Abstract
Background
A majority infections caused by dengue virus (DENV) are asymptomatic, but a higher incidence of severe illness, such as dengue hemorrhagic fever, is associated with secondary infections, suggesting that pre-existing immunity plays a central role in dengue pathogenesis. Primary infections are typically associated with a largely serotype-specific antibody response, while secondary infections show a shift to a broadly cross-reactive antibody response.
Methods/Principal findings
We hypothesized that the basis for the shift in serotype-specificity between primary and secondary infections can be found in a change in the antibody fine-specificity. To investigate the link between epitope- and serotype-specificity, we assembled the Dengue Virus Antibody Database, an online repository containing over 400 DENV-specific mAbs, each annotated with information on 1) its origin, including the immunogen, host immune history, and selection methods, 2) binding/neutralization data against all four DENV serotypes, and 3) epitope mapping at the domain or residue level to the DENV E protein. We combined epitope mapping and activity information to determine a residue-level index of epitope propensity and cross-reactivity and generated detailed composite epitope maps of primary and secondary antibody responses. We found differing patterns of epitope-specificity between primary and secondary infections, where secondary responses target a distinct subset of epitopes found in the primary response. We found that secondary infections were marked with an enhanced response to cross-reactive epitopes, such as the fusion-loop and E-dimer region, as well as increased cross-reactivity in what are typically more serotype-specific epitope regions, such as the domain I-II interface and domain III.
Conclusions/Significance
Our results support the theory that pre-existing cross-reactive memory B cells form the basis for the secondary antibody response, resulting in a broadening of the response in terms of cross-reactivity, and a focusing of the response to a subset of epitopes, including some, such as the fusion-loop region, that are implicated in poor neutralization and antibody-dependent enhancement of infection.
Author summary
Dengue virus (DENV) infections are typically asymptomatic, but severe and potentially lethal disease symptoms, such as dengue hemorrhagic fever, are associated with secondary infections. This suggests that pre-existing immunity from primary infection plays a central role in DENV pathogenesis. In order to characterize the antibody response in primary and secondary infections, we assembled the Dengue Virus Antibody Database, a freely accessible online repository (http://denvabdb.bhsai.org) storing over 400 unique monoclonal dengue-specific antibodies annotated by their 1) origin and host immune history, 2) activity information against all four dengue serotypes, and 3) epitope mapping information. Here we demonstrate the utility of the database by carrying out a large-scale analysis to characterize shifts in epitope fine-specificity and serotype cross-reactivity in primary and secondary infections. In particular, we show how the antibody response in secondary infections displays a systematic shift towards increased serotype cross-reactivity by focusing on a subset of cross-reactive epitopes on the dengue E protein. Our findings suggest a mechanistic basis for this shift in epitope and serotype specificity and demonstrate how a detailed understanding of the antibody response can provide insight into the mechanisms of dengue pathogenesis.
Introduction
Dengue virus (DENV), an arthropod-borne virus of the Flaviviridae family, infects an estimated 400 million people each year [1]. There are four antigenically related DENV serotypes, DENV 1–4, each capable of causing disease. DENV infections are often asymptomatic or result in an uncomplicated fever and can elicit life-long immunity to the infecting serotype and short-term cross-protection against heterotypic DENV infections [2–5]. Although, recent studies have demonstrated that homotypic DENV reinfection is possible [6]. Secondary infection with a heterotypic DENV serotype results in a higher incidence of more severe disease and cross-reactive antibodies are thought to contribute to this by a mechanism termed antibody-dependent enhancement (ADE) of infection [7–11]. The antibody response following secondary infection is broadly cross-reactive among DENV serotypes and longer periods of cross-protection are observed [3, 12]. Further characterizing differences in the antibody response to primary and secondary heterotypic DENV infections, and how these differences are associated with serotype-specificity and neutralization, is critical to understanding DHF pathogenesis and developing dengue vaccines.
The DENV virion consists of 180 copies of the envelope (E) protein, arranged in 90 dimers in an icosahedral ‘herring-bone’ geometry [13] and is the primary target of DENV neutralizing antibodies [14]. The soluble portion of the E protein consists of three distinct domains [15], termed Domain I (DI), Domain II (DII), and Domain III (DIII). Neutralizing antibodies (Abs) targeting E, reviewed in [16], are the main focus of current DENV vaccine development efforts. Not all E protein-specific Abs contribute equally to virus neutralization and neutralizing Ab potency is related to its epitope. Early work with mouse mAbs indicated that DIII was a major target of potently neutralizing DENV mAbs [17–27]. However, a low fraction of DIII-specific neutralizing Abs are found in human sera post-DENV infection and they only appear to make a minor contribution to DENV neutralization [28–31]. The human neutralizing Ab response appears to preferentially target the DI/DII hinge region of E protein monomers [32–34] and quaternary E protein epitopes that are only present in the context of intact virions [32, 35, 36]. Finally, DENV Abs can vary with respect to serotype cross-reactivity. Complex Abs bind or neutralize all four serotypes, type-specific Abs bind or neutralize only a single serotype, and sub-complex Abs bind or neutralize more than one, but not all four serotypes. It is important to note that there are significant strain and genotype-level differences in antibody neutralization within a serotype as well [27, 37–39].
The antibody response to dengue infection is a polyclonal response that is thought to consists of a repertoire of >103 unique monoclonal antibodies (mAbs) [40]. Previous studies from polyclonal sera [41, 42], and panels of monoclonal antibodies [36, 43–45], have shown that the serotype-specificity of the antibody response shifts between primary and secondary infections. Primary infections are characterized by a largely type-specific antibody response while secondary infections result in a broadly cross-reactive response.
We hypothesize that the basis for the shift in serotype-specificity between primary and secondary antibody responses can be found in a change in the fine-specificity—the relative response to different epitopes on the E protein. To investigate the link between epitope fine-specificity and serotype-specificity, we assembled the Dengue Virus Antibody Database (http://denvabdb.bhsai.org), an online repository containing 410 DENV-specific mAbs, each annotated with information on 1) its origin, including the immunogen, host immune history, and selection methods, 2) binding or neutralization data against four DENV serotypes, and 3) epitope mapping at the domain or residue level. Because the database contains information linking infection type (primary vs. secondary) and serotype-specificity (type-specific, sub-complex, complex), with residue or domain-level epitope mapping, it allows us to identify the epitope-level determinants of observed shifts in type-specificity associated with secondary infections. While analysis of any single study or panel of DENV mabs may reveal only a limited understanding of the polyclonal diversity of the DENV antibody response, we hypothesize that a large-scale analysis of hundreds of mAbs collected across dozens of diverse studies may be able to identify systematic trends in the DENV antibody repertoire and provide key insights into DENV antibody cross-reactivity and fine specificity. Finally, it is important to note that this study represents a meta-analysis of decades of previous studies on dengue mAbs, and as such seeks to provide quantitative basis for previously observed differences in cross-reactivity and fine-specificity in antibody responses to primary and secondary DENV infections.
Materials and methods
Database composition
We assembled a database of DENV mAbs described in literature that fulfill the following criteria: 1) has information on how the mAb was isolated, in terms of the immunogen, the host organism, and immune history; 2) has in vitro binding or neutralization data against all four DENV serotypes; and 3) binds to E protein, and has epitope mapping information with at least a domain-level resolution. Overall, we found 410 mAbs that matched these criteria.
There are three linked sections of the database: mAb, Activity, and Epitope (Fig 1). The mAb section contains a single record for each mAb in the database that includes the mAb name and information about its origin, including the host organism, the isotype, the immunogen, the exposure event that lead to the antibody, the host immune history, the selection criteria used to isolate or select that mAb for study, and the PubMedID of the reference that first reported the mAb. The Activity section can contain multiple records for each mAb and includes information on the activity assay details and data against all four DENV serotypes, and the corresponding PubMedID for its reference. This data could be qualitative, such as in the case of a Western Blot, or quantitative, as in the case of titers from a neutralization assay. The Epitope section also contains multiple records for each mAb and includes information on the epitope resolution or type (‘residue’ or ‘domain’), the epitope mapping method, the domain of E that the mAb was mapped to, the E amino acid sequence that describes that epitope, and the PubMedID of the corresponding reference. The Dengue Antibody Database is freely accessible online at http://denvabdb.bhsai.org. Through the web-based interface, users are able to search for particular mAbs, sort mAbs by various properties, and download any information on the database directly as a spreadsheet.
Fig 1. Dengue virus antibody database.
Each monoclonal antibody in the database is annotated with information about its origin and selection, activity against all four dengue serotypes, epitope mapping, and relevant references.
Antibody serotype-specificity classification
Based on the available activity information, we classified the serotype-specificity of each mAb in the database as ‘type,’ ‘sub-complex,’ and ‘complex’ based on the number of serotypes it was reactive towards. In the instance that different assays for the same mAb showed differing patterns of specificity, we classified the mAb based on the most serotype-restricted assay result. Among the cases where assays had conflicting serotype-specificity results, we found that, in general, neutralization assays tended to be more serotype-restricted than binding assays. We treated all activity methods equally to maximize the sample size for our analyses, however users are able to download the activity data from the database in spreadsheet form, and select subsets of mAbs that have data from certain activity methods. Finally, some studies also identify group-specific mAbs, antibodies that can bind to multiple different flaviviruses. However, since such characterization is available for only a limited number of mAbs in the database, we restricted our study to type, sub-complex, and complex mAbs. For many of the measurements, we focused our analysis on comparing type and complex mAbs. This was for two reasons: 1) the number of sub-complex mAbs was insufficient to generate statistically significant results for many measures, and 2) we found that sub-complex mAbs, in particular, were sensitive to assay-level variation in classification of their type-specificity, meaning they could not always be reliably distinguished from complex mAbs.
Determining epitope propensity
We developed a measure of epitope-level antigenicity to describe a set of mAbs in terms of ‘epitope propensity,’ or the probability that a given E protein residue is found within the epitope definitions that make up the set of mAbs. Briefly, a set of mAbs consists of Nr mAbs with residue-level epitope definitions and Nd mAbs with domain-level epitope definitions. Note that the same mAb may have both residue and domain-level definitions. We define the residue-level epitope definition of mAb j, as a set of residues, and the domain-level epitope definition of mAb j as or "DIII". Q(i) is the number of times residue i is found among all Nr residue-level epitope definitions in the data set (Eq 1):
| (1) |
The epitope propensity at residue i, P(i), defined in Eq 2, is the probability of finding residue i among the Nr residue-level epitopes that lie within the domain of residue i (di), multiplied by the probability of finding a epitope in di, as determined by Nd domain-level epitope definitions.
| (2) |
P(i | di) is calculated by dividing the total count of residue i, by the total count across all epitope residues that fall within the domain di, as shown in Eq 3. P(di) is determined by the total count of domain level epitopes that are the same domain as di divided by the total number of domain-level epitopes in the data set, as shown in Eq 4.
| (3) |
| (4) |
By defining epitope propensity as a function of P(i | di) and P(di) we can use the residue-level epitope definition to determine the high-resolution details of mAbs in the database, while using domain-level epitope definitions to determine the relative immunogenicity of larger segments of the antigen. In any empirical measure there is the risk of observation bias—that researchers intentionally studying antibodies to a particular epitope region will bias a propensity measure calculated from those observations. By including a separate domain-level term, we can, to some degree, account for this potential observation bias.
Calculating epitope-level cross reactivity
In addition to the epitope-level measure of propensity, we calculated aggregate epitope-level cross-reactivity from mAbs derived from primary and secondary infection. For each residue, we identified every mAb within the mAb subset (primary or secondary infection) that listed that residue as an epitope. We then determined the percentage of mAbs associated with that epitope residue that were classified as ‘complex’. If a residue had 60% or greater cross-reactivity, meaning at least 60% of all mAbs associated with that residue were classified as ‘complex’, we defined it as being of ‘high’ cross-reactivity. Residues with cross reactivity 30–60% cross-reactivity were defined as having ‘medium’ cross-reactivity; residues with <30% cross-reactivity were defined as having ‘low’ cross-reactivity.
Measuring sequence variation in DENV epitopes
We carried out two separate, but related, analyses to assess the degree of sequence variation in DENV epitopes on E: a residue-level measure of sequence conservation and an epitope-level measure of antigenic mismatch, known as pepitope [46, 47]. We carried out multiple sequence alignment using a set of 47 sequences of DENV across all four serotypes that were studied by Katzelnick et al. [37] for serum cross-reactivity and neutralization. We downloaded the E protein sequences for each strain from Genbank (see S2 Table). We then carried out a structure-based multiple sequence alignment using the Consurf algorithm [48] using the crystal structure of the DENV E protein [15] (PDB code: 1OKE). We defined sequence conservation at each residue position as the percentage of sequences that had the most common amino acid at that position.
| (5) |
pepitope is an epitope-level measure of antigenic mismatch between two viral strains and can be used to predict the likelihood that immunity to one strain would provide protection against the other. We used one representative strain of DENV for each of the four serotypes, which was the consensus sequence for the E protein for each of the four serotypes as determined by a previous bioinformatics study by Danecek et al. [49]. For a residue-level epitope definition for mAb j, , pepitope is calculated by dividing the number of mismatches between two strains (S1 and S2) along the residues defined by with the total number of residues that defines —describing a mismatch percentage along a defined set of epitope residues (Eq 5). We used ClustalW [50] to carry out the alignment and defined a mismatch as identified non-conserved or semi-conserved substitutions (denoted by ‘ ‘ and ‘.’, respectively, in the ClustalW sequence alignment file) in the alignment. For each mAb in the dataset with residue-level epitope definitions of five residues or more, we calculated pepitope for all pairwise comparisons between the four serotypes and report the average pepitope value as the pepitope for that mAb.
In addition to calculating pepitope values between representative sequences of DENV1-4, we also calculated pairwise pepitope values for each strain within each serotype for the 47 sequences in the Kaetzelnick data set in order to compare intra-serotype and inter-serotype sequence variation at the epitope level.
Results
Database composition
There are three linked sections of the database: mAb, Activity, and Epitope (Fig 1). The mAb section contains a single record for each mAb in the database that includes the mAb name and information about its origin, including the host organism, the isotype, the immunogen, the exposure event that lead to the antibody, the host immune history, the selection criteria used to isolate or select that mAb for study, and the PubMedID of the reference that first reported the mAb. The Activity section can contain multiple records for each mAb and includes information on the activity assay details and data against all four DENV serotypes, and the corresponding PubMedID for its reference.
The breakdown of the database is shown in Table 1. Overall, the database is approximately evenly divided between mouse mAbs and human mAbs, from both primary and secondary infection. ELISA and neutralization assays are the most common activity records, accounting for almost 75% of the activity information. In Epitope records, there are only eight cryo-EM structures and eighteen x-ray crystallographic structures, representing <4% of the mAbs in the database which reflects the relative paucity of high-resolution epitope information on DENV mAbs. Most mAbs in the database have either mutagenesis or yeast-display data, underscoring the value of synthesizing this sparse epitope information to build a more comprehensive picture of DENV E protein epitopes.
Table 1. Database composition.
| Host (Infection type) | # of mAbs |
| Mouse | 172 |
| Human (primary) | 99 |
| Human (secondary) | 138 |
| Activity Method | # of records |
| IFA | 41 |
| ELISA | 253 |
| ELISPOT | 18 |
| Neutralization | 163 |
| Flow cytometry | 87 |
| IP | 23 |
| Western blot | 10 |
| Epitope Mapping Method | # of records |
| X-ray crystallography | 18 |
| Cryo-EM | 8 |
| Mutagenesis | 264 |
| Yeast display | 184 |
| PepScan | 27 |
| Passaging | 11 |
| Western Blot | 30 |
It is important to note that factors such as the antigen used for B cell selection, and even the time point, post-infection, when cell samples were collected to isolate antigen-specific B cells, can play a major role in epitope fine-specificity and cross-reactivity. We analyzed the database for all human antibodies, and for human and mouse antibodies, irrespective of these factors, in order to maximize the sample size for the analysis. However, this information is included in the database, and attached in S2 Table, for use in any further study. In certain cases, outlined in their respective sections, we did look for biases that selection methods may have had on the results.
Serotype-specificity in primary and secondary dengue infections
Overall, 45% of published mAbs from human primary infection were type-specific, 21% were sub-complex, and 34% were complex, while for mAbs from human secondary infection, 4% were type-specific, 10% were sub-complex, and 86% were complex (Fig 2a). These results reflect findings from polyclonal sera reported elsewhere [41] and underscore the profound shift from a type-specific primary immune response to an almost entirely cross-reactive secondary immune response. We next sought to map the epitopes from primary and secondary responses and determine the link between the epitope fine-specificity of the antibody response and its serotype-specificity.
Fig 2. Serotype-specificity and epitope mapping in primary and secondary dengue infections.
A) Histograms of human mAbs in the primary (left) and secondary infection (right) data sets. B) Epitope propensity in primary (left) and secondary (right) infections across E protein amino acid sequence. Points are colored with respect to epitope cross-reactivity, or the proportion of mAbs that are associated with a given epitope residue that are classified as ‘complex’: red (>60%), orange (30–60%), or yellow (< 30%). C) A composite map of epitope propensity and cross-reactivity on the structure of the DENV E protein dimer for mAbs from primary (left) and secondary (right) infections. Epitope regions DI/DII interface, dimer interface, fusion loop, DII/DIII interface, and DIII-lateral ridge (LR) are highlighted. Spheres correspond to epitope residues. The size of the sphere corresponds to its epitope propensity: low propensity (<5%, small spheres), medium propensity (>5% and <10%; medium spheres), and high propensity (>10%, large spheres). The color of the sphere corresponds to the epitope cross-reactivity as described above.
Mapping fine-specificity and cross-reactivity at the epitope level
We calculated epitope propensity across all residues in the DENV E protein from published human mAbs from primary and secondary DENV infections (Fig 2b). Overall, the results show that mAbs from secondary infection bind to a subset of the epitope residues for mAbs from primary infections. We also calculated epitope cross-reactivity as the percentage of mAbs associated with a particular epitope residue that have a serotype-specificity classification of ‘complex.’ We found that epitope residues from primary infections had a mix of cross-reactivity, ranging from low (<30%), medium (30%-60%), and high (>60%), while epitope residues from secondary infections were almost entirely of high cross-reactivity.
We generated a ‘composite’ epitope map of DENV antibody responses by mapping the epitope propensity and cross-reactivity of human mAbs from primary and secondary infections to the structure of the DENV E protein dimer [15] (Fig 2c). We found three overall trends. First, the fusion loop region, which showed high cross-reactivity in both primary and secondary responses, show an enhanced immunogenicity in secondary infections. Second, the DIII region, which displayed comparable immunogenicity in primary and secondary infections, showed a marked shift in both cross-reactivity and fine-specificity. The DIII response in secondary infections, unlike in primary infections, was highly cross-reactive, and shifted away from the lateral-ridge epitopes and towards the A-strand and DII/DIII interface epitopes. Finally, we found that two other epitope regions, the dimer interface, and the DI/DII interface, showed moderate immunogenicity in primary infections, with a medium level of cross-reactivity. In secondary infections, both of these regions showed a shift to high cross-reactivity.
Sequence variation and serotype cross-reactivity
Katzelnick et al. found that although DENV strains cluster into discrete serotypes with respect to sequence similarity within the E protein, they do not cluster into discrete serotypes in terms of antigenic similarity [37]. We hypothesized that, among epitope residues identified in the database, the concordance between antigenic and sequence similarity might be greater. We used the set of 47 DENV strains studied by Katzelnick et al., to quantify the degree of sequence variation between the four serotypes to determine if epitopes associated with type-specific mAbs could be distinguished from epitopes associated with cross-reactive mAbs. We carried out a multiple sequence alignment using the Consurf algorithm [48] to align E protein sequences from all 47 DENV strains. For each residue in E, we calculated a sequence conservation measure as the percentage of the aligned sequences that had the most common amino acid at that position. In S1 Fig, we show the sequence diversity (defined as 1 − sequence conservation), across the E protein for all four serotypes. It is important to note that we sought specifically to compare intra-serotype variation from our epitope-based analysis with the results from the Katzelnick study, not characterize intra-serotype variation more generally.
We generated histograms with respect to sequence conservation among DENV 1–4 E protein epitope residues from type-specific and complex mAbs from primary and secondary infections (Fig 3A). Our results show that epitope residues for type-specific mAbs show a bimodal distribution with a majority of residues falling between 40% to 80% sequence conservation. By contrast, most epitope residues from complex-specific mAbs fall in the 80% to 100% sequence conservation range. For comparison, the sequence conservation for all residues shows that distribution if the respective type-specific and complex antibody responses were to target residues at random. These results show that primary type-specific antibodies preferentially target residues with lower sequence conservation. Furthermore, they show that complex antibodies do not have strong preference for conserved residues, as might be expected.
Fig 3. Sequence variation and serotype specificity in DENV mAbs.
A) Histogram of sequence conservation among DENV 1–4 E protein epitope residues for type-specific and complex mAbs from primary and secondary infections, compared with all residues in the E protein. B) Sequence variation between consensus sequences of DENV1-4, at the epitope level measured by the average pepitope for type-specific mAbs from primary infection (N = 45), complex mAbs from primary infection (N = 24), and complex mAbs from secondary infections (N = 25). Only mAbs with epitope definitions consisting of at least five residues were considered. Error bars correspond to the standard deviation of pepitope values. A statistically significant difference is indicated by ‘*’, corresponding to p < 0.001.
Previous research in antigenic variation in influenza virus identified a sequence-based measure, pepitope that was found to be correlated with antibody cross-neutralization [46, 47] between strains. pepitope is calculated by determining the proportion of sequence mismatches across a defined epitope region, between two strains. In order to calculate a reliable pepitope value, an epitope definition of a sufficient size is necessary. For each mAb in the database with a residue-level epitope definition of at least five residues, we calculated a pepitope value for that epitope based on the average pairwise pepitope across all possible pairs of the four DENV serotypes (Fig 3B). For reference, a distribution of defined epitope sizes is shown in S3 Fig. Our results show that pepitope successfully distinguishes between type-specific and complex mAbs and suggests that a threshold mismatch of 20% of an epitope is sufficient to result in type-specificity. Interestingly, Gupta et al. found that a similar threshold of pepitope = 20% corresponds to a loss of vaccine efficacy between two influenza strains [46]. For a typical mAb epitope of 25–35 residues in size, this would correspond to at least five amino acid mismatches among the epitope residues.
It is possible that antigenic variation among only a subset of epitope residues is responsible for type-specificity. For example, previous mutagenesis experiments have shown that only mutations at certain key epitope residues abrogate antibody binding, while mutations at other epitope residues have no effect [19, 24]. We extracted a subset of human mAbs in the database whose epitopes were defined exclusively by cell passaging or mutagenesis, and would thus reflect not just structural, but functionally significant epitope residues. When we generated a composite map of these epitopes on the E protein structure (S2 Fig), however, we found that the overall epitope map was similar to the epitope map using the entire database (Fig 2C), albeit more sparse. Likewise, when we looked at sequence conservation among this subset of functionally-significant epitope residues (S4 Fig), we saw similar results as when all epitope residues were considered (Fig 3A).
Finally, we compared pepitope values between serotypes (inter-serotype) with pepitope values within a serotype (intra-serotype), to determine if the high intra-serotype antigenic variation observed by Kaetzelnick et al. could be reflected in higher intra-serotype pepitope values. However, as shown in S5 Fig, this was not the case. pepitope values within each serotype (S5 Fig) were significantly lower than pepitope values between serotypes (Fig 3B), typically below 5%. Although we sought to determine whether type-specific epitopes might have more mismatches than complex epitopes, the number of significant mismatches within a serotype (see Methods) was too small to reliably determine average pepitope values. A more extensive analysis of intra-serotype variation was outside the scope of this study.
Differences in fine-specificity between mouse and human mAbs
Antibody responses to DENV have been most extensively characterized in humans and in mice. We sought to determine the degree to which there are systematic differences in epitope fine-specificity between mouse and human mAbs in the dataset. Towards that end, we calculated epitope propensity for mAbs from mouse and human separately (Fig 4). Our results show that the published mAbs from mice preferentially target DIII. Whereas, published human mAbs target epitopes on the DI/DII interface and the dimer interface. These findings suggest that there are systematic differences in epitope fine-specificity between published mouse and human mAbs—in particular that the type-specific mAbs in mice predominantly target the DIII region (>75%), while published type-specific mAbs from humans target the DI/DII interface. It is important to note, however, that DIII remains a major target in the human Ab response as 30–50% of the type-specific human mAbs in our database target this region.
Fig 4. Differences in epitope fine-specificity between mouse and human.
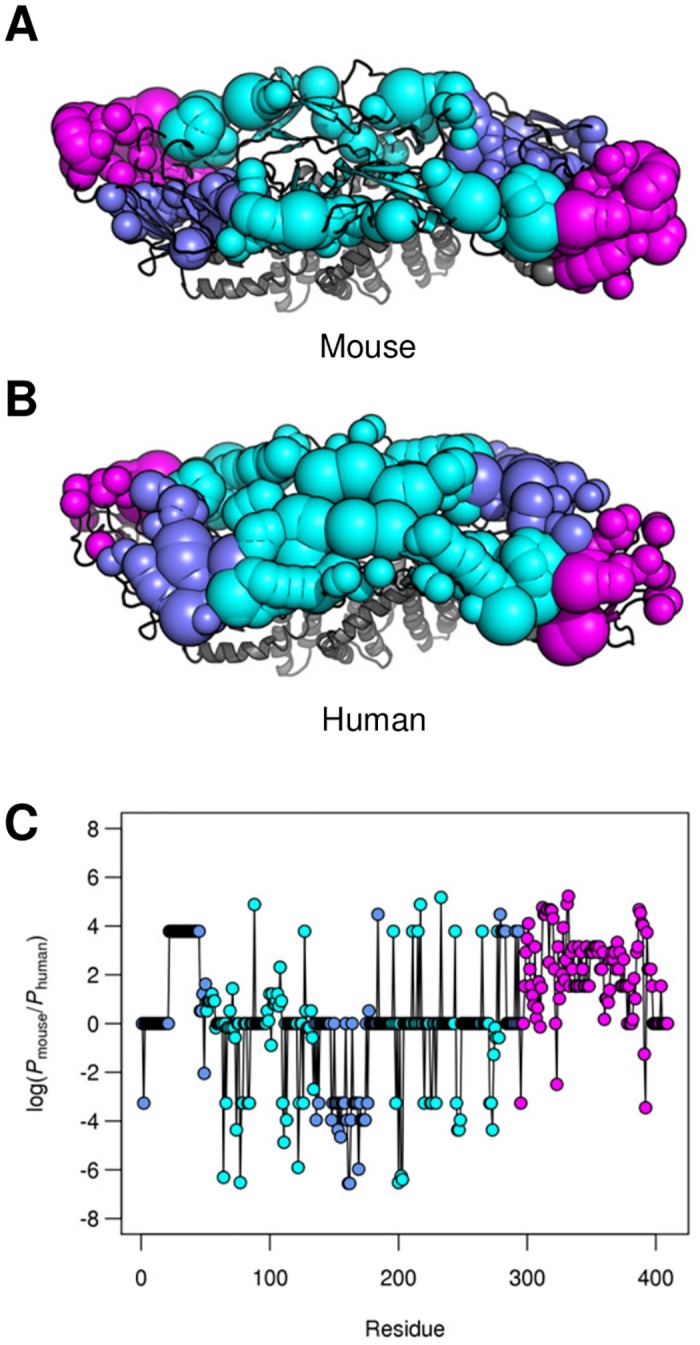
Mapping of epitope propensity on to the structure of the DENV E protein dimer for mAbs from mouse (A) and human (B). Spheres correspond to epitope residues. The size of the sphere corresponds to its epitope propensity, as described previously. C. Difference in epitope propensity between mouse and human across the E protein. Residue color corresponds to domain—purple, cyan, and magenta, for DI, DII, and DIII, respectively.
It is important to note that many mouse mAbs were collected in the 1990’s and 2000’s while many human mAbs were collected more recently. As such, any systematic differences in the methodologies used to select hybridomas in these studies may confound an analysis of host-level differences in the antibody responses. Indeed, 85% of human mAbs in the database were initially selected based on binding to whole-virus preparations, compared to 32% of mouse mAbs; most mouse mAbs were selected based on binding to recombinant E proteins. However, even among mouse mAbs selected based on binding to whole virus, 70% (33 of 51) targeted DIII.
Discussion
We hypothesized that the basis for the shift in cross-reactivity in the antibody response between primary and secondary infections could be found in the epitope-level fine-specificity of the respective antibody repertoires. We based our hypothesis on the theory of original antigenic sin that during secondary infection, pre-existing memory B cells specific to cross-reactive epitopes from the primary infection, would be selectively expanded to form the B cell and antibody repertoires in secondary infection [41, 51, 52]. Our comprehensive analysis of published epitopes showed that this was partially true. For example after secondary infection, we found that Abs to type-specific DIII epitopes, such as in the DIII-lateral ridge, are significantly diminished, while Abs to cross-reactive fusion loop epitopes are increased. However, we also found that epitopes, such as the DI/DII hinge region and the dimer interface, targeted by less cross-reactive Abs following primary infection were targeted by a higher proportion of cross-reactive Abs following secondary infection. It is important to note that an epitope region encompasses many overlapping epitopes, some of which are more conserved than others. During secondary infection it is these conserved epitopes within the epitope region that appear to be selected for, increasing the apparent cross-reactivity of that region compared to the primary infection.
Overall, these results indicate that secondary DENV infections increase the number of cross-reactive Abs that target regions of the E protein recognized by both cross-reactive and type-specific Abs elicited by primary infections. This result is reminiscent of previous modeling work done in our group on a polyvalent malaria vaccine which showed that polyvalent formulations not only enhance the Ab response to shared or cross-reactive epitopes within the vaccine, but enhance the cross-reactivity of what were considered type-specific epitopes as well [53, 54]. Either serially, as in the case of secondary DENV infection, or in parallel, as in the case with polyvalent vaccine formulations, this increased cross-reactivity results from a selective advantage of cross-reactive B cells over type-specific B cells. Whether similar effects on fine-specificity and cross-reactivity are present for polyvalent dengue vaccines remains to be seen. Furthermore, the immunological consequences of the shift in epitope-level fine-specificity in the antibody response to secondary DENV infection are still unclear.
We analyzed sequence variation across DENV sequences and found that epitopes from type-specific antibodies have significantly more variation than epitopes from complex antibodies. Furthermore, we showed that the epitope-level measure, pepitope, successfully distinguished between type-specific and complex antibodies. In a landmark study, Katzelnick et al. [37] showed that even though DENV strains cluster into discrete serotypes in terms of sequence similarity along the E protein, they do not cluster by discrete serotypes in terms of antigenic similarity—in many cases sera raised against one serotype shows higher neutralization to a genetically distant strain from a different serotype, than to genetically similar strain from the original infecting serotype. We hypothesized that, among epitope residues identified in the database, there might be greater concordance between sequence similarity and antigenic similarity. However, this did not turn out to be the case. When we calculated pepitope values across the 47 DENV strains tested in that study, we found that intra-serotype pepitope values were relatively low (<5%) and far exceeded by inter-serotype pepitope values, for epitopes from both type-specific and complex antibodies. Previous studies have found that a small number of mutations can result in significant strain-specific differences in neutralization [27, 39, 55], that sequence variation alone failed to predict strain-specific differences in neutralization [56], and that genotype differences in viral conformational dynamics and epitope accessibility may be responsible [57]. As of yet, the structural and immunological basis for why DENV strains do not cluster antigenically into discrete serotypes is still largely unknown.
Many previously published type-specific mouse mAbs predominantly target DIII of the E protein, while a lower proportion of characterized human mAbs (30–50% in the database) target this region [30, 31, 58]. Furthermore, type-specific human Abs predominately target other E protein regions such as the DI/DII hinge region that have not been described for mouse mAbs. Our findings suggest this might be the result of systematic differences in epitope fine specificity between the mouse and human antibody responses but may also reflect differences in experimental methods used to produce mAbs from mice versus humans.
We developed the DENV antibody database to provide a repository for activity and epitope information for DENV-specific mAbs in order to better characterize repertoire-level properties of the DENV antibody response. Here we provide an overview of the database and demonstrate how it can be used to analyze the relationship between epitope fine-specificity and serotype cross-reactivity in primary and secondary infections. We invite readers to explore the DENV antibody database (http://denvabdb.bhsai.org), use it both as repository for storing and accessing information on DENV mAbs, and as a means to systematically analyze and characterize DENV antibody responses.
Supporting information
Sequence diversity (1 − sequence conservation) was calculated from 47 DENV strains and is shown for DENV-1, DENV-2, DENV-3, and DENV-4 serotypes, across the E protein.
(TIF)
Composite epitope maps that were generated from epitopes defined exclusively from mutagenesis or cell passaging experiments from human mAbs from primary and secondary infections. Spheres correspond to epitope residues. The size of the sphere corresponds to its epitope propensity: low propensity (<5%, small spheres), medium propensity (>5% and <10%; medium spheres), and high propensity (>10%, large spheres). The color of the sphere corresponds to the epitope cross-reactivity as described above.
(TIF)
Histogram showing the the percentage of antibodies in the data set that have a poorly defined (5–10 residues), moderately well defined (10–20 residues), and well-defined epitopes (20+) in the data set.
(TIF)
Histogram of sequence conservation among DENV 1–4 E protein for epitope residues defined exclusively by cell passaging and mutagenesis experiments, for type-specific and complex human mAbs from primary and secondary infections.
(TIF)
Average and standard deviation of pairwise pepitope values within each serotype for primary type-specific mAbs, primary complex-specific mAbs, and secondary, complex-specific mAbs. Y-axis is scaled to the same range as Fig 3 and S4 Fig for comparison.
(TIF)
(PDF)
(XLSX)
Acknowledgments
The opinions and assertions contained herein are the private views of the authors and are not to be construed as official or as reflecting the views of the U.S. Army or the U.S. Department of Defense. This paper has been approved for public release with unlimited distribution.
Data Availability
All relevant data are within the paper and its Supporting Information files. The primary data used for the analysis in the paper are freely accessible at http://denvabdb.bhsai.org.
Funding Statement
The authors were supported by the U.S. Army Medical Research and Materiel Command (Ft. Detrick, MD) and the Military Infectious Disease Research Program (Grant# Z0010-TC-OC). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Bhatt S, Gething PW, Brady OJ, Messina JP, Farlow AW, Moyes CL, et al. The global distribution and burden of dengue. Nature. 2013;496(7446):504–7. 10.1038/nature12060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sabin AB. Research on dengue during World War II. Am J Trop Med Hyg. 1952;1(1):30–50. [DOI] [PubMed] [Google Scholar]
- 3.Anderson KB, Gibbons RV, Cummings DA, Nisalak A, Green S, Libraty DH, et al. A shorter time interval between first and second dengue infections is associated with protection from clinical illness in a school-based cohort in Thailand. J Infect Dis. 2014;209(3):360–8. 10.1093/infdis/jit436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Montoya M, Gresh L, Mercado JC, Williams KL, Vargas MJ, Gutierrez G, et al. Symptomatic versus inapparent outcome in repeat dengue virus infections is influenced by the time interval between infections and study year. PLoS Negl Trop Dis. 2013;7(8):e2357 10.1371/journal.pntd.0002357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sharp TM, Hunsperger E, Munoz-Jordan JL, Margolis HS, Tomashek KM. Sequential episodes of dengue—Puerto Rico, 2005–2010. Am J Trop Med Hyg. 2014;91(2):235–9. 10.4269/ajtmh.13-0742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Waggoner JJ, Balmaseda A, Gresh L, Sahoo MK, Montoya M, Wang C, et al. Homotypic Dengue Virus Reinfections in Nicaraguan Children. The Journal of infectious diseases. 2016;214(7):986–93. 10.1093/infdis/jiw099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Halstead SB. Neutralization and antibody-dependent enhancement of dengue viruses. Adv Virus Res. 2003;60:421–67. [DOI] [PubMed] [Google Scholar]
- 8.Kliks SC, Nimmanitya S, Nisalak A, Burke DS. Evidence that maternal dengue antibodies are important in the development of dengue hemorrhagic fever in infants. Am J Trop Med Hyg. 1988;38(2):411–9. [DOI] [PubMed] [Google Scholar]
- 9.Halstead SB, O'Rourke EJ. Dengue viruses and mononuclear phagocytes. I. Infection enhancement by non-neutralizing antibody. J Exp Med. 1977;146(1):201–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vaughn DW, Green S, Kalayanarooj S, Innis BL, Nimmannitya S, Suntayakorn S, et al. Dengue viremia titer, antibody response pattern, and virus serotype correlate with disease severity. J Infect Dis. 2000;181(1):2–9. 10.1086/315215 [DOI] [PubMed] [Google Scholar]
- 11.Burke DS, Nisalak A, Johnson DE, Scott RM. A prospective study of dengue infections in Bangkok. Am J Trop Med Hyg. 1988;38(1):172–80. [DOI] [PubMed] [Google Scholar]
- 12.Flipse J, Smit JM. The Complexity of a Dengue Vaccine: A Review of the Human Antibody Response. PLoS Negl Trop Dis. 2015;9(6):e0003749 10.1371/journal.pntd.0003749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kuhn RJ, Zhang W, Rossmann MG, Pletnev SV, Corver J, Lenches E, et al. Structure of dengue virus: implications for flavivirus organization, maturation, and fusion. Cell. 2002;108(5):717–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Roehrig JT. Antigenic structure of flavivirus proteins. Adv Virus Res. 2003;59:141–75. [DOI] [PubMed] [Google Scholar]
- 15.Modis Y, Ogata S, Clements D, Harrison SC. A ligand-binding pocket in the dengue virus envelope glycoprotein. Proc Natl Acad Sci U S A. 2003;100(12):6986–91. 10.1073/pnas.0832193100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pierson TC, Fremont DH, Kuhn RJ, Diamond MS. Structural insights into the mechanisms of antibody-mediated neutralization of flavivirus infection: implications for vaccine development. Cell Host Microbe. 2008;4(3):229–38. 10.1016/j.chom.2008.08.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gromowski GD, Roehrig JT, Diamond MS, Lee JC, Pitcher TJ, Barrett AD. Mutations of an antibody binding energy hot spot on domain III of the dengue 2 envelope glycoprotein exploited for neutralization escape. Virology. 2010;407(2):237–46. 10.1016/j.virol.2010.06.044 [DOI] [PubMed] [Google Scholar]
- 18.Matsui K, Gromowski GD, Li L, Schuh AJ, Lee JC, Barrett AD. Characterization of dengue complex-reactive epitopes on dengue 3 virus envelope protein domain III. Virology. 2009;384(1):16–20. 10.1016/j.virol.2008.11.013 [DOI] [PubMed] [Google Scholar]
- 19.Gromowski GD, Barrett ND, Barrett AD. Characterization of dengue virus complex-specific neutralizing epitopes on envelope protein domain III of dengue 2 virus. J Virol. 2008;82(17):8828–37. 10.1128/JVI.00606-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lok SM, Kostyuchenko V, Nybakken GE, Holdaway HA, Battisti AJ, Sukupolvi-Petty S, et al. Binding of a neutralizing antibody to dengue virus alters the arrangement of surface glycoproteins. Nat Struct Mol Biol. 2008;15(3):312–7. 10.1038/nsmb.1382 [DOI] [PubMed] [Google Scholar]
- 21.Sukupolvi-Petty S, Austin SK, Purtha WE, Oliphant T, Nybakken GE, Schlesinger JJ, et al. Type- and subcomplex-specific neutralizing antibodies against domain III of dengue virus type 2 envelope protein recognize adjacent epitopes. J Virol. 2007;81(23):12816–26. 10.1128/JVI.00432-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Roehrig JT, Bolin RA, Kelly RG. Monoclonal antibody mapping of the envelope glycoprotein of the dengue 2 virus, Jamaica. Virology. 1998;246(2):317–28. 10.1006/viro.1998.9200 [DOI] [PubMed] [Google Scholar]
- 23.Thullier P, Demangel C, Bedouelle H, Megret F, Jouan A, Deubel V, et al. Mapping of a dengue virus neutralizing epitope critical for the infectivity of all serotypes: insight into the neutralization mechanism. J Gen Virol. 2001;82(Pt 8):1885–92. 10.1099/0022-1317-82-8-1885 [DOI] [PubMed] [Google Scholar]
- 24.Gromowski GD, Barrett AD. Characterization of an antigenic site that contains a dominant, type-specific neutralization determinant on the envelope protein domain III (ED3) of dengue 2 virus. Virology. 2007;366(2):349–60. 10.1016/j.virol.2007.05.042 [DOI] [PubMed] [Google Scholar]
- 25.Lisova O, Hardy F, Petit V, Bedouelle H. Mapping to completeness and transplantation of a group-specific, discontinuous, neutralizing epitope in the envelope protein of dengue virus. J Gen Virol. 2007;88(Pt 9):2387–97. 10.1099/vir.0.83028-0 [DOI] [PubMed] [Google Scholar]
- 26.Matsui K, Gromowski GD, Li L, Barrett AD. Characterization of a dengue type-specific epitope on dengue 3 virus envelope protein domain III. J Gen Virol. 2010;91(Pt 9):2249–53. 10.1099/vir.0.021220-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wahala WM, Donaldson EF, de Alwis R, Accavitti-Loper MA, Baric RS, de Silva AM. Natural strain variation and antibody neutralization of dengue serotype 3 viruses. PLoS Pathog. 2010;6(3):e1000821 10.1371/journal.ppat.1000821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Midgley CM, Bajwa-Joseph M, Vasanawathana S, Limpitikul W, Wills B, Flanagan A, et al. An in-depth analysis of original antigenic sin in dengue virus infection. J Virol. 2011;85(1):410–21. 10.1128/JVI.01826-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moreland NJ, Susanto P, Lim E, Tay MY, Rajamanonmani R, Hanson BJ, et al. Phage display approaches for the isolation of monoclonal antibodies against dengue virus envelope domain III from human and mouse derived libraries. Int J Mol Sci. 2012;13(3):2618–35. 10.3390/ijms13032618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wahala WM, Kraus AA, Haymore LB, Accavitti-Loper MA, de Silva AM. Dengue virus neutralization by human immune sera: role of envelope protein domain III-reactive antibody. Virology. 2009;392(1):103–13. 10.1016/j.virol.2009.06.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wahala WM, Huang C, Butrapet S, White LJ, de Silva AM. Recombinant dengue type 2 viruses with altered e protein domain III epitopes are efficiently neutralized by human immune sera. J Virol. 2012;86(7):4019–23. 10.1128/JVI.06871-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.de Alwis R, Smith SA, Olivarez NP, Messer WB, Huynh JP, Wahala WM, et al. Identification of human neutralizing antibodies that bind to complex epitopes on dengue virions. Proc Natl Acad Sci U S A. 2012;109(19):7439–44. 10.1073/pnas.1200566109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Teoh EP, Kukkaro P, Teo EW, Lim AP, Tan TT, Yip A, et al. The structural basis for serotype-specific neutralization of dengue virus by a human antibody. Sci Transl Med. 2012;4(139):139ra83 10.1126/scitranslmed.3003888 [DOI] [PubMed] [Google Scholar]
- 34.Messer WB, de Alwis R, Yount BL, Royal SR, Huynh JP, Smith SA, et al. Dengue virus envelope protein domain I/II hinge determines long-lived serotype-specific dengue immunity. Proc Natl Acad Sci U S A. 2014;111(5):1939–44. 10.1073/pnas.1317350111 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 35.Smith SA, Zhou Y, Olivarez NP, Broadwater AH, de Silva AM, Crowe JE Jr. Persistence of circulating memory B cell clones with potential for dengue virus disease enhancement for decades following infection. J Virol. 2012;86(5):2665–75. 10.1128/JVI.06335-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dejnirattisai W, Wongwiwat W, Supasa S, Zhang X, Dai X, Rouvinski A, et al. A new class of highly potent, broadly neutralizing antibodies isolated from viremic patients infected with dengue virus. Nat Immunol. 2015;16(2):170–7. 10.1038/ni.3058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Katzelnick LC, Fonville JM, Gromowski GD, Bustos Arriaga J, Green A, James SL, et al. Dengue viruses cluster antigenically but not as discrete serotypes. Science. 2015;349(6254):1338–43. 10.1126/science.aac5017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sukupolvi-Petty S, Austin SK, Engle M, Brien JD, Dowd KA, Williams KL, et al. Structure and function analysis of therapeutic monoclonal antibodies against dengue virus type 2. J Virol. 2010;84(18):9227–39. 10.1128/JVI.01087-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Brien JD, Austin SK, Sukupolvi-Petty S, O'Brien KM, Johnson S, Fremont DH, et al. Genotype-specific neutralization and protection by antibodies against dengue virus type 3. J Virol. 2010;84(20):10630–43. 10.1128/JVI.01190-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Parameswaran P, Liu Y, Roskin KM, Jackson KK, Dixit VP, Lee JY, et al. Convergent antibody signatures in human dengue. Cell Host Microbe. 2013;13(6):691–700. 10.1016/j.chom.2013.05.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Toh YX, Gan V, Balakrishnan T, Zuest R, Poidinger M, Wilson S, et al. Dengue serotype cross-reactive, anti-e protein antibodies confound specific immune memory for 1 year after infection. Front Immunol. 2014;5:388 10.3389/fimmu.2014.00388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tsai WY, Durbin A, Tsai JJ, Hsieh SC, Whitehead S, Wang WK. Complexity of Neutralizing Antibodies against Multiple Dengue Virus Serotypes after Heterotypic Immunization and Secondary Infection Revealed by In-Depth Analysis of Cross-Reactive Antibodies. J Virol. 2015;89(14):7348–62. 10.1128/JVI.00273-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Beltramello M, Williams KL, Simmons CP, Macagno A, Simonelli L, Quyen NT, et al. The human immune response to Dengue virus is dominated by highly cross-reactive antibodies endowed with neutralizing and enhancing activity. Cell Host Microbe. 2010;8(3):271–83. 10.1016/j.chom.2010.08.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Smith SA, de Alwis R, Kose N, Durbin AP, Whitehead SS, de Silva AM, et al. Human monoclonal antibodies derived from memory B cells following live attenuated dengue virus vaccination or natural infection exhibit similar characteristics. The Journal of infectious diseases. 2013;207(12):1898–908. 10.1093/infdis/jit119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lin HE, Tsai WY, Liu IJ, Li PC, Liao MY, Tsai JJ, et al. Analysis of epitopes on dengue virus envelope protein recognized by monoclonal antibodies and polyclonal human sera by a high throughput assay. PLoS Negl Trop Dis. 2012;6(1):e1447 10.1371/journal.pntd.0001447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gupta V, Earl DJ, Deem MW. Quantifying influenza vaccine efficacy and antigenic distance. Vaccine. 2006;24(18):3881–8. 10.1016/j.vaccine.2006.01.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pan K, Subieta KC, Deem MW. A novel sequence-based antigenic distance measure for H1N1, with application to vaccine effectiveness and the selection of vaccine strains. Protein Eng Des Sel. 2011;24(3):291–9. 10.1093/protein/gzq105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ashkenazy H, Abadi S, Martz E, Chay O, Mayrose I, Pupko T, et al. ConSurf 2016: an improved methodology to estimate and visualize evolutionary conservation in macromolecules. Nucleic Acids Res. 2016;44(W1):W344–50. 10.1093/nar/gkw408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Danecek P, Lu W, Schein CH. PCP consensus sequences of flaviviruses: correlating variance with vector competence and disease phenotype. J Mol Biol. 2010;396(3):550–63. 10.1016/j.jmb.2009.11.070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Larkin MA, Blackshields G, Brown NP, Chenna R, McGettigan PA, McWilliam H, et al. Clustal W and Clustal X version 2.0. Bioinformatics. 2007;23(21):2947–8. 10.1093/bioinformatics/btm404 [DOI] [PubMed] [Google Scholar]
- 51.Zompi S, Montoya M, Pohl MO, Balmaseda A, Harris E. Dominant cross-reactive B cell response during secondary acute dengue virus infection in humans. PLoS Negl Trop Dis. 2012;6(3):e1568 10.1371/journal.pntd.0001568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Woda M, Friberg H, Currier JR, Srikiatkhachorn A, Macareo LR, Green S, et al. Dynamics of Dengue Virus (DENV)-Specific B Cells in the Response to DENV Serotype 1 Infections, Using Flow Cytometry With Labeled Virions. The Journal of infectious diseases. 2016;214(7):1001–9. 10.1093/infdis/jiw308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dutta S, Dlugosz LS, Drew DR, Ge X, Ababacar D, Rovira YI, et al. Overcoming antigenic diversity by enhancing the immunogenicity of conserved epitopes on the malaria vaccine candidate apical membrane antigen-1. PLoS Pathog. 2013;9(12):e1003840 10.1371/journal.ppat.1003840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chaudhury S, Reifman J, Wallqvist A. Simulation of B cell affinity maturation explains enhanced antibody cross-reactivity induced by the polyvalent malaria vaccine AMA1. J Immunol. 2014;193(5):2073–86. 10.4049/jimmunol.1401054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Messer WB, Yount B, Hacker KE, Donaldson EF, Huynh JP, de Silva AM, et al. Development and characterization of a reverse genetic system for studying dengue virus serotype 3 strain variation and neutralization. PLoS Negl Trop Dis. 2012;6(2):e1486 10.1371/journal.pntd.0001486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Shrestha B, Brien JD, Sukupolvi-Petty S, Austin SK, Edeling MA, Kim T, et al. The development of therapeutic antibodies that neutralize homologous and heterologous genotypes of dengue virus type 1. PLoS Pathog. 2010;6(4):e1000823 10.1371/journal.ppat.1000823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Austin SK, Dowd KA, Shrestha B, Nelson CA, Edeling MA, Johnson S, et al. Structural basis of differential neutralization of DENV-1 genotypes by an antibody that recognizes a cryptic epitope. PLoS Pathog. 2012;8(10):e1002930 10.1371/journal.ppat.1002930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Williams KL, Wahala WM, Orozco S, de Silva AM, Harris E. Antibodies targeting dengue virus envelope domain III are not required for serotype-specific protection or prevention of enhancement in vivo. Virology. 2012;429(1):12–20. 10.1016/j.virol.2012.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Sequence diversity (1 − sequence conservation) was calculated from 47 DENV strains and is shown for DENV-1, DENV-2, DENV-3, and DENV-4 serotypes, across the E protein.
(TIF)
Composite epitope maps that were generated from epitopes defined exclusively from mutagenesis or cell passaging experiments from human mAbs from primary and secondary infections. Spheres correspond to epitope residues. The size of the sphere corresponds to its epitope propensity: low propensity (<5%, small spheres), medium propensity (>5% and <10%; medium spheres), and high propensity (>10%, large spheres). The color of the sphere corresponds to the epitope cross-reactivity as described above.
(TIF)
Histogram showing the the percentage of antibodies in the data set that have a poorly defined (5–10 residues), moderately well defined (10–20 residues), and well-defined epitopes (20+) in the data set.
(TIF)
Histogram of sequence conservation among DENV 1–4 E protein for epitope residues defined exclusively by cell passaging and mutagenesis experiments, for type-specific and complex human mAbs from primary and secondary infections.
(TIF)
Average and standard deviation of pairwise pepitope values within each serotype for primary type-specific mAbs, primary complex-specific mAbs, and secondary, complex-specific mAbs. Y-axis is scaled to the same range as Fig 3 and S4 Fig for comparison.
(TIF)
(PDF)
(XLSX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files. The primary data used for the analysis in the paper are freely accessible at http://denvabdb.bhsai.org.





