | 26.1 | 29.4 | 31.8 | 26.0 | 22.6 | 22.9 | |
| History of cardiovascular disease, % | 20.4 | 30.2 | 18.2 | 16 | 23.7 | 17.8 | < 0.0001 |
aData are means ± standard deviation unless otherwise indicated
bMedian and interquartile range
The differences in characteristics between patients with and without newly developed DR are presented in Table 2. We found significant differences between the two groups in terms of age (p = 0.006), duration of diabetes (p < 0.0001), sBP (p = 0.0012), resting heart rate (p = 0.0408), eGFR (p = 0.0382), UACR (p = 0.0039), and HbA1c levels (p = 0.0006). No significant differences were evident between patients with and without newly developed DR in sex, BMI, PA levels, dBP, lipid profiles, serum creatinine levels, smoking status, medication use including ACE inhibitors, ARB, and past history of CVD.
Table 2. Differences between patients with and without newly developed diabetic retinopathy over 2 years of follow-upa.
| Patients without development of diabetic retinopathy | Patients with development of diabetic retinopathy | p-value | |
|---|---|---|---|
| Variables | n = 1630 | n = 184 | |
| Age, years | 65.3 (11.6) | 67.5 (10.6) | 0.0060 |
| Female, % | 37.2 | 37.5 | 0.936 |
| Duration of diabetes mellitus | 11.0 (8.4) | 14.7 (9.1) | <0.0001 |
| BMI, kg/m2 | 24.5 (4.3) | 24.3 (4.0) | 0.3620 |
| Physical activity levels, MET- hours/week b | 16.5 (5.5–38.5) | 11.6 (4.1–32.9) | 0.0581 |
| Systolic blood pressure, mm Hg | 135.1 (17.5) | 139.2 (17.3) | 0.0012 |
| Diastolic blood pressure, mm Hg | 73.7 (12.0) | 72.7 (11.2) | 0.1359 |
| Heart rate, /min | 73.4 (12.6) | 75.1 (11.8) | 0.0408 |
| HDL, mmol/L | 1.44 (0.40) | 1.41 (0.40) | 0.1501 |
| LDL, mmol/L | 2.69 (0.73) | 2.66 (0.65) | 0.2906 |
| Triglyceride, mmol/L b | 1.52 (1.04–2.12) | 1.46 (1.02–2.16) | 0.4872 |
| Creatinine, μmol/L | 69.5 (41.4) | 70.9 (26.3) | 0.3229 |
| eGFR, mL/min/1.73 m2 | 77.1 (22.1) | 74.0 (22.8) | 0.0382 |
| Urinary Albumin:creatinine ratio, mg/mmol b | 2.45 (1.39–6.08) | 4.00 (1.76–13.48) | 0.0039 |
| ALT, IU/L | 25.9 (20.1) | 24.0 (14.9) | 0.1058 |
| AST, IU/L | 27.1 (15.1) | 25.5 (11.6) | 0.0885 |
| Gamma GTP, IU/L | 46.5 (69.9) | 38.8 (46.2) | 0.0744 |
| Highly sensitive C-reactive protein, μg/Lb | 700 (100–1600) | 800 (100–2100) | 0.2787 |
| HbA1c | |||
| NGSP, % | 7.2 (1.1) | 7.5 (1.1) | 0.0006 |
| IFCC, mmol/mol | 53.7 (10.9) | 56.5 (11.7) | 0.0006 |
| Smoking, % | 0.735 | ||
| Never | 41.2 | 39.1 | |
| Past | 41.5 | 44.6 | |
| Current | 17.3 | 16.3 | |
| ACE inhibitor use, % | 4.9 | 6.5 | 0.370 |
| ARB use, % | 30.9 | 38.0 | 0.054 |
| History of cardiovascular disease, % | 20.4 | 20.7 | 0.923 |
a Data are means ± (standard deviation) unless otherwise indicated
b Median and interquartile range
In the cohort of patients who were followed up for a median of 700 days, 184 developed DR [incidence ratio, 54.3/1000 person-years (95% CI, 47.0–62.7); Table 3]. In almost all these patients, their DR was non-proliferative. HRs calculated using the Cox proportional hazards model for the association between PA categories and DR development are shown in Table 4. We observed a significant association between baseline PA and subsequent DR development in all three models. HRs for developing DR in the second, third, fourth, and fifth PA categories compared with the first PA category were 0.84 (95% CI, 0.57–1.24; p = 0.373), 0.75 (95% CI, 0.55–1.04; p = 0.081), 0.49 (95% CI, 0.30–0.80; p = 0.004), and 0.57 (95% CI, 0.41–0.79; p = 0.001), respectively, for the crude model; 0.92 (95% CI, 0.59–1.43; p = 0.697), 0.84 (95% CI, 0.58–1.22; p = 0.367), 0.53 (95% CI, 0.33–0.85; p = 0.009), and 0.61 (95% CI, 0.44–0.85; p = 0.004), respectively, for the age- and sex-adjusted model; 0.89 (95% CI, 0.54–1.44; p = 0.628), 0.85 (95% CI, 0.55–1.32; p = 0.464), 0.58 (95% CI, 0.35–0.94; p = 0.026), and 0.67 (95% CI, 0.48–0.93; p = 0.019), respectively, for the multivariable-adjusted model; and 0.87 (95% CI, 0.53–1.40; p = 0.557), 0.83 (95% CI, 0.52–1.31; p = 0.421), 0.58 (95% CI, 0.35–0.94; p = 0.027), and 0.63 (95% CI, 0.42–0.94; p = 0.025), respectively, for the multivariable-adjusted model with time-varying exposure information. During the follow-up period, 149 patients who did not undergo a fundus examination were not excluded from this study; however, the numbers of these patients in the first, second, third, fourth, and fifth PA categories were 33, 33, 28, 30, and 25, respectively, and the same associations were evident when we excluded these patients. For example, the HRs for developing DR in the second, third, fourth, and fifth PA categories compared with the first PA category were 0.83 (95% CI, 0.51–1.35; p = 0.463), 0.83 (95% CI, 0.51–1.35; p = 0.461), 0.53 (95% CI, 0.33–0.85; p = 0.008), and 0.60 (95% CI, 0.40–0.90; p = 0.013), respectively, for the multivariable-adjusted model with time-varying exposure information. We thought that excluding these patients may overestimate the incidence of DR, so we did not exclude them; patients with good glycemic control are less likely to see ophthalmologists.
Table 3. Baseline physical activity categories and incidence of diabetic retinopathy.
| Physical activity categories | Number of participants | Person-years (days) | Number of outcomes | Incidence ratio (95% CI) a |
|---|---|---|---|---|
| Category 1 | 245 | 451.1 | 32 | 70.9 (50.2–100.3) |
| Category 2 | 340 | 629.4 | 43 | 68.3 (50.7–92.1) |
| Category 3 | 431 | 803.3 | 48 | 59.8 (45–79.3) |
| Category 4 | 388 | 735.3 | 29 | 39.4 (27.4–56.8) |
| Category 5 | 410 | 770.9 | 32 | 41.5 (29.4–58.7) |
aIncidence of outcomes per 1000 person-years
Table 4. Association between physical activity levels and development of diabetic retinopathy.
| Physical activity categories | |||||
|---|---|---|---|---|---|
| Category 1 | Category 2 | Category 3 | Category 4 | Category 5 | |
| Number of participants | n = 245 | n = 340 | n = 431 | n = 388 | n = 410 |
| Physical activity levels, MET-hours/week | |||||
| Median (interquartile range) | 0 | 4.8 (3.3–6.6) | 13.2 (11–16.5) | 26.4 (23.1–34.6) | 77 (55.1–128.3) |
| Range, MET-hours/week | 0 | 0.3–8.24 | 8.25–19.82 | 19.83–41.99 | 42– |
| Hazard ratio for development (95% CI) | |||||
| Crude model | Ref. | 0.84 (0.57–1.24) | 0.75 (0.55–1.04) | 0.49 (0.30–0.80) | 0.57 (0.41–0.79) |
| Age- and sex-adjusted model | Ref. | 0.92 (0.59–1.43) | 0.84 (0.58–1.22) | 0.53 (0.33–0.85) | 0.61 (0.44–0.85) |
| aMultivariate-adjusted model | Ref. | 0.89 (0.54–1.44) | 0.85 (0.55–1.32) | 0.58 (0.35–0.94) | 0.67 (0.48–0.93) |
| bMultivariate-adjusted model | Ref. | 0.87 (0.53–1.40) | 0.83 (0.52–1.31) | 0.58 (0.35–0.94) | 0.63 (0.42–0.94) |
aAdjusted for age, sex, BMI, duration of diabetes mellitus, systolic blood pressure (sBP), diastolic blood pressure (dBP), heart rate (HR), Hemoglobin A1c (HbA1c) levels, high-density lipoprotein (HDL), low-density lipoprotein (LDL), triglyceride, estimated glomerular filtration rate (eGFR), diabetes therapy, and history of cardiovascular disease (CVD).
bAdjusted for age, sex, BMI, duration of diabetes mellitus, sBP, dBP, HR, HbA1c levels, HDL, LDL, triglyceride, eGFR, diabetes therapy, and history of CVD. SBP, dBP, BMI, and HbA1c were assumed as time-varying exposure information.
Discussion
In this study, higher PA was independently associated with a lower incidence of DR in patients with type 2 diabetes. To the best of our knowledge, this is the first large-scale prospective study to evaluate the influence of PA on DR development in patients with type 2 diabetes.
The first large-scale study to examine the association between PA and DR was the Pittsburgh Insulin-Dependent Diabetes Mellitus Morbidity and Mortality Study, which was published in 1986 [13]. Previous studies have likewise shown no significant association between PA and DR in patients with type 1 diabetes [13–15]. However, the data indicated that exercise had no adverse effect on the progression and development of DR. In contrast, WESDR reported protective associations between PA and proliferative DR. However, this association was shown in only female patients who were diagnosed with diabetes before the age of 14 years [16]. Moreover, the FinnDiane study showed a significant association between low PA and a higher incidence of proliferative DR in patients with type 1 diabetes [17]. Although the FinnDiane study was conducted at a larger scale than the previous studies on the association of PA and DR, the generalizability of its findings was limited by its cross-sectional study design. These two studies reported protective associations between PA and proliferative DR, but they did not indicate the association between PA and newly developed DR. The present study only included type 2 diabetic patients without DR and revealed the protective associations between PA and the consequent risk of newly developed DR.
Possible mechanisms that link PA to the prevention of the progression and remission of microvascular complications in patients with type 2 diabetes reportedly include the following: reduction of blood pressure, improvements in lipid profile, glycemic control, insulin sensitivity, and endothelial function [34–38]. However, our results show that the association is robust after adjusting for sBP, dBP, BMI, and HbA1c level as time-varying variables, suggesting that PA could lower the risk of developing DR via pathways other than blood pressure, BMI, and glycemic control. In Zucker diabetic rats, chronic running exercise alleviated the early progression of nephropathy with the upregulation of nitric oxide synthases and suppression of glycation [12]. This mechanism could contribute to the reduction of the risk of DR by higher PA in patients with type 2 diabetes.
In this study, 184 patients developed DR (incidence ratio, 54.3/1,000 person-years [95% CI, 47.0–62.7]; Table 3). As previously reported in the Japan Diabetes Complications Study [39], the incidence of diabetic retinopathy in Japanese patients with type 2 diabetes was 38.3/1,000 person-years. The HbA1c levels, age, and duration of diabetes of the patients in this study were 7.8%, 58.2 years old, and 9.8 years, respectively. Although our study participants had better baseline glycemic control, they tended to have a higher age and longer duration of diabetes. These factors could be responsible for the difference in the incidence of DR.
Our results were derived from an observational study, but it is intriguing to consider that PA may play a protective role in the pathogenesis of DR. This hypothesis should be tested in randomized controlled trials designed to examine the effects of altering PA on DR development. Further long-term studies, both clinical and experimental, are needed to establish the clinical utility of PA for the prediction of DR development and to understand the potential pathophysiologic roles of physical inactivity on DR.
Our study had some limitations. First, because this was an epidemiologic study, residual confounders may have existed in the association with DR development and PA that may have acted as bystanders or epiphenomena. Second, the data were derived from the registry of a single diabetes center in Japan, thereby raising concerns regarding generalizations derived from the results, particularly for multiethnic populations. Third, we evaluated only DR development in patients without DR, but did not evaluate changes in the severity of existing DR. Fourth, we were unable to investigate the prevalence of dementia among the participants in our study. Dementia may be related to PA levels or DR. Fifth, the relationship between baseline PA and DR development was estimated in a cohort of patients without DR at baseline. We did not evaluate PA as a time-varying variable because PA was not evaluated every year in this investigation. Moreover, we used subjective estimates to evaluate PA levels instead of assessing PA objectively, for example by using accelerometers. However, the IPAQ is a widely used objective questionnaire. The criterion validity of the short version of the IPAQ had a median rho of about 0.3 [31]. Thus, we believe that the data obtained were trustworthy.
Conclusion
In conclusion, higher PA was independently associated with a lower incidence of DR in patients with type 2 diabetes. More research needs to be performed to determine if effective strategies to increase PA will reduce the risk of DR in patients with diabetes.
Acknowledgments
We would especially like to thank Yukari Moritsuji, Yuki Fujita, Noriko Nakamura, and Yoko Sakamoto (Department of Endocrinology, Tenri Hospital) for their clerical support. The authors would like to thank Enago (www.enago.jp) for the English language review.
Members of the Diabetes Distress and Care Registry at Tenri Study Group:
Hitoshi Ishii, Hirohito Kuwata, and Tsuyoshi Mashitani (Department of Diabetology, Nara Medical University); Satoru Tsujii, Shintaro Okamura, Yasuaki Hayashino, Miyuki Furuya, and Masako Kitatani (Department of Endocrinology, Tenriyorozu Hospital); Satoshi Matsunaga (Department of Hematology, Endocrinology and Metabolism, Niigata University Faculty of Medicine); Yaeko Kondo and Naotaka Fujita (Department of Diabetes and Clinical Nutrition, Kyoto University); Rei Ueda (Second Department of Internal Medicine, Faculty of Medicine, University of the Ryukyus); Rie Kurokawa (Osaka Medical Center and Research Institute for Maternal and Child Health); and Masami Tanaka (Division of Endocrinology, Metabolism and Nephrology, Department of Internal Medicine, Keio University School of Medicine).
Data Availability
Data are available for researchers who meet the criteria for access to confidential data. We cannot provide individual data because we did not obtain informed consent from participants to provide data to anyone outside of the research group. Please contact the corresponding author to request access to confidential data.
Funding Statement
This study was partially supported by the Manpei Suzuki Diabetes Foundation, the grants and endowment from Takeda Pharmaceutical Company Limited, and JSPS KAKENHI (Grant Number 25460641); however, they played no role in the design, conduct, data collection, analysis, or interpretation of the study, or in the preparation, review, or approval of the manuscript.
References
- 1.Fenwick E, Rees G, Pesudovs K, Dirani M, Kawasaki R, Wong TY, et al. Social and emotional impact of diabetic retinopathy: a review. Clin Experiment Ophthalmol 2012;40:27–38 10.1111/j.1442-9071.2011.02599.x [DOI] [PubMed] [Google Scholar]
- 2.Buch H, Vinding T, La Cour M, Appleyard M, Jensen GB, Nielsen NV. Prevalence and causes of visual impairment and blindness among 9980 Scandinavian adults: the Copenhagen City Eye Study. Ophthalmology 2004;111:53–61 [DOI] [PubMed] [Google Scholar]
- 3.Davis MD, Fisher MR, Gangnon RE, Barton F, Aiello LM, Chew EY,et al. Risk factors for high-risk proliferative diabetic retinopathy and severe visual loss: Early Treatment Diabetic Retinopathy Study Report #18. Invest Ophthalmol Vis Sci 1998;39:233–252 [PubMed] [Google Scholar]
- 4.Klein R, Klein BE, Moss SE, Davis MD, DeMets DL. The Wisconsin epidemiologic study of diabetic retinopathy. II. Prevalence and risk of diabetic retinopathy when age at diagnosis is less than 30 years. Arch Ophthalmol 1984;102:520–526 [DOI] [PubMed] [Google Scholar]
- 5.Lu B, Wen J, Song XY, Dong XH, Yang YH, Zhang ZY, et al. High prevalence of albuminuria in population-based patients diagnosed with type 2 diabetes in the Shanghai downtown. Diabetes Res Clin Pract 2007;75:184–192 10.1016/j.diabres.2006.06.024 [DOI] [PubMed] [Google Scholar]
- 6.Wong TY, Klein R, Islam FM, Cotch MF, Folsom AR, Klein BE, et al. Diabetic retinopathy in a multi-ethnic cohort in the United States. Am J Ophthalmol 2006;141:446–455 10.1016/j.ajo.2005.08.063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pradeepa R, Anjana RM, Unnikrishnan R, Ganesan A, Mohan V, Rema M. Risk factors for microvascular complications of diabetes among South Indian subjects with type 2 diabetes—the Chennai Urban Rural Epidemiology Study (CURES) Eye Study-5. Diabetes Technol Ther 2010;12:755–761 10.1089/dia.2010.0069 [DOI] [PubMed] [Google Scholar]
- 8.Sclavo M. [Physical activity and risk for cardiovascular events in diabetic women]. Ital Heart J Suppl 2001;2:563–565 [PubMed] [Google Scholar]
- 9.Blomster JI, Chow CK, Zoungas S, Woodward M, Patel A, Poulter NR, et al. The influence of physical activity on vascular complications and mortality in patients with type 2 diabetes mellitus. Diabetes Obes Metab 2013;15:1008–1012 10.1111/dom.12122 [DOI] [PubMed] [Google Scholar]
- 10.Wei M, Gibbons LW, Kampert JB, Nichaman MZ, Blair SN. Low cardiorespiratory fitness and physical inactivity as predictors of mortality in men with type 2 diabetes. Ann Intern Med 2000;132:605–611 [DOI] [PubMed] [Google Scholar]
- 11.Zethelius B, Gudbjörnsdottir S, Eliasson B, Eeg-Olofsson K, Cederholm J. Level of physical activity associated with risk of cardiovascular diseases and mortality in patients with type-2 diabetes: report from the Swedish National Diabetes Register. Eur J Prev Cardiol 2014;21:244–251 10.1177/2047487313510893 [DOI] [PubMed] [Google Scholar]
- 12.Ito D, Cao P, Kakihana T, Sato E, Suda C, Muroya Y, et al. Chronic running exercise alleviates early progression of nephropathy with upregulation of nitric oxide synthases and suppression of glycation in Zucker diabetic rats. PLoS One 2015;10:e0138037 10.1371/journal.pone.0138037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.LaPorte RE, Dorman JS, Tajima N, Cruickshanks KJ, Orchard TJ, Cavender DE, et al. Pittsburgh insulin-dependent diabetes mellitus morbidity and mortality study: physical activity and diabetic complications. Pediatrics 1986;78:1027–1033 [PubMed] [Google Scholar]
- 14.Kriska AM, LaPorte RE, Patrick SL, Kuller LH, Orchard TJ. The association of physical activity and diabetic complications in individuals with insulin-dependent diabetes mellitus: The epidemiology of diabetes complications study—VII. J Clin Epidemiol 1991;44:1207–1214 [DOI] [PubMed] [Google Scholar]
- 15.Cruickshanks KJ, Moss SE, Klein R, Klein BE. Physical activity and the risk of progression of retinopathy or the development of proliferative retinopathy. Ophthalmology 1995;102:1177–1182 [DOI] [PubMed] [Google Scholar]
- 16.Cruickshanks KJ, Moss SE, Klein R, Klein BE. Physical activity and proliferative retinopathy in people diagnosed with diabetes before age 30 years. Diabetes Care 1992;15:1267–1272. [DOI] [PubMed] [Google Scholar]
- 17.Wadén J, Forsblom C, Thorn LM, Saraheimo M, Rosengård-Bärlund M, Heikkilä O, et al. FinnDiane Study Group. Physical activity and diabetes complications in patients with type 1 diabetes: the Finnish Diabetic Nephropathy (FinnDiane) Study. Diabetes Care 2008;31:230–232 10.2337/dc07-1238 [DOI] [PubMed] [Google Scholar]
- 18.Tsujii S, Hayashino Y, Ishii H. Diabetes distress, but not depressive symptoms, is associated with glycaemic control among Japanese patients with type 2 diabetes: Diabetes Distress and Care Registry at Tenri (DDCRT 1). Diabet Med 2012;29:1451–1455 10.1111/j.1464-5491.2012.03647.x [DOI] [PubMed] [Google Scholar]
- 19.Hayashino Y, Okamura S, Matsunaga S, Tsujii S, Ishii H. The association between problem areas in diabetes scale scores and glycemic control is modified by types of diabetes therapy: diabetes distress and care registry in Tenri (DDCRT 2). Diabetes Res Clin Pract 2012;97:405–410 10.1016/j.diabres.2012.04.005 [DOI] [PubMed] [Google Scholar]
- 20.Mashitani T, Hayashino Y, Okamura S, Kitatani M, Furuya M, Matsunaga S, et al. Patient-reported adherence to insulin regimen is associated with glycemic control among Japanese patients with type 2 diabetes: Diabetes Distress and Care Registry at Tenri (DDCRT 3). Diabetes Res Clin Pract 2013;100:189–194 10.1016/j.diabres.2013.03.006 [DOI] [PubMed] [Google Scholar]
- 21.Hayashino Y, Tsujii S, Ishii H. High frequency of non-nocturnal hypoglycemia was associated with poor sleep quality measure by Pittsburg Sleep Quality Index in patients with diabetes receiving insulin therapy: Diabetes Distress and Care Registry at Tenri (DDCRT 4). Exp Clin Endocrinol Diabetes 2013;121:628–634 10.1055/s-0033-1355424 [DOI] [PubMed] [Google Scholar]
- 22.Mashitani T, Hayashino Y, Okamura S, Tsujii S, Ishii H. Correlations between serum bilirubin levels and diabetic nephropathy progression among Japanese type 2 diabetic patients: a prospective cohort study (Diabetes Distress and Care Registry at Tenri [DDCRT 5]). Diabetes Care 2014;37:252–258 10.2337/dc13-0407 [DOI] [PubMed] [Google Scholar]
- 23.Hayashino Y, Mashitani T, Tsujii S, Ishii H. Elevated levels of hs-CRP are associated with high prevalence of depression in japanese patients with type 2 diabetes: the Diabetes Distress and Care Registry at Tenri (DDCRT 6). Diabetes Care 2014;37:2459–2465 10.2337/dc13-2312 [DOI] [PubMed] [Google Scholar]
- 24.Mashitani T, Hayashino Y, Okamura S, Kitatani M, Furuya M, Iburi T, et al. Diabetes treatment-related quality of life is associated with levels of self-care activities in insulin injection among Japanese patients with type 2 diabetes: Diabetes Distress and Care Registry at Tenri (DDCRT 8). Acta Diabetol 2015;52:639–647 10.1007/s00592-015-0725-0 [DOI] [PubMed] [Google Scholar]
- 25.Hayashino Yasuaki, Tsujii Satoru, Ishii Hitoshi. Serum uric acid levels are associated with high risk of progressing diabetic nephropathy among Japanese type 2 diabetes patients: A prospective cohort study [Diabetes Distress and Care Registry at Tenri (DDCRT 10)]. Acta Diabetol, 2016. March 3. [DOI] [PubMed] [Google Scholar]
- 26.Kuwata H, Okamura S, Hayashino Y, Ishii H, Tsujii S. Serum uric acid levels are associated with a high risk of rapid chronic kidney disease progression among patients with type 2 diabetes: a prospective cohort study [Diabetes Distress and Care Registry at Tenri (DDCRT 12)]. Diabetol Int, 03 February 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Committee of the Japan Diabetes Society on the Diagnostic Criteria of Diabetes Mellitus, Seino Y, Nanjo K, Tajima N, Kadowaki T, Kashiwagi A, Araki E, et al. Report of the committee on the classification and diagnostic criteria of diabetes mellitus. J Diabetes Investig 2010;1:212–228 10.1111/j.2040-1124.2010.00074.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Matsuo S, Imai E, Horio M, Yasuda Y, Tomita K, Nitta K, et al. Collaborators developing the Japanese equation for estimated GFR. Revised equations for estimated GFR from serum creatinine in Japan. Am J Kidney Dis 2009;53:982–992 10.1053/j.ajkd.2008.12.034 [DOI] [PubMed] [Google Scholar]
- 29.Fukuda M. Clinical arrangement of classification of diabetic retinopathy. Tohoku J Exp Med. 1983;141 Suppl:331–335 [DOI] [PubMed] [Google Scholar]
- 30.Murase N, Katsumura T, Ueda C, Inoue S ST. Reliability and validity study of the Japanese version of the International Physical Activity Questionnaire (IPAQ). J Heal Welf Stat 2002;49:1–9 [Google Scholar]
- 31.Craig CL, Marshall AL, Sjöström M, Bauman AE, Booth ML, Ainsworth BE, et al. International physical activity questionnaire: 12-country reliability and validity. Med Sci Sports Exerc 2003;35:1381–1395 10.1249/01.MSS.0000078924.61453.FB [DOI] [PubMed] [Google Scholar]
- 32.Kamada M, Kitayuguchi J, Lee IM, Hamano T, Imamura F, Inoue S, et al. Relationship between physical activity and chronic musculoskeletal pain among community-dwelling Japanese adults. J Epidemiol 2014;24:474–483 10.2188/jea.JE20140025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.(4)Foundations of care: education, nutrition, physical activity, smoking cessation, psychosocial care, and immunization. Diabetes Care 2015;38 Suppl:S20–S30 [DOI] [PubMed] [Google Scholar]
- 34.Lehmann R, Vokac A, Niedermann K, Agosti K, Spinas GA. Loss of abdominal fat and improvement of the cardiovascular risk profile by regular moderate exercise training in patients with NIDDM. Diabetologia 1995;38:1313–1319 [DOI] [PubMed] [Google Scholar]
- 35.Boulé NG, Haddad E, Kenny GP, Wells GA, Sigal RJ. Effects of exercise on glycemic control and body mass in type 2 diabetes mellitus. JAMA 2001;286:1218 [DOI] [PubMed] [Google Scholar]
- 36.Coker RH, Williams RH, Yeo SE, Kortebein PM, Bodenner DL, Kern PA, et al. The impact of exercise training compared to caloric restriction on hepatic and peripheral insulin resistance in obesity. J Clin Endocrinol Metab 2009;94:4258–4266 10.1210/jc.2008-2033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stehouwer CDA, Gall MA, Twisk JWR, Knudsen E, Emeis JJ, Parving HH. Increased urinary albumin excretion, endothelial dysfunction, and chronic low-grade inflammation in type 2 diabetes: progressive, interrelated, and independently associated with risk of death. Diabetes 2002;51:1157–1165 [DOI] [PubMed] [Google Scholar]
- 38.Wadén J, Tikkanen HK, Forsblom C, Harjutsalo V, Thorn LM, Saraheimo M, et al. FinnDiane Study Group. Leisure-time physical activity and development and progression of diabetic nephropathy in type 1 diabetes: the FinnDiane Study. Diabetologia 2015;58:929–936 10.1007/s00125-015-3499-6 [DOI] [PubMed] [Google Scholar]
- 39.Kawasaki R, Tanaka S, Tanaka S, Yamamoto T, Sone H, Ohashi Y, et al. Japan Diabetes Complications Study Group: Incidence and progression of diabetic retinopathy in Japanese adults with type 2 diabetes: 8 year follow-up study of the Japan Diabetes Complications Study (JDCS). Diabetologia 2011; 54: 2288–94 10.1007/s00125-011-2199-0 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are available for researchers who meet the criteria for access to confidential data. We cannot provide individual data because we did not obtain informed consent from participants to provide data to anyone outside of the research group. Please contact the corresponding author to request access to confidential data.