Abstract
The introduction of omics technologies in the field of Transfusion Medicine has significantly advanced our understanding of the red cell storage lesion. While the clinical relevance of such a lesion is still a matter of debate, quantitative and redox proteomics approaches, as well quantitative metabolic flux analysis and metabolic tracing experiments promise to revolutionise our understanding of the role of blood processing strategies, inform the design and testing of novel additives or technologies (such as pathogen reduction), and evaluate the clinical relevance of donor and recipient biological variability with respect to red cell storability and transfusion outcomes. By reviewing existing literature in this rapidly expanding research endeavour, we highlight for the first time a correlation between metabolic markers of the red cell storage age and protein markers of haemolysis. Finally, we introduce the concept of metabolic linkage, i.e. the appreciation of a network of highly correlated small molecule metabolites which results from biochemical constraints of erythrocyte metabolic enzyme activities. For the foreseeable future, red cell studies will advance Transfusion Medicine and haematology by addressing the alteration of metabolic linkage phenotypes in response to stimuli, including, but not limited to, storage additives, enzymopathies (e.g. glucose 6-phosphate dehydrogenase deficiency), hypoxia, sepsis or haemorrhage.
Keywords: mass spectrometry, advanced omics, Transfusion Medicine, blood, storage
The red blood cell storage lesion(s) and clinical trials
Over the past ten years, concerns have been raised upon the publication of retrospective clinical evidence1 suggesting the potential negative association between storage “age of blood” and transfusion outcomes. Following these controversial observations, literature has burgeoned around the description of the so-called storage lesion(s), a wide series of biochemical and morphological alterations red blood cells (RBCs)2 undergo during storage in the blood bank. Many groups (as extensively reviewed3–6), including ours7–20, have contributed to document the energy and oxidative lesions targeting stored RBCs. Compelling biochemical rationale and laboratory evidence14–16,21–24 in vitro and in vivo (animal models25–27) have been produced to support the hypothesis that prolonged storage does not only negatively affect RBC physiology and functionality (e.g. gas transport18,22,28), but it also influences RBC survival in animal models in vivo29. These observations have led to question whether the storage lesion could thus impair the effectiveness of the transfusion therapy and likely mediate untoward transfusion-related events (e.g. transfusion-related acute lung injury [TRALI], transfusion-related immune modulation [TRIM]30–33) or aggravate underlying conditions (e.g. sepsis26,33). However, reassuring evidence from independent randomised clinical trials (RCTs)34–38 showed no detectable difference between fresh blood and standard of care at the limits of the statistical power of these studies, which prompted the field to conclude (as summarised in the most recent American Association of Blood Banks [AABB] guidelines39) that the general standard of care will not be improved by preferentially issuing fresh blood, at least to the specific categories of recipients enrolled in those RCTs. Many have noted the limitations of the RCTs40, as elegantly described in several of the papers appearing in this issue of Blood Transfusion. For example, none of the RCTs performed to date has actually compared the transfusion of fresh blood products vs products close to the end of their shelf-life owing to ethical concerns40. However, the general take home message from the RCTs is overall comforting and suggests that, and to quote AABB guidelines, “a restrictive transfusion threshold is safe in most clinical settings and the current blood banking practices of using standard-issue blood should be continued”39. Still, quoting Zimring and Spitalnik in this issue41, “when approximately 80 million RBC units are transfused annually worldwide, even vanishingly small (transfusion-associated negative) events, if they are real, can affect actual human lives; it then becomes a question of ethics and economics whether it is ‘worthwhile’ to study and attempt to prevent them”.
In the light of these considerations, welcoming the challenge to further advance the field of Transfusion Medicine, in 2016 the National Heart, Lung, Blood Institutes and Food and Drug Administration gathered leading experts in the field to identify current issues associated with blood storage and define an agenda to pursue the amelioration of blood storage strategies42,43. Discussions in these meetings highlighted the lack of consensus in the definition of parameters of transfusion efficacy, while classic parameters necessary to obtain FDA clearance for new packed RBC storage additives (haemolysis and 24-hour in vivo survival in autologous healthy volunteers) have been unanimously regarded as necessary, but not sufficient, to predict transfusion efficacy in the clinical setting. Several studies suggest that useful, often orthogonal, parameters can be obtained through modern omics technologies.
Omics markers of the RBC storage lesion
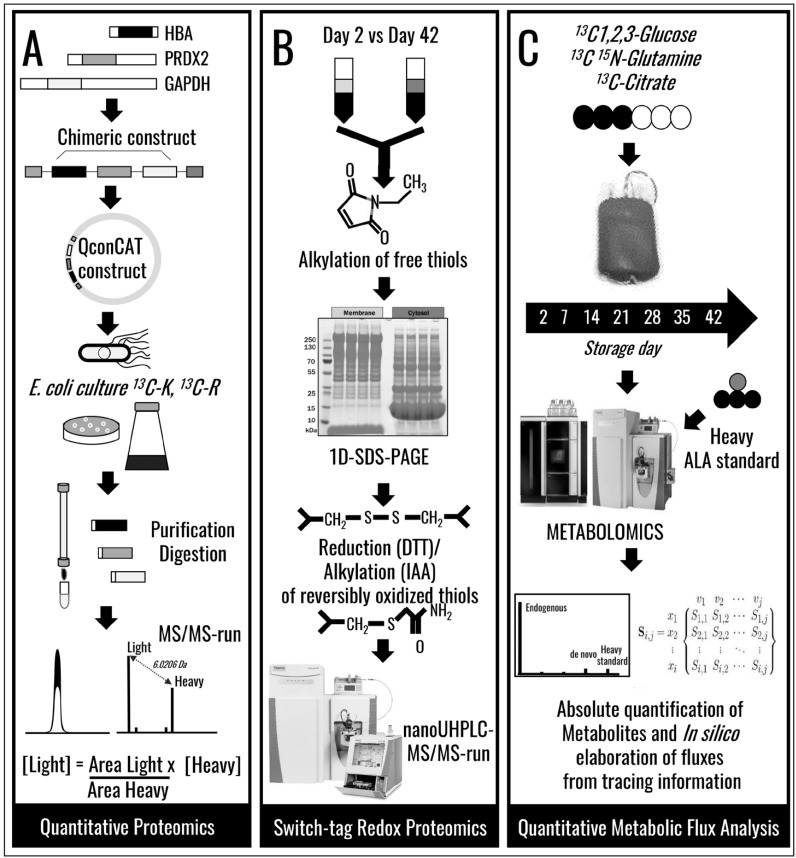
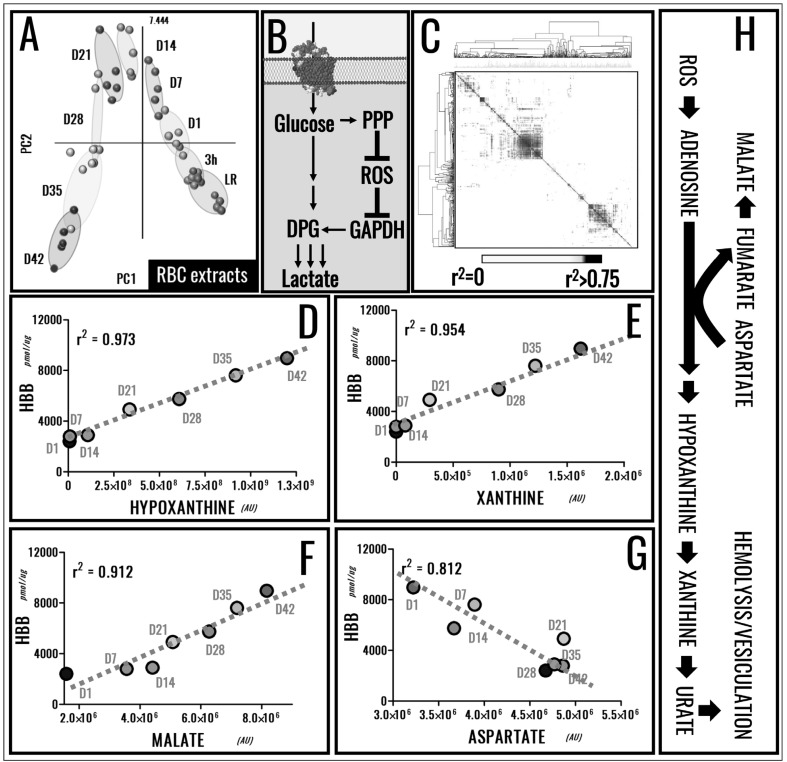
Over the last ten years44, the introduction of omics technologies in the field of Transfusion Medicine has brought about significant advancements in the understanding of the RBC storage lesion2,4,6,45. We can now perform state-of-the-art quantitative proteomics approaches (see Figure 1A for the QconCAT approach10,15,46,47) or redox proteomics analyses (e.g. switch-tag redox proteomics15,18; Figure 1B) to highlight the proteomics storage lesion with unprecedented specificity and sensitivity. In addition, recent advancements in the field of metabolomics enabled us not just to expand on our understanding of the metabolic storage lesion2,7,8,11,12,14,17,19,21,45,48–53, but also to perform ultra-high throughput54 quantitative tracing experiments with heavy labelled substrates14,15,55 to inform biomarker16 and metabolic flux analyses14,15,21,55 (Figure 1C). However, we17 and others21 have recently acknowledged that molecular appreciation of the storage lesion is but the first step in defining novel strategies to improve storage quality. Protein8,10,15,24 and metabolic markers13,14,16,21,51 of the RBC storage lesion have been proposed by us and others. The metabolic phenotype of stored RBCs follows a specific 3-stage sequence, as gleaned through multivariate analysis of metabolomics data of SAGM and AS-3 RBCs (Figure 2A)16. Metabolic reprogramming of stored RBCs has been associated with the necessity to restore reducing equivalents in order to counteract oxidative stress to functional proteins, such as haemoglobins18 and anti-oxidant enzymes (e.g. peroxiredoxin 28,24). Of note, these redox systems appear to be linked, in that irreversible thiol oxidation of cysteine 93 of haemoglobin beta (a residue necessary for haemoglobin S-nitrosylation and thus haemoglobin-mediated nitric oxide signalling) impairs recycling of the oxidised active site cysteine of peroxiredoxin 2, inhibiting catalysis22. Oxidative lesion(s) to stored RBCs also affect redox sensitive amino acid residues in active site pocket of glyceraldheyde 3-phosphate dehydrogenase (GAPDH)15,23, a biochemical adaptation that promotes RBC metabolic reprogramming from glycolysis to NADPH-generating pentose phosphate pathway (PPP) in response to oxidative stress (Figure 2B). However, these salvage mechanisms appear to be insufficient to fully counteract the oxidative lesion, resulting in the progressive release of irreversibly oxidised/functionally impaired proteins and small molecule metabolites (including oxidised lipids) into packed RBC supernatants8,15,18,23,24,31,48. Of note, protein and metabolite markers of the RBC storage lesion show significant correlations among each other (Figure 2C–G), resulting from the elaboration of data from our previous publications10,13,16. Importantly, there is a significant correlation between the levels of metabolic markers of the storage age and the absolute concentrations of supernatant haemoglobin (Figure 2D–G), a marker of RBC storage haemolysis/vesiculation, as we recently proposed10. Though merely correlative, these observations are relevant in that they suggest that monitoring RBC markers of the metabolic age could provide information about the quality of stored RBCs and potentially predict the effectiveness of the transfusion therapy, in addition to guiding the design and development of novel storage strategies/additives to decrease RBC storage haemolysis.
Figure 1.
Advancements in omics technologies for Transfusion Medicine applications.
(A) An overview of the QconCAT approach for quantitative proteomics10 and (B) switch-tag approach for redox proteomics applications15,18. (C) An overview of a tracing quantitative metabolic experiment, a workflow that can be exploited to inform quantitative metabolic flux analysis elaboration with systems biology tools.
Figure 2.
Metabolic markers of the red blood cell (RBC) storage lesion have been identified through statistical analysis, such as Partial least-square discriminant analysis (A) and receiver operating characteristic curves (ROC)16,21.
A combination of redox proteomics, quantitative proteomics and metabolic flux analyses has revealed a role for the oxidative stress-dependent regulation of glyceraldehyde 3-phosphate dehydrogenase (GAPDH) activity in mediating the activation of the pentose phosphate pathway (PPP) to generate reducing equivalents in the attempt to counteract oxidative stress over storage15,23. (B). Correlative analysis of metabolic13 and protein markers10 of the storage lesion was here performed and plotted as a heat map (black = R>0.75) (C). Of note, metabolic markers of the RBC storage age correlated with the absolute concentration of supernatant haemoglobin over storage, a marker of RBC vesiculation and haemolysis (D–G). Though only correlative analyses are here provided, it is interesting to note that all the metabolites showing the highest positive correlations with supernatant haemoglobin were part of the purine catabolism/salvage pathway, a pathway that is activated by oxidative lesion to the purine nucleoside pool and is in part counteracted by salvage reactions fueled by aspartate consumption and resulting in fumaratemalate accumulation (H).
From omics markers of the storage lesion to in vivo survival and haemolysis
Some of the metabolic markers of the storage lesion are not just related to energy metabolism, but also to purine homeostasis and oxidation, such as the adenosine triphosphate catabolites hypoxanthine and xanthine16,51. Oxidised lipids and purines that accumulate in packed RBC supernatants during refrigerated storage correlate with in vivo performances of transfused cells in animal models29. These observations are suggestive of the likely clinical relevance of the storage lesion, in that it is a safe statement to conclude that, to function, RBCs must at least circulate. Similarly, the metabolic phenotypes (especially in terms of energy and redox homeostasis) correlate with RBC storage haemolysis, both parameters being affected by the genetic phenotype of the donor56–58. Studies on RBC storage haemolysis and 24-hour in vivo survival suggest that there is room for improvement of the current processing and storage strategies. A large retrospective study of radiolabelled RBC recoveries in autologous healthy volunteers (n=641) by Dumont and Aubuchon reported that end-of-storage RBCs have recoveries of 82.4±6.7%59. These numbers indicate that approximately 17% of the RBCs in a transfused unit are lost during storage and transfusion to healthy volunteers59. In the light of these data, it is reasonable to assume that heterologous chronically or massively transfused recipients would respond to blood transfusion differently to autologous healthy volunteer recipients owing to their repeated exposure to allogeneic cells or the underlying pro-inflammatory/metabolically-deranged physiology, respectively. Donor and recipient biological variability have often been overlooked in laboratory and clinical studies of the RBC storage lesion, a trend that has been rapidly changing in recent years60–64. It is still not fully understood whether omics phenotypes of stored RBCs are affected by donor variability and whether that relates to RBC in vivo performances and resistance to the storage lesion, such as in particular haemolysis. Studies such as the REDS III Omics initiative will tackle this important issue in the coming years65.
Biological variability, metabolic poise and enzymatic constraints: introducing the concept of metabolic linkage
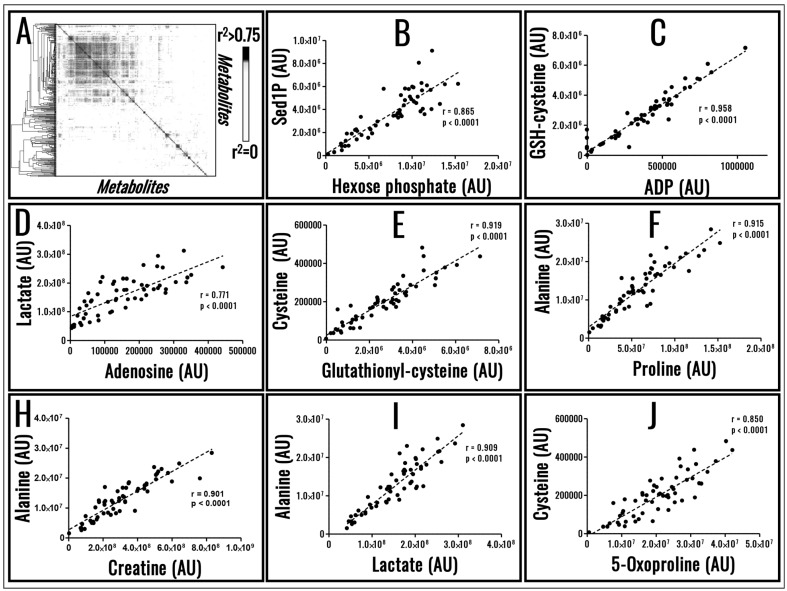
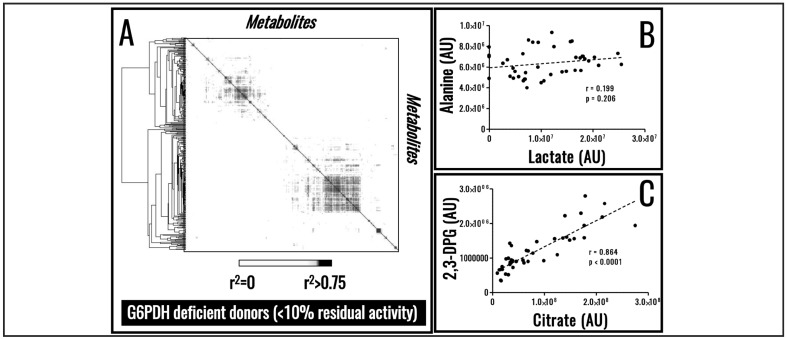
Despite biological variability, RBC metabolism has evolved to preserve the metabolic poise. To achieve this task, RBC metabolism is tightly regulated by biochemical constraints as a result of evolutionarily conserved enzymatic activities, a phenomenon that can be appreciated through systems biology in silico elaboration of RBC metabolism66. Owing to such biochemical constraints, small molecule metabolites do not just show extremely high correlations with storage age16, but also among each other (Figure 3A–J), a phenomenon we refer to as metabolic linkage. We believe that future advancements in the field of Transfusion Medicine17, personalised medicine and clinical biochemistry67 will be driven by the appreciation of the metabolic linkage and how such linkage is affected by various stimuli; e.g. metabolic responses to hypoxia under physiological (high altitude68–70) or pathological (haemorrhagic shock71 or sepsis72) conditions. Similar considerations may also apply with respect to enzymopathies and the role they may play within the framework of the RBC storage lesion. One paradigmatic example is the case of glucose 6-phosphate dehydrogenase deficiency, the most common human enzymopathy that, depending on the variant and thus specific enzymatic mutation, results in different metabolic reprogramming that can affect RBC capacity to cope with stresses upon storage and transfusion into recipients73,74. Elaboration of data available in the literature reveals that glucose 6-phosphate dehydrogenase deficient donors are characterised by a re-arranged metabolic linkage phenotype (Figure 4A in comparison to Figure 3A), resulting in the loss of significant correlations between some of the metabolites whose level are linked in healthy donors (e.g. lactate and alanine; Figure 4B in comparison to Figure 3I). On the other hand, as a result of a metabolic re-wiring as previously suggested74, novel significant correlations emerge (e.g. citrate and 2,3-diphosphoglycerate; Figure 4C), suggesting that recently appreciated metabolic pathways in stored RBCs, such as cytosolic metabolism of citrate in mitochondria-deficient RBCs14, may play a key role in preserving reducing equivalent homeostasis through alternate pathways in RBCs from glucose 6-phosphate dehydrogenase individuals.
Figure 3.
Metabolic linkage. Metabolite levels in stored red blood cells (RBCs) are significantly correlated, a phenomenon that is explained by enzymatic biochemical constraints and here defined as metabolic linkage (A).
A few examples of metabolites showing significant correlations among each other in 60 packed RBC extracts sampled at random storage time points is shown in (B–J). Figures and panels are the result of the elaborations of metabolomics analyses of samples kindly provided by Dr. Eldad Hod at Columbia University, NY, USA.
Figure 4.
Enzymopathies affect the metabolic linkage.
Determination of the metabolic linkage in stored red blood cells (RBCs) from glucose 6-phosphate dehydrogenase deficient donors reveals a re-wiring of RBC metabolism (A). As a result, significant correlations observed in healthy volunteers are lost (B), while novel ones appear (C). Figures and panels are the result of the elaborations of freely available data from Tzounakas et al.74.
Thesis, antithesis and synthesis
As for the three moments of Hegelian philosophy, the transfusion community at first hypothesised that storage age affected the safety and effectiveness of the transfusion therapy (thesis). Despite laboratory and retrospective clinical evidence, reassuring reports from the recent randomised clinical trials have suggested that the current standard of care is not inferior to the transfusion of the freshest units available (antithesis). For the foreseeable future, the combination of omics technologies and clinical evidence, through ambitious studies like the REDS III Omics trial65, will enhance our understanding of the effects of handling processes (e.g. leucoreduction, storage additives) and donor/recipient biological variability, and likely reconcile (synthesis) the apparent inconsistencies of the past ten years of Transfusion Medicine research.
Acknowledgements
ADA received funds from the National Blood Foundation, Early Career Grant 2016 cycle. We would like to acknowledge that Figure 4 of this paper results from an elaboration of freely accessible online data kindly made available by Dr. Antoleou’s group, as referenced. Figure 3 is based on elaborations of metabolomics analyses of samples kindly provided by Dr. Eldad Hod at Columbia University, NY, USA.
Footnotes
Disclosure of conflicts of interest
Though unrelated to the contents of the manuscript, the Authors disclose that ADA, TN and KCH are part of Endura LLC. ADA is a consultant for New Health Sciences Inc. The other Authors declare no conflicts of interest.
References
- 1.Koch CG, Li L, Sessler DI, et al. Duration of Red-Cell Storage and Complications after Cardiac Surgery. N Engl J Med. 2008;358:1229–39. doi: 10.1056/NEJMoa070403. [DOI] [PubMed] [Google Scholar]
- 2.D’Alessandro A, Kriebardis AG, Rinalducci S, et al. An update on red blood cell storage lesions, as gleaned through biochemistry and omics technologies. Transfusion. 2015;55:205–19. doi: 10.1111/trf.12804. [DOI] [PubMed] [Google Scholar]
- 3.Zolla L, D’alessandro A, Rinalducci S, et al. Classic and alternative red blood cell storage strategies: seven years of “-omics” investigations. Blood Transfus. 2015;13:21–31. doi: 10.2450/2014.0053-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Glynn SA, Klein HG, Ness PM. The red blood cell storage lesion: the end of the beginning. Transfusion. 2016;56:1462–8. doi: 10.1111/trf.13609. [DOI] [PubMed] [Google Scholar]
- 5.Zimring JC. Established and theoretical factors to consider in assessing the red cell storage lesion. Blood. 2015;125:2185–90. doi: 10.1182/blood-2014-11-567750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hess JR. RBC storage lesions. Blood. 2016;128:1544–5. doi: 10.1182/blood-2016-08-729541. [DOI] [PubMed] [Google Scholar]
- 7.D’Alessandro A, Hansen KC, Silliman CC, et al. Metabolomics of AS-5 RBC supernatants following routine storage. Vox Sang. 2015;108:131–40. doi: 10.1111/vox.12193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.D’Alessandro A, D’Amici GM, Vaglio S, Zolla L. Time-course investigation of SAGM-stored leukocyte-filtered red bood cell concentrates: from metabolism to proteomics. Haematologica. 2012;97:107–15. doi: 10.3324/haematol.2011.051789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.D’Alessandro A, Mirasole C, Zolla L. Haemoglobin glycation (Hb1Ac) increases during red blood cell storage: a MALDI-TOF mass-spectrometry-based investigation. Vox Sang. 2013;105:177–80. doi: 10.1111/vox.12029. [DOI] [PubMed] [Google Scholar]
- 10.D’Alessandro A, Dzieciatkowska M, Hill RC, Hansen KC. Supernatant protein biomarkers of red blood cell storage hemolysis as determined through an absolute quantification proteomics technology. Transfusion. 2016;56:1329–39. doi: 10.1111/trf.13483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.D’Alessandro A, Gevi F, Zolla L. Red blood cell metabolism under prolonged anaerobic storage. Mol Biosyst. 2013;9:1196–209. doi: 10.1039/c3mb25575a. [DOI] [PubMed] [Google Scholar]
- 12.D’Alessandro A, Nemkov T, Hansen KC, et al. Red blood cell storage in additive solution-7 preserves energy and redox metabolism: a metabolomics approach. Transfusion. 2015;55:2955–66. doi: 10.1111/trf.13253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.D’Alessandro A, Nemkov T, Kelher M, et al. Routine storage of red blood cell (RBC) units in additive solution-3: a comprehensive investigation of the RBC metabolome. Transfusion. 2015;55:1155–68. doi: 10.1111/trf.12975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.D’Alessandro A, Nemkov T, Yoshida T, et al. Citrate metabolism in red blood cells stored in additive solution-3. Transfusion. 2016 doi: 10.1111/trf.13892. [DOI] [PubMed] [Google Scholar]
- 15.Reisz JA, Wither MJ, Dzieciatkowska M, et al. Oxidative modifications of glyceraldehyde 3-phosphate dehydrogenase regulate metabolic reprogramming of stored red blood cells. Blood. 2016;128:e32–42. doi: 10.1182/blood-2016-05-714816. [DOI] [PubMed] [Google Scholar]
- 16.Paglia G, D’Alessandro A, Rolfsson Ó, et al. Biomarkers defining the metabolic age of red blood cells during cold storage. Blood. 2016;128:e43–50. doi: 10.1182/blood-2016-06-721688. [DOI] [PubMed] [Google Scholar]
- 17.Nemkov T, Hansen KC, Dumont LJ, D’Alessandro A. Metabolomics in transfusion medicine. Transfusion. 2016;56:980–93. doi: 10.1111/trf.13442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wither M, Dzieciatkowska M, Nemkov T, et al. Hemoglobin oxidation at functional amino acid residues during routine storage of red blood cells. Transfusion. 2016;56:421–6. doi: 10.1111/trf.13363. [DOI] [PubMed] [Google Scholar]
- 19.Dumont LJ, D’Alessandro A, Szczepiorkowski ZM, Yoshida T. CO2-dependent metabolic modulation in red blood cells stored under anaerobic conditions. Transfusion. 2016;56:392–403. doi: 10.1111/trf.13364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Blasi B, D’Alessandro A, Ramundo N, Zolla L. Red blood cell storage and cell morphology. Transfus Med Oxf Engl. 2012;22:90–6. doi: 10.1111/j.1365-3148.2012.01139.x. [DOI] [PubMed] [Google Scholar]
- 21.Bordbar A, Johansson PI, Paglia G, et al. Identified metabolic signature for assessing red blood cell unit quality is associated with endothelial damage markers and clinical outcomes. Transfusion. 2016;56:852–62. doi: 10.1111/trf.13460. [DOI] [PubMed] [Google Scholar]
- 22.Harper VM, Oh JY, Stapley R, et al. Peroxiredoxin-2 recycling is inhibited during erythrocyte storage. Antioxid Redox Signal. 2015;22:294–307. doi: 10.1089/ars.2014.5950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rinalducci S, Marrocco C, Zolla L. Thiol-based regulation of glyceraldehyde-3-phosphate dehydrogenase in blood bank-stored red blood cells: a strategy to counteract oxidative stress. Transfusion. 2015;55:499–506. doi: 10.1111/trf.12855. [DOI] [PubMed] [Google Scholar]
- 24.Rinalducci S, D’Amici GM, Blasi B, et al. Peroxiredoxin-2 as a candidate biomarker to test oxidative stress levels of stored red blood cells under blood bank conditions. Transfusion. 2011;51:1439–49. doi: 10.1111/j.1537-2995.2010.03032.x. [DOI] [PubMed] [Google Scholar]
- 25.Gilson CR, Kraus TS, Hod EA, et al. A novel mouse model of red blood cell storage and posttransfusion in vivo survival. Transfusion. 2009;49:1546–53. doi: 10.1111/j.1537-2995.2009.02173.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hod EA, Zhang N, Sokol SA, et al. Transfusion of red blood cells after prolonged storage produces harmful effects that are mediated by iron and inflammation. Blood. 2010;115:4284–92. doi: 10.1182/blood-2009-10-245001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Solomon SB, Wang D, Sun J, et al. Mortality increases after massive exchange transfusion with older stored blood in canines with experimental pneumonia. Blood. 2013;121:1663–72. doi: 10.1182/blood-2012-10-462945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tsai AG, Hofmann A, Cabrales P, Intaglietta M. Perfusion vs. oxygen delivery in transfusion with “fresh” and “old” red blood cells: the experimental evidence. Transfus Apher Sci. 2010;43:69–78. doi: 10.1016/j.transci.2010.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.de Wolski K, Fu X, Dumont LJ, et al. Metabolic pathways that correlate with post-transfusion circulation of stored murine red blood cells. Haematologica. 2016;101:578–86. doi: 10.3324/haematol.2015.139139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zubair AC. Clinical impact of blood storage lesions. Am J Hematol. 2010;85:117–22. doi: 10.1002/ajh.21599. [DOI] [PubMed] [Google Scholar]
- 31.Silliman CC, Voelkel NF, Allard JD, et al. Plasma and lipids from stored packed red blood cells cause acute lung injury in an animal model. J Clin Invest. 1998;101:1458–67. doi: 10.1172/JCI1841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Silliman CC, Ambruso DR, Boshkov LK. Transfusion-related acute lung injury. Blood. 2005;105:2266–73. doi: 10.1182/blood-2004-07-2929. [DOI] [PubMed] [Google Scholar]
- 33.Janz DR, Zhao Z, Koyama T, et al. Longer storage duration of red blood cells is associated with an increased risk of acute lung injury in patients with sepsis. Ann Intensive Care. 2013;3:33. doi: 10.1186/2110-5820-3-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dhabangi A, Ainomugisha B, Cserti-Gazdewich C, et al. Effect of transfusion of red blood cells with longer vs shorter storage duration on elevated blood lactate levels in children with severe anemia: the TOTAL randomized clinical trial. JAMA. 2015;314:2514–23. doi: 10.1001/jama.2015.13977. [DOI] [PubMed] [Google Scholar]
- 35.Fergusson DA, Hébert P, Hogan DL, et al. Effect of fresh red blood cell transfusions on clinical outcomes in premature, very low-birth-weight infants: the ARIPI randomized trial. JAMA. 2012;308:1443–51. doi: 10.1001/2012.jama.11953. [DOI] [PubMed] [Google Scholar]
- 36.Heddle NM, Cook RJ, Arnold DM, et al. Effect of short-term vs. long-term blood storage on mortality after transfusion. N Engl J Med. 2016;375:1937–45. doi: 10.1056/NEJMoa1609014. [DOI] [PubMed] [Google Scholar]
- 37.Lacroix J, Hébert PC, Fergusson DA, et al. Age of transfused blood in critically ill adults. N Engl J Med. 2015;372:1410–8. doi: 10.1056/NEJMoa1500704. [DOI] [PubMed] [Google Scholar]
- 38.Steiner ME, Ness PM, Assmann SF, et al. Effects of red-cell storage duration on patients undergoing cardiac surgery. N Engl J Med. 2015;372:1419–29. doi: 10.1056/NEJMoa1414219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Carson JL, Guyatt G, Heddle NM, et al. Clinical practice guidelines from the AABB: red blood cell transfusion thresholds and storage. JAMA. 2016;316:2025–35. doi: 10.1001/jama.2016.9185. [DOI] [PubMed] [Google Scholar]
- 40.Chin-Yee IH, Chin-Yee BH, Pereira A. Clinical trials and the age of blood: ABLE but still wanting. Transfus Med Oxf Engl. 2015;25:349–50. doi: 10.1111/tme.12252. [DOI] [PubMed] [Google Scholar]
- 41.Zimring JC, Spitalnik SL. Large retrospective effects, clear differences in animals, and multiple negative RCTs: this is exactly how it is supposed to work. Blood Transfus. 2017;15:104–6. doi: 10.2450/2017.0307-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.National Heart, Lung, and Blood Institute, National Institutes of Health. 2016 Scientific Priorities in Pediatric Transfusion Medicine. [Accessed on 18/01/2017]. Available at: https://www.nhlbi.nih.gov/research/reports/2016-scientific-priorities-pediatric-transfusion-medicine. [DOI] [PMC free article] [PubMed]
- 43.Spitalnik SL, Triulzi D, Devine DV, et al. 2015 proceedings of the National Heart, Lung, and Blood Institute’s State of the Science in Transfusion Medicine symposium. Transfusion. 2015;55:2282–90. doi: 10.1111/trf.13250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liumbruno G, D’Alessandro A, Grazzini G, Zolla L. Blood-related proteomics. J Proteomics. 2010;73:483–507. doi: 10.1016/j.jprot.2009.06.010. [DOI] [PubMed] [Google Scholar]
- 45.Zimring JC. Widening our gaze of red blood storage haze: a role for metabolomics. Transfusion. 2015;55:1139–42. doi: 10.1111/trf.13071. [DOI] [PubMed] [Google Scholar]
- 46.Dzieciatkowska M, D’Alessandro A, Burke TA, et al. Proteomics of apheresis platelet supernatants during routine storage: Gender-related differences. J Proteomics. 2015;112:190–209. doi: 10.1016/j.jprot.2014.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dzieciatkowska M, D’Alessandro A, Hill RC, Hansen KC. Plasma QconCATs reveal a gender-specific proteomic signature in apheresis platelet plasma supernatants. J Proteomics. 2015;120:1–6. doi: 10.1016/j.jprot.2015.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fu X, Felcyn JR, Odem-Davis K, Zimring JC. Bioactive lipids accumulate in stored red blood cells despite leukoreduction: a targeted metabolomics study. Transfusion. 2016;56:2560–70. doi: 10.1111/trf.13748. [DOI] [PubMed] [Google Scholar]
- 49.Gevi F, D’Alessandro A, Rinalducci S, Zolla L. Alterations of red blood cell metabolome during cold liquid storage of erythrocyte concentrates in CPD-SAGM. J Proteomics. 2012;76:168–80. doi: 10.1016/j.jprot.2012.03.012. [DOI] [PubMed] [Google Scholar]
- 50.Abonnenc M, Crettaz D, Marvin L, et al. Metabolomic profiling highlights oxidative damages in platelet concentrates treated for pathogen inactivation and shows protective role of urate. Metabolomics. 2016;12:188. [Google Scholar]
- 51.Casali E, Berni P, Spisni A, et al. Hypoxanthine: a new paradigm to interpret the origin of transfusion toxicity. Blood Transfus. 2015;14:555–6. doi: 10.2450/2015.0177-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pertinhez TA, Casali E, Baroni F, et al. A comparative study of the effect of leukoreduction and pre-storage leukodepletion on red blood cells during storage. Front Mol Biosci. 2016;3:13. doi: 10.3389/fmolb.2016.00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pertinhez TA, Casali E, Lindner L, et al. Biochemical assessment of red blood cells during storage by 1H nuclear magnetic resonance spectroscopy. Identification of a biomarker of their level of protection against oxidative stress. Blood Transfus. 2014;12:548–56. doi: 10.2450/2014.0305-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nemkov T, D’Alessandro A, Hansen KC. Three-minute method for amino acid analysis by UHPLC and high-resolution quadrupole orbitrap mass spectrometry. Amino Acids. 2015;47:2345–57. doi: 10.1007/s00726-015-2019-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Paglia G, Sigurjónsson ÓE, Bordbar A, et al. Metabolic fate of adenine in red blood cells during storage in SAGM solution. Transfusion. 2016;56:2538–47. doi: 10.1111/trf.13740. [DOI] [PubMed] [Google Scholar]
- 56.van ‘t Erve TJ, Doskey CM, Wagner BA, et al. Heritability of glutathione and related metabolites in stored red blood cells. Free Radic Biol Med. 2014;76:107–13. doi: 10.1016/j.freeradbiomed.2014.07.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.van ‘t Erve TJ, Wagner BA, Martin SM, et al. The heritability of metabolite concentrations in stored human red blood cells. Transfusion. 2014;54:2055–63. doi: 10.1111/trf.12605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.van ‘t Erve TJ, Wagner BA, Martin SM, et al. The heritability of hemolysis in stored human red blood cells. Transfusion. 2015;55:1178–85. doi: 10.1111/trf.12992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dumont LJ, AuBuchon JP. Evaluation of proposed FDA criteria for the evaluation of radiolabeled red cell recovery trials. Transfusion. 2008;48:1053–60. doi: 10.1111/j.1537-2995.2008.01642.x. [DOI] [PubMed] [Google Scholar]
- 60.Chassé M, Tinmouth A, English SW, et al. Association of Blood Donor Age and Sex With Recipient Survival After Red Blood Cell Transfusion. JAMA Intern Med. 2016;176:1307–14. doi: 10.1001/jamainternmed.2016.3324. [DOI] [PubMed] [Google Scholar]
- 61.Kanias T, Sinchar D, Osei-Hwedieh D, et al. Testosterone-dependent sex differences in red blood cell hemolysis in storage, stress, and disease. Transfusion. 2016;56:2571–83. doi: 10.1111/trf.13745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tzounakas VL, Georgatzakou HT, Kriebardis AG, et al. Uric acid variation among regular blood donors is indicative of red blood cell susceptibility to storage lesion markers: A new hypothesis tested. Transfusion. 2015;55:2659–71. doi: 10.1111/trf.13211. [DOI] [PubMed] [Google Scholar]
- 63.Tzounakas VL, Georgatzakou HT, Kriebardis AG, et al. Donor variation effect on red blood cell storage lesion: a multivariable, yet consistent, story. Transfusion. 2016;56:1274–86. doi: 10.1111/trf.13582. [DOI] [PubMed] [Google Scholar]
- 64.Tzounakas VL, Kriebardis AG, Papassideri IS, Antonelou MH. Donor-variation effect on red blood cell storage lesion: a close relationship emerges. Proteomics Clin Appl. 2016;10:791–804. doi: 10.1002/prca.201500128. [DOI] [PubMed] [Google Scholar]
- 65.Kleinman S, Busch MP, Murphy EL, et al. The National Heart, Lung, and Blood Institute Recipient Epidemiology and Donor Evaluation Study (REDS-III): a research program striving to improve blood donor and transfusion recipient outcomes. Transfusion. 2014;54:942–55. doi: 10.1111/trf.12468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bordbar A, Jamshidi N, Palsson BO. iAB-RBC-283: a proteomically derived knowledge-base of erythrocyte metabolism that can be used to simulate its physiological and patho-physiological states. BMC Syst Biol. 2011;5:110. doi: 10.1186/1752-0509-5-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.D’Alessandro A, Giardina B, Gevi F, et al. Clinical metabolomics: the next stage of clinical biochemistry. Blood Transfus. 2012;10:s19–24. doi: 10.2450/2012.005S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.D’Alessandro A, Nemkov T, Sun K, et al. AltitudeOmics: red blood cell metabolic adaptation to high altitude hypoxia. J Proteome Res. 2016;15:3883–95. doi: 10.1021/acs.jproteome.6b00733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Liu H, Zhang Y, Wu H, et al. beneficial role of erythrocyte adenosine A2B receptor-mediated AMP-activated protein kinase activation in high-altitude hypoxia. Circulation. 2016;134:405–21. doi: 10.1161/CIRCULATIONAHA.116.021311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sun K, Zhang Y, D’Alessandro A, et al. Sphingosine-1-phosphate promotes erythrocyte glycolysis and oxygen release for adaptation to high-altitude hypoxia. Nat Commun. 2016;7:12086. doi: 10.1038/ncomms12086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.D’Alessandro A, Moore HB, Moore EE, et al. Early hemorrhage triggers metabolic responses that build up during prolonged shock. Am J Physiol Regul Integr Comp Physiol. 2015;308:R1034–44. doi: 10.1152/ajpregu.00030.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Doctor A, Spinella P. Effect of processing and storage on red blood cell function in vivo. Semin Perinatol. 2012;36:248–59. doi: 10.1053/j.semperi.2012.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Francis RO, Jhang JS, Pham HP, et al. Glucose-6-phosphate dehydrogenase deficiency in transfusion medicine: the unknown risks. Vox Sang. 2013;105:271–82. doi: 10.1111/vox.12068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tzounakas VL, Kriebardis AG, Georgatzakou HT, et al. Glucose 6-phosphate dehydrogenase deficient subjects may be better “storers” than donors of red blood cells. Free Radic Biol Med. 2016;96:152–65. doi: 10.1016/j.freeradbiomed.2016.04.005. [DOI] [PubMed] [Google Scholar]