SUMMARY
Regenerative medicine is predicated on understanding the mechanisms regulating development and applying these conditions to direct stem cell fate. Embryogenesis is guided by cell-cell and cell-matrix interactions, but it is unclear how these physical cues influence stem cells in culture. We used human embryonic stem cells (hESCs) to examine whether mechanical features of the extracellular microenvironment could differentially modulate mesoderm specification. We found that, on a hydrogel-based compliant matrix, hESCs accumulate β-catenin at cell-cell adhesions and show enhanced Wnt-dependent mesoderm differentiation. Mechanistically, Src-driven ubiquitination of E-cadherin by Cbl-like ubiquitin ligase releases P120-catenin to facilitate transcriptional activity of β-catenin, which initiates and reinforces mesoderm differentiation. By contrast, on a stiff hydrogel matrix, hESCs show elevated integrin-dependent GSK3 and Src activity that promotes β-catenin degradation and inhibits differentiation. Thus, we found that mechanical features of the microenvironmental matrix influence tissue-specific differentiation of hESCs by altering the cellular response to morphogens.
Graphical abstract

INTRODUCTION
Regenerative medicine is predicated on understanding the molecular basis of tissue-specific differentiation and then judiciously applying the appropriate soluble and physical cues to optimize stem cell fate. Although much is known about the role of morphogens and transcription factors in stem cell fate specification, less is known about how physical cues modulate stem cell behavior. Thus, while it is appreciated that mechanical cues can dramatically affect cell fate (McBeath et al., 2004), little is known about how extracellular forces are translated into intracellular signals to direct tissue-specific differentiation and development. Cells undergoing the coordinated processes involved in embryonic development require dynamic and reciprocal changes in cytoskeletal organization, which affect mechanical inputs via changes in tension at cell adhesions between cells and between cells and their extracellular matrix (ECM). Indeed, many developmental processes require mechanical signaling at cell-cell adhesions (Simôes et al., 2014; Weber et al., 2012) and tension at the cell-ECM interface (Crawford et al., 2003; Pines et al., 2012). Consistently, knockout of E-cadherin in cell-cell adhesions is embryonic lethal during epiblast formation before implantation (Riethmacher et al., 1995), while loss of integrin β1-containing cell-ECM adhesions causes embryos to degenerate soon after implantation and inhibits mesoderm formation (Stephens et al., 1995). These studies imply that coordination of cell adhesions is likely a key mechanism whereby mechanics regulate tissue-specific development, though the molecular mechanisms that link cell adhesion to mechanics to direct development remain unclear. A more thorough understanding of how these signals guide developmental processes will inform the generation of differentiation strategies that faithfully recapitulate the complexity of mature tissues and organs.
Tissue architecture during morphogenesis is controlled in part by the mechanical properties of the tissue substrate, and this effect and the underlying mechanisms can be studied using engineered matrices of tunable stiffness (Paszek et al., 2005; Yeung et al., 2005). Functionalized polyacrylamide substrates (PA gels) have revealed a role for ECM stiffness in directing the neurogenic, myogenic, or osteogenic fate of mesenchymal stem cells (Engler et al., 2006; Gobaa et al., 2015), and they have demonstrated that substrate stiffness affects the self-renewal potential of pluripotent stem cells (Chowdhury et al., 2010). Our studies using optimized functionalized PA gels showed that human embryonic stem cells (hESCs) exhibit striking changes in actin organization in response to ECM stiffness, consistent with differences in cell-cell and cell-ECM adhesions (Lakins et al., 2012; Przybyla et al., 2016), and differential cytoskeletal organization within hESC colonies has been linked to changes in differentiation potential (Rosowski et al., 2015). The tissue-level architecture of embryoid bodies (EBs) made from pluripotent stem cells can also influence differentiation potential (Bauwens et al., 2008), so the ability to control tissue architecture via altering substrate stiffness could avoid the heterogeneity frequently encountered using EBs while providing a tunable parameter for optimization of pluripotent stem cell differentiation.
The embryonic process of mesoderm specification during gastrulation requires mechanically driven coordinated cell rearrangements as cells undergo an epithelial-mesenchymal transition (EMT) to migrate into the primitive streak. Mesoderm specification requires many spatiotemporally regulated signals, including Wnts, which are expressed at the primitive streak (McMahon, 1992) and are required for both mesoderm differentiation and EMT (Howard et al., 2011; Lindsley et al., 2006). The Drosophila homolog of β-catenin, a downstream effector of Wnt, is a mechanoregulator of transcription during gastrulation (Farge, 2003) and β-catenin is activated by mechanical loading during bone development (Hens et al., 2005; Kang and Robling, 2015), indicating its mechanosensitivity (Ou and Weaver, 2015). Accordingly, the association of β-catenin with cell-cell adhesions and its regulation by cell-ECM signaling suggests that it may transduce adhesion-dependent force cues within the cell as a critically conserved node in mechanotransduction during development. The involvement of Wnt/β-catenin signaling in mediating mechanosignaling during mesoderm differentiation may, therefore, represent a system in which to study how changes in cytoskeletal organization affect cell-cell and cell-matrix contacts to influence response to differentiation cues and regulate developmental fate. Protocols exist to induce mesoderm specification of hESCs (Evseenko et al., 2010), and geometric confinement of pluripotent stem cells was found to trigger differentiation into regionally organized ectoderm and mesendoderm (Warmflash et al., 2014). Here we leverage our ability to grow and differentiate hESCs on PA gels to explore how mechanical signals can integrate with soluble signals to regulate embryonic specification by differentially modulating cell-cell versus cell-ECM adhesion.
RESULTS
Substrate Compliance Does Not Affect hESC Self-Renewal
We first examined the impact of ECM stiffness on hESC phenotype using gels functionalized with reconstituted basement membrane (rBM) that spanned a broad range of stiffness using an approach we previously described (Lakins et al., 2012; Przybyla et al., 2016). Briefly, hESCs were plated at high cell densities using 3D printed guides to rapidly form adherent, monolayered circular colonies with a diameter similar to the size of gastrulation-stage embryos (3 mm).This approach reduced the time to form coherent colonies from 8 to 10 days on the softest gels (Lakins et al., 2012) and normalized it to 3 days across the stiffness range. We found that hESCs remained viable, but they exhibited differences in morphology in response to stiffnesses that ranged from a soft substrate analogous to the stiffness of the gastrulation-stage embryo (E = 400 Pa) (Majkut et al., 2013) to much stiffer gels (E = 60 kPa) on which hESCs morphologically recapitulated hESCs cultured on tissue culture plastic (TCP; E = ~3 GPa, Hollinger, 2011). On the softest gels, hESCs assembled a columnar epithelium with extensive apical circumferential actin networks and pronounced cell-cell associations, while they spread appreciably and formed abundant basal actin stress fibers that emanated from cell-ECM contacts in response to the stiffest gel (Figure 1A). Although we observed a modest but significant enhancement of cell viability in the hESCs plated on the softest gels (Figure S1A), their proliferation was similar regardless of substrate stiffness (Figure 1B) and their rate of cell growth was equivalent (Figure S1B).
Figure 1. Substrate Stiffness Does Not Affect hESC Self-Renewal.
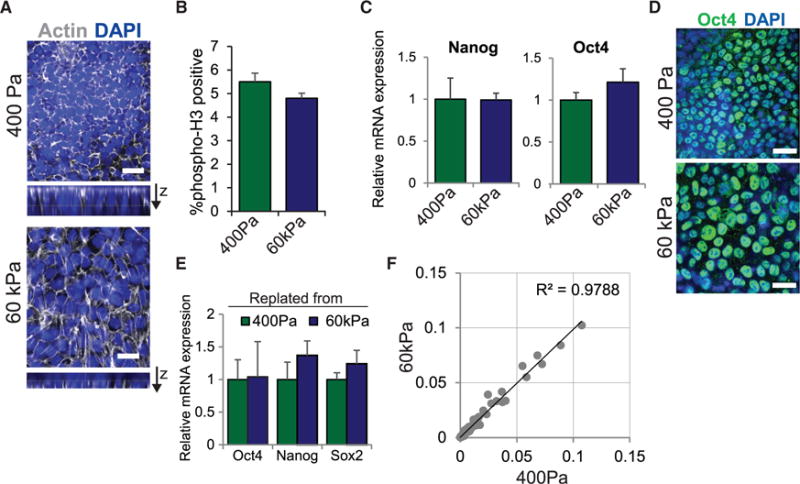
(A) Actin staining in hESCs on soft versus stiff substrates is shown.
(B) Percentage of cells positive for phosphorylated Histone H3 in hESCs on soft or stiff substrates is shown.
(C) mRNA expression of self-renewal markers in hESCs grown on soft or stiff substrates for 5 days is shown.
(D) Immunofluorescent staining in hESCs grown in the indicated conditions for 5 days is shown.
(E) mRNA expression of self-renewal markers in hESCs replated from soft or stiff gels onto Matrigel-coated tissue culture plates cultured for two passages is shown.
(F) Panel analysis data of mRNA expression levels of 77 genes involved in self-renewal and lineage specification compared in hESCs grown on soft or stiff substrates for 5 days. Linear regression analysis yields an R2 value of 0.9788.
Data are represented as mean of at least three independent experiments ± SD. All scale bars, 20 μm. See also Figure S1.
Importantly, 5 days after culture, gross measures of pluripotency, including mRNA levels of the self-renewal markers Nanog and Oct4 (Figures 1C and S1C) and Oct4 protein (Figure 1D), remained unchanged. Moreover, hESCs re-plated onto fibroblast feeders from either the soft substrate or the stiff substrate adhered, spread, and retained pluripotency and viability for multiple passages (Figures 1E and S1D). Consistently, analysis of changes in genes involved in hESC self-renewal and lineage commitment, including ectoderm, mesoderm, and endoderm markers, were similar in cells grown on soft and stiff gels (Figures 1F and S1E). These findings indicate that, despite a marked effect on cell morphology, ECM stiffness has little to no direct effect on hESC differentiation or pluripotency.
Compliant Substrates Enhance Mesoderm Differentiation Potential
Although substrate compliance did not directly affect the differentiation of hESCs, the striking similarity in cytoarchitecture between hESCs on 400 Pa substrates to cells in the pregastrulation epiblast prompted us to ask whether hESCs would differentiate differently in response to exogenous morphogens associated with gastrulation. Directed differentiation protocols have been amended to include strategies that tune substrate stiffness to physiological levels to improve the differentiation potential of human mesenchymal stem cells and adult neural stem cells (Gobaa et al., 2015; Keung et al., 2011) and to enhance the propagation potential of adult satellite stem cells (Gilbert et al., 2010). Accordingly, we asked if the differentiation behavior of hESCs could be modified by culture on a substrate that approximated the stiffness of the gastrulation-stage embryo (400 Pa). Because mesoderm is specified early during gastrulation and represents a motile population that is responsive to mechanical cues, we applied a differentiation protocol designed to generate multipotent mesoderm progenitors (Evseenko et al., 2010) to hESCs plated as monolayers on rBM (Figure 2A). On day 3.5 after the induction of mesoderm differentiation, we noted a significant increase in the percentage of cells expressing NCAM, a marker of mesoderm progenitor differentiation, in the hESCs on soft substrates as compared to those on the stiffer substrates (Figure 2B). Transcriptome-wide RNA-sequencing (RNA-seq) analysis confirmed that the increase in NCAM expression was accompanied by the upregulation of several mesoderm markers as well as genes reflecting the associated EMT (Figure 2C; Table S1). When we grouped the differential gene expression into gene ontology categories, we clearly observed a significant upregulation of terms involving mesoderm progenitor differentiation, morphogenesis, and development (Figure S2A), but not endoderm or ectoderm differentiation (Figure S2B).
Figure 2. The Induction of Mesoderm Differentiation in hESCs Is Enhanced on Compliant Matrices.
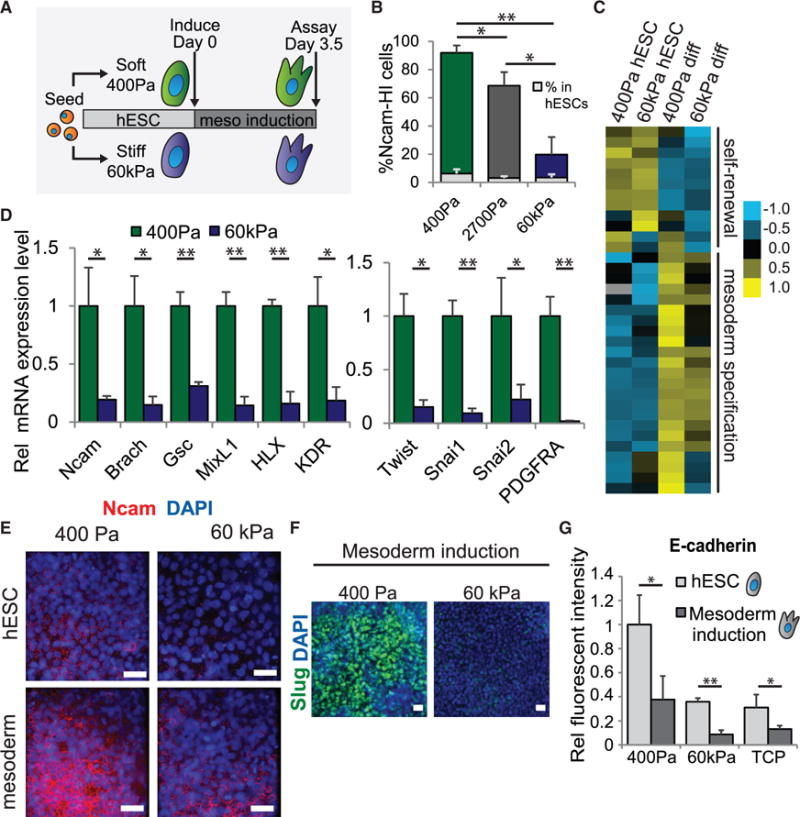
(A) Time course of differentiation for hESCs on soft and stiff hydrogels is shown.
(B) Percentage of NCAM-high cells as analyzed by flow cytometry in cells differentiated on gels of the indicated stiffness. Light gray bars indicate percentages in uninduced cells on the corresponding stiffness. Statistics refer to the bars depicting differentiated cells.
(C) Heatmap showing a subset of genes from RNA-seq analysis that are significantly upregulated and correspond to gene ontology designations for self-renewal (top) or mesoderm differentiation (bottom). Table S1 includes associated reads per kilobase per million mapped reads (RPKM) values.
(D) mRNA expression of genes involved in mesoderm differentiation (left) and EMT (right) in hESCs differentiated on soft or stiff gels is shown.
(E) Immunofluorescent staining in cells grown in the indicated conditions is shown.
(F) Immunofluorescent staining in cells grown in the indicated conditions is shown.
(G) Relative E-cadherin protein expression levels as analyzed by flow cytometry in cells grown in the indicated conditions.
Data are represented as mean of at least three independent experiments ± SD (**p < 0.001 and *p < 0.05). TCP, tissue culture plastic. All scale bars, 20 μm. See also Figure S2, Table S1, and Movie S1.
We validated the downregulation of self-renewal markers (Figure S2C) and the upregulation of a panel of mesoderm and EMT markers at the mRNA (Figure 2D) and protein levels (Figures 2E and 2F) in hESCs differentiated on soft, embryo-like as compared to stiff substrates. We also demonstrated that our observation was not confined to only one hESC line (Figures S2D and S2E). We found that E-cadherin levels dropped significantly following differentiation on hydrogels or on TCP (Figure 2G), consistent with the loss of E-cadherin expression observed upon initiation of EMT (Huber et al., 1996; Takeichi, 1995). We found no evidence for differences in cell proliferation, as indicated by phospho-HistoneH3 staining in differentiating hESCs on soft versus stiff matrices (Figure S2F). Moreover, prolonged incubation of morphogen-differentiated cells did not enhance mesoderm or EMT marker induction in the hESCs on the stiff matrices, thereby confirming the specificity of the enhanced differentiation induced on the compliant ECMs (Figure S2G). Importantly, the mesoderm progenitor cells generated on soft substrates could be further differentiated to generate mesenchymal stromal cells (Figure S2H) and cardiomyocytes (Figures S2I and S2J; Movie S1), emphasizing the functionality of the resulting mesoderm progenitors, as has been demonstrated previously (Evseenko et al., 2010). These findings not only reveal that recreating the substrate compliance of the developing human embryo significantly enhances the ability of hESCs to differentiate toward mesoderm progenitors, they argue that substrate stiffness per se is insufficient and must synergize with developmental morphogens. Likewise, developmental morphogens are similarly insufficient and also must synergize with substrate stiffness, since lineages such as cardiomyocytes are clearly suppressed by stiff substrates, particularly with respect to their contractility dynamics (Majkut et al., 2014).
Wnt Signaling Is a Necessary Component of Compliance-Mediated Mesoderm Differentiation
We next explored how the tissue-level organization dictated by substrate compliance potentiated mesoderm progenitor differentiation. Noting that the Wnt family of secreted proteins is involved in both mesoderm differentiation (Lindsley et al., 2006) and EMT (Howard et al., 2011), we checked whether secreted Wnts were upregulated upon mesoderm induction of hESCs to potentiate autocrine signaling (Figure 3A). We quantified higher levels of Wnt mRNAs in cells differentiated on soft embryo-like versus stiff gels (Figures 3B and S3A), corresponding to an upregulation in protein expression of Wnt3a (Figure 3C), an essential mesoderm-inducing morphogen (Takada et al., 1994). Evidence of Wnt functionality was indicated using a 7×TCF-GFP reporter hESC line to assess transcriptional activity downstream of Wnt signaling. We found higher Wnt target transcriptional activity in hESCs differentiated on soft versus stiff gels, and we noted that addition of the Wnt inhibitor IWP2 repressed this activity (Figure 3D).
Figure 3. Cell-Secreted Wnts Are Critically Involved in Mechanically Mediated Mesoderm Differentiation.
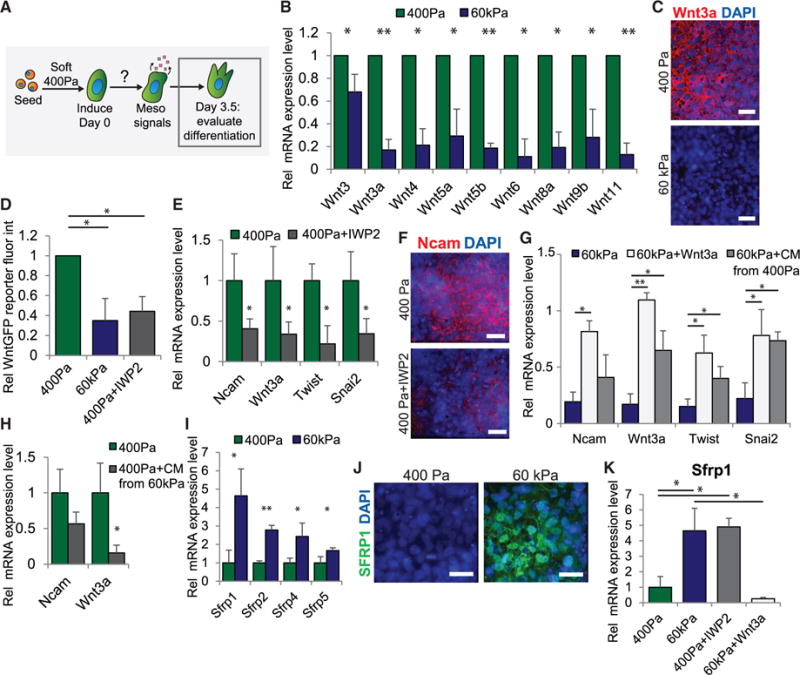
(A) Diagram depicting the mechanistic question addressed in this figure. All figure panels are from data obtained on day 3.5 at the end of mesoderm induction.
(B) mRNA expression of Wnt genes in cells differentiated on soft or stiff gels is shown.
(C) Immunofluorescent staining in cells differentiated on soft or stiff gels is shown.
(D) Relative average fluorescent intensity in 7×TCF-GFP Wnt activity reporter hESCs differentiated in the indicated conditions is shown.
(E) mRNA expression of mesoderm and EMT markers in cells differentiated in the indicated conditions. IWP2 is a Wnt inhibitor.
(F) Immunofluorescent staining for NCAM for cells differentiated in the indicated conditions is shown.
(G) mRNA expression in cells differentiated in the indicated conditions. All bars are set relative to expression levels in cells differentiated on 400-Pa gels (= 1). CM, conditioned media.
(H) mRNA expression in cells differentiated in the indicated conditions is shown. CM, conditioned media.
(I) mRNA expression of SFRP family proteins in cells differentiated on soft or stiff gels is shown.
(J) Immunofluorescent staining in cells differentiated in the indicated conditions is shown.
(K) mRNA expression of Sfrp1 in cells differentiated in the indicated conditions.
Data are represented as mean of at least three independent experiments ± SD (**p < 0.001 and *p < 0.05). All scale bars, 20 μm. See also Figure S3.
To directly implicate Wnt signaling in mesoderm differentiation, we assessed the ability of IWP2 to inhibit mesoderm differentiation. Consistently, we noted that Wnt inhibition caused a decrease in mRNA and protein mesoderm marker induction (Figures 3E and 3F). Conversely, adding recombinant Wnt3a to cells on stiff substrates enhanced their ability to differentiate toward mesoderm (Figure 3G). Moreover, consistent with the notion that functional Wnts are secreted by cells on soft substrates, when we added conditioned media from cells grown on soft gels to cells on stiff gels, we noted a significant enhancement of their differentiation (Figure 3G). Likewise conditioned media from cells on stiff substrates decreased mesoderm markers in hESCs induced to differentiate on soft gels (Figure 3H), findings suggesting that cells on stiff gels may secrete factors that are inhibitory to Wnt signaling or mesoderm induction.
Indeed, we detected elevated mRNA and protein levels of the Sfrp family of soluble secreted Wnt inhibitors in hESCs differentiated on the stiff gels (Figures 3I, 3J, and S3B). Of these, Sfrp1, which binds and inhibits Wnt3a extracellularly (Wawrzak et al., 2007), was the most dramatically increased. Consistently, we quantified higher levels of Sfrp1 in hESCs differentiated on compliant gels when they were treated with IWP2, whereas we detected a decrease in cells on stiff gels in the presence of recombinant Wnt3a (Figure 3K). Further analysis revealed that multiple secreted factors that function as autocrine regulators of mesoderm differentiation also were induced in differentiating cells on soft gels (Figure S3C). These findings indicate that a substrate of compliance similar to that of an embryo enables the development of a Wnt-dependent feedforward signaling cascade that strengthens and reinforces mesoderm differentiation by enhancing the expression of Wnt activators and reducing the transcription of Wnt inhibitors. The data argue that, in the absence of a permissive microenvironment, such as on a stiff ECM, this feedforward mechanism does not become activated. This example of a self-reinforcing signaling mechanism is highly reminiscent of classic developmental programs, which frequently rely on robust, stable signal propagation to orchestrate coordinated tissue movements and to pattern tissue-specific differentiation and organization (Freeman and Gurdon, 2002).
Stabilization of Adherens Junctions Is Required to Prime Cells for Mesoderm Differentiation
Gastrulation is a highly orchestrated process that requires precisely timed changes in intracellular protein expression and localization. We observed high Wnt transcript levels in hESC colonies but only following their morphogen-induced differentiation (Figures 4A and S4A), analogous to the strong upregulation of Wnt3a observed in the differentiating mesoderm cells migrating into the primitive streak during gastrulation (Chapman et al., 2004). Wnt acts canonically in vivo to stabilize β-catenin and enable its nuclear translocation and gene transcription activity, and, consistently, we observed a significant increase in nuclear β-catenin in differentiating hESC colonies (Figure 4B). We therefore asked whether hESCs on the soft gels were primed to upregulate Wnts prior to mesoderm differentiation (Figure 4C).
Figure 4. E-Cadherin-Based AJs Are Required for Compliance-Driven Mesoderm Induction.
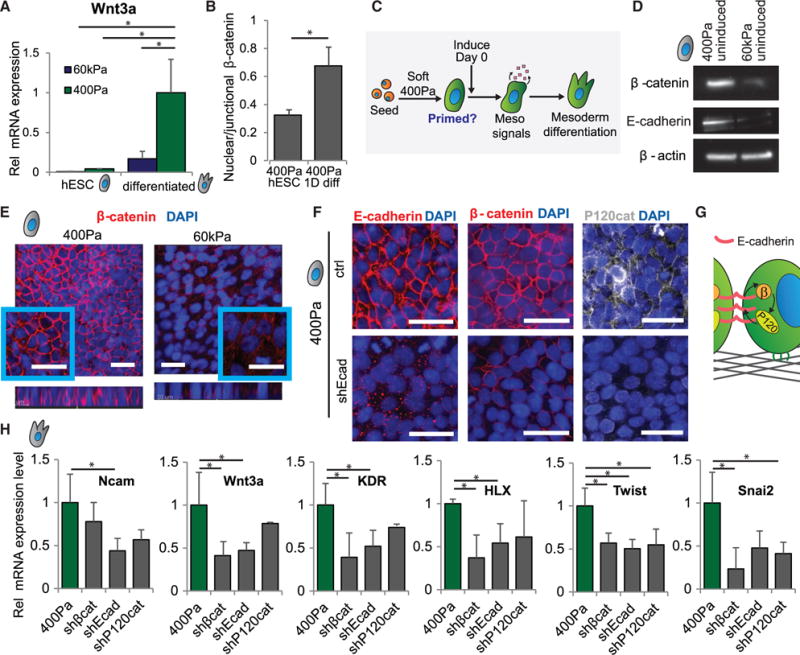
(A) mRNA expression of Wnt3a in hESCs and mesoderm-induced cells on soft or stiff gels is shown.
(B) Immunofluorescent quantification of nuclear divided by junctional β-catenin levels in uninduced hESCs on soft gels and in cells induced for 1 day on soft gels is shown.
(C) The proposed pathway involved in enhancing mesoderm differentiation on soft gels is shown.
(D) Western blot analysis of protein levels of β-catenin and E-cadherin with β-actin as a loading control in hESCs on soft or stiff gels is shown.
(E) Immunofluorescent staining in hESCs on soft or stiff gels is shown.
(F) Immunofluorescent staining shows localization of E-cadherin, β-catenin, and P120-catenin in hESCs on soft gels with and without induction of an shRNA against E-cadherin.
(G) hESCs on soft gels have reciprocal junctional stabilization of E-cadherin, β-catenin, and P120-catenin.
(H) mRNA expression in cells differentiated in the indicated conditions. Differentiation was performed on soft gels in each case.
Data are represented as mean of at least three independent experiments ± SD (*p < 0.05). All scale bars, 20 μm. See also Figure S4.
The Wnt/β-catenin axis plays a critical role throughout early embryogenesis, where the levels and function of β-catenin are regulated through several mechanisms, including its spatial localization, stabilization, and degradation (Wang and Wynshaw-Boris, 2004). Thus, while β-catenin is required for mesoderm differentiation (Haegel et al., 1995), β-catenin transcripts also are expressed earlier, throughout pre-streak and gastrulation-stage chick embryos (Schmidt et al., 2004), and β-catenin is involved in the initiation of primitive streak formation (Skromne and Stern, 2001). Therefore, we explored the role and status of β-catenin in undifferentiated hESCs cultured on substrates that recapitulate soft, embryo-like mechanical conditions. We found that hESCs on soft substrates had higher levels of β-catenin protein than hESCs on stiff gels (Figure 4D) and that β-catenin was primarily localized at cell-cell junctions (Figure 4E). Interestingly, the expression of β-catenin in hESCs cultured on TCP was similar to that found in hESCs plated on the stiff gels, and it was considerably reduced when compared to the levels found in hESCs plated on the soft gels and the levels observed in EBs of hESCs (Figure S4B).
We previously noted that hESCs cultured on soft substrates exhibit strong cell-cell adhesive forces (Lakins et al., 2012), so we checked whether β-catenin co-localized with E-cadherin and P120-catenin at cell-cell junctions. We observed localization of E-cadherin and P120-catenin together with β-catenin at cell-cell junctions in undifferentiated hESC colonies plated on compliant substrates (Figure 4F). In agreement with adherens junction (AJ)-mediated stabilization of β-catenin and P120-catenin in hESCs on soft gels, small hairpin RNA (shRNA) knockdown of E-cadherin (Figure S4C) disrupted β-catenin and P120-catenin localization at cell-cell junctions (Figure 4F), while knockdown of either β-catenin or P120-catenin (Figures S4D and S4E) correspondingly disrupted E-cadherin localization at cell-cell junctions (Figure S4F), suggesting a reciprocal stabilization of cell-cell AJs (Figure 4G).
Though E-cadherin is required for formation of the embryonic epiblast (Riethmacher et al., 1995), cells that undergo EMT and ingress into the primitive streak to differentiate into mesoderm lose E-cadherin protein expression (Dady et al., 2012) and shift from strong cell-cell to strong cell-ECM adhesions (Burdsal et al., 1993). In keeping with the induction of embryonic mesoderm in our system, we observed a marked downregulation of E-cadherin following differentiation of hESCs on soft substrates (Figure 2G). We therefore explored the molecular mechanisms regulating the cell-cell to cell-ECM adhesion switch accompanying mesoderm differentiation. We added differentiation media to hESCs in which β-catenin, E-cadherin, or P120-catenin had been knocked down to determine whether AJ stabilization was critical for mesoderm specification. Consistent with this idea, we found that loss of any one of these AJ-associated proteins diminished mesoderm differentiation of the hESC colonies (Figures 4H and S4G). These findings suggest that hESCs on compliant substrates assemble prominent AJs that then prime them for morphogen-induced, Wnt/β-catenin-dependent mesoderm differentiation, possibly by amassing a critical pool of β-catenin and facilitating β-catenin-dependent transcription.
Protein Expression and Localization Is Compliance Dependent and Functional
To explore whether a soft matrix primes hESCs for mesoderm differentiation by altering cell-cell and cell-ECM adhesion composition, we assessed the proteins associated with the cell-cell and cell-ECM adhesions in hESCs cultured on soft versus stiff gels (Figure 5A). Consistent with a reciprocal interplay between cell-cell adhesion stability and cell-ECM tension controlled by substrate stiffness, we observed significantly lower E-cadherin levels in hESCs on stiff gels (Figures 4D and 5B) and more activated integrin β1 (Figure 5B). The activated integrin β1 was localized to the cell-ECM interface in the same region as actin stress fibers, reminiscent of the enhanced integrin-mediated cell contractility previously documented at focal adhesions in cells on stiff gels (Paszek et al., 2005). Moreover, knockdown of E-cadherin in hESCs on soft gels not only disrupted AJs (Figure 4F) but also increased the level of activated integrin β1 at the cell-ECM adhesions (Figure 5C). Conversely, disrupting cell-ECM contacts in hESCs on stiff gels using a neutralizing antibody (AIIB2) that blocks integrin β1 activity not only inhibited cell-ECM adhesion but also enhanced cell-cell interactions, as indicated by increased E-cadherin and β-catenin protein localized to the cell-cell junctions (Figure 5D) and higher total β-catenin (Figure 5E). Importantly, inhibiting β1 integrin adhesions in hESC colonies on stiff gels significantly enhanced their mesoderm differentiation (Figure 5F), whereas disrupting cell-cell adhesions in hESC colonies on soft gels reduced their differentiation (Figure 4H). Thus, by reducing cell-ECM adhesions and stabilizing E-cadherin-containing AJs, hESCs plated on soft gels are primed for Wnt/β-catenin-dependent mesoderm differentiation.
Figure 5. Elaboration of Cell-Cell versus Cell-ECM Contacts Varies across Substrate Stiffness.
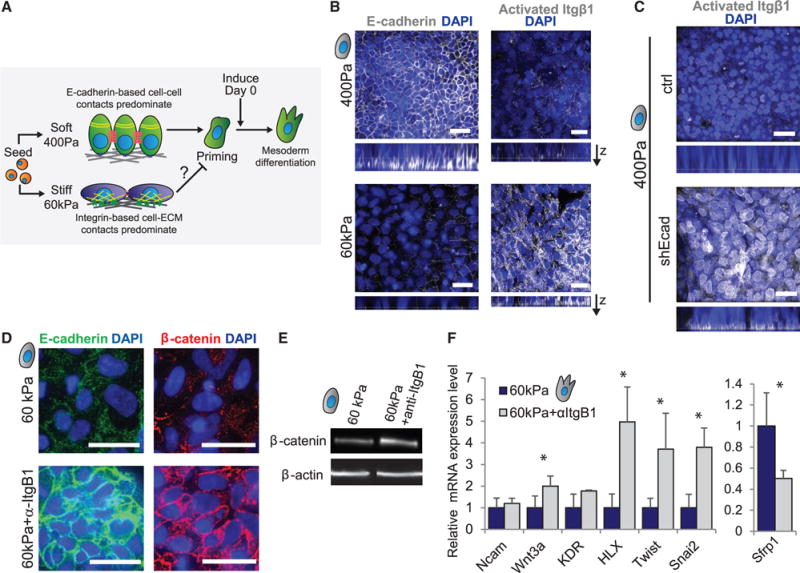
(A) The proposed relationship between cell coordination in hESCs on soft versus stiff gels and the ability of cells to become primed for mesoderm differentiation are shown.
(B) Immunofluorescent staining in hESCs on soft and stiff gels is shown.
(C) Immunofluorescent staining in hESCs on soft gels with and without induction of an shRNA against E-cadherin is shown.
(D) Immunofluorescent staining in hESCs on stiff gels with or without an integrin β1-blocking antibody (AIIB2) is shown.
(E) Western blot for β-catenin with β-actin as a loading control in hESCs on stiff gels with or without an integrin β1-blocking antibody is shown.
(F) mRNA expression in cells differentiated in the indicated conditions.
Data are represented as mean of at least three independent experiments ± SD (*p < 0.05). All scale bars, 20 μm.
β-Catenin Is Destabilized and Degraded in Cells on Stiff Substrates
To determine how a soft ECM could prime hESCs for mesoderm differentiation, we explored whether matrix compliance influences β-catenin levels. β-catenin is primarily regulated post-transcriptionally during development (Wodarz and Nusse, 1998); therefore, we first assessed the impact of ECM stiffness on β-catenin degradation (Figure 6A). Consistently, although β-catenin protein levels were substantially lower in hESC colonies on stiff versus soft substrates (Figure 4D), mRNA levels were not significantly different (Figure 6B). Moreover, treatment of hESCs with a low dose of the multi-catalytic proteasome inhibitor MG132 increased cellular β-catenin protein in the hESCs on the stiff gels (Figure 6C) and enhanced morphogen-stimulated mesoderm differentiation (Figure 6D). These data reveal that a stiff ECM compromises hESC mesoderm differentiation by enhancing β-catenin degradation.
Figure 6. β-Catenin Is Actively Degraded in hESCs on Stiff Gels.
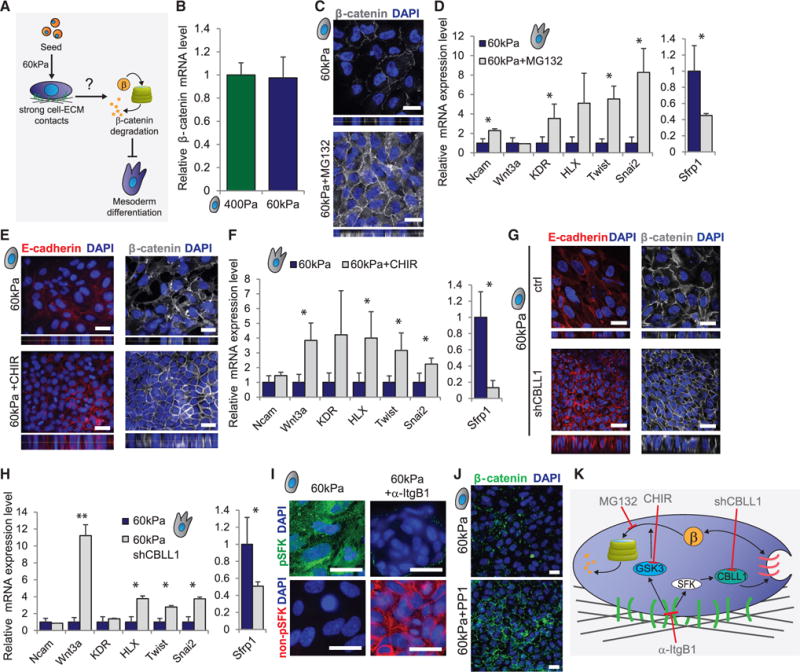
(A) Diagram depicts the question addressed in this figure.
(B) mRNA expression of β-catenin in hESCs on soft or stiff gels is shown.
(C) Immunofluorescent staining in hESCs on stiff gels with or without proteasome inhibitor MG132 is shown.
(D) mRNA expression in hESCs cultured on stiff gels with or without proteasome inhibitor MG132 and then induced to differentiate is shown.
(E) Immunofluorescent staining in hESCs on stiff gels with or without the GSK3β inhibitor CHIR99021 is shown.
(F) mRNA expression levels in cells differentiated in the indicated conditions are shown.
(G) Immunofluorescent staining in hESCs on stiff gels with or without induction of an shRNA against CBLL1 is shown.
(H) mRNA expression levels in cells differentiated in the indicated conditions are shown.
(I) Immunofluorescent staining in hESCs on stiff gels with or without an integrin β1-blocking antibody is shown.
(J) Immunofluorescent staining in hESCs on stiff gels with or without addition of the SFK inhibitor PP1 is shown.
(K) Depiction of the proteins activated in hESCs on stiff gels that are responsible for enhancing the degradation of β-catenin.
Data are represented as mean of at least three independent experiments ± SD (**p < 0.001 and *p < 0.05). All scale bars, 20 μm. See also Figure S5.
Proteasomal degradation of ubiquitinated β-catenin proceeds once free β-catenin is phosphorylated by GSK3β at ubiquitin ligase recognition sites (Stamos and Weis, 2013). We found a higher fraction of active (unphosphorylated at S9) GSK3β in hESCs on stiff substrates (Figure S5A). Moreover, blocking GSK3β activity by addition of the inhibitor CHIR99021 in hESCs on stiff gels increased the amount of β-catenin and E-cadherin localized at cell-cell junctions (Figure 6E) and, in response to morphogens, elevated nuclear β-catenin (Figure S5B) to potentiate mesoderm differentiation (Figure 6F). However, GSK3β has not been reported to phosphorylate β-catenin residing within AJs, suggesting that additional mechanisms must exist to destabilize AJs in hESCs on the stiff gels. In keeping with this prediction, we noted that knockdown of Cbl-Like 1 (CBLL1, also known as HAKAI; Figure S5C), an E3 ubiquitin ligase that promotes the internalization of E-cadherin (Fujita et al., 2002), increased levels of E-cadherin and β-catenin (Figure 6G) and enhanced the efficiency of morphogen-stimulated mesoderm differentiation in hESCs on stiff gels (Figure 6H). These findings imply that, on a stiff substrate, elevated CBLL1 activity targets E-cadherin internalization to destabilize AJs and release β-catenin into the cytoplasm, where it is rapidly marked for proteasomal degradation and rendered non-available for mesoderm differentiation induction.
CBLL1 is activated by Src-family kinases (SFKs) (Fujita et al., 2002), and SFKs are stimulated by integrin adhesion-associated kinases downstream of myosin-based cell contractility (Huveneers and Danen, 2009; Webb et al., 2004). We detected abundant activated (phosphorylated) SFKs in hESCs on stiff gels and low levels of inactive SFKs, and we noted that this could be reversed by treatment with a function-blocking antibody to β1 integrin (Figure 6I), consistent with our prior observations that hESCs on stiff gels assemble prominent stress fibers and have high activated β1 integrin at the cell-ECM interface (Figures 1A and 5B). By contrast, the majority of the SFKs in the hESCs on the soft gels were inactive and localized at cell-cell junctions (Figure S5D). Directly inhibiting SFKs in the hESCs on the stiff gels using a pharmaceutical inhibitor (PP1) not only ablated stress fibers (Figure S5E) but also increased β-catenin (Figure 6J), further implicating integrin-dependent SFK activation of CBLL1 in AJ destabilization (Figure 6K). These data mechanistically illustrate why cell-ECM adhesions inhibit priming of hESCs for mesoderm differentiation.
Reorganization of Junctionally Localized Proteins upon Induction Drives Mesoderm Differentiation
Thus far we established that AJ stabilization generates a critical pool of β-catenin required to prime hESCs for morphogen-stimulated mesoderm differentiation. We next explored how the junctionally stabilized pool of β-catenin was released following morphogen stimulation to initiate the mesoderm gene transcription critical for tissue-specific differentiation. Prior studies demonstrated strong synergy between SFK activation and growth factor receptor signaling (Wang et al., 2005). We therefore assessed whether morphogen stimulation of hESCs on soft gels was sufficient to activate SFKs and could induce the release of β-catenin from AJs through CBLL ubiquitin ligase-mediated E-cadherin endocytosis. In keeping with this idea, we observed the rapid phosphorylation (within 2 hr) of junctional SFKs in hESCs on soft gels following morphogen stimulation (Figure 7A). As an in vivo correlate, we monitored SFK activation in a gastrulation-stage chick embryo, focusing on the region where mesoderm cells were specified as they ingressed into the primitive streak (Figure 7B). Consistent with our results in differentiating hESCs, we observed that the chick cells in the epiblast displayed junctionally localized, non-phosphorylated SFKs, whereas cells ingressing to form mesoderm instead upregulated the level of phosphorylated SFKs (Figure 7C). Expression of inactive SFKs in the epiblast localized with actin at cell-cell junctions (Figure S6A), whereas activation of SFKs in the ingressing cells occurred in tandem with an upregulation of NCAM in this population (Figure S6B). Consistent with a link between SFK activation and CBLL1-mediated AJ breakdown, CBLL1 knockdown in differentiating hESCs on soft gels prevented E-cadherin downregulation (Figure 7D), indicating a role for CBLL1 in initiating the EMT associated with mesoderm induction. Similarly, we observed that CBLL1 was expressed in both the epithelial and ingressing cells of the gastrulating chick embryo (Figure S6C), consistent with its role in EMT.
Figure 7. SFKs Induce E-Cadherin Internalization to Initiate the Signaling Cascade Associated with EMT and Mesoderm Differentiation.
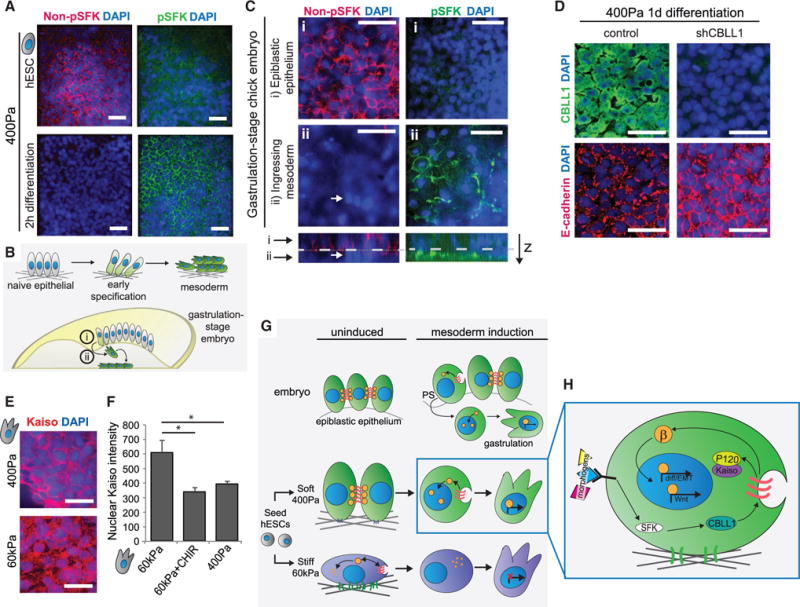
(A) Immunofluorescent staining of non-phosphorylated (left) and phosphorylated (right) SFKs in cells on soft gels that were never induced for differentiation (top) or that were induced for 2 hr (bottom) is shown.
(B) Diagram depicts corresponding cell types to those differentiating to mesoderm on a soft gel in an appropriately staged chicken embryo.
(C) Immunofluorescent staining of cells in a gastrulating chick embryo (stage HH4). Top layer of cells (i) has high levels of unphosphorylated SFKs, while cells that have ingressed below the epithelial sheet (ii, white arrow) lose expression (left panels). This loss is anticorrelated to the upregulation of phosphorylated SFKs in cells that have ingressed (right panels).
(D) Immunofluorescent staining in cells induced for differentiation on soft gels for 1 day with or without induction of an shRNA against CBLL1. Top and bottom panels are from the same image.
(E) Immunofluorescent staining in cells differentiated on soft or stiff gels is shown.
(F) Immunofluorescent quantification of nuclear Kaiso levels in cells differentiated on stiff gels with or without CHIR99021 or on soft gels is shown.
(G) Model figure depicts how cells are primed to undergo differentiation on soft gels and the relationship to mesoderm differentiation in vivo.
(H) Detailed signaling pathways that act upon differentiation of hESCs cultured on soft gels.
Data are represented as mean of at least three independent experiments ± SD (*p < 0.05). All scale bars, 20 μm. See also Figure S6.
When AJs break down and β-catenin is released in hESCs differentiating on soft substrates, it is not degraded as in hESCs on stiff gels, but instead it is able to enter the nucleus (Figure 4B) and transcribe target genes (Figure 3D). This is due in part to less active GSK3β in cells on soft gels (Figure S5A), and also to the release of P120-catenin from AJs into the cytoplasm, where it sequesters Kaiso, a transcriptional inhibitor of β-catenin (Del Valle-Pérez et al., 2011; Kourtidis et al., 2013). Indeed, Kaiso was excluded from the nucleus in cells differentiated on soft substrates, whereas it was both cytoplasmic and nuclear in hESCs on stiff substrates unless GSK3 also was inhibited (Figures 7E and 7F). Thus, hESCs on soft gels stabilize β-catenin and also potentiate its transcriptional activity, and these two effects combine to reinforce mesoderm differentiation (Figures 7G and 7H). By contrast, mesoderm differentiation efficiency is significantly compromised in hESCs plated on a stiff gel because β-catenin levels are neither stabilized nor is its transcriptional activity enhanced; rather, β-catenin transcriptional activity is actively repressed in hESCs on a stiff gel through the accumulation of nuclear Kaiso. Importantly, however, mesoderm differentiation can be driven in hESCs on a stiff gel either through pharmacological intervention or via genetic modification of key pathways that increase β-catenin levels and/or activity (Figure 6K). These findings illustrate how substrate stiffness dictates the temporally coordinated interplay between cell-cell and cell-ECM adhesions to orchestrate the rapid and synchronous induction of mesoderm progenitor differentiation through a series of interwoven, reinforcing pathways that converge on the engagement of a Wnt/β-catenin-signaling circuit.
DISCUSSION
Here we identified tissue-level organization as a key regulator of morphogen-dependent hESC fate. Using BM-conjugated PA gels of tunable stiffness, we showed that culture conditions that favor cell-cell adhesion over cell-ECM adhesion permit the synchronous and efficient induction of Wnt-dependent mesoderm progenitor differentiation of hESCs. Importantly, hESC mesoderm differentiation was not influenced solely by ECM stiffness, but rather it was amplified in response to soluble cues, thus reinforcing earlier work suggesting that tissue tension modifies cell behavior by altering the context of cell signaling (Levental et al., 2009; Mouw et al., 2014). Through the identification of molecular mechanisms whereby tissue tension directs stem cell fate and via prudent genetic and pharmacologic manipulation, we were able to demonstrate how hESC differentiation protocols can be optimized to direct early progenitor specification.
Our findings obtained using this simplified hESC system not only build on prior knowledge of Wnt-dependent mesoderm specification during embryogenesis derived from model organisms but also definitively link Wnt-stimulated developmental programs to a tightly coordinated cytoskeletal-linked adhesion-directed mechanosignaling pathway. Indeed, we were able to validate our findings linking Src signaling in hESC mesoderm differentiation in an in vivo chick model of gastrulation, thereby gaining new insight into embryonic development. Accordingly, our work illustrates and validates the utility of the ECM gel-hESC model for interrogating the underlying mechanisms whereby tissue organization and cytoskeletal tension within an embryo direct tissue development. Our study therefore emphasizes the power of this simplified system to identify mechanosensitive molecules involved in early differentiation, to inform directed differentiation protocols required for tissue engineering, and to gain a deeper understanding of regulatory systems in embryonic development.
We found that morphogen stimulation of hESCs on soft substrates induced SFK activation at the cell-cell interface, and we determined that this led to the disassembly of AJs through activation of the ubiquitin ligase CBLL1. AJ destabilization released P120-catenin to mediate β-catenin nuclear translocation, resulting in mesoderm-reinforcing transcriptional activity. These findings are consistent with studies in Drosophila, Xenopus, and zebrafish, which showed that CBLL1 is required for mesoderm morphogenesis (Kaido et al., 2009) and that SFKs, P120-catenin, and β-catenin are involved in gastrulation (Denoyelle et al., 2001; Fang et al., 2004; Haegel et al., 1995). By connecting the developmental roles of these molecules to a mechanically regulated pathway, our study builds on previous work demonstrating that a β-catenin homolog acts as a mechano-transducer (Farge, 2003) and that β-catenin is translocated to the nucleus downstream of mechanically regulated Src (Desprat et al., 2008). Our finding that the ubiquitin ligase CBLL1 is involved in mesoderm specification is particularly interesting given that prior studies have shown that proteasome inhibition is detrimental to development (Shin et al., 2010; Velentzas et al., 2011). These data raise the intriguing possibility that additional ubiquitin ligases may play a critical role in embryogenesis and warrant further investigation. Together, these data connect and extend known findings to establish a mechanically regulated pathway that is involved in amniote mesoderm differentiation, thereby demonstrating how our system can uncover novel mechanisms that regulate embryogenesis.
Our work indicates that, by optimizing the mechanical properties of the extracellular substrate, mesoderm differentiation of hESCs can be enhanced and the relevant signaling pathways identified, manipulated, and potentiated to direct cell fate specification. We showed that AJs formed readily in hESCs on soft substrates, yielding a population that was competent for efficient differentiation. Practically, such an approach would be difficult to scale up for therapeutic applications. However, we also showed that treatment of hESCs grown on stiff substrates with a GSK3β inhibitor to stabilize β-catenin significantly augmented AJ formation and increased the efficiency of subsequent mesoderm induction, thereby providing an approach that could be implemented for hESCs differentiating on TCP, where baseline β-catenin levels are similar to those on stiff substrates. In addition, our results provide a potential mechanistic framework for understanding a classic approach to ex vivo differentiation of ESCs, the formation of multicellular EBs. Although differentiation of EBs may be augmented by the addition or withdrawal of exogenous morphogens, it is primarily spontaneous and begins once cells are aggregated in suspension. This implies that endogenous signals are sufficient to initiate differentiation, as shown when endogenous Wnts caused local differentiation of mesendodermal progenitors in EBs (ten Berge et al., 2008). Cells in EBs have maximal levels of cell-cell interactions reflected in their prominent AJs, and, at this extreme, we conjecture that cells in EBs may be poised to respond to endogenous differentiation-inducing signals at lower levels than those required to drive differentiation in hESCs grown on soft substrates. Therefore, the use of tuned ECM substrates and uniform populations of hESCs provides a pluripotently metastable population that can be induced by exogenous morphogens to achieve synchronous, uniform mesoderm progenitor differentiation. Accordingly, our model provides a facile approach with which to manipulate and optimize hESC differentiation and to generate mature cell types for therapeutic applications as well as a tractable system in which to study early tissue development.
Our results also have potential implications for understanding early embryonic development. During development, the apolar inner cell mass (ICM) gives rise to two apposed epithelial layers, the primitive endoderm and the apico-basally polarized, pseudostratified columnar pluripotent epiblast (Rossant, 2004). In our experimental model, single epiblastic hESCs were reaggregated at high density to form an apico-basally polarized monolayer similar in size and shape to the epiblast in vivo. In this system, AJ re-formation was rate limited post-transcriptionally in cells on stiff substrates due to low β-catenin and E-cadherin levels, which resulted in the failure of hESCs to effectively differentiate to mesoderm. This bottleneck was rapidly overcome in hESCs on soft substrates, indicating that we can use this system to mimic the organization of cells in the pluripotent epiblast in a controlled way. These insights underscore the likely direct interdependence among the acquisition of apico-basal polarity, AJ formation, and the competence of the epiblast in vivo to undergo gastrulation, and they suggest that, in large part, the mesoderm-inducing and/or -amplifying activity of β-catenin in vivo is supplied by β-catenin released from disassembling AJs. Under this view, the epithelialization of the epiblast from the apolar ICM may largely control the timing of gastrulation. Together, these results demonstrate that understanding how fundamental developmental processes are regulated via mechanical cues at both the tissue and the molecular levels provides novel biological insights that can be exploited to enhance therapeutically relevant directed differentiation protocols.
EXPERIMENTAL PROCEDURES
Cell Culture
The hESCs (H9 and H7 lines) were routinely cultured in media consisting of KO-DMEM supplemented with 20% knockout serum replacement (Life Technologies), 2 mM L-glutamine, 1 mM non-essential amino acids, 1× penicillin-streptomycin, 100 μM β-mercaptoethanol, and 10 ng/ml bFgf (Global Stem). Routine passaging was performed with collagenase IV (Life Technologies) at 1 mg/ml. Mouse embryonic fibroblasts (MEFs) were cultured on gelatin-coated TCP substrates in DMEM supplemented with 10% fetal bovine serum (HyClone), 4 mM L-glutamine, and 1× penicillin-streptomycin. WntGFP reporter hESC line was generated using a 7×TCF-nuclear GFP construct adapted from (Fuerer and Nusse, 2010) to include a nuclear localized H2B-GFP reporter. Cells were grown at 37°C in a humidified incubator with 5% CO2. Work with human stem cell lines was approved by the University of California, San Francisco (UCSF) Stem Cell Research Oversight Committee study number 11-05439.
Cell Differentiation
Mesoderm differentiation was performed as previously reported (Evseenko et al., 2010), by the addition of HSC differentiation media (Sigma) supplemented with Activin, bFgf, VEGF, and BMP4 (all PeproTech). EB formation protocol was adapted from Przybyla and Voldman (2012) for use with hESCs, using the same culture conditions as in hESC hydrogel culture. Cardiomyocyte differentiation was performed on cells differentiated to mesoderm progenitors on 400-Pa gels by adapting from Lian et al. (2012) to begin at the Wnt inhibition stage of the differentiation time course. Differentiation to mesenchymal stromal cells was performed according to Evseenko et al. (2010).
Hydrogel Preparation
PA hydrogels were prepared according to Przybyla et al. (2016). Briefly, PA gels were cast on 18-mm circular coverslips using recipes calibrated for stiffness and functionalized to provide a surface for ligand to bind. Gels were coated with Matrigel (225 μg/ml) and collagen (25 μg/ml) overnight. After washing gels, cells were plated in 3-mm diameter colonies using 3D-printed plating guides (Przybyla et al., 2016) at 20,000 cells/colony. The hESCs on gels were cultured in MEF-conditioned KO-DMEM/KSR media. Rock inhibitor Y27632 was added at 20 μM when cells were plated, then progressively removed. TCP or glass coated with diluted gelatin or Matrigel was considered to have essentially the same stiffness as noncoated substrates, as the thickness of these coatings are lower than the threshold (~50 μm) at which cells can no longer sense the stiffness of an underlying hard substrate (Buxboim et al., 2010).
Chick Embryo Manipulation
Embryos were dissected from fertilized chicken eggs (Petaluma Farms) and cultured according to Chapman et al. (2001) until Hamburger and Hamilton (HH) stage 3+ to 4, when the primitive streak is fully formed and mesodermal cells are actively ingressing (Hamburger and Hamilton, 1992; Voiculescu et al., 2014). Gastrulation-stage embryos were fixed and stained using fluorescently conjugated phalloidin (Life Technologies), non-phosphorylated SFK antibody (Cell Signaling Technology), phosphorylated SFK antibody (Cell Signaling Technology), NCAM antibody (EMD Millipore), or CBLL1 antibody (AVIVA Biosciences) followed by fluorescently conjugated secondary antibodies (Life Technologies).
Supplementary Material
Highlights.
Compliant hydrogel substrates enhance mesoderm differentiation of human ESCs
Stabilization of adherens junctions primes hESCs for mesoderm differentiation
Junctional reorganization and Src activity promote nuclear translocation of β-catenin
On stiff gels, β-catenin degradation inhibits mesodermal differentiation
Acknowledgments
We would like to acknowledge funding from CIRM grants RB5-07409 and TR3-05542. L.P. acknowledges California Institute of Regenerative Medicine (CIRM) training grant TG2-01153. We thank Luke Cassereau and Xcell Biosciences for providing support with the Cellular Research gene expression panels and Russell Bainer for providing support with the RNA-sequencing dataset. We also thank Takashi Mikawa for assistance with chick embryo manipulation protocols.
Footnotes
ACCESSION NUMBERS
The accession number for the RNA-seq dataset reported in this paper is GEO: GSE83584.
SUPPLEMENTAL INFORMATION
Supplemental Information includes Supplemental Experimental Procedures, six figures, one table, and one movie and can be found with this article online at http://dx.doi.org/10.1016/j.stem.2016.06.018.
AUTHOR CONTRIBUTIONS
Conceptualization, V.M.W., J.N.L., and L.P.; Methodology, L.P. and J.N.L.; Formal Analysis, L.P.; Investigation, L.P.; Writing, L.P., J.N.L., and V.M.W.; Funding Acquisition, V.M.W.
References
- Bauwens CL, Peerani R, Niebruegge S, Woodhouse KA, Kumacheva E, Husain M, Zandstra PW. Control of human embryonic stem cell colony and aggregate size heterogeneity influences differentiation trajectories. Stem Cells. 2008;26:2300–2310. doi: 10.1634/stemcells.2008-0183. [DOI] [PubMed] [Google Scholar]
- Burdsal CA, Damsky CH, Pedersen RA. The role of E-cadherin and integrins in mesoderm differentiation and migration at the mammalian primitive streak. Development. 1993;118:829–844. doi: 10.1242/dev.118.3.829. [DOI] [PubMed] [Google Scholar]
- Buxboim A, Rajagopal K, Brown AEX, Discher DE. How deeply cells feel: methods for thin gels. J Phys Condens Matter. 2010;22:194116. doi: 10.1088/0953-8984/22/19/194116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman SC, Collignon J, Schoenwolf GC, Lumsden A. Improved method for chick whole-embryo culture using a filter paper carrier. Dev Dyn. 2001;220:284–289. doi: 10.1002/1097-0177(20010301)220:3<284::AID-DVDY1102>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- Chapman SC, Brown R, Lees L, Schoenwolf GC, Lumsden A. Expression analysis of chick Wnt and frizzled genes and selected inhibitors in early chick patterning. Dev Dyn. 2004;229:668–676. doi: 10.1002/dvdy.10491. [DOI] [PubMed] [Google Scholar]
- Chowdhury F, Li Y, Poh YC, Yokohama-Tamaki T, Wang N, Tanaka TS. Soft substrates promote homogeneous self-renewal of embryonic stem cells via downregulating cell-matrix tractions. PLoS ONE. 2010;5:e15655. doi: 10.1371/journal.pone.0015655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford BD, Henry CA, Clason TA, Becker AL, Hille MB. Activity and distribution of paxillin, focal adhesion kinase, and cadherin indicate cooperative roles during zebrafish morphogenesis. Mol Biol Cell. 2003;14:3065–3081. doi: 10.1091/mbc.E02-08-0537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dady A, Blavet C, Duband JL. Timing and kinetics of E- to N-cadherin switch during neurulation in the avian embryo. Dev Dyn. 2012;241:1333–1349. doi: 10.1002/dvdy.23813. [DOI] [PubMed] [Google Scholar]
- Del Valle-Pérez B, Casagolda D, Lugilde E, Valls G, Codina M, Dave N, de Herreros AG, Duñach M. Wnt controls the transcriptional activity of Kaiso through CK1ε-dependent phosphorylation of p120-catenin. J Cell Sci. 2011;124:2298–2309. doi: 10.1242/jcs.082693. [DOI] [PubMed] [Google Scholar]
- Denoyelle M, Vallés AM, Lentz D, Thiery JP, Boyer B. Mesoderm-independent regulation of gastrulation movements by the src tyrosine kinase in Xenopus embryo. Differentiation. 2001;69:38–48. doi: 10.1046/j.1432-0436.2001.690104.x. [DOI] [PubMed] [Google Scholar]
- Desprat N, Supatto W, Pouille PA, Beaurepaire E, Farge E. Tissue deformation modulates twist expression to determine anterior midgut differentiation in Drosophila embryos. Dev Cell. 2008;15:470–477. doi: 10.1016/j.devcel.2008.07.009. [DOI] [PubMed] [Google Scholar]
- Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126:677–689. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- Evseenko D, Zhu Y, Schenke-Layland K, Kuo J, Latour B, Ge S, Scholes J, Dravid G, Li X, MacLellan WR, Crooks GM. Mapping the first stages of mesoderm commitment during differentiation of human embryonic stem cells. Proc Natl Acad Sci USA. 2010;107:13742–13747. doi: 10.1073/pnas.1002077107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang X, Ji H, Kim SW, Park JI, Vaught TG, Anastasiadis PZ, Ciesiolka M, McCrea PD. Vertebrate development requires ARVCF and p120 catenins and their interplay with RhoA and Rac. J Cell Biol. 2004;165:87–98. doi: 10.1083/jcb.200307109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farge E. Mechanical induction of Twist in the Drosophila foregut/stomodeal primordium. Curr Biol. 2003;13:1365–1377. doi: 10.1016/s0960-9822(03)00576-1. [DOI] [PubMed] [Google Scholar]
- Freeman M, Gurdon JB. Regulatory principles of developmental signaling. Annu Rev Cell Dev Biol. 2002;18:515–539. doi: 10.1146/annurev.cellbio.18.012502.083458. [DOI] [PubMed] [Google Scholar]
- Fuerer C, Nusse R. Lentiviral vectors to probe and manipulate the Wnt signaling pathway. PLoS ONE. 2010;5:e9370. doi: 10.1371/journal.pone.0009370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita Y, Krause G, Scheffner M, Zechner D, Leddy HEM, Behrens J, Sommer T, Birchmeier W. Hakai, a c-Cbl-like protein, ubiquitinates and induces endocytosis of the E-cadherin complex. Nat Cell Biol. 2002;4:222–231. doi: 10.1038/ncb758. [DOI] [PubMed] [Google Scholar]
- Gilbert PM, Havenstrite KL, Magnusson KEG, Sacco A, Leonardi NA, Kraft P, Nguyen NK, Thrun S, Lutolf MP, Blau HM. Substrate elasticity regulates skeletal muscle stem cell self-renewal in culture. Science. 2010;329:1078–1081. doi: 10.1126/science.1191035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gobaa S, Hoehnel S, Lutolf MP. Substrate elasticity modulates the responsiveness of mesenchymal stem cells to commitment cues. Integr Biol (Camb) 2015;7:1135–1142. doi: 10.1039/c4ib00176a. [DOI] [PubMed] [Google Scholar]
- Haegel H, Larue L, Ohsugi M, Fedorov L, Herrenknecht K, Kemler R. Lack of beta-catenin affects mouse development at gastrulation. Development. 1995;121:3529–3537. doi: 10.1242/dev.121.11.3529. [DOI] [PubMed] [Google Scholar]
- Hamburger V, Hamilton HL. A series of normal stages in the development of the chick embryo. 1951. Dev Dyn. 1992;195:231–272. doi: 10.1002/aja.1001950404. [DOI] [PubMed] [Google Scholar]
- Hens JR, Wilson KM, Dann P, Chen X, Horowitz MC, Wysolmerski JJ. TOPGAL mice show that the canonical Wnt signaling pathway is active during bone development and growth and is activated by mechanical loading in vitro. J Bone Miner Res. 2005;20:1103–1113. doi: 10.1359/JBMR.050210. [DOI] [PubMed] [Google Scholar]
- Hollinger JO. An Introduction to Biomaterials. Second. CRC Press; 2011. [Google Scholar]
- Howard S, Deroo T, Fujita Y, Itasaki N. A positive role of cadherin in Wnt/β-catenin signalling during epithelial-mesenchymal transition. PLoS ONE. 2011;6:e23899. doi: 10.1371/journal.pone.0023899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber O, Bierkamp C, Kemler R. Cadherins and catenins in development. Curr Opin Cell Biol. 1996;8:685–691. doi: 10.1016/s0955-0674(96)80110-4. [DOI] [PubMed] [Google Scholar]
- Huveneers S, Danen EHJ. Adhesion signaling - crosstalk between integrins, Src and Rho. J Cell Sci. 2009;122:1059–1069. doi: 10.1242/jcs.039446. [DOI] [PubMed] [Google Scholar]
- Kaido M, Wada H, Shindo M, Hayashi S. Essential requirement for RING finger E3 ubiquitin ligase Hakai in early embryonic development of Drosophila. Genes Cells. 2009;14:1067–1077. doi: 10.1111/j.1365-2443.2009.01335.x. [DOI] [PubMed] [Google Scholar]
- Kang KS, Robling AG. New insights into Wnt–Lrp5/6–β-catenin signaling in mechanotransduction. Front Endocrinol (Lausanne) 2015;5:246. doi: 10.3389/fendo.2014.00246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keung AJ, de Juan-Pardo EM, Schaffer DV, Kumar S. Rho GTPases mediate the mechanosensitive lineage commitment of neural stem cells. Stem Cells. 2011;29:1886–1897. doi: 10.1002/stem.746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kourtidis A, Ngok SP, Anastasiadis PZ. p120 catenin: an essential regulator of cadherin stability, adhesion-induced signaling, and cancer progression. Prog Mol Biol Transl Sci. 2013;116:409–432. doi: 10.1016/B978-0-12-394311-8.00018-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakins JN, Chin AR, Weaver VM. Exploring the link between human embryonic stem cell organization and fate using tension-calibrated extracellular matrix functionalized polyacrylamide gels. Methods Mol Biol. 2012;916:317–350. doi: 10.1007/978-1-61779-980-8_24. [DOI] [PubMed] [Google Scholar]
- Levental KR, Yu H, Kass L, Lakins JN, Egeblad M, Erler JT, Fong SFT, Csiszar K, Giaccia A, Weninger W, et al. Matrix crosslinking forces tumor progression by enhancing integrin signaling. Cell. 2009;139:891–906. doi: 10.1016/j.cell.2009.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lian X, Hsiao C, Wilson G, Zhu K, Hazeltine LB, Azarin SM, Raval KK, Zhang J, Kamp TJ, Palecek SP. Robust cardiomyocyte differentiation from human pluripotent stem cells via temporal modulation of canonical Wnt signaling. Proc Natl Acad Sci USA. 2012;109:E1848–E1857. doi: 10.1073/pnas.1200250109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsley RC, Gill JG, Kyba M, Murphy TL, Murphy KM. Canonical Wnt signaling is required for development of embryonic stem cell-derived mesoderm. Development. 2006;133:3787–3796. doi: 10.1242/dev.02551. [DOI] [PubMed] [Google Scholar]
- Majkut S, Idema T, Swift J, Krieger C, Liu A, Discher DE. Heart-specific stiffening in early embryos parallels matrix and myosin expression to optimize beating. Curr Biol. 2013;23:2434–2439. doi: 10.1016/j.cub.2013.10.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majkut S, Dingal PCDP, Discher DE. Stress sensitivity and mechanotransduction during heart development. Curr Biol. 2014;24:R495–R501. doi: 10.1016/j.cub.2014.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBeath R, Pirone DM, Nelson CM, Bhadriraju K, Chen CS. Cell shape, cytoskeletal tension, and RhoA regulate stem cell lineage commitment. Dev Cell. 2004;6:483–495. doi: 10.1016/s1534-5807(04)00075-9. [DOI] [PubMed] [Google Scholar]
- McMahon AP. The Wnt family of developmental regulators. Trends Genet. 1992;8:236–242. [Google Scholar]
- Mouw JK, Yui Y, Damiano L, Bainer RO, Lakins JN, Acerbi I, Ou G, Wijekoon AC, Levental KR, Gilbert PM, et al. Tissue mechanics modulate microRNA-dependent PTEN expression to regulate malignant progression. Nat Med. 2014;20:360–367. doi: 10.1038/nm.3497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ou G, Weaver VM. Tumor-induced solid stress activates β-catenin signaling to drive malignant behavior in normal, tumor-adjacent cells. BioEssays. 2015;37:1293–1297. doi: 10.1002/bies.201500090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paszek MJ, Zahir N, Johnson KR, Lakins JN, Rozenberg GI, Gefen A, Reinhart-King CA, Margulies SS, Dembo M, Boettiger D, et al. Tensional homeostasis and the malignant phenotype. Cancer Cell. 2005;8:241–254. doi: 10.1016/j.ccr.2005.08.010. [DOI] [PubMed] [Google Scholar]
- Pines M, Das R, Ellis SJ, Morin A, Czerniecki S, Yuan L, Klose M, Coombs D, Tanentzapf G. Mechanical force regulates integrin turnover in Drosophila in vivo. Nat Cell Biol. 2012;14:935–943. doi: 10.1038/ncb2555. [DOI] [PubMed] [Google Scholar]
- Przybyla LM, Voldman J. Attenuation of extrinsic signaling reveals the importance of matrix remodeling on maintenance of embryonic stem cell self-renewal. Proc Natl Acad Sci USA. 2012;109:835–840. doi: 10.1073/pnas.1103100109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Przybyla L, Lakins JN, Sunyer R, Trepat X, Weaver VM. Monitoring developmental force distributions in reconstituted embryonic epithelia. Methods. 2016;94:101–113. doi: 10.1016/j.ymeth.2015.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riethmacher D, Brinkmann V, Birchmeier C. A targeted mutation in the mouse E-cadherin gene results in defective preimplantation development. Proc Natl Acad Sci USA. 1995;92:855–859. doi: 10.1073/pnas.92.3.855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosowski KA, Mertz AF, Norcross S, Dufresne ER, Horsley V. Edges of human embryonic stem cell colonies display distinct mechanical properties and differentiation potential. Sci Rep. 2015;5:14218. doi: 10.1038/srep14218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossant J. Lineage development and polar asymmetries in the peri-implantation mouse blastocyst. Semin Cell Dev Biol. 2004;15:573–581. doi: 10.1016/j.semcdb.2004.04.003. [DOI] [PubMed] [Google Scholar]
- Schmidt M, Patterson M, Farrell E, Münsterberg A. Dynamic expression of Lef/Tcf family members and β-catenin during chick gastrulation, neurulation, and early limb development. Dev Dyn. 2004;229:703–707. doi: 10.1002/dvdy.20010. [DOI] [PubMed] [Google Scholar]
- Shin SW, Tokoro M, Nishikawa S, Lee HH, Hatanaka Y, Nishihara T, Amano T, Anzai M, Kato H, Mitani T, et al. Inhibition of the ubiquitin-proteasome system leads to delay of the onset of ZGA gene expression. J Reprod Dev. 2010;56:655–663. doi: 10.1262/jrd.10-104m. [DOI] [PubMed] [Google Scholar]
- Simôes S de M, Mainieri A, Zallen JA. Rho GTPase and Shroom direct planar polarized actomyosin contractility during convergent extension. J Cell Biol. 2014;204:575–589. doi: 10.1083/jcb.201307070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skromne I, Stern CD. Interactions between Wnt and Vg1 signalling pathways initiate primitive streak formation in the chick embryo. Development. 2001;128:2915–2927. doi: 10.1242/dev.128.15.2915. [DOI] [PubMed] [Google Scholar]
- Stamos JL, Weis WI. The β-catenin destruction complex. Cold Spring Harb Perspect Biol. 2013;5:a007898. doi: 10.1101/cshperspect.a007898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens LE, Sutherland AE, Klimanskaya IV, Andrieux A, Meneses J, Pedersen RA, Damsky CH. Deletion of beta 1 integrins in mice results in inner cell mass failure and peri-implantation lethality. Genes Dev. 1995;9:1883–1895. doi: 10.1101/gad.9.15.1883. [DOI] [PubMed] [Google Scholar]
- Takada S, Stark KL, Shea MJ, Vassileva G, McMahon JA, McMahon AP. Wnt-3a regulates somite and tailbud formation in the mouse embryo. Genes Dev. 1994;8:174–189. doi: 10.1101/gad.8.2.174. [DOI] [PubMed] [Google Scholar]
- Takeichi M. Morphogenetic roles of classic cadherins. Curr Opin Cell Biol. 1995;7:619–627. doi: 10.1016/0955-0674(95)80102-2. [DOI] [PubMed] [Google Scholar]
- ten Berge D, Koole W, Fuerer C, Fish M, Eroglu E, Nusse R. Wnt signaling mediates self-organization and axis formation in embryoid bodies. Cell Stem Cell. 2008;3:508–518. doi: 10.1016/j.stem.2008.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velentzas PD, Velentzas AD, Mpakou VE, Papassideri IS, Stravopodis DJ, Margaritis LH. Proteasome inhibition induces developmentally deregulated programs of apoptotic and autophagic cell death during Drosophila melanogaster oogenesis. Cell Biol Int. 2011;35:15–27. doi: 10.1042/CBI20100191. [DOI] [PubMed] [Google Scholar]
- Voiculescu O, Bodenstein L, Lau IJ, Stern CD. Local cell interactions and self-amplifying individual cell ingression drive amniote gastrulation. eLife. 2014;3:e01817. doi: 10.7554/eLife.01817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Wynshaw-Boris A. The canonical Wnt pathway in early mammalian embryogenesis and stem cell maintenance/differentiation. Curr Opin Genet Dev. 2004;14:533–539. doi: 10.1016/j.gde.2004.07.013. [DOI] [PubMed] [Google Scholar]
- Wang Y, Botvinick EL, Zhao Y, Berns MW, Usami S, Tsien RY, Chien S. Visualizing the mechanical activation of Src. Nature. 2005;434:1040–1045. doi: 10.1038/nature03469. [DOI] [PubMed] [Google Scholar]
- Warmflash A, Sorre B, Etoc F, Siggia ED, Brivanlou AH. A method to recapitulate early embryonic spatial patterning in human embryonic stem cells. Nat Methods. 2014;11:847–854. doi: 10.1038/nmeth.3016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wawrzak D, Métioui M, Willems E, Hendrickx M, de Genst E, Leyns L. Wnt3a binds to several sFRPs in the nanomolar range. Biochem Biophys Res Commun. 2007;357:1119–1123. doi: 10.1016/j.bbrc.2007.04.069. [DOI] [PubMed] [Google Scholar]
- Webb DJ, Donais K, Whitmore LA, Thomas SM, Turner CE, Parsons JT, Horwitz AF. FAK-Src signalling through paxillin, ERK and MLCK regulates adhesion disassembly. Nat Cell Biol. 2004;6:154–161. doi: 10.1038/ncb1094. [DOI] [PubMed] [Google Scholar]
- Weber GF, Bjerke MA, DeSimone DW. A mechanoresponsive cadherin-keratin complex directs polarized protrusive behavior and collective cell migration. Dev Cell. 2012;22:104–115. doi: 10.1016/j.devcel.2011.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wodarz A, Nusse R. Mechanisms of Wnt signaling in development. Annu Rev Cell Dev Biol. 1998;14:59–88. doi: 10.1146/annurev.cellbio.14.1.59. [DOI] [PubMed] [Google Scholar]
- Yeung T, Georges PC, Flanagan LA, Marg B, Ortiz M, Funaki M, Zahir N, Ming W, Weaver V, Janmey PA. Effects of substrate stiffness on cell morphology, cytoskeletal structure, and adhesion. Cell Motil Cytoskeleton. 2005;60:24–34. doi: 10.1002/cm.20041. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


