Abstract
Estrogens are important for bone metabolism via a variety of mechanisms in osteoblasts, osteocytes, osteoclasts, immune cells and other cells to maintain bone mineral density. Estrogens bind to estrogen receptor alpha (ERα) and ERβ, and the roles of each of these receptors are beginning to be elucidated through whole body and tissue-specific knockouts of the receptors. In vitro and in vivo experiments have shown that ERα and ERβ antagonize each other in bone and in other tissues. This review will highlight the role of these receptors in bone, with particular emphasis on their antagonism.
Keywords: Estrogen; estrogen receptor alpha (ERα, ESR1); estrogen receptor beta (ERβ, ESR2); bone
Estrogen Receptors
Estrogens have physiological functions in almost all tissues in the body in both males and females [1]. Estrogens, including the most abundant estrogen 17β-estradiol (E2), interact with estrogen receptors (ER) alpha and beta (ERα and ERβ) [2]. ERα was identified in the 1960s [3] (and cloned in 1986 [4]) and is best studied in the female reproductive system and in breast cancer. ERβ was identified and cloned in 1996 [5] and is less well characterized. ERα and ERβ are highly conserved in the DNA binding domain (95%) and ligand binding domain (60%), but the NH2-terminal domains, including the transcriptional activation domain AF-1, are only 20% conserved. While the role of estrogen in bone has been reviewed (for example, refs. [6–8],) this review will highlight the role of these receptors in bone, with particular emphasis on their antagonism.
ERα and ERβ Antagonism
It is beginning to become clear that ERα and ERβ antagonize each other’s actions in many tissues. In breast cancer cell lines, such as T47D cells, overexpression of ERβ inhibits E2-mediated proliferation and gene expression [9]. In the prostate, ERα also promotes cell proliferation and survival, whereas ERβ is protective and pro-apoptotic [10]. Another example of their antagonism in vivo is observed in behavior; compared to WT mice, ERβKO mice have an increase in sexual aggression, whereas ERαKO mice have a decrease in such behavior, compared to control WT mice [11]. At the molecular level, ERα and ERβ have been observed to signal in opposite ways (activation vs. repression of transcription) at an AP1 site [12]. However, the roles of ERα and ERβ in different tissues and under different conditions remain to be further elucidated, particularly in bone biology.
Estrogens in Bone
Estrogens are important for maintaining bone mineral density in both mice and humans. When women go through menopause estrogen levels decrease and there is a decrease in bone mineral density, along with an increased risk for fractures, particularly in the hip, vertebrae and wrist [13]. Treatment of women with hormone replacement therapy (HRT) (either estrogen alone or estrogen plus progesterone) has been shown to prevent this bone loss [14]. In 2002 the Women’s Health Initiative (WHI) showed that HRT prevents bone fractures [14]. However, the routine use of HRT has diminished significantly due to the results of the WHI suggesting an increased risk of breast cancer, heart disease and stroke in women taking HRT. While we have long known the beneficial effects of estrogen in bone, surprisingly the molecular mechanism for the role of estrogen in bone cells is only beginning to be unraveled.
The skeleton is constantly being remodeled. Osteoblasts lay down the matrix for bone and osteoclasts degrade bone. If there is an increase in osteoblast number and/or activity, especially if coupled with a decrease in osteoclast activity, there is overall building of bone, such as occurs during E2-driven acquisition of bone mass during puberty. On the other hand, if there is a decrease in osteoblast number or activity and that is coupled to no change or an increase in osteoclast number or activity, a decrease in bone mineral density will occur. Thus, it is the balance between osteoblast and osteoclast numbers and activity that determines the quality and quantity of bone.
The protective effects of E2 in bone are due to many mechanisms. For example, repression of pro-osteoclastic cytokines, such as IL-1, IL-6, IL-7 and TNF, in T cells and osteoblasts have been well documented to promote increased bone mass [15–17]. The mechanism of E2 repression of proinflammatory cytokines in osteoblasts has not been as well characterized, but is thought to be via inhibition of the nuclear factor κB (NFκB) pathway and its multicomplex effects on a wide-variety of cellular and molecular processes [18].
E2 exposure not only represses pro-osteoclastic cytokines, but it induces apoptosis in bone resorbing osteoclasts [19, 20]. Mechanistically, E2, via ERα activation, induces transcription of Fas Ligand (FasL) in osteoblasts. FasL is cleaved from the cell surface by MMP3, and the soluble FasL induces osteoclast apoptosis [21, 22]. A third mechanism of estrogen-mediated suppression of osteoclasts involves the regulation of the RANKL/(OPG) ratio. Receptor activator of nuclear factor κB ligand (RANKL) is an essential cytokine for osteoclast differentiation. The RANKL pathway can be inhibited by OPG, which acts as a decoy receptor for RANKL. Thus, the RANKL:OPG ratio is critical for osteoclastogenesis. E2 has been shown to increase the transcription of OPG [23] and to affect RANKL localization at the cell surface of osteoblasts [24].
Furthermore, E2 is pro-osteoblastic, leading to a net increase in bone building. E2 is anti-apoptotic in osteoblasts [25], and the anti-apoptotic protein Bcl2 is regulated by E2 in osteoblasts [26]. Ovariectomy leads to an increase in osteoblast and osteocyte apoptosis, and conversely E2 can prevent etoposide, dexamethasone or TNFα-induced apoptosis in osteoblasts [25]. E2 also induces the transcription of alkaline phosphatase, a marker of osteoblast differentiation [27]. There are very few other known direct targets of E2 in normal osteoblasts and little to no information on the targets of ERβ [7].
The Role of Estrogen Receptors in Bone in vivo
ERα and ERβ have been detected by immunohistochemistry in osteoblasts [28–30], osteocytes [28, 30], and osteoclasts [28–30]. ERα and ERβ are also expressed in immune cells, such as T cells and monocytes [31–33], which are important in bone regulation. By IHC, ERβ is expressed at higher levels in trabecular bone than in cortical bone. Interestingly, in this same study ERα was detected in the opposite pattern: higher in cortical bone than in trabecular bone [28].
Both ERα and ERβ polymorphisms have been shown to correlate with bone mass in humans [34]. The so-called “PvuII” and “XbaI” haplotypes of ERα are well-studied at the population level. More recently, two genome-wide association studies have correlated SNPs upstream of the ERα promoter with bone mineral density [35, 36]. However, these polymorphisms have no known function to explain their effects on bone mineral density. Similarly, three intronic variations in ERβ are associated with femoral bone mineral density, but the mechanism is unknown. While an ESR1 mutation in a man has been described in the literature [37], no known ESR2 mutations have been reported. The patient had an ERα truncating mutation that led to incomplete epiphyseal closure and low bone mineral density.
Mouse models of the function of ERα and ERβ have been generated by several labs and provided us with some insights to their individual functions (Table 1). For example, ERαKO mice were generated in the Korach and Smithies laboratories (K/G-ERαKO) [38] and Chambon laboratory (C-ERβKO) [39]. The K/G ERαKO is not a complete knockout and expresses some ERα due to splicing of the Neo cassette. The female and male C-ERαKO mice have a decrease in cortical bone mineral density, and an increase in trabecular bone mineral density [40].
Table 1.
Tissue specific ER knockout mice
| Mice genotype | Cortical | Trabecular |
|---|---|---|
| Menopausal women |
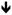
|
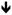
|
| OVX in mice |
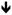
|
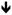
|
| C-ERαKO [40] |
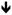
|

|
| C-ERβKO [40] | NC | NC |
| C-ERαβKO [40] |
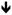
|
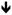
|
| ERαf/f;Prx1-cre [54] |
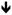
|
NC |
| ERαf/f;Osx1-cre [54] | females
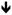
|
NC |
| ERαf/f;Col1a1-cre [54] | NC | NC |
| ERαf/f;OCN-cre [55] | females
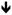
|
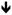
|
| ERαf/f;OCN-cre [56] | females
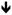
|
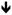
|
| ERαf/f;DMP1-cre [57] | NC | males
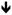
|
| ERα−/NERKI [59] | NC |
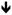
|
| ERαf/f;LysM-cre [58] | NC |
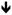
|
| ERαf/f;Nestin-cre [58] |

|

|
| ERβf/f;PRX1-cre [70] | NC |

|
Several ERβ knockout mice have also been created. ERβKO mice were first developed in the Korach, Gustafsson and Smithies laboratories (K/G-ERβKO) [41] and the Chambon laboratory (C-ERβKO) [39]. The female K/G-ERβKO mice have an increased cortical BMD at 11 weeks of age [42] and both cortical and trabecular BMD increases by 12 months of age [43], whereas the BMD in the Chambon ERβKO female mice is unchanged compared to WT controls. The Chambon group claimed that the phenotype of the K/G-ERβKO knockout mouse is a result of the neo selection cassette inserted into the ERβ gene and is not due to loss of ERβ itself. Furthermore, these mice express truncations in the ERβ transcript that might contribute to the phenotypes [44]. In 2007 Antal, et al., published an ERβ knockout (ERβSTL-/L-) that is not thought to have any ERβ splice forms. This mouse had reproductive abnormalities, but no phenotype in the prostate or other tissues shown to have an effect in the other ERβKO strains [44]. However, the bone phenotype for the ERβSTL-/L- mouse has not been published.
The C-ERβKO mice have no difference in the femoral length, but the K/G-ERβKO mice have longer femurs than WT mice, whereas ERαKO mice have shorter femurs than WT mice. However, the male C-ERβKO mice do have an increase in femoral width compared to WT mice and the C-ERαKO mice have a decrease in femoral width [40]. Together, this data demonstrate opposing effects of ERα and ERβ on femoral size [42, 45].
Estrogen and estrogen receptors play a critical role in the normal adaptation of bone to loading. The C-ERαKO and C-ERβKO knockout mice show opposite osteogenic responses to loading in the cortical bone. Whereas the ERαKO mice had a lower response, the ERβKO had a higher response to loading when compared to their respective wildtype mice [46]. In vitro, ERα and ERβ also differentially regulate SOST, which is involved in the osteoblast response to strain [47]. Loading experiments have shown that the ulnae of female ERβKO mice bone were stiffer than those from WT mice when subjected to mechanical strain. This increase in stiffness was due to the increase in the periosteal bone formation per unit increase in strain; however, there was no difference in periosteal bone formation per unit increase in strain reported in male ERβKO mice and WTs [48]. These results suggest that ERβ reduces response to mechanical loading at periosteal bone surface; interestingly these results are opposite to the effect of ERα. Cultured bone cells from ERβKO mice subjected to mechanical strain resulted in an increase in the number of osteoblast-like cells, while cells from ERαKO did not increase in number in response to mechanical loading [49]. This suggests that signaling through ERα increases the bones’ response to mechanical strain (increases cell number) while ERβ suppresses it.
In female mice there was no difference reported for trabecular bone mineral density between C-ERαKO and their age matched wildtype controls or C-ERβKO and their age matched wildtype controls, while the C-ERαβKO double knockout mice showed a decrease in trabecular BMD that mimics loss of estrogen in mice by ovariectomy or in humans by menopause [6, 40]. E2 can prevent ovariectomized trabecular bone mineral density loss in wildtype and ERβKO mice, and E2 can moderately rescue bone loss in ERαKO mice, indicating that ERβ can partially compensate for ERα [50]. Thus, it is thought that ERα and ERβ have redundant functions in trabecular bone, but opposing functions in femur length and response to mechanical strain.
The study of ERs in these KO mice is complicated by the facts that female ERαKO mice have high levels of serum estrogen and testosterone [51] and there are endocrine, paracrine and autocrine effects of estrogen receptors. Therefore, in the past decade many labs have created cell type specific knockouts of ERs.
Osteoblast-Specific ERαKO Mice
The Cre-lox system has been extensively used to characterize temporal and cell-specific deletions of genes involved in osteoblastogenesis [52]. The PRX-1 promoter drives Cre recombinase in osteoblast progenitors (limb bud mesenchyme). The OSX1 promoter drives Cre recombinase in osteoblast precursors. The 2.3 kb promoter of Col1a1 drives Cre recombinase in osteoblasts precursors (but later than OSX1-Cre). The osteocalcin promoter drives cre-recombinase in mature osteoblasts. The DMP1 promoter expresses Cre recombinase in osteocytes and some bone lining cells [53]. Each of these has been used to characterize the role of ERα in osteoblasts at various stages in bone development to elucidate its function in bone biology.
ERα deleted from limb bud mesenchyme (ERαf/f;PRX1-Cre) and cells expressing osterix1 (ERαf/f;Osx1-Cre) (osteoblast progenitors) both revealed low femoral BMD in adult females measured by DEXA, as compared to wildtype mice [54]. The trabecular bone volume in the femur of both ERαf/f;PRX1-Cre and ERαf/f;Osx1-Cre were both indistinguishable from their littermate controls. In line with reduced femoral BMD, mice with ERαf/f;PRX1-Cre and ERαf/f;Osx1-cre both had reduced cortical thickness.
In contrast to the early deletion of ERα, no effect on trabecular bone volume or cortical thickness was reported when ERα was deleted from mature osteoblasts during the matrix maturation phase (ERαfl/fl;Col1a1-Cre) [54]. These data suggest that ERα is responsible for maintaining optimal periosteal bone formation through osteoblasts progenitors and not via mature osteoblasts.
Two conditional models were described in which ERα was deleted by Osteocalcin-Cre. Osteocalcin is expressed by mature osteoblasts. Almeida and colleagues suggested that ERα does not play a role in mature osteoblasts, based on their ERαfl/fl;Col1a1-Cre model. However, Melville, et al., and Määttä, et al., both showed that in ERαfl/fl;OCN-Cre mice deletion of ERα led to a decrease in both cortical and trabecular bone parameters [55, 56], suggesting a role for ERα in mature osteoblasts
Deletion of ERα in osteocytes, using the DMP1-Cre mouse (ERαfl/fl;DMP1-Cre), revealed a decrease in trabecular bone mass phenotype, but only in male mice [57]. Female ERαfl/fl;DMP1-Cre mice did have a reduced response to E2 after ovariectomy. Interestingly ERαfl/fl;DMP1-Cre mice had reduced expression of both early (Runx2 and Sp7 (osterix)) and late (Ibsp) osteoblast markers, suggesting that osteocytes communicate with osteoblasts. Female ERαKO mice had a reduced response to mechanical loading [46], and since osteocytes are thought to respond to mechanical loading, it was thought that female ERαfl/fl;DMP1-cre would have reduced bone mineral density.
Osteoclast-Specific ERα Knockout Mice
ERα has also been specifically knocked out of osteoclasts, using the promoter of the LysM gene that regulates cre expression in monocytes and macrophages. The bone phenotype of a female osteoclast specific ERαKO mice was similar to that of an osteoporotic woman with low trabecular bone mass due to high bone turnover rate and decreased apoptosis of osteoclasts [58]. This suggests that ERα is necessary in both osteoblasts and osteoclasts, although the protective effects of ERα in cortical bone is via osteoblasts while in trabecular bone it’s through osteoclasts.
Other Models of ERα Function in Bone
Other interesting mouse models of ERα have been created to elucidate the molecular mechanism of ERα, especially to decipher the “classical” ERE-mediated signaling and “non-classical” non-ERE functions. Syed et al. described the consequences of either partial (ERα+/NERKI) or complete (ERα−/NERKI) loss of classical ERα signaling on the male and female skeleton due to substitution mutations (E207A/G208A) in the first zinc finger of the DNA binding domain. The NERKI (non-classical ERα knock-in) ERα can still regulate transcription through protein-protein interactions, for example at AP-1 elements, but cannot bind DNA at classical estrogen response elements (ERE). ERα−/NERKI mice had decreased cortical bone, but normal trabecular bone [59]. Interestingly, after ovariectomy the ERα−/NERKI mice gained bone, in contrast to wildtype mice that lose bone mass. These results not only suggest the importance of both ERα and the classical nuclear signaling pathway for bone homeostasis, but also indicate that classical and non-classical signaling have different effects of estrogen on bone cells.
Other mutations in ERα have been modeled in mice. A mouse in which ERα is transcriptionally constitutively active due to a substitution of tyrosine to serine at amino acid 537 reveals an increase in bone mineral density [60]. The membrane only estrogen receptor alpha (MOER) mouse has a mutant ERα that is localized to the cell membrane only [61], with no nuclear or cytoplasmic ERα detected. The nuclear-only ERα (NOER) mouse has a mutant ERα that has a mutation at amino acid 451 that prevents its palmitoylation and membrane localization [62]. The bone phenotypes of the MOER and NOER mice have not been analyzed yet.
In addition to the DNA binding domain and the membrane localization domain, ERα has other functional domains, including a ligand-independent activation function (AF)-1 domain near the N terminus and a ligand-dependent AF-2 domain near the C-terminus of the protein. The Ohlsson lab generated mice that are missing either the AF-1 or the AF-2 domain of ERα to determine the effects of ERα domains in male mice [63]. Wildtype mice and mice with a mutant AF-1 that were orchidectomized (orx) and treated with E2 had an increase in BMD compared to vehicle treated mice, but mice with a mutant AF-2 did not have an increase in BMD. In contrast, all three mutants (total ERα, AF-1mut and AF-2mut) did not have an increase in trabecular bone volume after orx and E2 treatment. Thus, there are cell-type (location) specific effects of ERα functional domains.
ERα has also been specifically ablated from nestin-positive cells using the nestin –cre driver. ERα was demonstrated to be deleted from the brain, but not the bone [64], although one must consider that MSCs are nestin-positive [65]. The ERαfl/fl;nestin-Cre mice showed an increase in both trabecular and cortical bone mineral density [64]. Leptin levels were higher in the serum of ERαfl/fl;nestin-Cre mice, which could explain the high bone mass phenotype. Osteoblasts express the leptin receptor, and studies have shown that leptin signaling can increase bone mass [66–68].
Transplantation experiments using ERαKO or WT hematopoietic stem cells into either ERαKO or WT mice were employed to control for tissue specific cre deletion and incomplete deletion. E2 restored ovariectomy-induced cortical and trabecular bone loss in WT mice receiving WT bone marrow or ERαKO bone marrow, implicating a non-hematopoietic cell (most likely an osteoblast) [69].
Osteoblast-specific ERβ Knockout Mice
ERβ was recently deleted in osteoprogenitor cells using the Prx1-Cre [70]. Six and 12 week old female ERβfl/fl;PRX1-Cre had an increase in trabecular bone, compared to wildtype mice, while there were no changes in cortical bone properties, as is seen in aged ERβKO mice [43]. The expression of ERβ has been reported to be localized to trabecular bone [28], and thus the phenotype correlates with the expression pattern. The authors report a similar phenotype with the Col2.3kb-Cre driver. Together, these experiments are in line with the hypothesis that ERβ antagonizes ERα action. Loss of ERβ in other cell types and later in osteoblast differentiation, will be informative, and will probably follow a similar trend.
ERα-Regulated Genes in Bone
Many genes have been reported to be regulated by E2 [7], but the receptors responsible for up-regulating these genes have not been well characterized until recently. Roforth and colleagues performed RNA-sequencing in human fetal osteoblast (hFOB cells) overexpressing ERα [71]. They identified 4353 genes upregulated by E2. By also using mutant ERα constructs with nuclear only (NOER) functions or non-classical ERα knock in (NERKI) mutations they determined that 45% of the genes are nuclear ERE-independent, 27% are nuclear ERE-dependent and 28% are extra-nuclear. However, these experiments are not designed to determine the role of ERα vs. ERβ in regulating E2-mediated gene transcription.
One specific role for ERα is to induce Fas Ligand (FasL) in osteoblasts. ERαKO mice have an increase in the total number of osteoclasts due to the lack of E2-induced osteoclast apoptosis [72]. E2, via ERα and not ERβ, binds to enhancers near FasL and induces transcription of FasL in osteoblasts resulting in a paracrine signal to induce osteoclast apoptosis. Furthermore, E2 increases the transcription of MMP3, which cleaves FasL, creating a soluble form of FasL that is necessary for osteoclast apoptosis [21]. This work is supported by the fact that a non-hematopoietic cell is necessary for maintenance of bone mineral density [69].
ERβ-Regulated Genes in Bone
Specific ERβ ligands are valuable tools to dissect out the functions of ERα vs. ERβ. The soy isoflavone genistein binds with a 20-fold greater affinity for ERβ than ERα [73]. Soy supplements are marketed for bone health, and several trials have shown promising results with giving women soy isoflavones. However, a meta-analysis of randomized controlled trials showed no effect of soy isoflavones on bone mineral density [74]. In addition, genistein had no effects on the decreased bone mineral density of ovariectomized rats [75, 76]. Similarly, the ERβ ligand ERB-041, which has an over 200-fold affinity for ERβ, did not increase proximal tibial bone mineral density in ovariectomized rats [77].
The U2OS osteosarcoma cell line that stably over-expresses ERα, ERβ or both receptors are useful cell lines as a screen for osteoblast genes [27, 78]. Expression arrays show only 21% overlap between E2-regulated genes in U2OS-ERα and U2OS-ERβ cell lines [79], demonstrating that the two receptors have different functions in osteoblast-like cells. When ERα and ERβ are expressed together, a distinct set of E2-regulated genes was observed [80].
However, it may not be “different” functions, but that ERβ represses ERα gene transcription. Genes that are upregulated by E2 in WT bones, as determined by microarray analysis, are also increased by estrogen in ERβKO mice, but at an increased level, suggesting that ERβ is inhibitory to ERα [81]. And although with different genes, the same inhibitory pattern of ERβ was seen in ERβKO and WT liver cells after E2 treatment. Lindberg, et al. hypothesize that the inhibitory effect of ERβ might be explained mechanistically by the observation that ERβ does not contain a strong AF-1 domain, but, rather, contains a repressor domain [81]. They also demonstrate that in the absence of ERα, ERβ can partially replace ERα. ChIP-sequencing experiments could show if ERβ and ERα are binding at the same genomic locations.
Conclusions and Future Directions
Studies have confirmed the importance of estrogen and estrogen receptors, not only in cortical and trabecular bone, but also in different bone cell types. However, further work still needs to be done to identify different genes that are regulated by estrogen in each of these cell types during different stages of differentiation. The function of ERβ in different cell types and stages of differentiation can be elucidated with cell type specific knockouts, as has been done for ERα. The function of ERβ in bone cells may be to antagonize the effects of ERα, but this remains to be fully explained by molecular and cellular assays in bone cells.
Highlights.
This review will highlight the role of estrogen receptors in bone.
Estrogen receptors alpha and beta can partially compensate for each other
Estrogen receptor beta antagonizes many effects of estrogen receptor alpha in bone
Acknowledgments
Research reported in this publication was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health under Award Number AR-064354-01.
Footnotes
Disclosure Statement: The authors have nothing to disclose.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Wend K, Wend P, Krum SA. Tissue-Specific Effects of Loss of Estrogen during Menopause and Aging. Front Endocrinol (Lausanne) 2012;3:19. doi: 10.3389/fendo.2012.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Candelaria NR, Liu K, Lin CY. Estrogen receptor alpha: molecular mechanisms and emerging insights. J Cell Biochem. 2013;114(10):2203–8. doi: 10.1002/jcb.24584. [DOI] [PubMed] [Google Scholar]
- 3.Gorski J, et al. Hormone receptors: studies on the interaction of estrogen with the uterus. Recent Prog Horm Res. 1968;24:45–80. doi: 10.1016/b978-1-4831-9827-9.50008-3. [DOI] [PubMed] [Google Scholar]
- 4.Green S, et al. Cloning of the human oestrogen receptor cDNA. J Steroid Biochem. 1986;24(1):77–83. doi: 10.1016/0022-4731(86)90035-x. [DOI] [PubMed] [Google Scholar]
- 5.Kuiper GG, et al. Cloning of a novel receptor expressed in rat prostate and ovary. Proc Natl Acad Sci U S A. 1996;93(12):5925–30. doi: 10.1073/pnas.93.12.5925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Krum SA, Brown M. Unraveling estrogen action in osteoporosis. Cell Cycle. 2008;7(10):1348–52. doi: 10.4161/cc.7.10.5892. [DOI] [PubMed] [Google Scholar]
- 7.Krum SA. Direct transcriptional targets of sex steroid hormones in bone. J Cell Biochem. 2011;112(2):401–8. doi: 10.1002/jcb.22970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Khosla S. Update on estrogens and the skeleton. J Clin Endocrinol Metab. 2010;95(8):3569–77. doi: 10.1210/jc.2010-0856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Williams C, et al. A genome-wide study of the repressive effects of estrogen receptor beta on estrogen receptor alpha signaling in breast cancer cells. Oncogene. 2008;27(7):1019–32. doi: 10.1038/sj.onc.1210712. [DOI] [PubMed] [Google Scholar]
- 10.Nelson AW, et al. Estrogen receptor beta in prostate cancer: friend or foe? Endocr Relat Cancer. 2014;21(4):T219–34. doi: 10.1530/ERC-13-0508. [DOI] [PubMed] [Google Scholar]
- 11.Ogawa S, et al. Survival of reproductive behaviors in estrogen receptor beta gene-deficient (betaERKO) male and female mice. Proc Natl Acad Sci U S A. 1999;96(22):12887–92. doi: 10.1073/pnas.96.22.12887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Paech K, et al. Differential ligand activation of estrogen receptors ERalpha and ERbeta at AP1 sites. Science. 1997;277(5331):1508–10. doi: 10.1126/science.277.5331.1508. [DOI] [PubMed] [Google Scholar]
- 13.Cauley JA. Estrogen and bone health in men and women. Steroids. 2015;99(Pt A):11–15. doi: 10.1016/j.steroids.2014.12.010. [DOI] [PubMed] [Google Scholar]
- 14.Rossouw JE, et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results From the Women’s Health Initiative randomized controlled trial. JAMA. 2002;288(3):321–33. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- 15.Pacifici R, et al. Ovarian steroid treatment blocks a postmenopausal increase in blood monocyte interleukin 1 release. Proc Natl Acad Sci U S A. 1989;86(7):2398–402. doi: 10.1073/pnas.86.7.2398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jilka RL, et al. Increased osteoclast development after estrogen loss: mediation by interleukin-6. Science. 1992;257(5066):88–91. doi: 10.1126/science.1621100. [DOI] [PubMed] [Google Scholar]
- 17.Weitzmann MN, et al. Increased production of IL-7 uncouples bone formation from bone resorption during estrogen deficiency. J Clin Invest. 2002;110(11):1643–50. doi: 10.1172/JCI15687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Krum SA, et al. Novel functions for NFkappaB: inhibition of bone formation. Nat Rev Rheumatol. 2010;6(10):607–11. doi: 10.1038/nrrheum.2010.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kameda T, et al. Estrogen inhibits bone resorption by directly inducing apoptosis of the bone-resorbing osteoclasts. J Exp Med. 1997;186(4):489–95. doi: 10.1084/jem.186.4.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kousteni S, et al. Reversal of bone loss in mice by nongenotropic signaling of sex steroids. Science. 2002;298(5594):843–6. doi: 10.1126/science.1074935. [DOI] [PubMed] [Google Scholar]
- 21.Garcia AJ, et al. ERalpha signaling regulates MMP3 expression to induce FasL cleavage and osteoclast apoptosis. J Bone Miner Res. 2013;28(2):283–90. doi: 10.1002/jbmr.1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Krum SA, et al. Estrogen protects bone by inducing Fas ligand in osteoblasts to regulate osteoclast survival. EMBO J. 2008;27(3):535–45. doi: 10.1038/sj.emboj.7601984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bord S, et al. The effects of estrogen on osteoprotegerin, RANKL, and estrogen receptor expression in human osteoblasts. Bone. 2003;32(2):136–41. doi: 10.1016/s8756-3282(02)00953-5. [DOI] [PubMed] [Google Scholar]
- 24.Martin A, et al. Estrogens antagonize RUNX2-mediated osteoblast-driven osteoclastogenesis through regulating RANKL membrane association. Bone. 2015;75:96–104. doi: 10.1016/j.bone.2015.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kousteni S, et al. Nongenotropic, sex-nonspecific signaling through the estrogen or androgen receptors: dissociation from transcriptional activity. Cell. 2001;104(5):719–30. [PubMed] [Google Scholar]
- 26.Pantschenko AG, et al. Effect of osteoblast-targeted expression of bcl-2 in bone: differential response in male and female mice. J Bone Miner Res. 2005;20(8):1414–29. doi: 10.1359/JBMR.050315. [DOI] [PubMed] [Google Scholar]
- 27.Krum SA, et al. Unique ER{alpha} cistromes control cell type-specific gene regulation. Mol Endocrinol. 2008 doi: 10.1210/me.2008-0100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bord S, et al. Estrogen receptors alpha and beta are differentially expressed in developing human bone. J Clin Endocrinol Metab. 2001;86(5):2309–14. doi: 10.1210/jcem.86.5.7513. [DOI] [PubMed] [Google Scholar]
- 29.Braidman IP, et al. Localization of estrogen receptor beta protein expression in adult human bone. J Bone Miner Res. 2001;16(2):214–20. doi: 10.1359/jbmr.2001.16.2.214. [DOI] [PubMed] [Google Scholar]
- 30.Crusode de Souza M, Sasso-Cerri E, Cerri PS. Immunohistochemical detection of estrogen receptor beta in alveolar bone cells of estradiol-treated female rats: possible direct action of estrogen on osteoclast life span. J Anat. 2009;215(6):673–81. doi: 10.1111/j.1469-7580.2009.01158.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Harkonen PL, Vaananen HK. Monocyte-macrophage system as a target for estrogen and selective estrogen receptor modulators. Ann N Y Acad Sci. 2006;1089:218–27. doi: 10.1196/annals.1386.045. [DOI] [PubMed] [Google Scholar]
- 32.Pacifici R. Estrogen deficiency, T cells and bone loss. Cell Immunol. 2008;252(1–2):68–80. doi: 10.1016/j.cellimm.2007.06.008. [DOI] [PubMed] [Google Scholar]
- 33.Kovats S. Estrogen receptors regulate innate immune cells and signaling pathways. Cell Immunol. 2015;294(2):63–9. doi: 10.1016/j.cellimm.2015.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gennari L, et al. Estrogen receptor gene polymorphisms and the genetics of osteoporosis: a HuGE review. Am J Epidemiol. 2005;161(4):307–20. doi: 10.1093/aje/kwi055. [DOI] [PubMed] [Google Scholar]
- 35.Estrada K, et al. Genome-wide meta-analysis identifies 56 bone mineral density loci and reveals 14 loci associated with risk of fracture. Nat Genet. 2012;44(5):491–501. doi: 10.1038/ng.2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Paternoster L, et al. Genetic determinants of trabecular and cortical volumetric bone mineral densities and bone microstructure. PLoS Genet. 2013;9(2):e1003247. doi: 10.1371/journal.pgen.1003247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Smith EP, et al. Estrogen resistance caused by a mutation in the estrogen-receptor gene in a man. N Engl J Med. 1994;331(16):1056–61. doi: 10.1056/NEJM199410203311604. [DOI] [PubMed] [Google Scholar]
- 38.Couse JF, et al. Analysis of transcription and estrogen insensitivity in the female mouse after targeted disruption of the estrogen receptor gene. Mol Endocrinol. 1995;9(11):1441–54. doi: 10.1210/mend.9.11.8584021. [DOI] [PubMed] [Google Scholar]
- 39.Dupont S, et al. Effect of single and compound knockouts of estrogen receptors alpha (ERalpha) and beta (ERbeta) on mouse reproductive phenotypes. Development. 2000;127(19):4277–91. doi: 10.1242/dev.127.19.4277. [DOI] [PubMed] [Google Scholar]
- 40.Sims NA, et al. Deletion of estrogen receptors reveals a regulatory role for estrogen receptors-beta in bone remodeling in females but not in males. Bone. 2002;30(1):18–25. doi: 10.1016/s8756-3282(01)00643-3. [DOI] [PubMed] [Google Scholar]
- 41.Krege JH, et al. Generation and reproductive phenotypes of mice lacking estrogen receptor beta. Proc Natl Acad Sci U S A. 1998;95(26):15677–82. doi: 10.1073/pnas.95.26.15677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Windahl SH, et al. Increased cortical bone mineral content but unchanged trabecular bone mineral density in female ERbeta(−/−) mice. J Clin Invest. 1999;104(7):895–901. doi: 10.1172/JCI6730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Windahl SH, et al. Female estrogen receptor beta−/− mice are partially protected against age-related trabecular bone loss. J Bone Miner Res. 2001;16(8):1388–98. doi: 10.1359/jbmr.2001.16.8.1388. [DOI] [PubMed] [Google Scholar]
- 44.Antal MC, et al. Sterility and absence of histopathological defects in nonreproductive organs of a mouse ERbeta-null mutant. Proc Natl Acad Sci U S A. 2008;105(7):2433–8. doi: 10.1073/pnas.0712029105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vidal O, et al. Disproportional body growth in female estrogen receptor-alpha-inactivated mice. Biochem Biophys Res Commun. 1999;265(2):569–71. doi: 10.1006/bbrc.1999.1711. [DOI] [PubMed] [Google Scholar]
- 46.Saxon LK, et al. Estrogen receptors alpha and beta have different gender-dependent effects on the adaptive responses to load bearing in cancellous and cortical bone. Endocrinology. 2012;153(5):2254–66. doi: 10.1210/en.2011-1977. [DOI] [PubMed] [Google Scholar]
- 47.Galea GL, et al. Estrogen receptor alpha mediates proliferation of osteoblastic cells stimulated by estrogen and mechanical strain, but their acute down-regulation of the Wnt antagonist Sost is mediated by estrogen receptor beta. J Biol Chem. 2013;288(13):9035–48. doi: 10.1074/jbc.M112.405456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Saxon LK, et al. The skeletal responsiveness to mechanical loading is enhanced in mice with a null mutation in estrogen receptor-beta. Am J Physiol Endocrinol Metab. 2007;293(2):E484–91. doi: 10.1152/ajpendo.00189.2007. [DOI] [PubMed] [Google Scholar]
- 49.Lee KC, et al. The adaptive response of bone to mechanical loading in female transgenic mice is deficient in the absence of oestrogen receptor-alpha and -beta. J Endocrinol. 2004;182(2):193–201. doi: 10.1677/joe.0.1820193. [DOI] [PubMed] [Google Scholar]
- 50.Sims NA, et al. A functional androgen receptor is not sufficient to allow estradiol to protect bone after gonadectomy in estradiol receptor-deficient mice. J Clin Invest. 2003;111(9):1319–27. doi: 10.1172/JCI17246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Windahl SH, Andersson G, Gustafsson JA. Elucidation of estrogen receptor function in bone with the use of mouse models. Trends Endocrinol Metab. 2002;13(5):195–200. doi: 10.1016/s1043-2760(02)00594-5. [DOI] [PubMed] [Google Scholar]
- 52.vanKoevering KK, Williams BO. Transgenic Mouse Strains for Conditional Gene Deletion During Skeletal Development. IBMS BoneKEy. 2008;5(5):151–170. [Google Scholar]
- 53.Kim SW, et al. Intermittent parathyroid hormone administration converts quiescent lining cells to active osteoblasts. J Bone Miner Res. 2012;27(10):2075–84. doi: 10.1002/jbmr.1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Almeida M, et al. Estrogen receptor-alpha signaling in osteoblast progenitors stimulates cortical bone accrual. J Clin Invest. 2013;123(1):394–404. doi: 10.1172/JCI65910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Maatta JA, et al. Inactivation of estrogen receptor alpha in bone-forming cells induces bone loss in female mice. FASEB J. 2013;27(2):478–88. doi: 10.1096/fj.12-213587. [DOI] [PubMed] [Google Scholar]
- 56.Melville KM, et al. Female mice lacking estrogen receptor-alpha in osteoblasts have compromised bone mass and strength. J Bone Miner Res. 2014;29(2):370–9. doi: 10.1002/jbmr.2082. [DOI] [PubMed] [Google Scholar]
- 57.Windahl SH, et al. Estrogen receptor-alpha in osteocytes is important for trabecular bone formation in male mice. Proc Natl Acad Sci U S A. 2013;110(6):2294–9. doi: 10.1073/pnas.1220811110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Martin-Millan M, et al. The estrogen receptor-alpha in osteoclasts mediates the protective effects of estrogens on cancellous but not cortical bone. Mol Endocrinol. 2010;24(2):323–34. doi: 10.1210/me.2009-0354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Syed FA, et al. Skeletal effects of estrogen are mediated by opposing actions of classical and nonclassical estrogen receptor pathways. J Bone Miner Res. 2005;20(11):1992–2001. doi: 10.1359/JBMR.050713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ikeda K, et al. Conditional expression of constitutively active estrogen receptor alpha in osteoblasts increases bone mineral density in mice. FEBS Lett. 2011;585(9):1303–9. doi: 10.1016/j.febslet.2011.03.038. [DOI] [PubMed] [Google Scholar]
- 61.Pedram A, et al. Developmental phenotype of a membrane only estrogen receptor alpha (MOER) mouse. J Biol Chem. 2009;284(6):3488–95. doi: 10.1074/jbc.M806249200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pedram A, et al. Membrane and nuclear estrogen receptor alpha collaborate to suppress adipogenesis but not triglyceride content. FASEB J. 2015 doi: 10.1096/fj.15-274878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Borjesson AE, et al. The role of activation functions 1 and 2 of estrogen receptor-alpha for the effects of estradiol and selective estrogen receptor modulators in male mice. J Bone Miner Res. 2013;28(5):1117–26. doi: 10.1002/jbmr.1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ohlsson C, et al. Estrogen receptor-alpha expression in neuronal cells affects bone mass. Proc Natl Acad Sci U S A. 2012;109(3):983–8. doi: 10.1073/pnas.1111436109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mendez-Ferrer S, et al. Mesenchymal and haematopoietic stem cells form a unique bone marrow niche. Nature. 2010;466(7308):829–34. doi: 10.1038/nature09262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cornish J, et al. Leptin directly regulates bone cell function in vitro and reduces bone fragility in vivo. J Endocrinol. 2002;175(2):405–15. doi: 10.1677/joe.0.1750405. [DOI] [PubMed] [Google Scholar]
- 67.Thomas T, et al. Leptin acts on human marrow stromal cells to enhance differentiation to osteoblasts and to inhibit differentiation to adipocytes. Endocrinology. 1999;140(4):1630–8. doi: 10.1210/endo.140.4.6637. [DOI] [PubMed] [Google Scholar]
- 68.Williams GA, et al. Skeletal phenotype of the leptin receptor-deficient db/db mouse. J Bone Miner Res. 2011;26(8):1698–709. doi: 10.1002/jbmr.367. [DOI] [PubMed] [Google Scholar]
- 69.Henning P, et al. The effect of estrogen on bone requires ERalpha in nonhematopoietic cells but is enhanced by ERalpha in hematopoietic cells. Am J Physiol Endocrinol Metab. 2014;307(7):E589–95. doi: 10.1152/ajpendo.00255.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Nicks KM, et al. Deletion of Estrogen Receptor Beta in Osteoprogenitor Cells Increases Trabecular but Not Cortical Bone Mass in Female Mice. J Bone Miner Res. 2015 doi: 10.1002/jbmr.2723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Roforth MM, et al. Dissection of estrogen receptor alpha signaling pathways in osteoblasts using RNA-sequencing. PLoS One. 2014;9(4):e95987. doi: 10.1371/journal.pone.0095987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Parikka V, et al. Estrogen responsiveness of bone formation in vitro and altered bone phenotype in aged estrogen receptor-alpha-deficient male and female mice. Eur J Endocrinol. 2005;152(2):301–14. doi: 10.1530/eje.1.01832. [DOI] [PubMed] [Google Scholar]
- 73.Kuiper GG, et al. Interaction of estrogenic chemicals and phytoestrogens with estrogen receptor beta. Endocrinology. 1998;139(10):4252–63. doi: 10.1210/endo.139.10.6216. [DOI] [PubMed] [Google Scholar]
- 74.Ricci E, et al. Soy isoflavones and bone mineral density in perimenopausal and postmenopausal Western women: a systematic review and meta-analysis of randomized controlled trials. J Womens Health (Larchmt) 2010;19(9):1609–17. doi: 10.1089/jwh.2010.2021. [DOI] [PubMed] [Google Scholar]
- 75.Turner RT, et al. Genistein administered as a once-daily oral supplement had no beneficial effect on the tibia in rat models for postmenopausal bone loss. Menopause. 2013;20(6):677–86. doi: 10.1097/gme.0b013e31827d44df. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Picherit C, et al. Daidzein is more efficient than genistein in preventing ovariectomy-induced bone loss in rats. J Nutr. 2000;130(7):1675–81. doi: 10.1093/jn/130.7.1675. [DOI] [PubMed] [Google Scholar]
- 77.Harris HA, et al. Evaluation of an estrogen receptor-beta agonist in animal models of human disease. Endocrinology. 2003;144(10):4241–9. doi: 10.1210/en.2003-0550. [DOI] [PubMed] [Google Scholar]
- 78.Monroe DG, et al. Estrogen receptor isoform-specific regulation of endogenous gene expression in human osteoblastic cell lines expressing either ERalpha or ERbeta. J Cell Biochem. 2003;90(2):315–26. doi: 10.1002/jcb.10633. [DOI] [PubMed] [Google Scholar]
- 79.Monroe DG, et al. Estrogen receptor isoform-specific regulation of the retinoblastoma-binding protein 1 (RBBP1) gene: roles of AF1 and enhancer elements. J Biol Chem. 2006;281(39):28596–604. doi: 10.1074/jbc.M605226200. [DOI] [PubMed] [Google Scholar]
- 80.Monroe DG, et al. Estrogen receptor alpha and beta heterodimers exert unique effects on estrogen- and tamoxifen-dependent gene expression in human U2OS osteosarcoma cells. Mol Endocrinol. 2005;19(6):1555–68. doi: 10.1210/me.2004-0381. [DOI] [PubMed] [Google Scholar]
- 81.Lindberg MK, et al. Estrogen receptor (ER)-beta reduces ERalpha-regulated gene transcription, supporting a “ying yang” relationship between ERalpha and ERbeta in mice. Mol Endocrinol. 2003;17(2):203–8. doi: 10.1210/me.2002-0206. [DOI] [PubMed] [Google Scholar]


