Abstract
Phosphatidylethanolamine binding proteins (PEBP) represent a superfamily of proteins that are conserved from bacteria to humans. In mammals, four members have been identified, PEBP1–4. To determine the functional differences among PEBP1–4 and the underlying mechanism for their actions, we performed a sequence alignment and found that PEBP4 contains a signal peptide and potential glycosylation sites, whereas PEBP1–3 are intracellular proteins. To test if PEBP4 is secreted, we made constructs with Myc epitope at the amino (N) terminus or carboxyl (C) terminus to mask the signal sequence or keep it free, respectively. Our data revealed that both mouse and human PEBP4 were secreted when the epitope was tagged at their C-terminus. To our surprise, secretion was dependent upon the C-terminal conserved domain in addition to the N-terminal signal sequence. When the epitope was placed to the N-terminus, the recombinant protein failed to secrete and instead, was retained in the cytoplasm. Mass spectrometry detected asparagine (N)-glycosylation on the secreted PEBP4. Although overexpression of N-terminal tagged PEBP4 resulted in an inhibition of ERK activation by EGF that with a C-terminal epitope tag did not have such an effect. Likewise, transfection of PEBP4 shRNA did not appear to affect ERK activation, suggesting that PEBP4 does not participate in the regulation of this pathway. In contrast, PEBP4 siRNA suppressed phosphorylation of Act at S473. Therefore, our results suggest that PEBP4 is a multifunctional protein and can be secreted. It will be important to investigate the mechanism by which PEBP4 is secreted and regulates cellular events.
Keywords: PEBP4, signal peptide, secretion, glycosylation, ERK, Act
1. Introduction
Phosphatidylethanolamine binding protein (PEBP) represents a superfamily of more than 400 members and is evolutionally conserved from bacteria to humans (1). In mammalian cells, four members of PEBP, ranging from 21–25 kids, have been documented; PEBP1 is ubiquitously expressed at high levels in brain, adrenal gland and thyroid (2, 3), PEBP2 is mostly restricted to testis (4), PEBP3 has not yet been characterized, and PEBP4 is predominantly expressed in skeletal muscle, heart and thyroid (5). Multiple functions have been ascribed to PEBPs, including membrane biogenesis, fluidity, and formation of functional domains (6–9), stimulation of acetylcholine secretion during neuronal development (9, 10), serine protease inhibition in neuronal tissue (11), and regulation of MAPK pathway, cell proliferation and survival and spermatogenesis or sperm maturation (4, 12).
PEBP1 was first found in brain (13) and later on isolated as a Raff kinase inhibitory protein (RKIP) by the yeast two hybrid method (12). It has been shown that RKIP binds to Raf-1 and MEK1 at overlapping sites (14). As a result, binding to Raf-1 precludes the binding to MEK1, and vice versa. Disruption of the interaction between Raf-1 and MEK1, leads to an inhibition of MEK phosphorylation and activation by Raf-1. In addition, RKIP has also been reported to regulate other signaling pathways including β-adrenergic signaling, and NFκB signaling, culminating in inhibition of tumorigenesis, metastasis, or modulation of other cellular events (1).
Similarly to PEBP1, PEBP4 was reported to associate with Raf-1 and MEK1, blocking MEK/ERK activation by TNFα or TRAIL and thereby inhibiting apoptosis (5, 15). PEBP4 is highly expressed in muscle, which leads to speculation of functional interactions between PEBP4 and Raff/MEK during myoblast differentiation (16). Garcia et al. showed that PEBP4 acts a scaffold for Raf-1 and MEK1 and augments their interaction (16). The effect of PEBP4 on ERK activation depends on the expression levels; paradoxically, low expression enhances but high expression suppresses ERK activation (16). In addition, knockdown of PEBP4 inhibits myoblast differentiation, possibly due to increased activation of the Raff/MEK. Recent studies indicate that PEBP4 enhances Act activation while inhibiting that of ERK/JNK (17–20). Furthermore, a number of recent reports have documented increased expression of PEBP4 in a variety of cancer specimens, correlative to invasion and metastasis of cancer, suggesting that PEBP4 plays a role in cancer progression (21–24). In agreement with this, in vitro studies have shown that silencing PEBP4 induces apoptosis and reduces invasiveness of cancer cells whereas overexpression elicits the opposite changes (17, 23, 25, 26). Interestingly, a recent study has reported that PEBP4 is a secreted protein, suggesting a new function or mechanism (27).
To determine the functional differences among PEBP1–4 and the underlying mechanisms for their actions, we first performed a sequence alignment, which revealed that PEBP4 contains a signal peptide, while PEBP1–2 are intracellular proteins. To test if PEBP4 is secreted, we made constructs with Myc epitope at the N- or C-terminus of PEBP4, respectively. Our data revealed that both mouse and human PEBP4 were secreted when the epitope was tagged at their C-termini. Surprisingly, the secretion was also dependent on the C-terminal conserved domain. In contrast, when the epitope was placed at the N-terminus, the recombinant protein failed to secrete and was retained in the cytoplasm. Mass spectrometry detected N-glycosylation on PEBP4. Overexpression of N-terminal tagged PEBP4 resulted in an inhibition of ERK activation by EGF, whereas C-terminal tagged PEBP4 was without such an effect. Likewise, transfection of PEBP4 shRNA did not appear to inhibit ERK activation, suggesting that PEBP4 does not participate in the regulation of this pathway. However, PEBP4 siRNA suppresses phosphorylation of Akt at S473.
2. Materials and Methods
2.1. Reagents
Epidermal growth factor (EGF) was purchased from Promega Life Sciences (San Luis Obispo, CA). Antibodies against phospho-ERK1/2 T202/Y204, total ERK, phospho-Akt S473, total Akt, and β-actin were from Cell Signal Biotechnologies (Danvers, MA). Antibody against Myc epitope was purchased from Sigmaaldrich (St Louis, MO). Antibody for human PEBP4 was from Abcam (Cambridge, MA). NTA agarose was from Qiagen (Valencia, CA). Human PEBP4 cDNA in pCDNA3.1 and pEGFPC was gifted from Dr. Water Koch (University College Dublin) and mouse PEBP4 cDNA was synthesized by Life Technologies (Grand Island, NY).
2.2. Construction of expression plasmids
cDNA for human PEBP4 was amplified by PCR and subcloned into pCDNA3.1(−)MycHisB (Life Technologies) at EcoRI and HinDIII sites to express recombinant PEBP4 with MycHis tag at the C-terminus. PEBP4 cDNA was amplified by PCR and subcloned in pCDNA3.1(−) to tag Myc epitope at the N-terminus. The deletion mutations were made as illustrated in Figure 4. The point mutation for T171A was made by PCR amplification of two overlapping fragments containing T171A and secondary amplification of full length mutant cDNA. The cDNA was sequenced and then subcloned to pCDNA3.1(−)MycHisB.
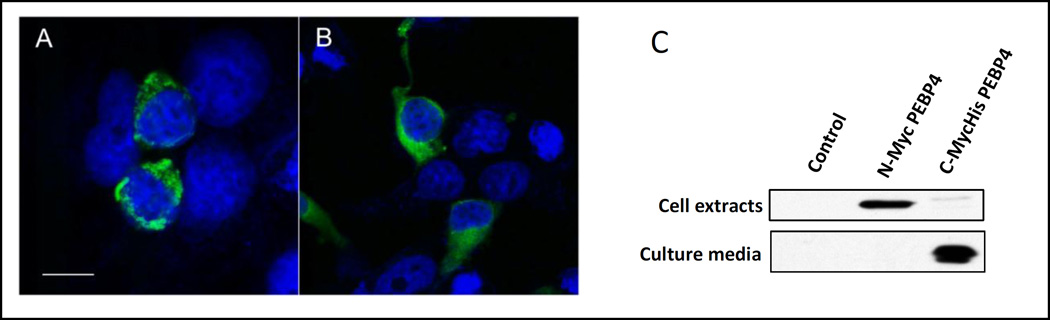
Figure 4. Secretion of PEBP4 variants to culture media.
PEBP4 full length, point mutation or truncation mutations were made at different sites and tagged with Myc or MycHis6, as indicated in (A). The recombinant proteins were transiently expressed in HEK293T cells and blotted with anti-Myc antibody in cell extracts or culture media (B).
2.3. Construction of shRNA plasmid for PEBP4
Oligonucleotides for shRNA were synthesized encompassing PEBP4 coding sequence bp 473–493 as underlined: Sense: 5’ GATCCCCGGAAAAGTCATCTCTCTCCttcaagagaGGAGAGAGATGACTTTTCCTTTTTA and antisense: agctTAAAAAGGAAAAGTCATCTCTCTCCTCTCTTGAAGGAGAGAGATGACTTTTCCGGG. The oligonucleotides were annealed and cloned into pSuperRetro at BglII and HindIII site.
2.4. Cell Culture and transfection
HEK293T cells were cultured in DMEM supplemented with 10% FBS and penicillin/streptomycin at 37°C, 5%CO2. Plasmid DNA was transfected into HEK293T cells by the calcium phosphate precipitation method. Two days after transfection, the cells were starved in 1% FBS-DMEM overnight and treated with EGF (10 ng/ml) for 10 min.
2.5. Purification of recombinant PEBP4
pCDNA3.1(−)MycHis expressing human or mouse PEBP4, respectively, was transfected into HEK293T cells as noted above. Two days after transfection, cell culture medium was harvested and passed through NTA agarose column. Nonspecific binding proteins were removed by washing and recombinant PEBP4 was eluted according to manufacturer’s protocol (Qiagen, Valencia, CA).
2.6. Western blot analysis
Cell extracts were prepared in lysis buffer (25 mM Tris-HCl, pH 7.8, 100 mM NaCl, 1 mM EDTA, 1 mM EGTA, 1 mMNa3VO4 and 25 mM β-glycerol-phosphate, 1 mM DTT, 1% NP-40 and protease inhibitors). The cell debris was removed by centrifugation at 14,000×g at 4°C for 15 min and protein concentration measured using a Bio-Rad Protein Assay kit. Protein samples (20 µg) were subjected to SDS–PAGE and electrophoretically transferred to PVDF membranes (EMD Millipore, Bedford, MA). The membranes were sequentially blotted with the first and second antibodies, and developed by the enhanced chemiluminescence (ECL) method (28).
2.7. Confocal microscopy examination
After transfection of PEBP4 tagged with GFP at the N-terminus or C-terminus into HEK293T cells on coverslips, the cells were fixed with 4% paraformaldehyde prepared in PBS and examined using a confocal microscope (Leica SP5) at 63× magnification.
2.8. Proteomics analysis
In-gel deglycosylation and trypsin digestion was performed on cut SDS-PAGE gel bands. In LC-MS/MS analysis, digestion products were separated by a C18 chromatography column (75 µm ID, 150 mm length; 120 min gradient elution at a flow rate 0.300 µL/min) with a Dionex 3000 nano-HPLC system which was interfaced with a Thermo-Fisher Scientific Q-Exactive mass spectrometer. Mobile phase A consisted of 0.1% formic acid, and mobile phase B consisted of 80% acetonitrile and 0.08% formic acid. The Q-Exactive mass spectrometer was operated in the data-dependent acquisition mode using Xcalibur2.1.3 software. For data acquisition, there was a single full-scan mass spectrum in the Orbitrap (400–1800 m/z, 70,000 resolution) followed by 20 data-dependent MS/MS scans. The tandem mass spectra from each LC-MS/MS run were searched against the selected database using the Proteome Discovery searching algorithm (version 1.4).
2.9. N-glycan analysis
In-gel PNGaseF digestion was performed for the cut band. The released N-glycans were analyzed by chip-based amide-HILIC LC/MS using an Agilent 6520 Q-TOF mass spectrometer (29).
3. Results
3.1. PEBP4 is a secreted protein
PEBP1 and PEBP4 apparently have different functions, as the former acts as a tumor suppressor while the latter promotes tumorigenesis and tumor progression. To decipher their functional differences, we performed sequence alignment of PEBP1–4 (Figure 1). Mouse PEBP1 displays 80% identity and 93.6% similarity to mouse PEBP2, and 86% identity and 95% similarity to rat PEBP3. Since no sequence for mouse PEBP3 was found in the database, we used the rat PEBP3 sequence, which shares 83% identity and 94% similarity to rat PEBP1, in the alignment. Mouse PEBP1 and PEBP4 exhibit 26.7% identity and 38.2% similarity (Figure 1). Thus, the sequence alignment data suggest that PEBP1–3 are functionally similar, while PEBP4 has unique functions. Interestingly, sequence alignment of mouse and human PEBP4 shows 45% identity and 59.5% similarity, and predicts that it contains a signal peptide (Figure 2). Furthermore, it predicts one N-glycosylation site with the NXT motif (asparagine 169, N169) on human PEBP4, and two such sites on mouse PEBP4 (N78, N140) (Figure 2).
Figure 1. Sequence alignment of PEPB1–4.
Access numbers are P70296 for mouse PEBP1 (mPEBP1), AF307146 for mouse PEBP2 (mPEBP2), AAB32786 for rat PEBP3 (rPEBP3), and NP_082802 for mouse PEBP4 (mPEBP4). Multiple sequence alignment was performed using ClusterW2 software. The degree of similarity is designated (*>:>.). PEBP1 and PEBP2 show 79.7% identity and 93.6% similarity; mouse PEBP1 and rat PEBP3 show 85.9% identity and 95.1% similarity; PEBP1 and PEBP4 show 26.7% identical and 38.2% similarity.
Figure 2. Sequence alignment of mouse and human PEBP4.
Sequence alignment shows 45.2% identify and 59.5% similarity between mouse and human PEBP4 and predicts signal peptide highlighted yellow and asparagine glycosylation sites (marked red N).
We hypothesized that the subcellular localization of PEBP4 might be different if we placed an epitope tag at the N-terminus, as opposed to the C-terminus. To test it, we made such constructs of human PEBP4 (hPEBP4) fused with green fluorescent protein (GFP) or a Myc tag. Indeed, when GFP was fused to the C-terminus of hPEBP4, the fluorescent signal displays punctate distribution centering near the perinucleus; in contrast, fusing GFP to the N-terminus resulted in a uniform signal distribution in the cytoplasm (Figure 3 A, B). We then examined the distribution of Myc-tagged hPEBP4 by immunoblotting. Most of C-terminal tagged hPEBP4 was secreted in the cell culture medium, while N-terminal tagged recombinant protein was retained in the cytoplasm (Figure 3C). Hence, our results suggest that hPEBP4 is a secreted protein and the secretion is disrupted by tagging a peptide to the N-terminus.
Figure 3. Effects of epitope tagging to different ends of PEBP4.
PEBP4 was tagged with GFP (A, B) or Myc epitope to at its N-terminus or C-terminus and transiently expressed in HEK293T cells. The cells were examined under confocal microscopy (A, B) or extracts and culture media subjected to western blot analysis using an anti-Myc antibody (C). The images in A and B were observed at 63×. Scale bar: 10 microns.
To further characterize the secreted nature of hPEBP4, we made a point mutation to convert T171 to alanine (T171A), thereby abolishing the N-glycosylation site, and two additional truncations at aa 172 and aa 188, where C-terminal 55 and 39 amino acids were deleted (Figure 4A), respectively. Mutant cDNA was transfected into HEK293T cells, and cell extracts and culture supernatant were collected for western blot analysis using the anti-Myc antibody. As shown in Figure 4B, both the C-terminal tagged full length PEBP4 and the T171A mutant, lacking the N-glycosylation site, were secreted into the cell culture media. To our surprise, deletion of the C-terminally conserved region between mouse and human PEBP4 abolished the secretion, suggesting that both N-terminus and C-terminal sequences are required for the secretion.
3.2. Human PEBP4 (hPEBP4) is glycosylated
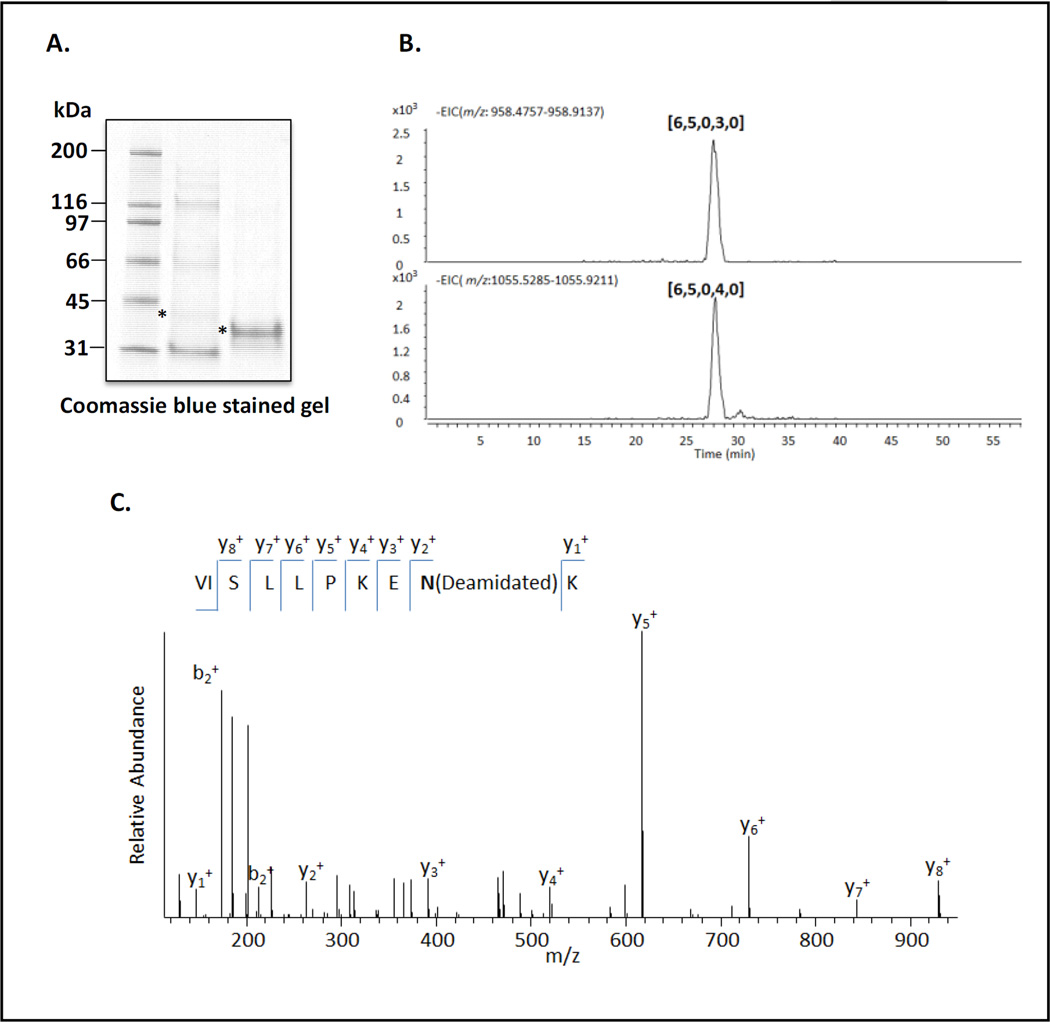
N-linked glycans are generally attached to the asparagine (Asn) residue as a part of Asn-X-Ser/Thr (N-X-S/T) consensus sequence, where X is any amino acid except proline. hPEBP4 contains only one N-glycosylation consensus sequence (N169KT), predicting potential N-glycan occupancy in hPEBP4. We compared the expression and secretion of the T171A mutant and wild type hPEBP4. As demonstrated in the western blotting (Figure 4B), the mutated sequences was smaller in the molecular weight than the wild type, indicating the blockade of N-glycosylation by mutation. Next, purified hPEBP4 sample was enzymatically deglycosylated using PNGase F, and then digested using trypsin. The released N-glycans from hPEBP4 was analyzed by mass spectrometer to further confirm that hPEBP4 is N-glycosylated. As illustrated in the Figure 5B, two major N-glycan compositions were detected, [6,5,0,3,0] and [6,5,0,4,0] [Hex, HexNAc, dHex, NeuAc, NeuGc] from hPEBP4. Tryptic peptides were analyzed using C18 LC-MS/MS analysis. The deglycosylated asparagine residue (N) was deamidated by the action of the PNGase F enzyme, resulting in a mass increase of 0.9840 Da. As shown in Figure 5C, a mass increase of 0.9840 and 90% sequence coverage was observed for the precursor VISLLPKENK, supporting that hPEBP4 is glycosylated. In summary, hPEBP4 contains a sialylated N-glycan. Mouse PEBP4 tryptic peptides were also analyzed by C18 LC-MS/MS and the data supported that mouse PEBP4 was N-glycosylated at two sites (N78IS and N140IT) (Supplemental Figure 1).
Figure 5. N-glycosylation of PEBP4.
A. Mouse and human PEBP4 tagged with MycHis6 at the C-terminus was expressed in HEK293T cells, respectively, purified by NTA affinity chromatography and resolved on SDS-PAGE. B. The PEBP4 bands in A were excised and digested with PNGaseF. The released N-glycans were analyzed and the compositions are listed C. Proteomics analysis (only the data with human PEBP4 were presented). The PEBP4 band was deglycosylated in-gel, digested with trypsin and the resulting peptides analyzed using LC-MS/MS analysis as described in the Materials and Methods section. Data interpretation is described in the text.
3.3. The role of PEBP4 in activation of ERK and Akt
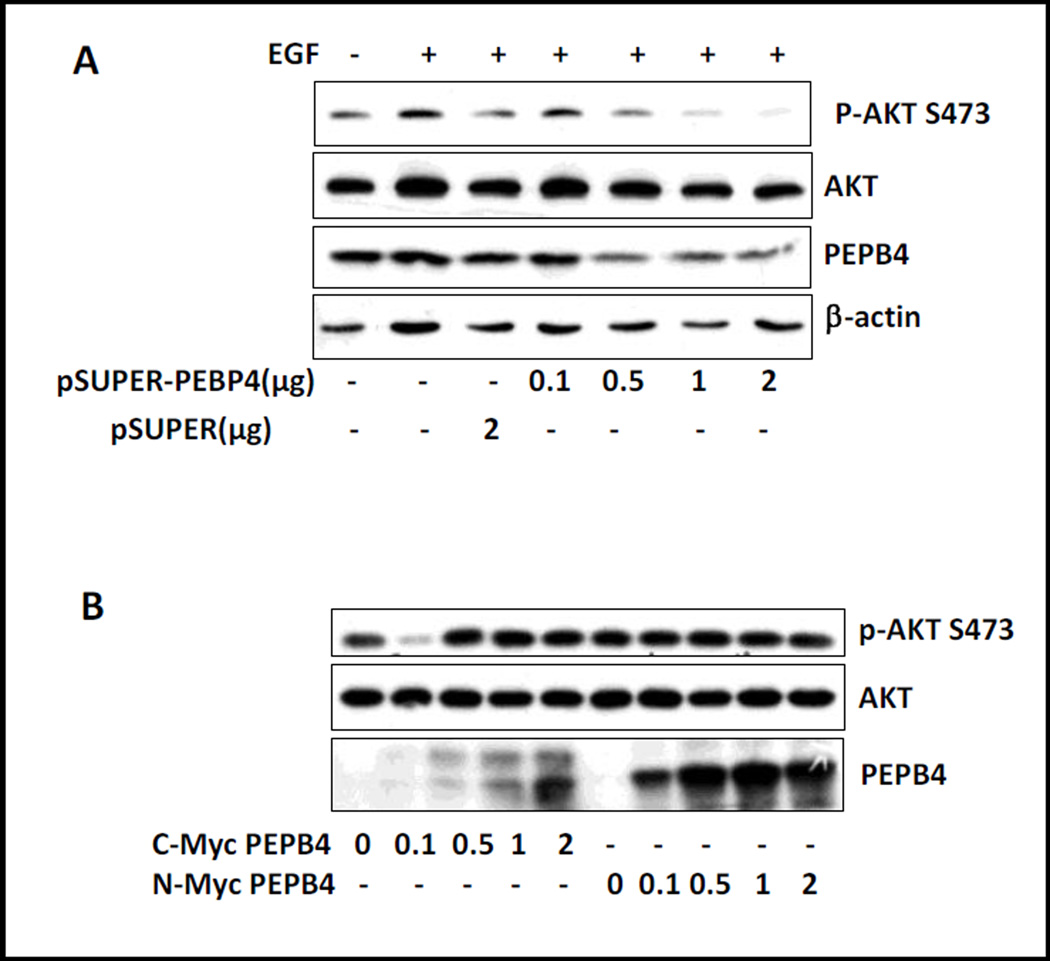
To assess the effect of PEBP4 on ERK activation, we transfected short hairpin RNA (shRNA) into HEK293T cells to silence the expression of PEBP4. As the dose of shRNA increased, the expression of PEBP4 progressively decreased. However, the activation of ERK by EGF was not affected (Figure 6A). We then transfected PEBP4 tagged at either the N- or C-terminus and treated with or without EGF. Transfection of C-terminal or N-terminal tagged PEBP4 did not affect the phosphorylation of ERK1/2 under baseline condition (Figure 6B). In addition, the C-terminal tagged PEBP4 did not show any inhibitory effect when cells were treated with EGF. However, the N-terminal tagged PEBP4 seemed to inhibit ERK activation at high doses (Figure 6C).
Figure 6. The effect of PEBP4 on ERK activation.
A. shRNA for PEBP4 or empty vector was transfected into HEK293T cells on 12 well plates, which were the treated with EGF (10 ng/ml) for 10 min. A. The cells were transfected with PEBP4 tagged at the N-terminus (N-Myc PEBP4) or C-terminus (C-Myc PEBP4) with Myc epitope at different doses. C. The experiments were conducted as B, except that the cells were treated with EGF. Cell extracts were analyzed by western blot with antibodies as indicated.
Next, we tested the effect of PEBP4 on Akt activity in response to EGF treatment. Consistent with previous reports, silencing PEBP4 did inhibit phosphorylation of Akt at S473 (Figure 7A). However, no evident effect on the phosphorylation of Akt was observed by overexpression of either N-terminally or C-terminally tagged PEBP4 (Figure 7B). This may result because Akt is constitutively activated in HEK293T cells.
Figure 7. The effect of PEBP4 on Akt activation.
A. HEK293T cells were transfected with PEBP4 shRNA or empty vector and treated as described in Figure 6A. B. The cells were transfected with C-Myc PEBP4 or N-Myc PEBP4. Western blot analysis was performed as indicated.
4. Discussion
PEBP4 appears to be a multifunctional protein. It was reported to be involved in activation of ERK/JNK and Akt. Our present study revealed that PEBP4 is a secreted and glycosylated protein. It contains a signal peptide at the N-terminus and consistently, addition of tags to the C-terminus allowed secretion of the protein, whereas masking the signal peptide by tagging an epitope to the N-terminus disabled the secretion. The mass spectrometric analysis identified N-glycosylation on both human and mouse PEBP4, exactly the same sites as predicted by motif alignment. Interestingly, our data indicate that the C-terminally conserved sequence is also required for secretion. Finally, our data showed that the native form of PEBP4 did not participate in ERK activation by EGF, although it seemed to be involved in the regulation of Akt activity.
PEBP4 was first identified as a regulator of the MAPK pathway (5, 20). Wang et al (5) have shown that PEBP4 interacts with Raf-1 and MEK1 and attenuates activation of MEK1. This role could lead to an inhibition of apoptosis induced by TNFα or TRAIL (20). Interestingly, it was demonstrated that PEBP4 was concentrated in the perinucleus, localized in lysosomes, and translocated to plasma membranes in response to TNFα (5). It has shown that this membrane localization signal is encompassed within the N-terminal 75 amino acids. In most scenarios, the Raf/MEK/ERK pathway promotes mitogenesis and is frequently activated in cancers, such as those with Ras mutation or amplification of Her2 and MET. If PEBP4 inhibits ERK activation by competitively interfering with the interaction between Raf and MEK, in a way similar to PEBP1/RKIP, we could speculate that it is a tumor suppressor. However, many recent studies support that PEBP4 acts as a promoting factor for cancer cell growth. First, in clinical specimens, PEBP4 is found increased in several types of cancer and the degree of expression correlates to pathological grade, progression and metastasis (21–24, 30). Second, transfection of interference RNA for PEBP4 into cancer cells inhibits cell proliferation, migration and invasion or induces apoptosis, whereas overexpression of this protein causes opposite changes (17, 19, 23–26). Third, the expression levels of PEBP4 correlates with the sensitivity of cancer cells to chemotherapy or radiotherapy in which the upregulation of PEBP4 confers resistance to these therapies while its downregulation enhances the sensitivity (15, 26, 31, 32). These properties of PEBP4 are consistent with a role in promoting tumor transformation and progression, but are inconsistent with the inhibitory effect on the activation of the Raf-1/MEK/ERK pathway. Two possibilities may explain this paradox. First, the precise role of PEBP4 in ERK activation depends on its level of expression. In keeping with this, Garcia et al have shown that low expression of PEBP4 enhances but high expression suppresses ERK activation (16). A second possibility is that PEBP4 regulates activity of some other molecules for cell growth/survival in addition to ERK/JNK. Indeed, several reports have shown that PEBP4 acts as a scaffold for Src, Akt, or estrogen receptor alpha (ERα), leading to increased activation of Akt or ERα (17–19, 24, 33). In keeping with these observations, our present study demonstrates that silencing PEBP4 leads to an inhibition of Akt phosphorylation at S473.
Our study using epitope tagging of PEBP4 yielded different results. Tagging epitopes (Myc and GFP) to the N-terminus altered subcellular localization and abolished secretion. We believe that tagging to the C-terminus does not disturb structure of native PEBP4. Additionally, although we did not refine specific subcellular localization, its punctate distribution of the protein C-terminal tagged with GFP was similar to that described previously at the endoplasmic reticulum/Golgi apparatus and lysosomes (5, 27, 34). Our findings that overexpression of only N-terminal tagged PEBP4 displayed an inhibitory effect on ERK activation but PEBP4 shRNA failed to affect ERK activation by EGF are different from previously published reports (5, 16, 20). The underlying reason is not clear and worth further investigating. It will be necessary to ascertain whether these differences result from the epitope tagging in our study and others.
The striking findings in the present study are that PEBP4 is a secreted protein and that two elements located at both the N-terminus and C-terminus contribute to secretion equally. Although PEBP4 contains a signal peptide, the signal strength might not be sufficient to allow transportation of the protein for secretion, thus requiring the C-terminal sequence. This is in line with the fact that not all signal peptides exhibit equal efficiencies to target proteins for secretion (35). Those signal sequences that are unable to direct protein secretion when engineered to other proteins may need to cooperate with another sequence from the original protein. To our knowledge, however, this possibility has never been examined. This would require engineering a fusion reporter that is inserted between the signal peptide and an additional carboxyl sequence of an original protein or the C-terminal conserved domain of PEBP4.
The weak strength of the signal peptide sequence of PEBP4 also suggests that it may possess additional intracellular functions. It is not unusual for intracellular proteins to execute functions with both secreted and cytoplasmic characteristics (36). For example, thioredoxin, a cytoplasmic protein involved in intracellular redox balance, can be secreted from T cells as an inflammatory cytokine in response to oxidative stress (37). A second protein is high mobility group box 1 (HMGB1), a nuclear protein that binds to chromatin and regulates gene expression. HMGB1 can be secreted from monocytes and macrophages as a pro-inflammatory cytokine upon induction by lipopolysaccharide and lysophosphatidylcholine (38). Results from the present study and others as noted above indicate that PEBP4 may have both extracellular and intracellular functions. Regarding the extracellular function, a recent paper determined that PEBP4 purified from swine seminal plasma stimulates motility of sperms (27). We attempted to examine if recombinant PEBP4 secreted from HEK293T cells can stimulate migration of cancer cells, but the result was negative. Whether the extracellular and intracellular functions of PEBP4 are independent, exclusive, or associated events is an interesting topic for future research.
5. Conclusion
Our present study suggests that PEBP4 is a multiple function protein. First, it is a secreted and glycosylated protein. Interestingly, in addition to the intact signal peptide, the C-terminal conserved domain is necessary for secretion, but the N-glycosylation is dispensable. Second, our data shows that PEBP4 does not participate in ERK activation by EGF. Third, PEBP4 appears to be required for Akt activation. It will be interesting to determine the extent to which secretion of PEBP4 is associated with the role in regulation of Akt. Therefore, we will further characterize the function of the secreted form of PEBP4 and correlate it to the role in Akt signaling and regulation of cancer cell growth and survival and even normal cellular events.
Supplementary Material
Highlights.
PEBP4 is a secreted and glycosylated protein
The carboxyterminal conserved sequence contributes to the secretion
PEBP4 does not participate in ERK activation by EGF,
PEBP4 is required for Akt activation
Acknowledgments
This work was supported in part by the National Institutes of Health (Grant R21EY024388) and by the National Nature Science Foundation of China (Grants 81572753, 81272926). H. He, H. Lin, and Y. Ying were supported by scholarships for postgraduate study from Nanchang University. S. Jiang was supported by scholarship from China Scholar Council. We were thankful to Dr. Water Kolch (University College Dublin) for providing expression plasmids encoding human PEBP4. J. Zaia and C. Shao were supported by NIH grant P41GM104603.
Abbreviations
- Act
protein kinase B
- DMEM
Dulbecco’s modified Eagle’s medium
- EGF
epidermal growth factor
- ERK
extracellular signal regulated kinase
- FBS
fetal bovine serum
- GFP
green fluorescent protein
- Her2
human epidermal growth factor receptor 2
- HMGB1
high mobility group box 1
- JNK
c-Jun N-terminal kinase
- MEK1
mitogen and extracellular signal activated kinase (mitogen activated kinase kinase)
- MET
hepatocyte growth factor receptor
- NFκB
nuclear factor kappa B
- PEBP
phosphatidylethanolamine binding protein
- Raf-1
proto-oncogenic protein kinase upstream of MEK1
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
The authors declare no conflicts of interest.
References
- 1.Al-Mulla F, Bitar MS, Taqi Z, Yeung KC. RKIP: much more than Raf kinase inhibitory protein. J Cell Physiol. 2013;228(8):1688–1702. doi: 10.1002/jcp.24335. PubMed PMID: 23359513. [DOI] [PubMed] [Google Scholar]
- 2.Frayne J, Ingram C, Love S, Hall L. Localisation of phosphatidylethanolamine-binding protein in the brain and other tissues of the rat. Cell Tissue Res. 1999;298(3):415–423. doi: 10.1007/s004419900113. PubMed PMID: 10639732. [DOI] [PubMed] [Google Scholar]
- 3.Katada E, Mitake S, Matsukawa N, Otsuka Y, Tsugu Y, Fujimori O, Ojika K. Distribution of hippocampal cholinergic neurostimulating peptide (HCNP)-like immunoreactivity in organs and tissues of young Wistar rats. Histochem Cell Biol. 1996;105(1):43–51. doi: 10.1007/BF01450877. PubMed PMID: 8824905. [DOI] [PubMed] [Google Scholar]
- 4.Hickox DM, Gibbs G, Morrison JR, Sebire K, Edgar K, Keah HH, Alter K, Loveland KL, Hearn MT, de Kretser DM, O'Bryan MK. Identification of a novel testis-specific member of the phosphatidylethanolamine binding protein family, pebp-2. Biol Reprod. 2002;67(3):917–927. doi: 10.1095/biolreprod.101.001446. PubMed PMID: 12193403. [DOI] [PubMed] [Google Scholar]
- 5.Wang X, Li N, Liu B, Sun H, Chen T, Li H, Qiu J, Zhang L, Wan T, Cao X. A novel human phosphatidylethanolamine-binding protein resists tumor necrosis factor alpha-induced apoptosis by inhibiting mitogen-activated protein kinase pathway activation and phosphatidylethanolamine externalization. The Journal of biological chemistry. 2004;279(44):45855–45864. doi: 10.1074/jbc.M405147200. Epub 2004/08/11. PubMed PMID: 15302887. [DOI] [PubMed] [Google Scholar]
- 6.Moore C, Perry AC, Love S, Hall L. Sequence analysis and immunolocalisation of phosphatidylethanolamine binding protein (PBP) in human brain tissue. Brain Res Mol Brain Res. 1996;37(1–2):74–78. doi: 10.1016/0169-328x(95)00285-z. PubMed PMID: 8738137. [DOI] [PubMed] [Google Scholar]
- 7.Frayne J, McMillen A, Love S, Hall L. Expression of phosphatidylethanolamine-binding protein in the male reproductive tract: immunolocalisation and expression in prepubertal and adult rat testes and epididymides. Mol Reprod Dev. 1998;49(4):454–460. doi: 10.1002/(SICI)1098-2795(199804)49:4<454::AID-MRD13>3.0.CO;2-U. doi: 10.1002/(SICI)1098-2795(199804)49:4<454::AID-MRD13>3.0.CO;2-U. PubMed PMID: 9508097. [DOI] [PubMed] [Google Scholar]
- 8.Perry AC, Hall L, Bell AE, Jones R. Sequence analysis of a mammalian phospholipid-binding protein from testis and epididymis and its distribution between spermatozoa and extracellular secretions. Biochem J. 1994;301(Pt 1):235–242. doi: 10.1042/bj3010235. PubMed PMID: 8037677; PMCID: PMC1137167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ojika K, Kojima S, Ueki Y, Fukushima N, Hayashi K, Yamamoto M. Purification and structural analysis of hippocampal cholinergic neurostimulating peptide. Brain research. 1992;572(1–2):164–171. doi: 10.1016/0006-8993(92)90465-l. Epub 1992/02/14. PubMed PMID: 1611510. [DOI] [PubMed] [Google Scholar]
- 10.Ojika K, Mitake S, Tohdoh N, Appel SH, Otsuka Y, Katada E, Matsukawa N. Hippocampal cholinergic neurostimulating peptides (HCNP) Progress in neurobiology. 2000;60(1):37–83. doi: 10.1016/s0301-0082(99)00021-0. Epub 2000/01/06. PubMed PMID: 10622376. [DOI] [PubMed] [Google Scholar]
- 11.Hengst U, Albrecht H, Hess D, Monard D. The phosphatidylethanolamine-binding protein is the prototype of a novel family of serine protease inhibitors. The Journal of biological chemistry. 2001;276(1):535–540. doi: 10.1074/jbc.M002524200. Epub 2000/10/18. PubMed PMID: 11034991. [DOI] [PubMed] [Google Scholar]
- 12.Yeung K, Seitz T, Li S, Janosch P, McFerran B, Kaiser C, Fee F, Katsanakis KD, Rose DW, Mischak H, Sedivy JM, Kolch W. Suppression of Raf-1 kinase activity and MAP kinase signalling by RKIP. Nature. 1999;401(6749):173–177. doi: 10.1038/43686. Epub 1999/09/18. PubMed PMID: 10490027. [DOI] [PubMed] [Google Scholar]
- 13.Bernier I, Jolles P. Purification and characterization of a basic 23 kDa cytosolic protein from bovine brain. Biochim Biophys Acta. 1984;790(2):174–181. doi: 10.1016/0167-4838(84)90221-8. PubMed PMID: 6435678. [DOI] [PubMed] [Google Scholar]
- 14.Yeung K, Janosch P, McFerran B, Rose DW, Mischak H, Sedivy JM, Kolch W. Mechanism of suppression of the Raf/MEK/extracellular signal-regulated kinase pathway by the raf kinase inhibitor protein. Molecular and cellular biology. 2000;20(9):3079–3085. doi: 10.1128/mcb.20.9.3079-3085.2000. Epub 2000/04/11. PubMed PMID: 10757792; PMCID: Pmc85596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang K, Jiang Y, Zheng W, Liu Z, Li H, Lou J, Gu M, Wang X. Silencing of human phosphatidylethanolamine-binding protein 4 enhances rituximab-induced death and chemosensitization in B-cell lymphoma. PLoS One. 2013;8(2):e56829. doi: 10.1371/journal.pone.0056829. PubMed PMID: 23451095; PMCID: PMC3581549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Garcia R, Grindlay J, Rath O, Fee F, Kolch W. Regulation of human myoblast differentiation by PEBP4. EMBO reports. 2009;10(3):278–284. doi: 10.1038/embor.2009.4. Epub 2009/02/07. PubMed PMID: 19197339; PMCID: Pmc2658556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li H, Huang F, Fan L, Jiang Y, Wang X, Li J, Wang Q, Pan H, Sun J, Cao X, Wang X. Phosphatidylethanolamine-binding protein 4 is associated with breast cancer metastasis through Src-mediated Akt tyrosine phosphorylation. Oncogene. 2014;33(37):4589–4598. doi: 10.1038/onc.2013.408. Epub 2013/11/28. PubMed PMID: 24276246. [DOI] [PubMed] [Google Scholar]
- 18.Qiu J, Yang G, Lin A, Shen Z, Wang D, Ding L. Human phosphatidylethanolamine-binding protein 4 promoted the radioresistance of human rectal cancer by activating Akt in an ROS-dependent way. PLoS One. 2014;9(3):e90062. doi: 10.1371/journal.pone.0090062. PubMed PMID: 24594691; PMCID: PMC3940727. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 19.Yu G, Huang B, Chen G, Mi Y. Phosphatidylethanolamine-binding protein 4 promotes lung cancer cells proliferation and invasion via PI3K/Akt/mTOR axis. J Thorac Dis. 2015;7(10):1806–1816. doi: 10.3978/j.issn.2072-1439.2015.10.17. PubMed PMID: 26623104; PMCID: PMC4635298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li P, Wang X, Li N, Kong H, Guo Z, Liu S, Cao X. Anti-apoptotic hPEBP4 silencing promotes TRAIL-induced apoptosis of human ovarian cancer cells by activating ERK and JNK pathways. Int J Mol Med. 2006;18(3):505–510. Epub 2006/07/26. PubMed PMID: 16865237. [PubMed] [Google Scholar]
- 21.Liu H, Kong Q, Li B, He Y, Li P, Jia B. Expression of PEBP4 protein correlates with the invasion and metastasis of colorectal cancer. Tumour Biol. 2012;33(1):267–273. doi: 10.1007/s13277-011-0279-x. Epub 2011/11/30. PubMed PMID: 22125029. [DOI] [PubMed] [Google Scholar]
- 22.Yu GP, Chen GQ, Wu S, Shen K, Ji Y. The expression of PEBP4 protein in lung squamous cell carcinoma. Tumour Biol. 2011;32(6):1257–1263. doi: 10.1007/s13277-011-0230-1. Epub 2011/09/03. PubMed PMID: 21887552. [DOI] [PubMed] [Google Scholar]
- 23.Yu GP, Huang B, Chen GQ, Wu S, Ji Y, Shen ZY. PEBP4 gene expression and its significance in invasion and metastasis of non-small cell lung cancer. Tumour Biol. 2012;33(1):223–228. doi: 10.1007/s13277-011-0265-3. Epub 2011/11/15. PubMed PMID: 22076923. [DOI] [PubMed] [Google Scholar]
- 24.Zhang D, Dai Y, Cai Y, Suo T, Liu H, Wang Y, Cheng Z, Liu H. PEBP4 promoted the growth and migration of cancer cells in pancreatic ductal adenocarcinoma. Tumour Biol. 2015 doi: 10.1007/s13277-015-3906-0. PubMed PMID: 26311050. [DOI] [PubMed] [Google Scholar]
- 25.Yu G, Shen Z, Chen G, Teng X, Hu Y, Huang B. PEBP4 enhanced HCC827 cell proliferation and invasion ability and inhibited apoptosis. Tumour Biol. 2013;34(1):91–98. doi: 10.1007/s13277-012-0514-0. Epub 2012/09/18. PubMed PMID: 22983920. [DOI] [PubMed] [Google Scholar]
- 26.Qiu J, Yang G, Shen Z, Xie Y, Wang L. hPEBP4 as a predictive marker for the pathological response of rectal cancer to preoperative radiotherapy. Int J Colorectal Dis. 2013;28(2):241–246. doi: 10.1007/s00384-012-1534-3. Epub 2012/07/18. PubMed PMID: 22801881. [DOI] [PubMed] [Google Scholar]
- 27.An LP, Maeda T, Sakaue T, Takeuchi K, Yamane T, Du PG, Ohkubo I, Ogita H. Purification, molecular cloning and functional characterization of swine phosphatidylethanolamine-binding protein 4 from seminal plasma. Biochem Biophys Res Commun. 2012;423(4):690–696. doi: 10.1016/j.bbrc.2012.06.016. Epub 2012/06/16. PubMed PMID: 22699120. [DOI] [PubMed] [Google Scholar]
- 28.Luo L, Huang W, Tao R, Hu N, Xiao ZX, Luo Z. ATM and LKB1 dependent activation of AMPK sensitizes cancer cells to etoposide-induced apoptosis. Cancer Lett. 328(1):114–119. doi: 10.1016/j.canlet.2012.08.034. doi: S0304-3835(12)00535-6 [pii] 10.1016/j.canlet.2012.08.034. PubMed PMID: 22960274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Staples GO, Bowman MJ, Costello CE, Hitchcock AM, Lau JM, Leymarie N, Miller C, Naimy H, Shi X, Zaia J. A chip-based amide-HILIC LC/MS platform for glycosaminoglycan glycomics profiling. Proteomics. 2009;9(3):686–695. doi: 10.1002/pmic.200701008. PubMed PMID: 19137549; PMCID: 2771417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang X, Li N, Li H, Liu B, Qiu J, Chen T, Cao X. Silencing of human phosphatidylethanolamine-binding protein 4 sensitizes breast cancer cells to tumor necrosis factor-alpha-induced apoptosis and cell growth arrest. Clin Cancer Res. 2005;11(20):7545–7553. doi: 10.1158/1078-0432.CCR-05-0879. PubMed PMID: 16243830. [DOI] [PubMed] [Google Scholar]
- 31.Zhao Z, Zhang L, Yao Q, Tao Z. miR-15b regulates cisplatin resistance and metastasis by targeting PEBP4 in human lung adenocarcinoma cells. Cancer Gene Ther. 2015;22(3):108–114. doi: 10.1038/cgt.2014.73. PubMed PMID: 25721211. [DOI] [PubMed] [Google Scholar]
- 32.Yu G, Zhong N, Chen G, Huang B, Wu S. Downregulation of PEBP4, a target of miR-34a, sensitizes drug-resistant lung cancer cells. Tumour Biol. 2014;35(10):10341–10349. doi: 10.1007/s13277-014-2284-3. Epub 2014/07/21. PubMed PMID: 25038915. [DOI] [PubMed] [Google Scholar]
- 33.Liu H, Qiu J, Li N, Chen T, Cao X. Human phosphatidylethanolamine-binding protein 4 promotes transactivation of estrogen receptor alpha (ERalpha) in human cancer cells by inhibiting proteasome-dependent ERalpha degradation via association with Src. The Journal of biological chemistry. 2010;285(29):21934–21942. doi: 10.1074/jbc.M110.109876. Epub 2010/05/13. PubMed PMID: 20460377; PMCID: Pmc2903382. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 34.Zhang Y, Wang X, Xiang Z, Li H, Qiu J, Sun Q, Wan T, Li N, Cao X, Wang J. Promotion of cellular migration and apoptosis resistance by a mouse eye-specific phosphatidylethanolamine-binding protein. Int J Mol Med. 2007;19(1):55–63. Epub 2006/12/05. PubMed PMID: 17143548. [PubMed] [Google Scholar]
- 35.Kober L, Zehe C, Bode J. Optimized signal peptides for the development of high expressing CHO cell lines. Biotechnol Bioeng. 2013;110(4):1164–1173. doi: 10.1002/bit.24776. PubMed PMID: 23124363. [DOI] [PubMed] [Google Scholar]
- 36.Nickel W, Seedorf M. Unconventional mechanisms of protein transport to the cell surface of eukaryotic cells. Annu Rev Cell Dev Biol. 2008;24:287–308. doi: 10.1146/annurev.cellbio.24.110707.175320. PubMed PMID: 18590485. [DOI] [PubMed] [Google Scholar]
- 37.Kondo N, Ishii Y, Kwon YW, Tanito M, Horita H, Nishinaka Y, Nakamura H, Yodoi J. Redox-sensing release of human thioredoxin from T lymphocytes with negative feedback loops. Journal of immunology (Baltimore, Md : 1950) 2004;172(1):442–448. doi: 10.4049/jimmunol.172.1.442. Epub 2003/12/23. PubMed PMID: 14688353. [DOI] [PubMed] [Google Scholar]
- 38.Gardella S, Andrei C, Ferrera D, Lotti LV, Torrisi MR, Bianchi ME, Rubartelli A. The nuclear protein HMGB1 is secreted by monocytes via a non-classical, vesicle-mediated secretory pathway. EMBO reports. 2002;3(10):995–1001. doi: 10.1093/embo-reports/kvf198. Epub 2002/09/17. PubMed PMID: 12231511; PMCID: Pmc1307617. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.