Abstract
What is following the impressive progress that has been made? During the last couple of years several tremors have shaken the field of Transfusion Medicine. The epicentres of those tremors were located on novel insights into the RBC storage lesion, on emerging connections between storage lesion and post-transfusion performance and effects, and on acknowledging that storage time is only one (rather than the most prominent) of the parameters which contribute to the progression of storage lesion in any given unit of blood. The optimisation of bio-preservation conditions emerged at the same time with all-new scientific knowledge gained by advances in research tools, implementation of technological innovations, and application of elegant in vitro and in vivo models of transfusion. Simultaneously, one after another, all the reported randomised clinical trials concluded, with spectacular consensus, that there is no significant difference in the rate of adverse clinical events (including death) among patients who underwent transfusion with fresh (and presumably good) or standard of care (and presumably bad) blood. The comparative analysis and comprehension of the aforementioned data would set the context for the next generation of research in blood transfusion science, since the need for safer and more efficient transfusions remains.
Keywords: red blood cell storage lesion, donor/recipient variation, transfusion outcome, clinical relevance, research opportunities
Back to basics
When Dern and his colleagues revealed a significant donor to donor variability in erythrocyte storage characteristics in 19661, they probably did not imagine that studying the impact of a donor's intrinsic biological "signature" would not only be relevant but truly revived almost 50 years later. Nowadays, increasingly more studies focus on the impact of donor characteristics on red blood cell storage lesion profile and transfusion outcomes2. The rationale behind those donor-oriented studies was similar to that widely adopted in solid organ or bone marrow transplantation research3. Moreover, metabolites crucial for red blood cell (RBC) physiology, such as glutathione, might be considered inherited characteristics that retain their donor-dependent pre-storage dynamics4. In the same context, several RBC and plasma characteristics, including osmotic fragility and plasma antioxidant capacity, share the ability to characterise in part the blood unit already at the time of donation, since they fluctuate during storage in close relation to their pre-storage and donor-dependent levels5. In addition, factors apparently irrelevant to storage quality, such as serum uric acid, can also affect storage lesion metrics in blood units6. Despite being based on low-quality evidence from 59 studies, female donor sex, positive white blood cell antibodies, HLA-DR and RBC antigen selection were identified as unique donor characteristics with potential impact on RBC transfusion recipient outcomes2. The realisation that distinct groups of donors demonstrate different degrees of storage lesion progression, or post-transfusion performance, offers the opportunity to design a blood logistics model for optimal selection of blood donors and donor-recipient matching. In fact, the donor's genetic background is not neutral in respect to storage lesion progression, as RBC units from donors with clinically silent familial pseudohyperkalaemia, for instance, exhibit increased potassium accumulation in the supernatant at the early storage period7, while RBC units from G6PDH deficient donors demonstrate poor post-transfusion performance in vitro8. Further proteomic analyses that produce large amounts of data have demonstrated donor-associated, storage induced changes that can be attributed to intrinsic variation in specific oxidative markers9. Apart from the genetic context, lifestyle factors, dietary habits and, probably, the frequency of blood donation are also strong contributing factors to RBC physiology, and probably to storage quality10. To sum up, the study of donor variation effects offers a golden opportunity to clarify those aspects of the storage lesion that are not related to the duration of storage and the conventional ageing of stored RBCs. Although seemingly opposed to the recently reported conclusions of the randomised clinical trials showing that the age of blood is not related to post-transfusion mortality and morbidity in those specific settings11, donor variation considerations stand side by side with those trials by appreciating time as only one among the many parameters that affect the quality of blood labile product. As a matter of fact, any study focused on donor properties has the ability to detect storage- and/or transfusion-associated differences between blood units of exactly the same age, although the evidence collected so far is insufficient to draw definite conclusions about the clinical relevance of those differences for any donor characteristics. Assessment of the relativity of time and of the cell-to-organism complexity of the biological systems would inevitably lead to the acknowledgement that donor variability stands hierarchically above the age of stored blood.
Gazing ahead to the future: (re)searching in the new era
Speaking of time and relativism, recent studies from pioneer groups in the field re-defined the meaning of time in respect to RBC ageing and storage lesion by using state-of-the-art metabolomic analyses12. Their work means we are now on the verge of establishing a new way of measuring stored RBCs' age, besides the conventional approach, by reading the signature of metabolic ageing in stored RBCs. After all, measuring storage time in μM/mM of equivalent metabolites instead of days seems extremely interesting. Extracting this kind of information would not be possible at all without the development of omics technologies and their application to blood storage. New generation proteomics platforms have provided the opportunity for absolute quantification that resulted in the introduction of novel candidate protein biomarkers of RBC haemolysis and vesiculation and, thus, of RBC quality during storage13. At the same time, metabolomics analyses have revealed an impairment of energy and redox metabolism in RBCs, like the storage-dependent reversible oxidation of glyceraldehyde-3-phosphate dehydrogenase (GAPDH), which promotes metabolic reprogramming during storage14, while several metabolic features of the storage lesion are found to be heritable (thus highly donor-related) in human or mouse models15,16. In addition, contemporary advances in optical and non-optical high-resolution technologies allow for the thorough study of extracellular vesicles generated in the bag in order to assess their possible clinical relevance17. This panoply of data acquired by the application of innovative techniques to blood units has offered challenging opportunities in Transfusion Medicine research and identified numerous parameters with a storage and/or transfusion-outcome biomarker potential. Even after sorting the data using bioinformatics tools, the need to link storage lesion variables with transfusion performance remains vital. Thus, the introduction of in vitro models of transfusion has been proved very helpful for a first-line evaluation of the post-transfusion phenotype8,18. In a second step, transition to the in vivo state, by using animal models of transfusion, provides further insight into the correlation between storage quality and transfusion effects19 and, eventually, both types of models fuel extended clinical trials in humans. Bearing in mind that all of the above-mentioned approaches for studying post-transfusion efficacy and effects have their own pros and cons, it would be really informative to combine them, focusing on what each of them can provide instead of what each of them may conceal based on their intrinsic limitations20. Therefore, future clinical trials designed on the basis of more reliable and upward tested/checked input (and output) parameters would help in clarifying current uncertainties and controversial issues. Advances in omics and small particle biology technologies might permit the establishment of a large donor-to-recipient data infrastructure to achieve a robust assessment of the clinical relevance of various blood donor characteristics. In this context, the Recipient Epidemiology and Donor Evaluation Study-III (REDS-III) premier research of the National Health, Lung and Blood Institute (NHLBI) programme21, which involves basic, translational and clinical research, has committed to the innovative development of comprehensive databases which will link information on donor/donation/components to that of transfused recipients (compared to untransfused controls) at all participating hospitals. These cumulative databases, will contribute to address key research questions in blood banking and Transfusion Medicine, and inform blood policy decisions.
The story of a dog chasing his own tail: the transfusion paradox
Both assessment and interpretation of clinical trials are of high importance for the evolution of Transfusion Medicine services. Despite research opportunities offered by the strictly controlled system of a blood unit to biomedical sciences, donated blood and its components represent precious therapeutic substances of human origin that are limited by their very nature. Consequently, it makes sense that the primary outcome measured by almost all of the recent randomised clinical trials was the ultimate human good, namely survival22,23. On the other hand, owing to the numerous systemic factors implicated, the outcome of a specific transfusion is by default a highly complex, multifaceted phenomenon. When evaluating the effects of a given transfusion therapy, one must take into account not only the variability of the blood components used (donor, processing and storage strategy variations), but also the specific biomedical context of the treated recipient in need (recipient variation), similar to the strategic planning of the REDS-III programme. As a result, the paradox lies in the heart of the approach chosen. Although in vivo studies aim to overcome the limitations of in vitro human models in evaluating post-transfusion effects, instead of this, they unintentionally feed and multiply the complexity of the findings and their interpretation. In other words, the combination of storage lesion variables (probably related to post-transfusion efficacy) with the infinite systemic factors of the recipient, results in an exponential output of possible conditions rather than a cumulative one. To support this concept, although lower 24-hour post-transfusion recovery of stored G6PDH-deficient RBCs was reported about fifty years ago, studies on post-transfusion haemolysis have provided contradictory data, highlighting the presence of an uncharted universe of interactions and crosstalk (between storage, processing, donor and recipient) that take place during or soon after transfusion therapy24. In that case, retrospective studies regarding the efficacy of transfusion or its adverse effects for distinct groups of recipients treated with standard practice or (as much as possible) "equal" blood units might be of great value. Moreover, in terms of transfusion, it is clear that "what you see (or measure) is not always what you get", as several aspects of an RBC storage lesion remain hidden. A set of sub-lethal lesions and defects are only evident under physiological or near-physiological levels of stress (osmotic, mechanical, biochemical, etc.) encountered in the recipient25. Nevertheless, it is really interesting that in our own studies, ex vivo haemolysis, the gold standard for blood bag quality assessment, and other haemolysis-related factors are found to be linked to donor-specific variation in almost 200 RBC or plasma parameters (Tzounakas M, unpublished data; 2017). These examples give only a glimpse of the complexity of transfusion-related research, pointing towards a more systemic approach to answer outstanding issues.
The end is the beginning is the end is…
Every end represents a new beginning and vice versa26. Novel means and new findings offer the chance for a new dawn in Transfusion Medicine research. Storage lesion and post-transfusion performance and effects represent two different worlds that are connected (?) by a still obscure link. Something has been lost in translation, since, to date, it still has to be proved that really important storage lesion parameters are crucial for post-transfusion metrics. This second paradox is partly owing to the swarm of pragmatic and multifaceted difficulties in the design and implementation of relevant studies. However, it is also well-fed by the strict, one-dimensional target orientation of the majority of the clinical trials performed. By representing the ultimate "gold" checkpoint for transfusion effects, they focus solely on the age of the blood component to measure obviously important things (such as mortality and other hospital metrics) but not on other metrics of patient status (tissue oxygenation, NO-biology issues, etc.) and established storage lesion metrics (e.g. extracellular vesicle levels and signalling) with probable severe effects on safety and efficacy issues27. Moreover, they did not take into account donor- and recipient-associated variables. As a result, such clinical trials cannot come to definitive conclusions on the impact of the storage lesion, even on the conventional age of stored blood, on transfusion outcomes, especially in massive transfusion and traumatic haemorrhagic shock contexts. For instance, what is the impact of transfusing a bolus of donor-specific communicable extracellular vesicles along with a set of vesiculation triggers in specific groups of patients well-characterised by overproduction of bio-active, pro-inflammatory/pro-coagulant vesicles or by a vesiculation-prone endothelium? A recent longitudinal cohort study focused on donor variation parameters such as age and sex showed that RBCs transfusions from younger or female donors were associated with increased mortality28. Moreover, another prospective, observational study in critically ill children found that post-transfusion haemolysis was independent of RBC storage duration; in contrast, most storage duration effects on haemolysis were overwhelmed by recipient and/or donor factors29.
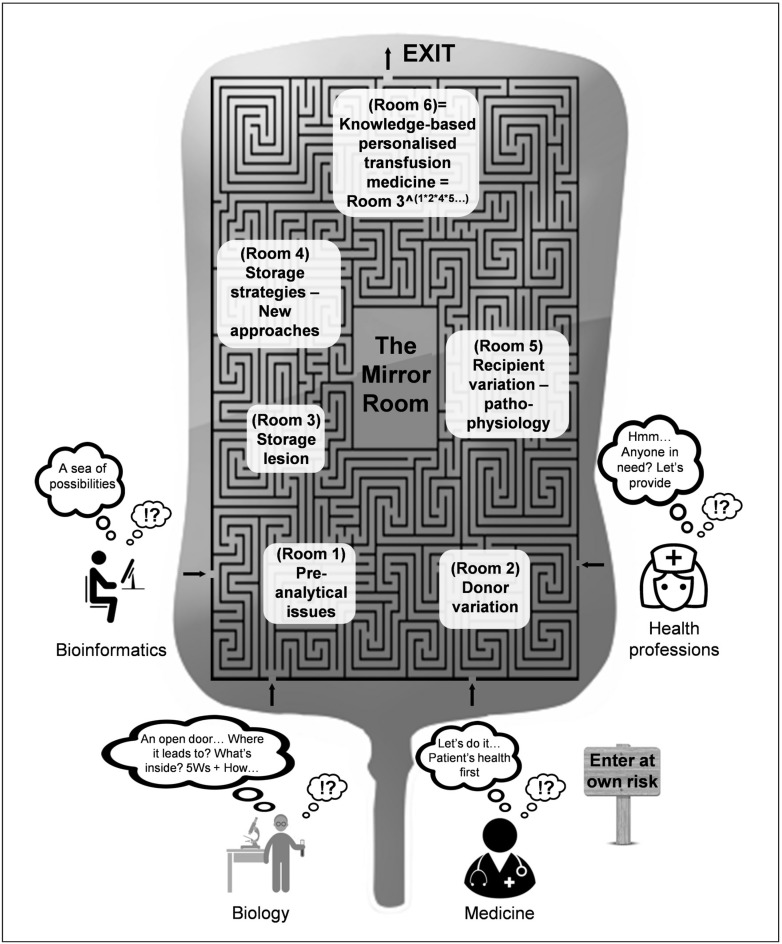
Given the influence and impact of donor characteristics on numerous bio-medical settings, it is probably time to re-evaluate research priorities. The novel technologies in combination with established post-transfusion research tools (including in vitro and in vivo models of transfusion), pave the way for a better understanding of the storage lesion and effects. The field is in constant evolution, from evidence-based, cohort Transfusion Medicine, to knowledge-based, personalised Transfusion Medicine. The path to the core of transfusion research resembles a labyrinth, since there are many ways of entry, but only one way out (Figure 1). This particular journey might prove to be more difficult than we anticipate, since several pieces of the labyrinth are in fact, a mirror maze. We have all entered through different doorways and we follow distinct scientific paths that (sometimes) cross each other. Nevertheless, we all seek the same exit. The odds are favourable for the members of the transfusion research community to find their way out of the labyrinth.
Figure 1.
The labyrinth of research in transfusion biology and medicine and its aspects.
Let us enjoy this fascinating, mind-opening journey. Let us be prepared for what we cannot see and expect the unexpected.
Footnotes
The Authors declare no conflicts of interest.
References
- 1.Dern RJ, Gwinn RP, Wiorkowski JJ. Studies on the preservation of human blood. I. Variability in erythrocyte storage characteristics among healthy donors. J Lab Clin Med. 1966;67:955–65. [PubMed] [Google Scholar]
- 2.Chasse M, McIntyre L, English SW, et al. Effect of blood donor characteristics on transfusion outcomes: a systematic review and meta-analysis. Transfus Med Rev. 2016;30:69–80. doi: 10.1016/j.tmrv.2016.01.002. [DOI] [PubMed] [Google Scholar]
- 3.Kollman C, Howe CW, Anasetti C, et al. Donor characteristics as risk factors in recipients after transplantation of bone marrow from unrelated donors: the effect of donor age. Blood. 2001;98:2043–51. doi: 10.1182/blood.v98.7.2043. [DOI] [PubMed] [Google Scholar]
- 4.van ’t Erve TJ, Wagner BA, Ryckman KK, et al. The concentration of glutathione in human erythrocytes is a heritable trait. Free Radic Biol Med. 2013;65:742–9. doi: 10.1016/j.freeradbiomed.2013.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tzounakas VL, Georgatzakou HT, Kriebardis AG, et al. Donor variation effect on red blood cell storage lesion: a multivariable, yet consistent, story. Transfusion. 2016;56:1274–86. doi: 10.1111/trf.13582. [DOI] [PubMed] [Google Scholar]
- 6.Tzounakas VL, Georgatzakou HT, Kriebardis AG, et al. Uric acid variation among regular blood donors is indicative of red blood cell susceptibility to storage lesion markers: A new hypothesis tested. Transfusion. 2015;55:2659–71. doi: 10.1111/trf.13211. [DOI] [PubMed] [Google Scholar]
- 7.Bawazir WM, Flatt JF, Wallis JP, et al. Familial pseudohyperkalemia in blood donors: a novel mutation with implications for transfusion practice. Transfusion. 2014;54:3043–50. doi: 10.1111/trf.12757. [DOI] [PubMed] [Google Scholar]
- 8.Tzounakas VL, Kriebardis AG, Georgatzakou HT, et al. Glucose 6-phosphate dehydrogenase deficient subjects may be better "storers" than donors of red blood cells. Free Radic Biol Med. 2016;96:152–65. doi: 10.1016/j.freeradbiomed.2016.04.005. [DOI] [PubMed] [Google Scholar]
- 9.Rinalducci S, D'Amici GM, Blasi B, et al. Peroxiredoxin-2 as a candidate biomarker to test oxidative stress levels of stored red blood cells under blood bank conditions. Transfusion. 2011;51:1439–49. doi: 10.1111/j.1537-2995.2010.03032.x. [DOI] [PubMed] [Google Scholar]
- 10.Tzounakas VL, Kriebardis AG, Papassideri IS, Antonelou MH. Donor-variation effect on red blood cell storage lesion: a close relationship emerges. Proteomics Clin Appl. 2016;10:791–804. doi: 10.1002/prca.201500128. [DOI] [PubMed] [Google Scholar]
- 11.Carson JL, Guyatt G, Heddle NM, et al. Clinical practice guidelines from the AABB: Red blood cell transfusion thresholds and storage. JAMA. 2016;316:2025–35. doi: 10.1001/jama.2016.9185. [DOI] [PubMed] [Google Scholar]
- 12.Paglia G, D'Alessandro A, Rolfsson O, et al. Biomarkers defining the metabolic age of red blood cells during cold storage. Blood. 2016;128:e43–50. doi: 10.1182/blood-2016-06-721688. [DOI] [PubMed] [Google Scholar]
- 13.D'Alessandro A, Dzieciatkowska M, Hill RC, Hansen KC. Supernatant protein biomarkers of red blood cell storage hemolysis as determined through an absolute quantification proteomics technology. Transfusion. 2016;56:1329–39. doi: 10.1111/trf.13483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Reisz JA, Wither MJ, Dzieciatkowska M, et al. Oxidative modifications of glyceraldehyde 3-phosphate dehydrogenase regulate metabolic reprogramming of stored red blood cells. Blood. 2016;128:e32–42. doi: 10.1182/blood-2016-05-714816. [DOI] [PubMed] [Google Scholar]
- 15.Zimring JC, Smith N, Stowell SR, et al. Strain-specific red blood cell storage, metabolism, and eicosanoid generation in a mouse model. Transfusion. 2014;54:137–48. doi: 10.1111/trf.12264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.D’Alessandro A, Nemkov T, Yoshida T, et al. Citrate metabolism in red blood cells stored in additive solution-3. Transfusion. 2016 doi: 10.1111/trf.13892. [DOI] [PubMed] [Google Scholar]
- 17.Antonelou MH, Seghatchian J. Update on extracellular vesicles inside red blood cell storage units: adjust the sails closer to the new wind. Transfus Apher Sci. 2016;55:92–104. doi: 10.1016/j.transci.2016.07.016. [DOI] [PubMed] [Google Scholar]
- 18.Mittag D, Sran A, Chan KS, et al. Stored red blood cell susceptibility to in vitro transfusion-associated stress conditions is higher after longer storage and increased by storage in saline-adenine-glucose-mannitol compared to AS-1. Transfusion. 2015;55:2197–206. doi: 10.1111/trf.13138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.de Wolski K, Fu X, Dumont LJ, et al. Metabolic pathways that correlate with post-transfusion circulation of stored murine red blood cells. Haematologica. 2016;101:578–86. doi: 10.3324/haematol.2015.139139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zimring JC, Spitalnik SL. Scientific advances: fallacy of perfection harms peer review. Nature. 2016;537:34. doi: 10.1038/537034a. [DOI] [PubMed] [Google Scholar]
- 21.National Heart, Lung and Blood Institute, REDS-III Phase 2 Studies. [Accessed on 23/12/2016]. Available at: https://reds-iii.rti.org/ResearchStudies/Phase2Studies.aspx.
- 22.Lacroix J, Hebert PC, Fergusson DA, et al. Age of transfused blood in critically ill adults. N Engl J Med. 2015;372:1410–8. doi: 10.1056/NEJMoa1500704. [DOI] [PubMed] [Google Scholar]
- 23.Heddle NM, Cook RJ, Arnold DM, et al. Effect of short-term vs. long-term blood storage on mortality after transfusion. N Engl J Med. 2016;375:1937–45. doi: 10.1056/NEJMoa1609014. [DOI] [PubMed] [Google Scholar]
- 24.Francis RO, Jhang JS, Pham HP, et al. Glucose-6-phosphate dehydrogenase deficiency in transfusion medicine: the unknown risks. Vox Sang. 2013;105:271–82. doi: 10.1111/vox.12068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bosman GJ. Survival of red blood cells after transfusion: processes and consequences. Front Physiol. 2013;4:376. doi: 10.3389/fphys.2013.00376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Glynn SA, Klein HG, Ness PM. The red blood cell storage lesion: the end of the beginning. Transfusion. 2016;56:1462–8. doi: 10.1111/trf.13609. [DOI] [PubMed] [Google Scholar]
- 27.Petrik J, Seghatchian J. Big things from small packages: the multifaceted roles of extracellular vesicles in the components quality, therapy and infection. Transfus Apher Sci. 2016;55:4–8. doi: 10.1016/j.transci.2016.07.008. [DOI] [PubMed] [Google Scholar]
- 28.Chasse M, Tinmouth A, English SW, et al. Association of blood donor age and sex with recipient survival after red blood cell transfusion. JAMA Intern Med. 2016;176:1307–14. doi: 10.1001/jamainternmed.2016.3324. [DOI] [PubMed] [Google Scholar]
- 29.L'Acqua C, Bandyopadhyay S, Francis RO, et al. Red blood cell transfusion is associated with increased hemolysis and an acute phase response in a subset of critically ill children. Am J Hematol. 2015;90:915–20. doi: 10.1002/ajh.24119. [DOI] [PMC free article] [PubMed] [Google Scholar]